Introduction
Pu-erh tea is a type of fermented tea that
incorporates microorganism metabolites during the fermentation
process (1). It is prepared from
processed leaves and buds of the broad-leaf variety of the tea
plant [Camellia sinensis var. assamica (L.) O. Kuntze;
Theaceae], primarily grown in Yunnan province of China (2). Pu-erh tea is widely consumed worldwide
as part of a normal diet. Its chemical components and properties
vary due to the time of year that the tealeaves are harvested and
the fermentation methods used. The main components of Pu-erh tea
extracts include tea polyphenol, tea pigment, tea polysaccharide
and alkaloids (3). In previous years,
studies on the potential health-beneficial effects of Pu-erh tea
have identified a range of biological activities, including
anti-oxidation (4), anti-obesity
(5), anti-inflammation,
anti-immunosenescence (6),
anti-hyperlipidemic (7), antitumor
(8), antiviral (9) and antibacterial (10) effects. However, its potential action
in reducing or limiting gastric mucosal injury and the mechanisms
associated with these effects have not been experimentally
clarified.
It is established that gastric mucosal injury occurs
due to an imbalance between mucosal defensive and aggressive
factors (11). Gastric mucosa is
frequently exposed to HCl, pepsin, bile acids, ethanol,
non-steroidal anti-inflammatory drugs (12), Helicobacter pylori toxins and other
noxious substances (13,14). Defensive mechanisms manifest through
the activation of various mucosal protection lines, including of
mucus and bicarbonate secretion, the mucosal barrier itself,
gastric microcirculation (15) and
the renin-angiotensin system (16).
The biological functions of Pu-erh tea in the
stomach are broad according to previous literature, and include
detoxification, promotion of food digestion, regulation of
gastrectasia and removal of fats (17). However, there is a lack of data on the
gastro-protective activity of Pu-erh tea. In the present study,
experiments were designed to investigate the effect and mechanisms
of Pu-erh tea extracts on preventing gastric mucosa injury in rats
induced with ethanol, namely by histopathological examination and
determination of the levels of myeloperoxidase (MPO) and asymmetric
arginine (ADMA) in the stomach tissue. MPO activity is regularly
used as an indicator for evaluating the progression of intestinal
ulcers: it is known to be increased in the ulcerated condition and
to be reduced through the healing process (18). Meanwhile, it has been demonstrated
that ADMA facilitates in gastric mucosal injury: acute
administration of high-dose ethanol significantly increased the
gastric ulcer index, which was concomitant with an increase of
ADMA, while the increased level of ADMA was suppressed by the
resveratrol analog BTM-0512 (19).
Therefore, these indicators were used in the present study to
determine the protective effect of Pu-erh tea extracts against
ethanol-induced gastric mucosal damage in rats.
Materials and methods
Animals
Sprague-Dawley male rats (n=126) were obtained from
the Vital River Laboratories Co., Ltd. (Beijing, China), the weight
of which ranged from 180 to 220 g. They were group-housed (5 rats
per cage) in standard and pathogen-free environmental conditions
(22±1°C, 60±5% humidity, 12-h light/dark cycle) with free access to
a standard commercial diet and water ad libitum. Animals were
acclimatized to the environment for at least one week. The study
protocols were approved by the Tasly Laboratory Animal Welfare and
Ethics Committee of Tasly Pharmaceuticals, Inc. (Tianjin, China),
and conducted according to the rules of animal experimentation and
the guide for the Care and Use of Laboratory Animals of Tasly
Pharmaceuticals, Inc. The rats were randomly divided into seven
groups (n=18 per group).
Chemicals and drugs
An aqueous extract of Pu-erh tea was provided by
Tasly Pharmaceuticals, Inc., which was dissolved in distilled water
and prepared as described previously (1).
The ingredients in fermented Pu-erh tea include
caffeine, polyphenols, γ-aminobutyric acid, theanine, statin,
polysaccharides (3,20–22),
theaflavins, thearubigins and theabrownins (23–26).
Cimetidine, used as a reference drug in this study,
was obtained from GlaxoSmithKline (Shanghai, China) and dissolved
in distilled water. A standardized powder of green tea was
purchased from Damin Foodstuff (Zhangzhou) Co., Ltd., (Zhangzhou,
China). Hematoxylin and eosin were provided by Muto Pure Chemicals
Co., Ltd. (Tokyo, Japan). Absolute ethanol, formalin, paraffin and
dimethylbenzene were supplied by Rionlon (Tianjin) Industry Co.,
Ltd. (Tianjin, China). ELISA kits for rat MPO (catalogue no.
CK-E30635) and rat ADMA (catalogue no. CK-E30769) were provided by
Shanghai Bogoo Biotechnology Co., Ltd. (Shanghai, China). Other
reagents used in the study were of analytical grade or higher
without further purification.
Ethanol-induced gastric lesion and
pharmacological intervention
The rats in normal and model groups were
intragastrically (i.g.) administered 10 ml/kg distilled water,
while those in other groups were administered 0.08 g/kg cimetidine
(i.g.), Pu-erh tea extracts at 0.50, 1.00 and 1.50 g/kg, and 1.00
g/kg green tea powder, respectively. The animals were administered
with the test drugs or distilled water between 8:00-9:00 am once a
day for 14 consecutive days. Acute gastric lesions were created by
intragastric application of absolute ethanol according to a common
method (27). The rats, excluding
those in the normal control group, were orally administrated
absolute ethanol (5 ml/kg) on day 15 after being fasted for 24 h
but with free access to water. At 60 min after ethanol
administration, the rats were euthanized by cervical dislocation
following an overdose of diethyl ether anesthesia (28–30) and
the stomachs were immediately excised. Each stomach was opened
along the greater curvature as described by Balan et al
(31) and rinsed in cold saline
solution. Half of the stomachs in each group were fixed in 10%
buffered formalin at room temperature for 15 min for anatomical
examination, while the others were immediately preserved in liquid
nitrogen (−196°C) for determination of MPO and ADMA.
Gross assessment of gastric
lesions
After 15 min, the stomachs in 10% buffered formalin
were removed. Photographs of the gastric mucosa were taken, and the
mucosal lesions were scored by a laboratory animal technician
blinded to the experimental protocol. The length and width of each
injured area of the gastric mucosa were measured with a vernier
caliper. Gastric mucosal ulcer index (UI) was determined according
to Table I, following the guidelines
of the Technical Standards for Testing and Assessment of Health
Food (32), and calculated according
to the following formula: UI = spot erosion point + erosion length
points + (erosion width points ×2). The inhibitory rate (I%) was
calculated by the formula: I (%) = [(UIcontrol -
UItreated)/UIcontrol] × 100% (33).
 | Table I.Gross scoring system for gastric
mucosal lesions. |
Table I.
