Introduction
Approximately 5.23–11.04% of newly diagnosed type 2
diabetes mellitus cases are complicated by cerebrovascular
disorders (1). Currently, CI is
divided into symptomatic and silent cerebral infarction (SCI)
according to clinical manifestations. Clinically, CI often occurs
after transient ischemic attack (TIA) and shows no obvious symptoms
or signs due to the small territory of infarction or involvement
outside areas associated with easily observable functional
manifestations. Thus, SCI is often misdiagnosed or missed in
routine examination. However, SCI may be detected by brain computed
tomography (CT) or magnetic resonance imaging (MRI) (2,3). Recurrent
SCI may cause cognitive decline, symptomatic cerebral infarction,
vascular dementia or Parkinsonism, affecting patient quality of
life and increasing family and social burdens (4).
The inhibition of dipeptidyl peptidase-IV (DPP-IV)
and increase of the activity of endogenous glucagon-like peptide-1
are new therapeutic targets of diabetes mellitus. DPP-IV inhibitors
have biological effects for reducing the incidence of ischemic
cerebral infarction and improving the cognitive function, in
addition to a hypoglycemic effect (5,6). However,
there is no study on the role of DPP-IV inhibitor alogliptin in the
treatment of diabetes nephropathy (DN) complicated with silent
cerebral infarction. Hyperbaric oxygen is one of the effective
methods to treat cerebral infarction (7). However, due to the special therapeutic
environment, patient compliance is poor and the curative effect is
easily affected. It was reported that stroke patients who received
motor imagery training reached a certain degree of athletic and
cognitive rehabilitation (8).
Thus, the present study is focused on whether
alogliptin in combination with motor imagery in a hyperbaric oxygen
chamber can effectively reduce blood glucose, overcome the
aforementioned shortcomings and improve the function impairment of
DN patients complicated with SCI.
Materials and methods
Patients
We enrolled 200 patients who where newly diagnosed
with diabetic nephropathy (DN) from the Chongqing General Hospital
and the First Affiliated Hospital of Chongqing Medical
University.
DN was diagnosed clinically if one or more of the
following criteria were fulfilled: i) Histological diagnosis by
renal biopsy; ii) presence of diabetic retinopathy; and iii)
history of type 2 diabetes mellitus at least 3 years before
enrollment. All participants had a glomerular filtration rate
(eGFR) of >30 ml/min/1.73 m2. The patients were
divided into the SCI group and without SCI (NSCI) group according
to radiological data and clinical features. The SCI group patients
were divided into two treatment groups: Alogliptin (group A, n=50)
and alogliptin combined with motor imagery under hyperbaric oxygen
(group B, n=50). Diagnostic criteria of SCI (9,10) were: i)
No neurological symptom or sign; ii) low signals on MRI T1WI and
high signals on T2WI, lesion diameter of >3 mm and no high
signal on MRI DWI. Inclusion criteria for the SCI group were: i)
Daily life and social activities were not obviously affected; ii)
SCI lesion was confirmed by MRI and no encephalatrophy was noted;
iii) the patients were right-handed.
Exclusion criteria were: i) Patients with a definite
history of cerebral infarction and dementia; ii) patients with a
definite history of other central nervous system diseases such as
infection and demyelinating disease; iii) patients with severe
physical diseases; iv) patients with a definite history of mental
and psychological diseases such as schizophrenia and major
depression; v) alcohol or drug addicts; vi) patients unable to
complete the scale of cognitive function due to severe diseases or
physical disabilities; vii) patients with allergic constitution;
viii) patients with active bleeding or bleeding tendency; ix)
patients on non-steroid anti-inflammatory drugs, glucocorticoids,
thyroid hormone tablets and other drugs; and x) patients with liver
disease. NSCI patients were those without cerebral infarction or
patients with symptomatic cerebral infarction. After the screening
period, patients received DPP-IV inhibitor alogliptin (25 mg once
daily; Takeda Pharmaceutical Company Ltd., Japan), in addition to
continuing their anticoagulation background treatment. The patients
in group B underwent motor imagery in the hyperbaric oxygen chamber
after taking alogliptin. Informed consent was obtained from all the
patients. The protocol was registered with the Chinese Clinical
Trial Registry (ChiCTR-INR-17012590). The hospital Ethics Committee
had reviewed our study.
Treatment of motor imagery in the
hyperbaric oxygen chamber
A hyperbaric oxygen chamber (Haux-Life-Support GmbH,
Baden-Wurttemberg, Germany) for 34 patients was used. At the
pressure of 0.2 MPa, the patients inhaled pure oxygen using a mask
for 30 min and rested for 10 min between two episodes of oxygen
inhalation. The pressure was increased and pressured at a uniform
speed for 15 min. The hyperbaric oxygen therapy was performed once
a day and 10 days were a course of treatment. There was an interval
of 5 days between each course of treatment. The patients were
required to perform intermittent motor imagery (11,12) during
the hyperbaric oxygen therapy. After the motor imagery, the
patients were asked to open their eyes, move their extremities and
joints following the rhythms of music and carry out alternative
muscular tension-relaxation activities for 5–10 min.
Neurocognitive dysfunction degree and
clinical curative effect evaluation
Two physicians carried out the evaluation according
to the National Institutes of Health Stroke Scale (NIHSS) (13). Scores of 0–1 were considered normal or
near-normal range and 1–4 were considered mild. Scores of 5–15 were
considered moderate; 15–20 were considered moderate-severe; and
21–42 were severe. Evaluation criteria of the clinical curative
effect were: Basic cure (decrease of scores >90%), significant
change (decrease of scores 46–89%), change (decrease of scores
18–45%), no change (decrease of scores ≤17%) and deterioration
(scores increase). When the scores were reduced by >18%, it
indicated that the treatment was effective. The total effective
rate was calculated as: (cases of basic cure + cases of significant
change + cases of change)/total cases × 100%.
Montreal cognitive assessment (MoCA) (14) was performed from eight aspects of
visuospatial/executive ability, attention, memory, language,
abstraction, naming, calculation and orientation. The scale was
completed within 10 min and the total scores were 30. Scores of
>26 were considered normal cognition. In case of the education
years being ≤12 year, the scores were added to be 1.
Thromboelastograms (TEGs) mapping
Blood samples were collected from the antecubital
vein and tested with Thrombelastograph Analyzer TEG-500 (15). The tested parameters included: i)
Reaction time (R), which indicated the time from the test start to
the formation of the clot or fibrin. Normal R values ranged between
5 and 10 min; ii) clotting time (K), considered from the clotting
start to the time when TEG amplitude reached 20 mm. Normal K values
ranged between 1 and 3 min; iii) α-angle, which was the angle
between the tangent line and the horizontal line of the maximum
curve when TEG reached the maximum curve from clot formation,
representing the speed of clot formation and the speed of
hemocoagulase formation. Normal α-angle was between 53 and 72°; iv)
maximum amplitude (MA), which was the maximum solidness of
thrombus, reflecting the absolute clot intensity. Normal values
were between 50 and 70 mm; v) clot strength (G): G value was a
measure of clot strength or clot firmness, and was calculated based
on the amplitude value until the MA was reached. Normal G values
were between 4.6 and 11 kDa; vi) coagulation index (CI): −3<
normal <+3; index <-3 indicated low coagulation; index >-3
indicated high coagulation. If two or more of the following
conditions were met, it was diagnosed as hypercoagulable state: R
and K were shortened and α-angle and/or MA were increased.
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qRCR) detection glycoprotein VI
(GPVI) mRNA expression
Total RNA was extracted with TRIzol (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's instructions.
For RT-qPCR, cDNA was synthesized using the PrimeScript RT-PCR kit
(Takara Bio, Inc.). The primers (Shanghai Biotechnology, Shanghai,
China) used to detect human GPVI and β-actin (internal control)
were: GPVI forward, 5′-GCCAAGCTATTGCGACATGA-3′ and reverse,
5′-AAAAGAATCTCAATGTCCGAGACTTT-3′; β-actin forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The cDNAs were amplified using the
following thermal cycle: Denaturation at 95°C for 1 min, followed
by 35 cycles of denaturation at 95°C for 30 sec, annealing for 1
min at 58°C, polymerization for 1 min at 70°C, and brief detection
at 70°C. The signal was expressed relative to β-actin (relative
ratio = average copy number of target gene in the sample/average
copy number of β-actin).
Enzyme-linked immunosorbent assay
(ELISA) detection levels of 11-DH-TXB2
The production of 11-DH-TXB2 in the urine
after 24 h (overnight) of treatment was quantified with ELISA
detection kits (Quantikine; R&D Systems, Abingdon, UK)
according to the manufacturer's instructions. Optical densities
were measured at 450 nm. Each assay was performed in
triplicate.
Statistical analysis
Data were analyzed with SPSS v23.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Values are expressed as
mean ± standard deviation throughout the text and figures, and the
enumeration data were expressed by Chi-square test. Comparison
among groups was conducted by t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Basic clinical characteristics of
patients