Gross scoring system for gastric
mucosal lesions.
|
| Points |
|---|
|
|
|
|---|
| Gastric mucosal
lesion | 1 | 2 | 3 | 4 |
|---|
| Spot erosion
(no.) | 1 | – | – | – |
| Erosion length
(mm) | 1–5 | 6–10 | 10–15 | >15 |
| Erosion width
(mm) | 1–2 | >2 | – | – |
Histopathological examination
The formalin-fixed stomach tissues were embedded in
paraffin wax and gradient dehydrated in increasing concentrations
of ethanol (70–100% v/v). The specimens were sectioned (4-µm thick)
and stained with hematoxylin (~1.5 min) and eosin (30 sec) at room
temperature for histopathological examination. An epithelial damage
scoring system (34) was applied to
rate histopathological changes, including congestion, edema,
hemorrhage, degeneration and necrosis in the gastric mucosa, by
light microscopy (×400), as described in Table II. The total score of pathological
changes was calculated by the formula: Total score = hyperemia
points + (bleeding points ×2) + (degeneration and necrosis points
×3) (34).
 | Table II.Scoring system for histopathological
changes in gastric mucosal epithelia. |
Table II.
Scoring system for histopathological
changes in gastric mucosal epithelia.
|
| Points |
|---|
|
|
|
|---|
| Pathology, area
affected | 1 | 2 | 3 | 4 | 5 |
|---|
| Hyperemia | <1/5 | 1/5-2/5 | 2/5-3/5 | 3/5-4/5 | All over the
epithelia |
| Bleeding | <1/5 | 1/5-2/5 | 2/5-3/5 | 3/5-4/5 | All over the
epithelia |
| Degeneration and
necrosis | <1/5 | 1/5-2/5 | 2/5-3/5 | 3/5-4/5 | All over the
epithelia |
Determination of MPO and ADMA
The segments of stomach tissue with ulcers were
processed into 20% tissue homogenate in cold saline with a UP400S
ultrasonic processor (Ningbo Xinzhi Bio-tech Co., Ltd., Ningbo,
China). The rat MPO and ADMA ELISA kits were then used to determine
MPO activity and ADMA concentration in the homogenate, according to
the manufacturer's protocols. Optical absorbance (O.D.) at 450 nm
was recorded with a Tecan Infinite 200 Microplate Reader (Tecan