Among DN patients, there was no significant
difference in age, sex and risk factors (history of smoking, atrial
fibrillation, hyperlipidemia, hypertension, duration of diabetes
and HbA1c) between the SCI and NSCI groups (P>0.05). However,
there was significant difference in homeostasis model assessment
insulin resistance (HOMA-IR), homeostasis model assessment for β
cell function (HOMA-β) between the SCI and NSCI groups (P<0.05).
IMT, fibrinogen (FIB), and body fat mass of the NSCI group were
lower than those of the SCI group with significant inter-group
difference (P<0.05). Ankle brachial index (ABI) of the NSCI
group was higher than that of SCI group, also with significant
inter-group difference (P<0.05) (Table
I).
 | Table I.Basic clinical and laboratory
characteristics of diabetic nephropathy with and without SCI. |
Table I.
Basic clinical and laboratory
characteristics of diabetic nephropathy with and without SCI.
| Characteristics | NSCI group
(n=100) | SCI group
(n=100) | P-value |
|---|
| Age (years) | 61±5 | 62±4 | 0.793 |
| Sex
(male:female) | 51:49 | 50:50 | 0.886 |
| Smoking | 14 (14%) | 15 (15%) | 0.852 |
| Hyperlipidemia | 8 (8%) | 10 (10%) | 0.847 |
| Atrial
fibrillation | 5 (5%) | 7 (7%) | 0.765 |
| Systolic pressure
(mmHg) | 131.66±4.52 | 133.00±5.04 | 0.694 |
| Diastolic pressure
(mmHg) | 79.92±4.69 | 81.20±3.88 | 0.703 |
| Duration of
diabetes (year) | 3.70±0.80 | 3.50±1.10 | 0.778 |
| Duration of
diabetic nephropathy (year) | 0.50±0.10 |
1.10±0.00a | 0.049 |
| Intima-media
thickness (mm) | 1.18±0.06 |
1.41±0.08a | 0.040 |
| HOMA-IR | 3.85±0.72 |
5.86±0.51a | 0.045 |
| HOMA-β | 36.50±2.42 |
28.60±5.36a | 0.043 |
| Glycosylated
hemoglobin A1c (%) | 6.81±0.25 | 7.09±0.05 | 0.848 |
| Fibrinogen | 3.32±0.38 |
4.66±0.35a | 0.048 |
| ABI | 0.91±0.06 |
0.55±0.07a | 0.049 |
| Body fat mass
(%) | 26.10±4.09 |
31.80±4.10a | 0.032 |
MRI features and clinical
manifestation
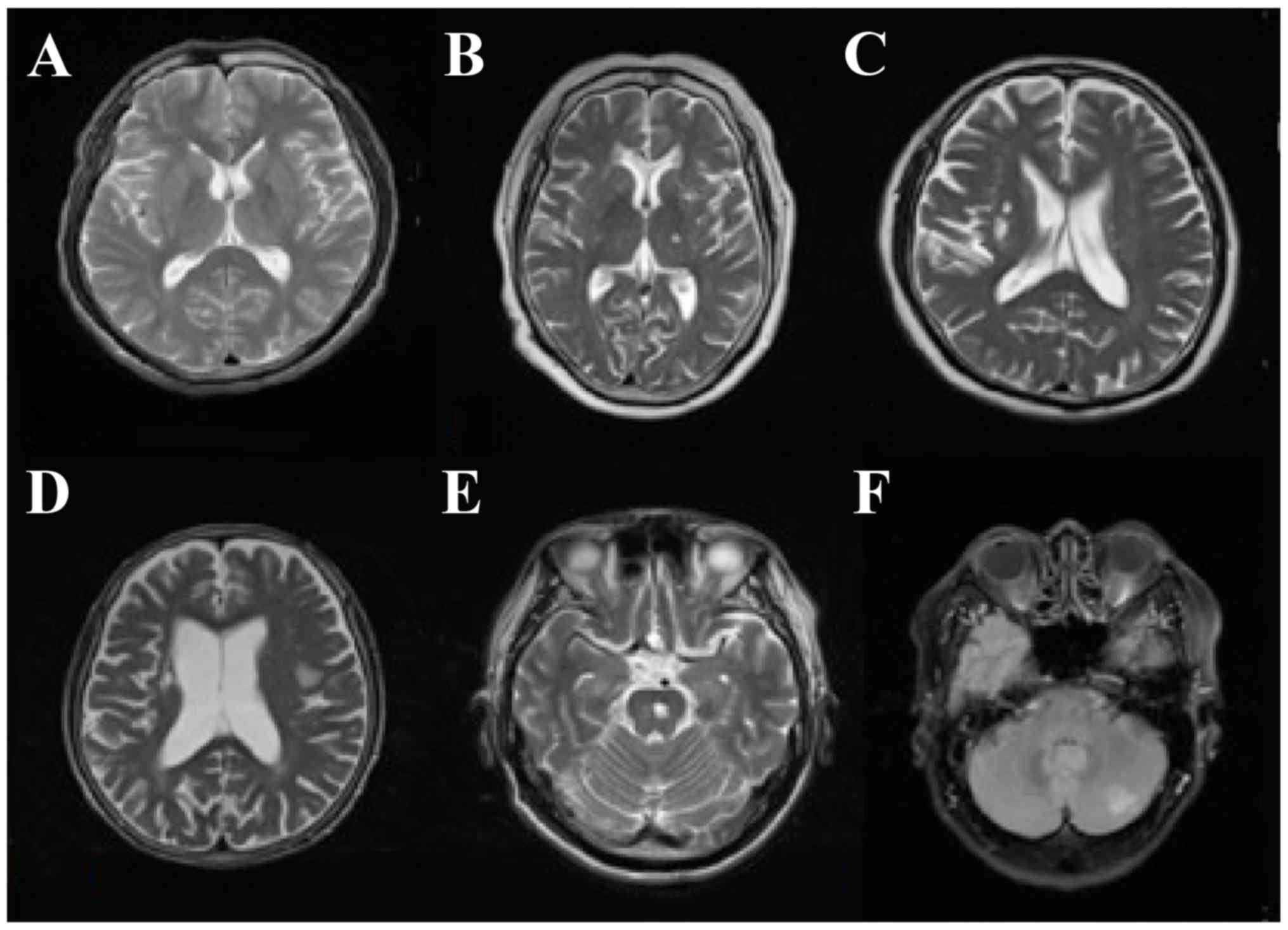
The infarction foci of DN patients with SCI
presented as different sizes and shapes. Among SCI patients, 30
patients had lacunar infarction (diameter ≤20 mm), 22 had a single
lesion and 28 had multiple lesions. The infarction foci of 28
patients were identified in the basal ganglia region and others
were evident in the internal capsule, ventricle, thalamus, left
temporal lobe, radial crown, bridge brain and insular lobe.
Generally, SCI patients had no focal neurological signs.
Non-specific signs included headache, dizziness, fatigue, impaired
memory, slow response and subjective feeling and limb numbness
(Fig. 1).
Neurocognitive dysfunction degree
score and clinical curative effect
Compared with the NSCI group, the neurological
deficit scores of the SCI group were increased (4.43±1.12 vs.
8.48±3.69, P<0.05). Before treatment, the comparison between
groups A and B showed no significant difference in neurological
deficit scores (8.47±3.72 vs. 8.49±3.68, P>0.05). Six months
after treatment, the neurological deficit scores of group B were
lower than those of group A, with a significant difference
(6.88±2.20 vs. 4.58±2.17, P<0.05). The inter-group compared
before and after treatment showed that the neurological deficit was
improved (P<0.05). As shown in Table
II, the curative effect of group B was better than that of
group A (P<0.05).
 | Table II.Clinical curative effect of SCI
patients between the treatment groups. |
Table II.
Clinical curative effect of SCI
patients between the treatment groups.
| Groups | n | Basic cure | Significant
change | Change | No change | Deterioration | Total effective
rate (%) |
|---|
| A | 50 | 4 | 18 | 8 | 18 | 2 | 60 |
| B | 50 | 9 | 26 | 6 | 9 | 0 | 82 |
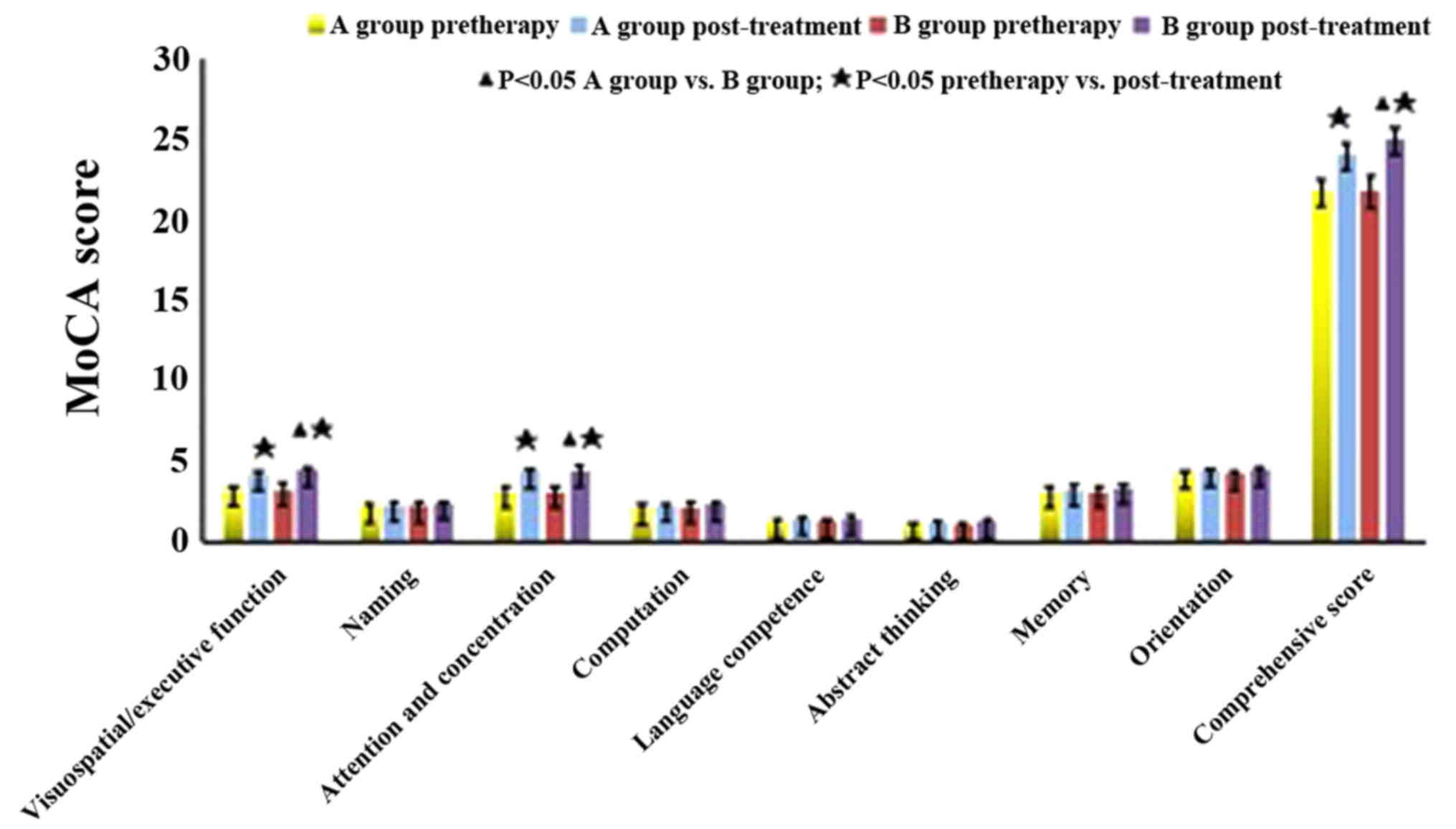
MoCA score
Compared with the NSCI group, the MoCA scores of the
SCI group were decreased (P<0.05). As shown in Table III, before treatment, the comparison
between groups A and B showed no significant difference in MoCA
scores (P>0.05). MoCA scores of group B were higher than those
of group A after a 6-month treatment. The subscores of
visuospatial/executive function, attention and concentration and
comprehensive score were significantly increased (P<0.05), while
the subscores of computation, abstract thinking, language
competence, memory, orientation and memory were also increased,
albeit the difference was not statistically significant (P>0.05)
(Fig. 2).
 | Table III.Comparison of Montreal cognitive
assessment score between treatment groups of diabetic nephropathy
with SCI. |
Table III.
Comparison of Montreal cognitive
assessment score between treatment groups of diabetic nephropathy
with SCI.
| Groups | Treatment |
Visuospatial/executive ability | Naming | Attention | Calculation | Language | Abstraction | Memory | Orientation | Comprehensive
score |
|---|
| A (n=50) | Pretherapy |
3.20±0.26 |
2.21±0.19 |
3.15±0.27 |
2.12±0.23 |
1.29±0.10 |
1.11±0.06 |
3.11±0.36 |
4.26±0.17 |
21.80±0.69 |
|
| Post-treatment |
4.20±0.21b |
2.31±0.15 |
4.35±0.22b |
2.28±0.11 |
1.41±0.13 |
1.22±0.11 |
3.27±0.22 |
4.40±0.15 |
24.06±0.72b |
| B (n=50) | Pretherapy |
3.21±0.40 |
2.20±0.18 |
3.13±0.32 |
2.13±0.28 |
1.28±0.15 |
1.10±0.05 |
3.10±0.35 |
4.26±0.15 |
21.78±0.92 |
|
| Post-treatment |
4.48±0.20a,b |
2.35±0.16 |
4.43±0.28a,b |
2.34±0.10 |
1.46±0.16 |
1.24±0.13 |
3.38±0.18 |
4.45±0.12 |
25.02±0.66a,b |
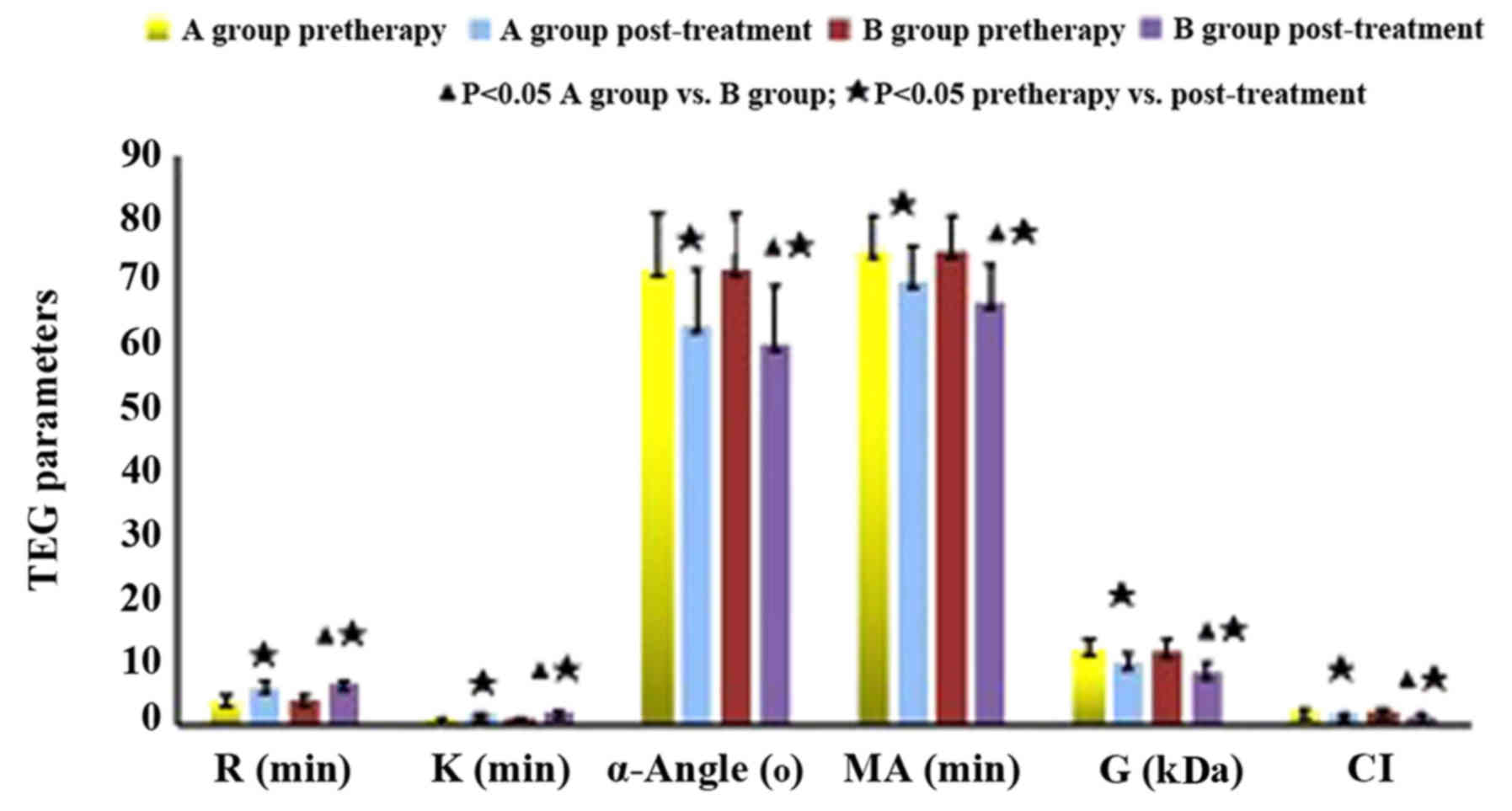
TEGs
As shown in Fig. 3,
there was no statistical significance of single and multiple
lesions in SCI patients (P>0.05). Before treatment, the
decreased R and K values, the increased MA value, α-angle and G
value as well as the prolonged CI indicated DN patients with silent
cerebral infarction were in an hypercoagulable state (Table IV). Compared with those before
treatment, the TEG indexes of both groups after treatment were
improved, manifesting as increased R and K values, decreased MA
value, α-angle and G, and shortened CI. The comparison between
groups A and B after treatment showed significant difference
(P<0.05) (Table V).
 | Table IV.Comparison of TEG parameters of
single lesion and multiple lesions in SCI patients. |
Table IV.
Comparison of TEG parameters of
single lesion and multiple lesions in SCI patients.