Group, Ltd., Mannedorf, Switzerland). MPO activity or ADMA
concentration in the samples was then determined by comparing the
O.D. value of the samples to the standard curve.
Statistical analysis
The results from each group were expressed as the
mean ± standard error of mean. The data were analyzed by one-way
analysis of variance with Fisher's least significant difference
post hoc analysis. Statistical analysis was performed with SPSS
16.0 (SPSS, Inc., Chicago, IL, USA), and P<0.05 was considered
to indicate statistical significance.
Results
Protective effect of the extracts in
gastric lesions based on gross evaluation
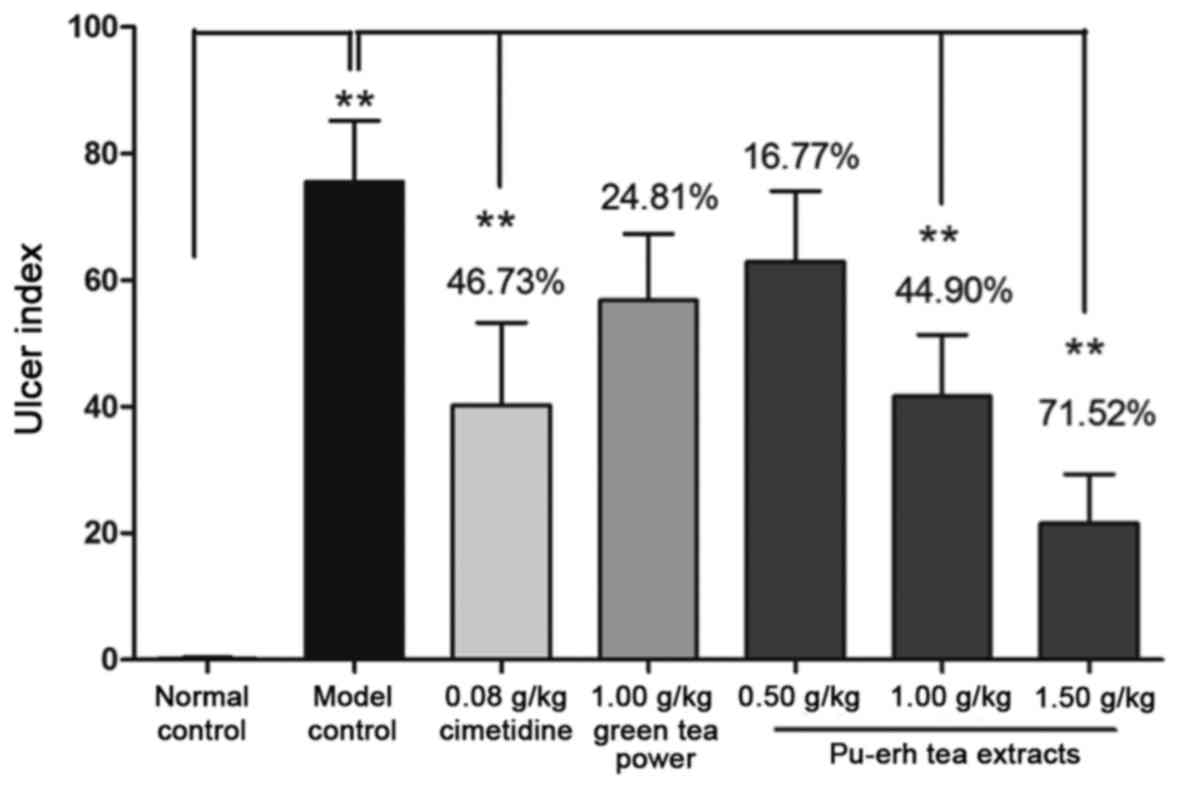
Cimetidine and moderate-to-high-dose Pu-erh tea
extracts (1.00 and 1.50 g/kg) administered prior to alcohol-induced
gastric injury significantly decreased the ulcer index in rat
gastric mucosa, compared with the model control (P<0.01;
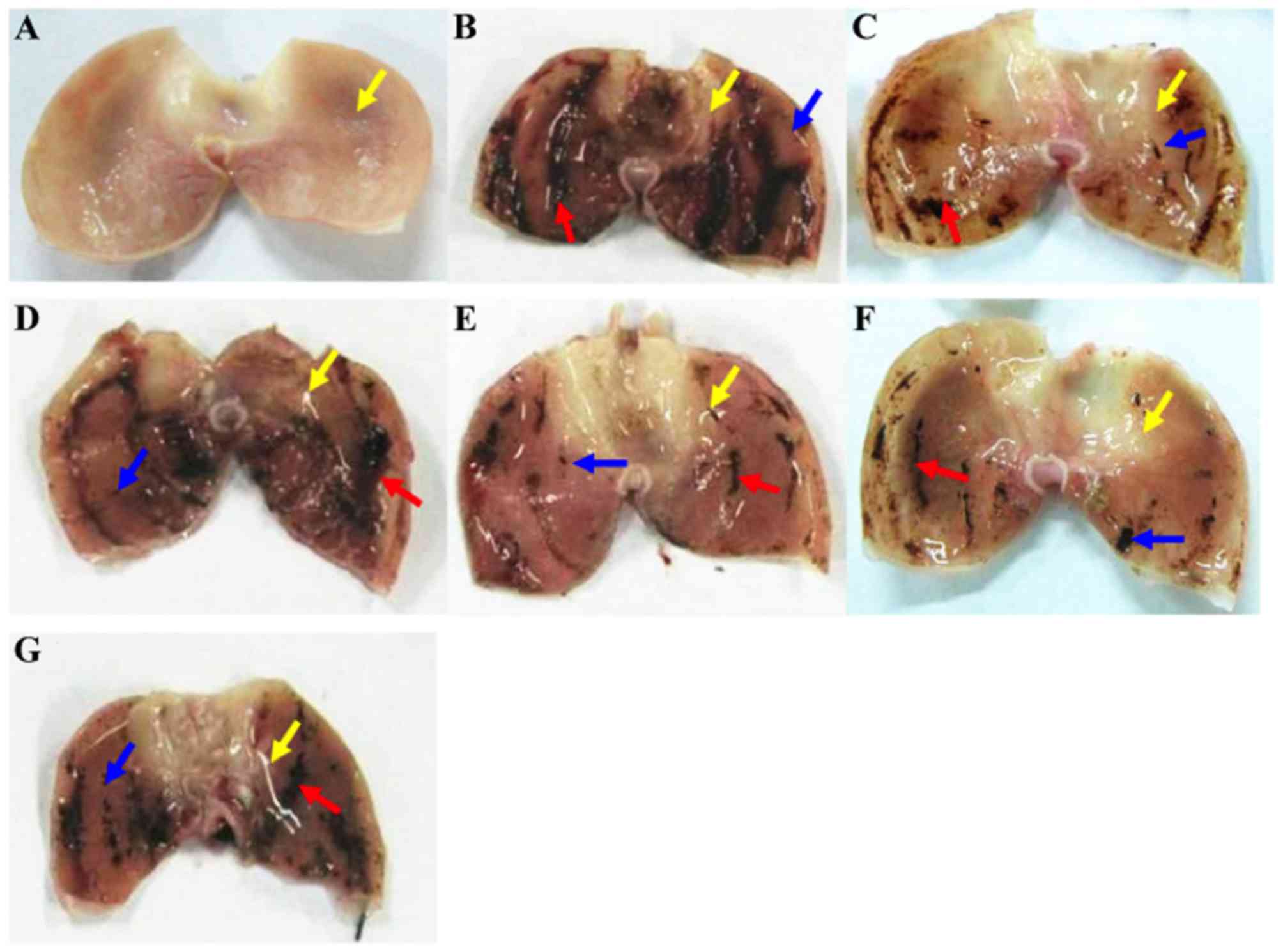
Figs. 1 and 2). From initial observations, it was noted
that the middle-to-high doses of Pu-erh tea extracts markedly
suppressed the formation of damage; a notable phenomenon was that
mucosal folds in the rat stomach became flatter following treatment
with the extracts (0.50–1.50 g/kg; Fig.
1). Additionally, the severity of ethanol-induced gastric
mucosal damage appeared to be dose-dependently reduced by the
pretreatment with Pu-erh tea extracts (Figs. 1 and 2).
The inhibitory rate of the high-dose Pu-erh tea extracts (1.50
g/kg) was 71.52%, which was higher than that of cimetidine
(46.73%). Meanwhile, gastric mucosal injury in rats pre-treated
with green tea powder (1.00 g/kg) was not significantly improved,
though its inhibitory rate was 24.81%.
Protective effect of the extracts
against in gastric lesions based on histopathological
evaluation
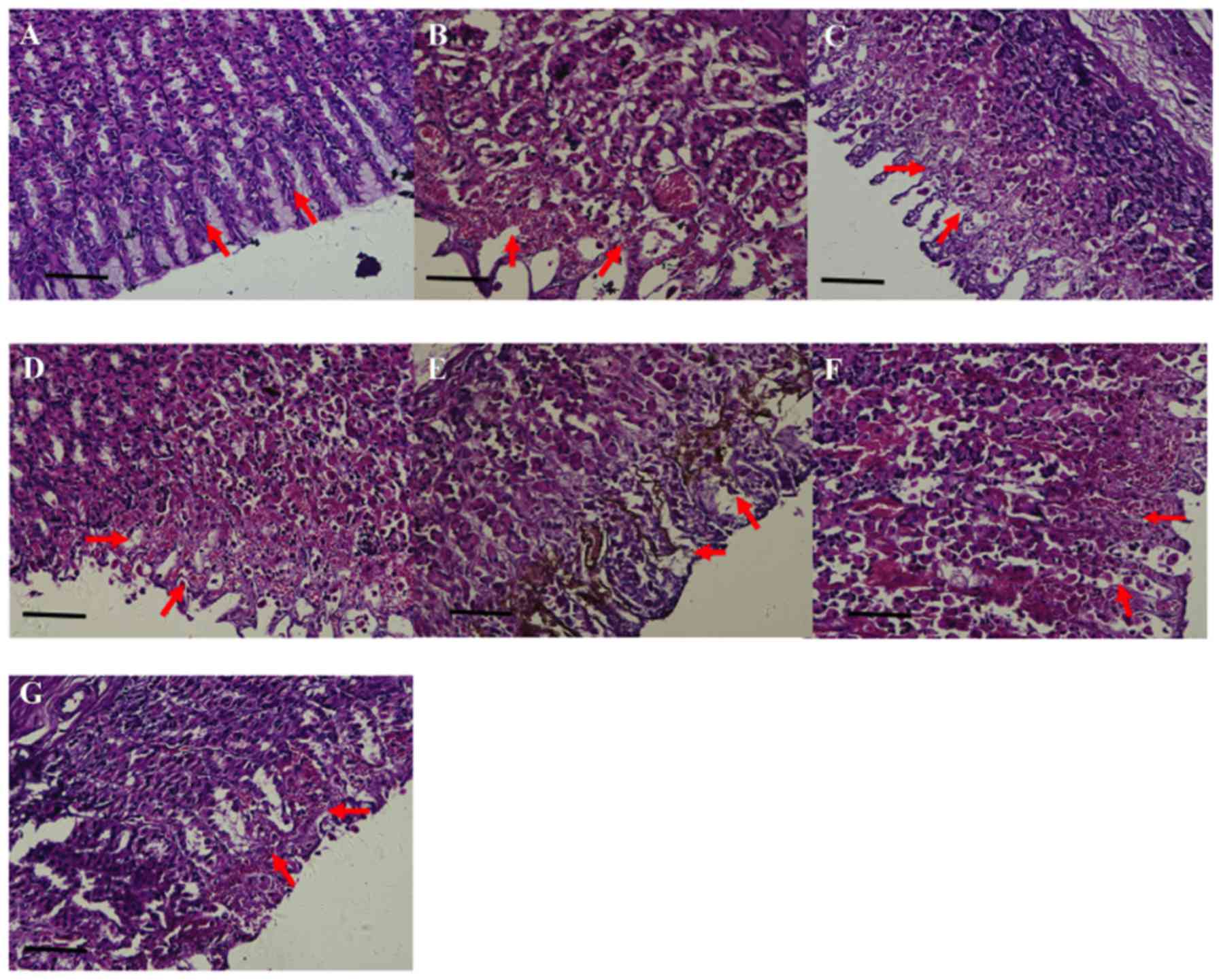
Histopathological assessment of the gastric tissues
was subsequently performed (Fig. 3).
Under high-power light microscopy, the gastric mucosa of rats in
the normal control was smooth and flat. The layers of the gastric
mucosa exhibited clear boundaries, and there were no signs of
pathological changes such as hyperemia, bleeding or epithelial cell
degeneration or necrosis (Fig. 3A).
In the model control, however, relatively increased damage to the
gastric mucosa was identified (Fig.
3B): The surface of the gastric mucosa was uneven and exhibited
erosion, ulcers and bleeding; furthermore, marked pathological
changes including hyperemia, bleeding and epithelial cell
degeneration and necrosis were observed. Gastric mucosal damage in
rats pre-treated with cimetidine or high-dose Pu-erh tea extracts
(1.50 g/kg) was clearly alleviated (Fig.
3C and G), compared with that of the model control. Notably,
the mucosal superficial layer of rats pre-treated with high-dose
Pu-erh tea extracts was intact and the submucosal layers were only
slightly congested (Fig. 3G). On
scoring of the damage, the model group was determined to have a
score for hyperemia of 1.22±0.16, for bleeding of 2.11±0.37, and
for epithelial cell degeneration or necrosis of 3.33±0.25 (Table III). Thus, the score for total
pathological change in the model control was 15.44±0.56, which was
significantly higher than that of the normal control (0.38±0.20;
P<0.05); whereas, the total scores for rats in the Pu-erh tea
extract groups were significantly decreased compared with the model
group (P<0.05), to 9.75±1.16 in the middle-dose group (1.00g/kg)
and 6.22±0.77 in the high-dose group (1.50 g/kg; Table III). Green tea powder at the dose of
1.00 g/kg did not exert significant protection in the gastric
mucosa (Fig. 3D and Table III).
 | Table III.Histopathological scores of the
gastric mucosa of rats with alcohol-induced gastric lesions
pre-treated with the tested drugs. |
Table III.
Histopathological scores of the
gastric mucosa of rats with alcohol-induced gastric lesions
pre-treated with the tested drugs.
| Group | Hyperemia | Bleeding | Epithelial cell
degeneration/necrosis | Pathological
changes, total score |
|---|
| Normal | 0.38±0.20 |
0.00±0.00d |
0.00±0.00d |
0.38±0.20d |
| Model |
1.22±0.16a |
2.11±0.37b |
3.33±0.25b |
15.44±0.56b |
| Cimetidine
(g/kg) | 1.11±0.12 | 2.00±0.31 |
1.56±0.31d |
9.78±0.79c |
| Green tea powder
(g/kg) | 1.11±0.12 | 1.67±0.35 | 3.11±0.41 | 13.78±1.50 |
| Pu-erh tea
extracts(g/kg) |
|
|
|
|
|
0.5 | 1.11±0.12 | 1.78±0.42 | 3.44±0.26 | 13.89±1.26 |
|
1.0 | 1.25±0.25 | 1.25±0.37 |
2.00±0.76c |
9.75±1.16c |
|
1.5 | 1.11±0.12 |
0.56±0.19c |
1.33±0.18d |
6.22±0.77d |
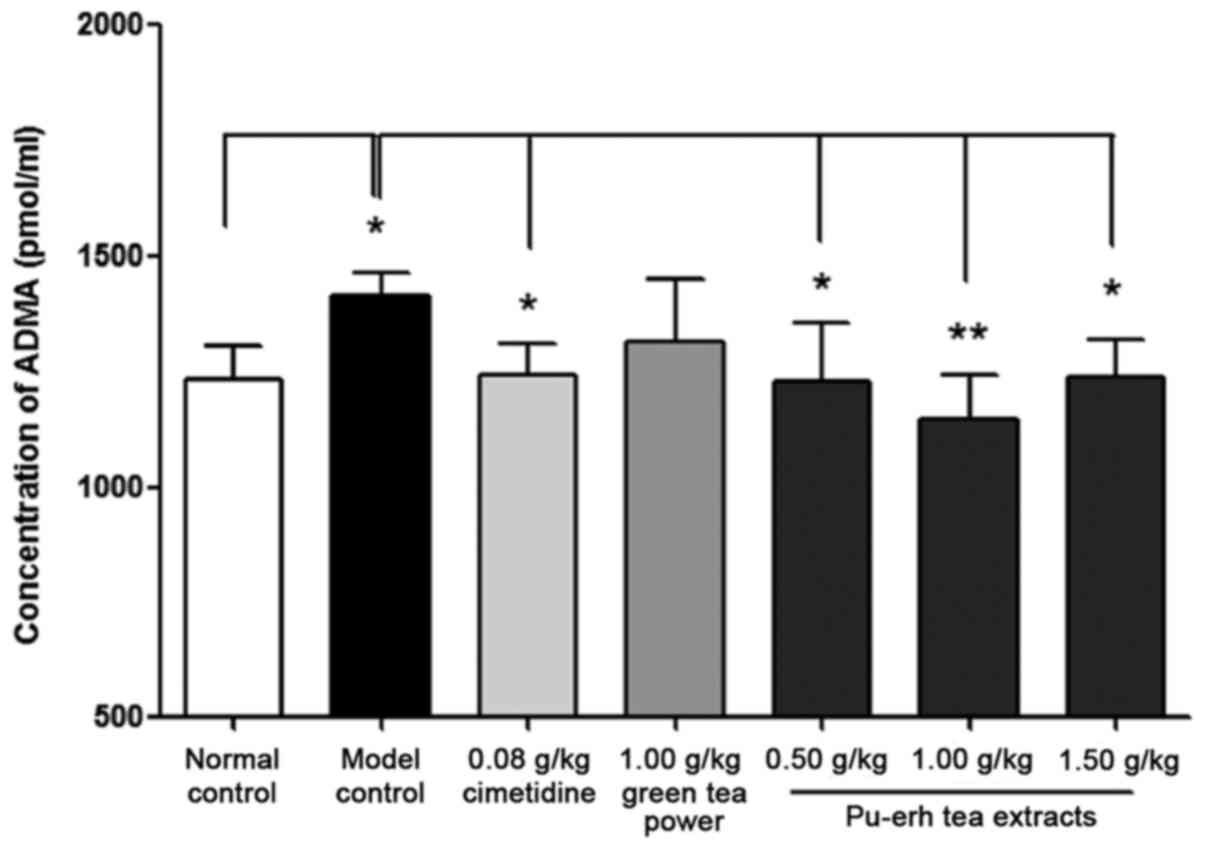
Effect on MPO activity and ADMA
concentration in mucosal tissue
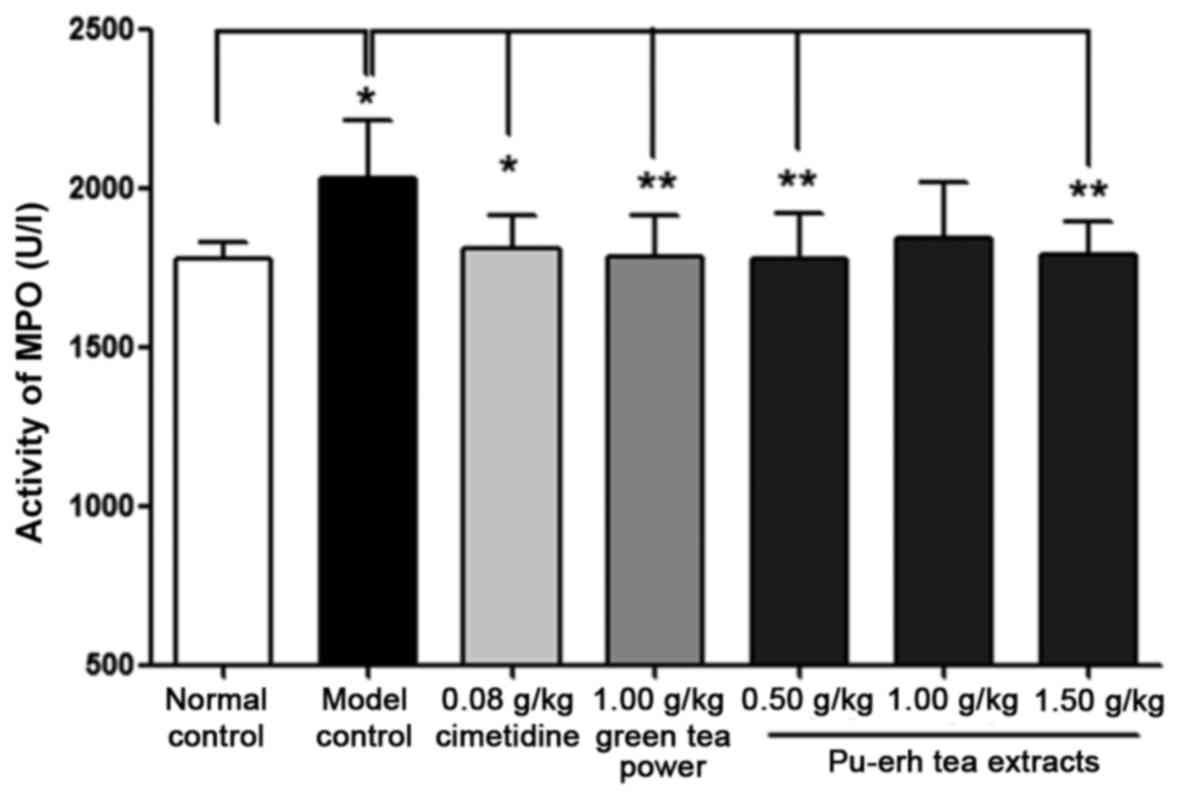
MPO activity in gastric tissue homogenate of the