| Parameters | R (min) | K (min) | α-angle (°) | MA (mm) | G (kDa) | CI |
|---|
| Single lesion |
5.55±1.53 |
1.52±0.82 |
68.92±11.0 |
72.07±5.71 |
11.60±1.74 |
2.20±0.90 |
| Multiple
lesions |
5.83±1.39 |
1.57±0.68 |
69.89±9.63 |
73.35±5.32 |
11.43±2.01 |
2.04±0.96 |
 | Table V.Comparison of TEG parameters between
treatment groups of diabetic nephropathy with SCI. |
Table V.
Comparison of TEG parameters between
treatment groups of diabetic nephropathy with SCI.
| Groups | Treatment | R (min) | K (min) | α-angle (°) | MA (mm) | G (kDa) | CI |
|---|
| A (n=50) | Pretherapy |
4.04±0.82 |
1.20±0.19 |
72.15±8.91 |
75.06±5.46 |
12.30±1.42 |
2.93±0.52 |
|
| Post-treatment |
6.20±0.84b |
1.96±0.23b |
63.22±9.04b |
70.40±5.35b |
10.12±1.38b |
1.85±0.32b |
| B (n=50) | Pretherapy |
4.05±0.86 |
1.21±0.20 |
72.00±9.00 |
75.00±5.62 |
11.93±1.67 |
2.94±0.50 |
|
| Post-treatment |
6.78±0.32a,b |
2.28±0.19a,b |
60.38±9.21a,b |
66.90±5.93a,b |
8.62±1.62a,b |
1.56±0.39a,b |
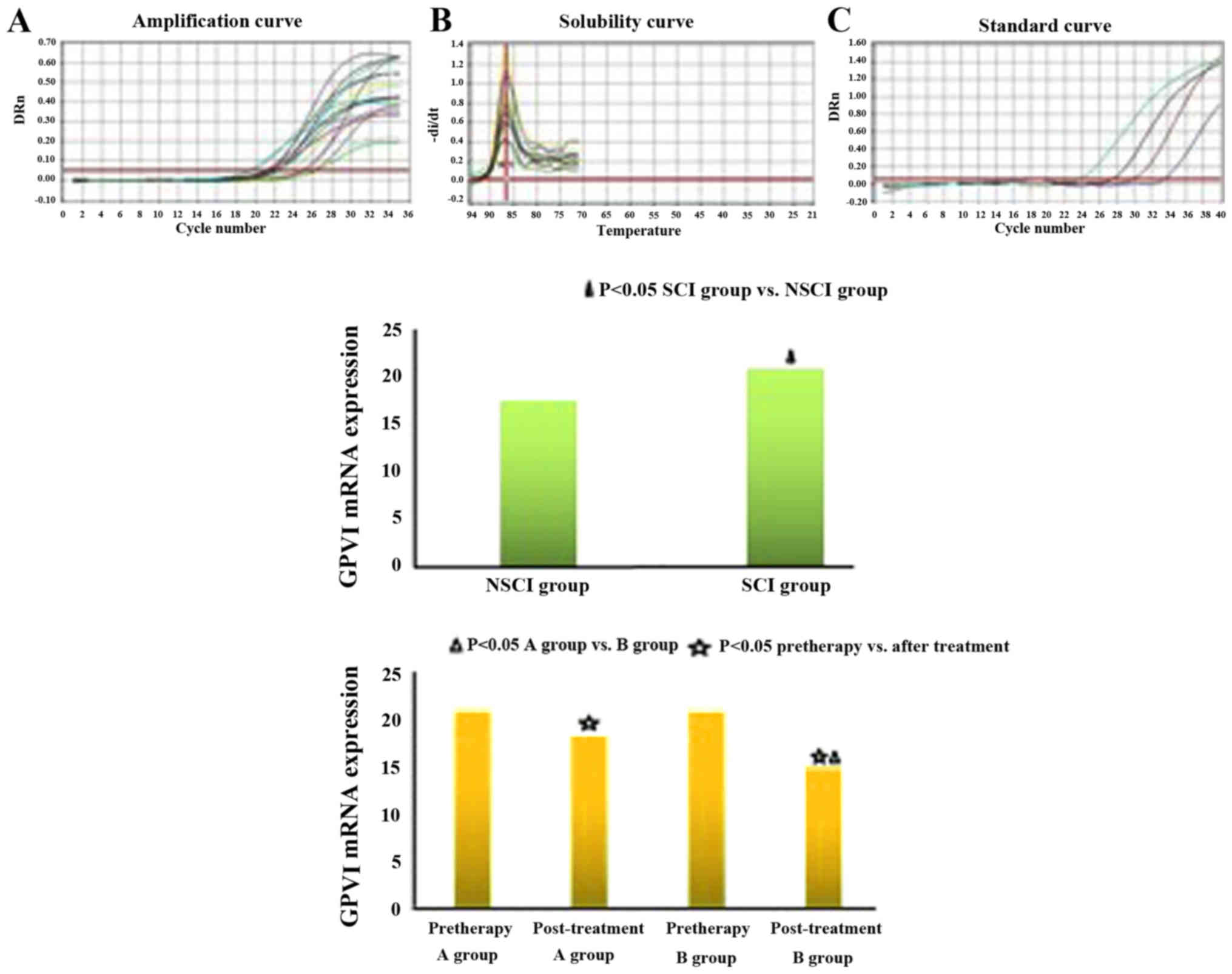
mRNA expression of GPVI and level of
11-DH-TXB2
Compared with the NSCI group, GPVI expression and
11-DH-TXB2 level of the SCI group were increased (GPVI:
17.50±4.50 vs. 20.86±4.62, P<0.05; 11-DH-TXB2:
43.21±6.38 vs. 58.39±7.22, P<0.05). Before treatment, the
comparison between groups A and B showed no significant difference
in GPVI expression and 11-DH-TXB2 levels (GPVI:
20.91±4.83 vs. 20.80±4.66, P>0.05; 11-DH-TXB2:
58.55±7.15 vs. 58.28±7.34, P>0.05). Six months after treatment,
the GPVI expression and 11-DH-TXB2 level of group B were
lower than those of group A, with a significant difference (GPVI:
18.11±4.26 vs. 14.81±4.08, P<0.05; 11-DH-TXB2:
46.80±6.69 vs. 39.30±6.25, P<0.05). As shown in the inter-group
comparison before and after treatment, the difference of GPVI
expression and 11-DH-TXB2 level was statistically
significant (P<0.05) (Fig. 4).
Discussion
DN is one of the independent risk factors for
cerebral infarction. The clinical manifestations of cerebral
infarction perform as SCI and recurrent transient ischemic attack.
SCI is short for silent cerebral infarction and it is known as
occult cerebral infarction or subclinical stroke (16,17). It is a
particular type of cerebral infarction and the most common SCI is
lacunar infarction, which has a small range of cerebral infarction
or involved cerebral tissues far from the functional area (18,19). The
abovementioned study results are consistent with those obtained in
the present study.
The prevention and treatment of DN complicated with
SCI has become a research hotspot. Incretin drugs are new
hypoglycemic drugs, mainly including glucagon-like peptide 1 (GLP1)
receptor agonist and DPP-IV inhibitors. DPP-IV inhibitors have
other biological effects besides a hypoglycemic effect. The
combination of incretin drugs and GLP-1R may activate adenylate
cyclase, upregulate cyclic adenosine monophosphate levels, trigger
Ca2+ inflow, enhance synaptic plasticity, increase
neurotransmitter release and improve learning, memory and cognitive
function (20,21). Hyperbaric oxygen therapy may increase
the oxygen saturation of patients, reduce intracranial pressure,