model control group was significantly increased compared with that
of the normal control group (2,032.59±69.63 vs. 1,778.13±20.58 U/l,
P<0.05; Fig. 4). The concentration
of ADMA was also significantly higher in the model group compared
with that in the normal control group (1,411.25±20.85 vs.
1,233.39±27.30 pmol/ml, P<0.05; Fig.
5). The activity of MPO and the concentration of ADMA in rats
pre-treated with cimetidine was 1,811.07±32.50 U/l (Fig. 4) and 1,242.37±24.76 pmol/ml (Fig. 5), respectively, which were reduced
significantly compared with that of the model control group
(P<0.05). Similarly, MPO activity in the low and high-dose
Pu-erh tea extract groups (P<0.01), and ADMA concentration in
all three extract treatment groups (0.50 and 1.50 g/kg: P<0.05;
1.00 g/kg: P<0.01) were also significantly attenuated compared
with their levels in the model control group (Figs. 4 and 5).
Discussion
Gastric mucosal damage is caused by an imbalance
between the protective and aggressive mechanisms in the mucosa, and
is considered the net result of the actions of several endogenous
factors and aggressive exogenous factors (11). The integrity of the gastric mucosa
primarily depends on efficient protection of the gastric mucosal
barrier, through maintaining defenses such as the gastric mucus
layer, the mucus-bicarbonate barrier and mucosal microcirculation
(14), which may be damaged by
internal factors and external stimuli factors (11). Damage to the mucosa caused by internal
factors and external stimuli is accompanied with the production of
a number of inflammatory mediators and cytokines (35). Gastric mucosal injury may occur when
noxious factors attack the intact mucosal defense, or when the
mucosal defensive mechanisms are impaired (14). The gastric mucus layer is the first
line of defense that serves to protect stomach tissue from external
stimuli (14). Mucosal defensive
factors enable the mucosa to remain intact despite its frequent
exposure to external substances and ranging temperature, pH and
osmolarity, and notably, to substances with detergent or cytotoxic
action and bacterial products that induce local and systemic
inflammatory reactions (36,37).
Ethanol is commonly used to induce ulcers in
experimental animals, and causes acute gastric mucosal damage
(38). Oral administration of
absolute ethanol to rats produces typical characteristics of
alcohol injury including linear hemorrhagic lesions, extensive
submucosal edema, inflammatory cell infiltration and epithelial
cell loss in stomach tissue (39).
Ethanol produces necrotic lesions in the gastric mucosa through its
direct toxic effect, as well as by reducing the secretion of
bicarbonates and the production of mucus and defensive factors
(40,41).
Gastric mucus when secreted in sufficient quantity
is an important factor for the functioning of the gastric mucosa.
It consists of a viscous, elastic, adherent and transparent gel,
formed by water and glycoproteins, that covers the surface of the
gastrointestinal mucosa (42). The
protective properties of the mucus barrier depend not only on the
gel structure but also on the amount or thickness of the layer
covering the mucosal surface (28).
In the present study, it was notable that folds of gastric mucosa
were flattened and gastric damage was markedly reduced in rats
pretreated with Pu-erh tea extracts, compared with the model
control. Furthermore, the pretreatment with Pu-erh tea extracts at
moderate and high-dose caused significant reduction in the UI and
inhibition of gastric mucosal injury. A study by Scoparo et
al (43) demonstrated that the
fraction of green and black tea containing heteropolysaccharides
reduced gastric lesions induced with ethanol and protected gastric
mucosa tissue. In the current study, it was identified that Pu-erh
tea extracts clearly suppressed the formation of the gastric ulcer,
and exhibited a higher inhibitory rate on gastric ulcer formation
than green tea. This observed effect is probably associated with
the high content of polysaccharides present in Pu-erh tea extracts
(43).
MPO, a biomarker for neutrophil-dependent
inflammation, is mainly released from neutrophils, and therefore is
also an essential marker for normal neutrophil function. MPO and
other tissue-damaging substances including reactive oxygen
metabolites and cytotoxic proteins are released into the
extracellular space when neutrophils are stimulated (44,45).
Ethanol administration causes an increase of mucosal MPO activity,
which thus indicates that the level of activated neutrophils
secreting oxygen radicals is increased (46,47). In
the present study, absolute ethanol induced an increase of MPO
activity in the model control compared with the normal control. The
pretreatment of Pu-erh tea extracts prior to this alcohol induction
suppressed the release of MPO, compared with the model control,
which may indicate that the degree of inflammation induced by
neutrophils was also inhibited.
ADMA is the endogenous inhibitor of nitric oxide
synthase (NOS), and has been implicated in pathophysiologies of the
upper gastrointestinal tract (48).
The generation of high levels of ADMA suppresses nitric oxide (NO)
production through inhibition of NOS activity (49). NO, when serving as a potent
vasodilator, increases blood flow in the gastric mucosa, inhibits
the secretion of gastric acid and potentiates the secretion of
mucus and bicarbonate, which thus protect the gastric mucosa
against damage induced by a variety of corrosive substances and
noxious agents (48). However, the
biological life of ADMA is limited, as it may be hydrolyzed into
L-citrulline and dimethylamine by dimethylarginine
dimethylaminohydrolase (DDAH) excreted from the kidneys (50). A previous study focused on the effect
of ADMA in gastric mucosal injury induced by ethanol, cold stress
and indomethacin, and identified that ADMA levels were increased in
gastric juice in animal models of gastric mucosal lesions (51). Furthermore, NO biosynthesis and DDAH
activity in the stomach may be significantly inhibited in animals
exposed to ethanol, stress and indomethacin (50–52). A
study by Yi et al (53) study
demonstrated that there were crude polyphenols in Dragon-pearl
green tea, which increased the level of NO, improved
microcirculation in the gastric mucosa, cleared oxygen free
radicals and strengthened the protective function of the mucosal
barrier. Adhikary et al (54)
observed that both black tea and theaflavins suppressed various
inflammatory modulators and inducible NOS-mediated nitric oxide
synthesis during gastric ulcer healing. In the current study, the
concentration of ADMA in the model control was significantly
increased, compared with that in the normal control. The
administration of Pu-erh tea extracts prior to ethanol-induced
injury exerted clear protective effects against the gastric mucosal
injury, and the concentration of ADMA was significantly decreased,
compared with the model control. Furthermore Pu-erh tea extracts
were identified to decrease the concentration ADMA to a greater
extent than green tea, which is probably due to the higher content
of theaflavins in Pu-erh tea extracts (54).
In conclusion, the study present focused on the
protective effect of Pu-erh tea extracts in the gastric mucosa.
Absolute ethanol was used to induce gastric mucosal injury and the
action of Pu-erh tea extracts was investigated. The extracts
exerted significant protective effects against the gastric mucosal
damage. The human equivalent of the rat dose 1.00 g/kg is 0.16
g/kg, according to the dose conversion relationship of the Food and
Drug Administration (55), which is
equivalent to physiological tea consumption in humans. The effect
of Pu-erh tea extracts was greater than that of green tea powder.
Pretreatment with Pu-erh tea extracts prior to the intragastrical
administration of absolute ethanol inhibited the activity of MPO
and decreased the concentration of ADMA compared with the
pre-administration of distilled water. These protective effects of
Pu-erh tea extracts against gastric mucosal damage may be due to
its preservation of the gastric mucus layer as well as roles in
decreasing inflammation and increasing NO production.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China
National Science and Technology Support Program (grant no.
2013Bad26q01).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JY and WZ were the principal investigators
responsible for the study and contributed to the design of the
experiments, and JY wrote the original manuscript. YG and JD were
responsible for analyzing the experimental data. XL, PT and YL were
responsible for collecting experimental data. XM and YZ was the
leader of the research group, and was responsible for the study
design and guidance, and for checking the accuracy and authenticity
of the manuscript.
Ethics approval and consent to
participate
The study protocols were approved by the Tasly
Laboratory Animal Welfare and Ethics Committee of Tasly
Pharmaceuticals, Inc. (Tianjin, China), and conducted according to
the rules of animal experimentation and the guide for the Care and
Use of Laboratory Animals of Tasly Pharmaceuticals, Inc.
Consent for publication
Not applicable.
Competing interests
WZ, XL, PT and YL are research fellows and XM is
vice director of Tasly Pharmaceuticals, Inc., Tianjin, China. All
other authors declare no competing interests.
References
|
1
|
Zhao M, Zhang DL, Su XQ, Duan SM, Wan JQ,
Yuan WX, Liu BY, Ma Y and Pan YH: An Integrated
Metagenomics/Metaproteomics Investigation of the Microbial
Communities and Enzymes in Solid-state Fermentation of Pu-erh tea.
Sci Rep. 5:101172015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao L, Jia S, Tang W, Sheng J and Luo Y:
Pu-erh tea inhibits tumor cell growth by down-regulating mutant
p53. Int J Mol Sci. 12:7581–7593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Syu KY, Lin CL, Huang HC and Lin JK:
Determination of theanine, GABA, and other amino acids in green,
oolong, black, and Pu-erh teas with dabsylation and
high-performance liquid chromatography. J Agric Food Chem.