relieve inflammatory reaction and edema degree, decrease infarction
size (22), use ‘reverse steal
phenomenon’ to increase blood and oxygen supply in the focal area,
increase the mitochondrial membrane permeability of brain cells,
reduce neutrophil apoptosis and promote neural functional recovery
and regeneration (23). Thus, it is an
effective means to treat cerebral infarction. However, patients may
generate fear, anxiety irritability and other negative emotions
during the treatment due to the closed environment. Therefore, we
utilized the DPP-IV inhibitor alogliptin in combination with motor
imagery in a hyperbaric oxygen chamber to treat DN patients with
SCI. Motor imagery may be used to reinforce and improve exercise
plans, enhance sensory information input, promote active latent
pathways and dormant synapse, accelerate reperfusion of ischemic
penumbra and improve cerebral blood flow by repeatedly simulating
and rehearsing exercises in heart without obvious body movement
according to the exercise plans stored in a certain active brain
area in order to repair the neurologic impairment to a certain
degree (24,25).
The Montreal cognitive assessment (MoCA) is more
sensitive in testing mild vascular cognitive impairment than MMSE
(26–28). In the present study, the neurocognitive
dysfunction of SCI patients was more severe than that of NSCI
patients. The naming and language functioning capabilities of SCI
patients were lower than those of NSCI patients, but the difference
had no statistical significance. It indicates that it is rare for
SCI patients to develop language dysfunction at an early stage. The
neurologic dysfunction of patients in group B was significantly
lower than those of patients in group A, while MoCA scores and
clinical effectiveness were higher than group A. In MoCA scale, the
combined treatment with alogliptin and motor imagery in hyperbaric
oxygen was excellent regarding the function of
visuospatial/executive, attention and concentration, which has much
better improvement on neurocognitive function.
Previous findings have show that the platelet
adhesion, aggregation and release of diabetic patients are
enhanced, thus the risk of thrombosis is increased (29). Moreover, the platelets significantly
change before and after the onset of cerebral infarction. Thus, the
abnormality of platelet function may induce cerebral infarction.
The changes in platelet count and platelet volume may be important
factors for inducing cerebral infarction. The more severe the
conditions and the larger the infarction area, the more obvious the
change in platelet parameters (30,31). In our
preliminary study, in comparison with the NSCI group, the platelet
count of SCI group decreased while the platelet distribution width
increased (32). Poor blood glucose
control may lead to the increase of HbA1c, histanoxia, the
glycation of plasma, low-density lipoprotein-cholesterol,
fibrinogen and platelets, the increase of blood glucose viscosity
and free radical generation and the acceleration of
atherosclerosis, which may induce cerebral infarction (33).
We also studied the evaluation of platelet function
in patients by TEG and the secretion of platelet-associated
cytokines. The mechanism of TEG occurs throughout the whole
coagulation process, from coagulation to fibrinolysis. It is mainly
applied to detect blood coagulation disorder and can
comprehensively reflect the physical features of blood clot
(including the rate of blood clot formation as well as the strength
and stability of blood clot). Thus, it is an effective method to
test coagulation changes of patients, and is characterized by small
blood use, simple operation, short testing time and permanent
preservation. Thus, it can be used to guide treatment and judge
curative effect in clinic. In our study, SCI patients experienced a
hypercoagulable state, and the combined treatment with alogliptin
improved this hypercoagulable state.
GPVI is a platelet collagen receptor, that plays a
key role in mediating signal transduction in platelets after
collagen adhesion, activating GPIa-IIa and GPIIb-IIIa, promoting
activity expression as well as accelerating platelet adhesiveness
and thrombosis. GPVI levels of patients with acute coronary
syndrome were higher than those of patients with stable angina
pectoris (34,35). The monitoring of GPVI levels in
platelets may predict the risk for thrombosis (36,37).
11-DH-TXB2 is mainly from thromboxane A generated by
platelets and is not formed in vitro. The content of
11-DH-TXB2 is stable in urine. Urine
11-DH-TXB2 is the best index for reflecting the release
level of TXA2 in vivo, which may indirectly reflect the
levels of platelet aggregation and activation (38). We found that the combined treatment of
alogliptin and motor imagery in hyperbaric oxygen chamber can
better promote thrombolysis. The GPVI expression and
11-DH-TXB2 level of combined treatment group B were
significantly lower than those of group A.
In conclusion, the condition assessment and
management of SCI patients greatly affects prognosis. SCI patients
should have early prevention and early diagnosis. In particaular,
diabetic patients with smoking, hyperlipidemia, hypertension,
atrial fibrillation and carotid artery stenosis should regularly be
followed-up irrespective of whether the desease is complicated with
SCI. The treatment of DPP-IV inhibitor alogliptin combined with
motor imagery in hyperbaric oxygen can better promote thrombolysis
absorption, restore brain damage and improve neurocognitive
dysfunction in DN patients with SCI. However, our study also has
disadvantages. Due to small sample size, the curative effect on SCI
patients at different infraction sites is not compared. The
aforementioned disadvantages will be deeply discussed in subsequent
studies in order to provide more scientific theoretical basis for
the prevention and treatment of silent cerebral infraction in
clinic.
Acknowledgements
We thank Mrs. Weixue Tang for technical assistance.
She is work in the departments of Pathophysiology, Basic Medical
College, Chongqing Medical University.
References
|
1
|
Dedov II and Shestakova MV: Cerebral
vascular lesions in diabetes mellitus: Solved and unresolved
questions. Zh Nevrol Psikhiatr Im S S Korsakova. 115:79–82.
2015.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yi C, Liu W and Zhang Y: Correlation
between metabolic syndrome and silent cerebral infarction: A
cross-sectional study. J Third Mil Med Univ. 33:408–410. 2011.
|
|
3
|
Hashimoto M, Takashima Y, Uchino A,
Yuzuriha T and Yao H: Dual task walking reveals cognitive
dysfunction in community-dwelling elderly subjects: The Sefuri
brain MRI study. J Stroke Cerebrovasc Dis. 23:1770–1775. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacquin A, Binquet C, Rouaud O,
Graule-Petot A, Daubail B, Osseby GV, Bonithon-Kopp C, Giroud M and
Béjot Y: Post-stroke cognitive impairment: High prevalence and
determining factors in a cohort of mild stroke. J Alzheimers Dis.
40:1029–1038. 2014.PubMed/NCBI
|
|
5
|
Ma M, Hasegawa Y, Koibuchi N, Toyama K,
Uekawa K, Nakagawa T, Lin B and Kim-Mitsuyama S: DPP-4 inhibition
with linagliptin ameliorates cognitive impairment and brain atrophy
induced by transient cerebral ischemia in type 2 diabetic mice.
Cardiovasc Diabetol. 14:542015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan SY, Ou SM, Chen YT and Shih CJ:
Effects of DPP-4 inhibitors on cardiovascular outcomes in patients
with type 2 diabetes and end-stage renal disease. Int J Cardiol.
218:170–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen CH, Chen SY, Wang V, Chen CC, Wang
KC, Chen CH, Liu YC, Lu KC, Yip PK, Ma WY, et al: Effects of
repetitive hyperbaric oxygen treatment in patients with acute
cerebral infarction: A pilot study. ScientificWorldJournal.
2012:6947032012.doi: 10.1100/2012/694703. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan J, Guo X, Jin Z, Sun J, Shen L and
Tong S: Cognitive alterations in motor imagery process after left
hemispheric ischemic stroke. PLoS One. 7:e429222012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu YC, Dufouil C, Tzourio C and Chabriat
H: Silent brain infarcts: A review of MRI diagnostic criteria.
Stroke. 42:1140–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Auriel E, Westover MB, Bianchi MT, Reijmer
Y, Martinez-Ramirez S, Ni J, Van Etten E, Frosch MP, Fotiadis P,
Schwab K, et al: Estimating total cerebral microinfarct burden from
diffusion-weighted imaging. Stroke. 46:2129–2135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Page SJ: Mental practice: A promising
restorative technique in stroke rehabilitation. Top Stroke Rehabil.
8:54–63. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petit LS, Pegna AJ, Mayer E and Hauert CA:
Representation of anatomical constraints in motor imagery: Mental
rotation of a body segment. Brain Cogn. 51:95–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bushnell CD, Johnston DC and Goldstein LB:
Retrospective assessment of initial stroke severity: Comparison of
the NIH Stroke Scale and the Canadian Neurological Scale. Stroke.
32:656–660. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt WP, Roesler A, Kretzschmar K,
Ladwig KH, Junker R and Berger K: Functional and cognitive
consequences of silent stroke discovered using brain magnetic
resonance imaging in an elderly population. J Am Geriatr Soc.
52:1045–1050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yatsu FM and Shaltoni HM: Implications of
silent strokes. Curr Atheroscler Rep. 6:307–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nasreddine ZS, Phillips NA, Bédirian V,
Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H:
The Montreal Cognitive Assessment, MoCA: A brief screening tool for
mild cognitive impairment. J Am Geriatr Soc. 53:695–699. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akay OM, Ustuner Z, Canturk Z, Mutlu FS
and Gulbas Z: Laboratory investigation of hypercoagulability in
cancer patients using rotation thrombelastography. Med Oncol.