56:7637–7643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan JP, Fan C, Dong WM, Gao B, Yuan W and
Gong JS: Free radical scavenging and anti-oxidative activities of
an ethanol-soluble pigment extract prepared from fermented Zijuan
Pu-erh tea. Food Chem Toxicol. 59:527–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oi Y, Hou IC, Fujita H and Yazawa K:
Antiobesity effects of Chinese black tea (Pu-erh tea) extract and
gallic acid. Phytother Res. 26:475–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Shao WF, Yuan LF, Tu PF and Ma
ZZ: Decreasing pro-inflammatory cytokine and reversing the
immunosenescence with extracts of Pu-erh tea in senescence
accelerated mouse (SAM). Food Chem. 135:2222–2228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou Y, Shao W, Xiao R, Xu K, Ma Z,
Johnstone BH and Du Y: Pu-erh tea aqueous extracts lower
atherosclerotic risk factors in a rat hyperlipidemia model. Exp
Gerontol. 44:434–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao X, Qian Y, Zhou YL, Wang R, Wang Q
and Li GJ: Pu-erh tea has in vitro anticancer activity in TCA8113
cells and preventive effects on buccal mucosa cancer in U14 cells
injected mice in vivo. Nutr Cancer. 66:1059–1069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pei S, Zhang Y, Xu H, Chen X and Chen S:
Inhibition of the replication of hepatitis B virus in vitro by
pu-erh tea extracts. J Agric Food Chem. 59:9927–9934. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su Y, Zhang C, Wang Y and Li P:
Antibacterial property and mechanism of a novel Pu-erh tea
nanofibrous membrane. Appl Microbiol Biotechnol. 93:1663–1671.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Liao H, Liu Y, Zheng Y, Wu X and
Su Z, Zhang X, Lai Z, Lai X, Lin ZX and Su Z: Protective effects of
pogostone from Pogostemonis Herba against ethanol-induced gastric
ulcer in rats. Fitoterapia. 100:110–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas D, Govindhan S, Baiju EC,
Padmavathi G, Kunnumakkara AB and Padikkala J: Cyperus rotundus L.
prevents non-steroidal anti-inflammatory drug-induced gastric
mucosal damage by inhibiting oxidative stress. J Basic Clin Physiol
Pharmacol. 26:485–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
deFoneska A and Kaunitz JD: Gastroduodenal
mucosal defense. Curr Opin Gastroenterol. 26:604–610. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laine L, Takeuchi K and Tarnawski A:
Gastric mucosal defense and cytoprotection: Bench to bedside.
Gastroenterology. 135:41–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tarnawski AS, Ahluwalia A and Jones MK:
The mechanisms of gastric mucosal injury: Focus on microvascular
endothelium as a key target. Curr Med Chem. 19:4–15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brzozowski T, Ptak-Belowska A, Kwiecien S,
Krzysiek-Maczka G, Strzalka M, Drozdowicz D, Pajdo R, Olszanecki R,
Korbut R, Konturek SJ, et al: Novel concept in the mechanism of
injury and protection of gastric mucosa: Role of renin-angiotensin
system and active metabolites of angiotensin. Curr Med Chem.
19:55–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo KL, Weng MS, Chiang CT, Tsai YJ,
Lin-Shiau SY and Lin JK: Comparative studies on the hypolipidemic
and growth suppressive effects of oolong, black, pu-erh, and green
tea leaves in rats. J Agric Food Chem. 53:480–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonisamy P, Kannan P, Aravinthan A,
Duraipandiyan V, Arasu MV, Ignacimuthu S, Al-Dhabi NA and Kim JH:
Gastroprotective activity of violacein isolated from
Chromobacterium violaceum on indomethacin-induced gastric lesions
in rats: Investigation of potential mechanisms of action. Sci World
J. 2014:6164322014. View Article : Google Scholar
|
|
19
|
Li L, Luo XJ, Liu YZ, Zhang YS, Yuan Q,
Tan N, Xiang DX and Peng J: The role of the DDAH-ADMA pathway in
the protective effect of resveratrol analog BTM-0512 on gastric
mucosal injury. Can J Physiol Pharmacol. 88:562–567. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou CW, Jeng KC and Chen YS: Enhancement
of fermentation process in Pu-erh tea by tea-leaf extract. J Food
Sci. 75:H44–H48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeng KC, Chen CS, Fang YP, Hou RC and Chen
YS: Effect of microbial fermentation on content of statin, GABA,
and polyphenols in Pu-Erh tea. J Agric Food Chem. 55:8787–8792.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duh PD, Yen GC, Yen WJ, Wang BS and Chang
LW: Effects of pu-erh tea on oxidative damage and nitric oxide
scavenging. J Agric Food Chem. 52:8169–8176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Zhao H, Zhang M, Li CJ, Lin XZ,
Sheng J and Shi W: Variations of antioxidant properties and NO
scavenging abilities during fermentation of tea. Int J Mol Sci.
12:4574–4590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YS, Liu BL and Chang YN:
Bioactivities and sensory evaluation of Pu-erh teas made from three
tea leaves in an improved pile fermentation process. J Biosci
Bioeng. 109:557–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Gong J, Chisti Y and
Sirisansaneeyakul S: Fungal isolates from a Pu-erh type tea
fermentation and their ability to convert tea polyphenols to
theabrownins. J Food Sci. 80:M809–M817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Y, Wang G, Li C, Zhang M, Zhao H, Sheng
J and Shi W: Pu-erh tea reduces nitric oxide levels in rats by
inhibiting inducible nitric oxide synthase expression through
toll-like receptor 4. Int J Mol Sci. 13:7174–7185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu CF, Lin CC, Ng LT and Lin SC:
Protection by tetramethylpyrazine in acute absolute ethanol-induced
gastric lesions. J Biomed Sci. 9:395–400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua HE: Application of Anaesthesia with
Combined Ether Inhalation and Sodium Pentobarbital Intraperitoneal
Injection to Rat Liver Transplantation. Chin J Comp Med. 18:30–31.
2008.(In Chinese).
|
|
29
|
Halim SZ, Zakaria ZA, Omar MH, Mohtarrudin
N, Wahab IRA and Abdullah MNH: Synergistic gastroprotective
activity of methanolic extract of a mixture of Melastoma
malabathricum and Muntingia calabura leaves in rats. BMC Complement
Altern Med. 17:4882017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balan T, Sani MH, Mumtaz Ahmad SH,
Suppaiah V, Mohtarrudin N and Zakaria ZA: Antioxidant and
anti-inflammatory activities contribute to the prophylactic effect
of semi-purified fractions obtained from the crude methanol extract
of Muntingia calabura leaves against gastric ulceration in rats. J
Ethnopharmacol. 164:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balan T, Mohd Sani MH, Suppaiah V,
Mohtarrudin N, Suhaili Z, Ahmad Z and Zakaria ZA: Antiulcer
activity of Muntingia calabura leaves involves the modulation of
endogenous nitric oxide and nonprotein sulfhydryl compounds. Pharm
Biol. 2013.PubMed/NCBI
|
|
32
|
Technical Standards for Testing and
Assessment of Health Food: Document Guidelines of the Ministry of
health of the People's Republic of China. 23–28. 2012.
|
|
33
|
Guth PH, Aures D and Paulsen G: Topical
aspirin plus HCl gastric lesions in the rat. Cytoprotective effect
of prostaglandin, cimetidine, and probanthine. Gastroenterology.