26:358–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohnishi J and Kudo Y: Clinical aspects of
silent cerebral infarction - MRI findings and its distribution.
Rinsho Shinkeigaku. 31:610–615. 1991.(In Japanese). PubMed/NCBI
|
|
19
|
Shintani S, Shiigai T and Arinami T:
Silent lacunar infarction on magnetic resonance imaging (MRI): Risk
factors. J Neurol Sci. 160:82–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mabuchi T, Kitagawa K, Kuwabara K,
Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M and
Matsumoto M: Phosphorylation of cAMP response element-binding
protein in hippocampal neurons as a protective response after
exposure to glutamate in vitro and ischemia in vivo. J Neurosci.
21:9204–9213. 2001.PubMed/NCBI
|
|
21
|
Gault VA, Lennox R and Flatt PR:
Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves
recognition memory, oxidative stress and hippocampal neurogenesis
and upregulates key genes involved in cognitive decline. Diabetes
Obes Metab. 17:403–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
James PB: Hyperbaric oxygenation in fluid
microembolism. Neurol Res. 29:156–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nazario J and Kuffler DP: Hyperbaric
oxygen therapy and promoting neurological recovery following nerve
trauma. Undersea Hyperb Med. 38:345–366. 2011.PubMed/NCBI
|
|
24
|
Gaggioli A, Meneghini A, Morganti F,
Alcaniz M and Riva G: A strategy for computer-assisted mental
practice in stroke rehabilitation. Neurorehabil Neural Repair.
20:503–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walbruch B and Kalliainen L: The
optimization of peripheral nerve recovery using cortical
reorganization techniques: A retrospective study of wrist level
nerve repairs. J Hand Ther. 28:341–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pendlebury ST, Cuthbertson FC, Welch SJ,
Mehta Z and Rothwell PM: Underestimation of cognitive impairment by
Mini-Mental State Examination versus the Montreal Cognitive
Assessment in patients with transient ischemic attack and stroke: A
population-based study. Stroke. 41:1290–1293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai JC, Chen CW, Chu H, Yang HL, Chung
MH, Liao YM and Chou KR: Comparing the sensitivity, specificity,
and predictive values of the Montreal Cognitive Assessment and
Mini-Mental State Examination when screening people for mild
cognitive impairment and dementia in Chinese population. Arch
Psychiatr Nurs. 30:486–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lees RA, Hendry Ba K, Broomfield N, Stott
D, Larner AJ and Quinn TJ: Cognitive assessment in stroke:
Feasibility and test properties using differing approaches to
scoring of incomplete items. Int J Geriatr Psychiatry. Aug
16–2016.(Epub ahead of print). PubMed/NCBI
|
|
29
|
Yamasaki Y, Kim YS and Kawamori R:
Rationale and protocol of a trial for prevention of diabetic
atherosclerosis by using antiplatelet drugs: Study of Diabetic
Atherosclerosis Prevention by Cilostazol (DAPC study). Cardiovasc
Diabetol. 5:162006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JH, Kwon KY, Yoon SY, Kim HS and Lim
CS: Characteristics of platelet indices, neutrophil-to-lymphocyte
ratio and erythrocyte sedimentation rate compared with C reactive
protein in patients with cerebral infarction: A retrospective
analysis of comparing haematological parameters and C reactive
protein. BMJ Open. 4:e0062752014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du J, Wang Q, He B, Liu P, Chen JY, Quan H
and Ma X: Association of mean platelet volume and platelet count
with the development and prognosis of ischemic and hemorrhagic
stroke. Int J Lab Hematol. 38:233–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen D, Huang X, Lu S, Deng H, Gan H, Du X
and Weixue T: RBP4/Lp-PLA2/Netrin-1 signaling regulation
of cognitive dysfunction in diabetic nephropathy complicated with
silent cerebral infarction. Exp Clin Endocrinol Diabetes. Jul
13–2017.(Epub ahead of print).
|
|
33
|
Dahl A, Lund C and Russell D:
Atherosclerosis and cerebral infarction. Tidsskr Nor Laegeforen.
127:892–896. 2007.PubMed/NCBI
|
|
34
|
Samaha FF, Hibbard C, Sacks J, Chen H,
Varello MA, George T and Kahn ML: Density of platelet collagen
receptors glycoprotein VI and alpha2beta1 and prior myocardial
infarction in human subjects, a pilot study. Med Sci Monit.
11:CR224–CR229. 2011.
|
|
35
|
Kassaian SE, Fathi Y, Lotfi-Tokaldany M,
Salarifar M, Alidoosti M, Haji-Zeinali AM, Aghajani H, Amirzadegan
A, Nozari Y, Mortazavi SH, et al: Comparison of 1-year major
adverse cardiac events in patients undergoing primary percutaneous
coronary intervention receiving intracoronary bolus only versus
intracoronary bolus plus infusion of glycoprotein IIb/IIIa
inhibitors. Crit Pathw Cardiol. 15:89–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Motovska Z, Kvasnicka J, Widimsky P, Petr
R, Hajkova J, Bobcikova P, Osmancik P, Odvodyova D and Katina S:
Platelet glycoprotein GP VI 13254C allele is an independent risk
factor of premature myocardial infarction. Thromb Res. 125:e61–e64.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ali-Hasan-Al-Saegh S, Mirhosseini SJ,
Shahidzadeh A, Rahimizadeh E, Sarrafan-Chaharsoughi Z, Ghodratipour
Z, Lotfaliani M, Rezaeisadrabadi M, Dehghan HR, Bireta C, et al:
Appropriate bolus administration of glycoprotein IIb/IIIa
inhibitors for patients with acute coronary syndromes undergoing
percutaneous coronary intervention: Intracoronary or intravenous? A
comprehensive and updated meta-analysis and systematic review.
Kardiol Pol. 74:104–118. 2016.PubMed/NCBI
|
|
38
|
Dharmasaroja PA and Sae-Lim S: Comparison
of aspirin response measured by urinary 11-dehydrothromboxane B2
and VerifyNow aspirin assay in patients with ischemic stroke. J
Stroke Cerebrovasc Dis. 23:953–957. 2014. View Article : Google Scholar : PubMed/NCBI
|