76:88–93. 1979.PubMed/NCBI
|
|
34
|
Li Q, Yang LL, Fan LL, Liang C, Wang QJ,
Wen HM, Dai JW, Li X and Zhang YY: Activity of Brucea javanica oil
emulsion against gastric ulcers in rodents. Asian J Pharmaceutical
Sci. (In press).
|
|
35
|
Mei X, Xu D and Xu S, Zheng Y and Xu S:
Novel role of Zn(II)-curcumin in enhancing cell proliferation and
adjusting proinflammatory cytokine-mediated oxidative damage of
ethanol-induced acute gastric ulcers. Chem Biol Interact.
197:31–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alrashdi AS, Salama SM, Alkiyumi SS,
Abdulla MA, Hadi AH, Abdelwahab SI, Taha MM, Hussiani J and Asykin
N: Mechanisms of gastroprotective effects of ethanolic leaf extract
of Jasminum sambac against HCl/ethanol-induced gastric mucosal
injury in rats. Evid Based Complement Alternat Med.
2012:7864262012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashim H, Mughrabi FF, Ameen M, Khaledi H
and Ali HM: Cytoprotective effect of benzyl
N′-(5-chloro-indol-3-yl-methylidene)-hydrazinecarbodithioate
against ethanol-induced gastric mucosal injury in rats. Molecules.
17:9306–9320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park CH, Nam DY, Son HU, Lee SR, Lee HJ,
Heo JC, Cha TY, Baek JH and Lee SH: Polymer fraction of Aloe vera
exhibits a protective activity on ethanol-induced gastric lesions.
Int J Mol Med. 27:511–518. 2011.PubMed/NCBI
|
|
39
|
Gazzieri D, Trevisani M, Springer J,
Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S
and Nosi D: Substance P released by TRPV1-expressing neurons
produces reactive oxygen species that mediate ethanol-induced
gastric injury. Free Radic Biol Med. 43:581–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luiz-Ferreira A, Almeida AC, Cola M,
Barbastefano V, Almeida AB, Batista LM, Farias-Silva E, Pellizzon
CH, Hiruma-Lima CA, Santos LC, et al: Mechanisms of the gastric
antiulcerogenic activity of Anacardium humile St. Hil on
ethanol-induced acute gastric mucosal injury in rats. Molecules.
15:7153–7166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ibrahim IA, Qader SW, Abdulla MA, Nimir
AR, Abdelwahab SI and Al-Bayaty FH: Effects of Pithecellobium
jiringa ethanol extract against ethanol-induced gastric mucosal
injuries in Sprague-Dawley rats. Molecules. 17:2796–2811. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tulassay Z and Herszényi L: Gastric
mucosal defense and cytoprotection. Best Pract Res Clin
Gastroenterol. 24:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scoparo CT, Souza LM, Dartora N, Sassaki
GL, Santana-Filho AP, Werner MF, Borato DG, Baggio CH and Iacomini
M: Chemical characterization of heteropolysaccharides from green
and black teas (Camellia sinensis) and their anti-ulcer effect. Int
J Biol Macromol. 86:772–781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nartey ET, Ofosuhene M and Agbale CM:
Anti-ulcerogenic activity of the root bark extract of the African
laburnum ‘Cassia sieberiana’ and its effect on the anti-oxidant
defence system in rats. BMC Complement Altern Med. 12:2472012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao W, Zhu F, Shen W, Fu A, Zheng L, Yan
Z, Zhao L and Fu G: Protective effects of DIDS against
ethanol-induced gastric mucosal injury in rats. Acta Biochim
Biophys Sin (Shanghai). 41:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Devi RS, Narayan S, Vani G and Shyamala
Devi CS: Gastroprotective effect of Terminalia arjuna bark on
diclofenac sodium induced gastric ulcer. Chem Biol Interact.
167:71–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Santos Cerqueira G, dos Santos e Silva G,
Rios Vasconcelos E, Fragoso de Freitas AP, Arcanjo Moura B,
Silveira Macedo D, Lopes Souto A, Barbosa Filho JM, de Almeida Leal
LK, de Castro Brito GA, et al: Effects of hecogenin and its
possible mechanism of action on experimental models of gastric
ulcer in mice. Eur J Pharmacol. 683:260–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Szlachcic A, Krzysiek-Maczka G, Pajdo R,
Targosz A, Magierowski M, Jasnos K, Drozdowicz D, Kwiecien S and
Brzozowski T: The impact of asymmetric dimethylarginine (ADAMA),
the endogenous nitric oxide (NO) synthase inhibitor, to the
pathogenesis of gastric mucosal damage. Curr Pharm Des. 19:90–97.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tran CT, Leiper JM and Vallance P: The
DDAH/ADMA/NOS pathway. Atheroscler Suppl. 4:33–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Z, Zou YY, Li FJ and Hu CP:
Asymmetric dimethylarginine: A novel biomarker of gastric mucosal
injury? World J Gastroenterol. 17:2178–2180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Z, Zhou Y, Zou YY, Wang L, Yang ZC,
Guo R, Li D, Peng J and Li YJ: Detrimental effects of nicotine on
the acute gastric mucosal injury induced by ethanol: Role of
asymmetric dimethylarginine. Can J Physiol Pharmacol. 86:835–840.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kwiecien S, Ptak-Belowska A,
Krzysiek-Maczka G, Targosz A, Jasnos K, Magierowski M, Szczyrk U,
Brzozowski B, Konturek SJ, Konturek PC, et al: Asymmetric
dimethylarginine, an endogenous inhibitor of nitric oxide synthase,
interacts with gastric oxidative metabolism and enhances
stress-induced gastric lesions. J Physiol Pharmacol. 63:515–524.
2012.PubMed/NCBI
|
|
53
|
Yi R, Wang R, Sun P and Zhao X:
Antioxidant-mediated preventative effect of Dragon-pearl tea crude
polyphenol extract on reserpine-induced gastric ulcers. Exp Ther
Med. 10:338–344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Adhikary B, Yadav SK, Chand S,
Bandyopadhyay SK and Chattopadhyay S: Black tea and theaflavins
suppress various inflammatory modulators and i-NOS mediated nitric
oxide synthesis during gastric ulcer healing. Free Radic Res.
45:767–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Food and Drug Administration (FDA), .
Estimating the safe starting dose in clinical trials for
therapeutics in adult healthy volunteersGuidance for Industry and
Reviewers. FDA; Rockville, MD: pp. 262002
|