Introduction
Type 2 diabetes mellitus (T2DM) is a chronic
metabolic abnormality that is characterized by hyperglycemia due to
decreased insulin secretion and/or insulin resistance (1). The disease typically starts in middle
age and its incidence is associated with certain risk factors,
including obesity, hypertension, physical inactivity and family
history of T2DM (1). To prevent
persistent hyperglycemia, patients with T2DM should adhere to their
prescribed pharmacological treatment and recommended lifestyle
modifications (2), and regularly
monitor their blood glucose measurements (3). Otherwise, uncontrolled T2DM may result
in long-term macro- and microvascular complications, including
atherosclerosis, retinopathy, nephropathy and neuropathy (4). One of the suggested mechanisms that may
be implicated in the development of microvascular complications is
the formation of advanced glycation end products (AGEs) (5). Of note, a growing body of evidence
suggests that increased levels of AGEs, in addition to persistent
hyperglycemia, may predispose patients to stiffness of connective
tissues, leading to painful musculoskeletal manifestations
(6).
Musculoskeletal pain is common in patients with
T2DM, and its occurrence is considered to be multifactorial
(7). One of the contributing factors
is increased body weight (8). T2DM
patients are usually obese, and increased body mass index (BMI) has
been identified to be associated with musculoskeletal pain,
particularly in the lower extremities (8). In addition, T2DM patients may suffer
from symptoms of neuropathy as a complication of the disease
itself; these symptoms include numbness, tingling, burning
sensation, muscle weakness and pain (9). Musculoskeletal manifestations in
patients with T2DM may also result from vitamin D deficiency
(10). This vitamin is involved in
maintaining calcium and phosphate homeostasis by controlling
intestinal absorption of both minerals (11). Thus, vitamin D deficiency may cause
defective bone mineralization, leading to bone pain, tenderness,
muscle weakness and myopathy (12).
In addition, vitamin D deficiency has been observed to be
associated with the development of anxiety and depression (13,14), which
may also cause musculoskeletal pain (15). Vitamin D supplementation is being
increasingly investigated in the context of chronic musculoskeletal
pain and even peripheral neuropathic pain relief, and results so
far suggest that these types of pain may be relieved by normalizing
vitamin D levels (16,17).
Vitamin D deficiency is a global health concern,
being highly prevalent among various populations, and can be caused
by reduced exposure to direct sunlight and/or reduced dietary
intake (18). In the present study,
it was hypothesized that musculoskeletal pain in patients with T2DM
is inversely associated with serum 25-hydroxyvitamin D levels. The
aims were i) to investigate the frequency of musculoskeletal pain
in patients with T2DM according to body site; ii) to determine
their serum 25-hydroxyvitamin D levels; iii) to assess their
anxiety, depression and neuropathy scores; and iv) to investigate
any association between pain measurements and serum
25-hydroxyvitamin D concentration, anxiety, depression and
neuropathy scores.
Materials and methods
Participants
Patients with a confirmed diagnosis of T2DM were
recruited from the King Abdullah University Hospital in Ramtha,
Jordan between February and August 2016. Patients who had received
vitamin D supplementation in the previous 3 months and patients
with chronic renal failure and/or chronic liver disease were
excluded from the study. All participants agreed to participate in
the study by signing informed consent forms after discussing the
study purpose and procedure. The study protocol received ethics
approval by the Institutional Research Board of King Abdullah
University Hospital and Jordan University of Science and Technology
(Irbid, Jordan; approval no. 20150266).
Sample size
The number of study subjects was calculated based on
other studies (19,20) that determined the prevalence of
vitamin D deficiency and insufficiency in patients with T2DM. In
the Middle East, the prevalence of vitamin D deficiency and
insufficiency in patients with T2DM between 2010 and 2013 ranged
from 98.5% in Saudi Arabia (19) to
89.7% in Iran (20). The following
formula was used: Sample size =
(t)2(p)(q)/d2, where t=1.96 (t-value, 95%
confidence interval), p=0.02–0.10 [estimated prevalence of vitamin
D deficiency and insufficiency based on other studies (19,20)],
q=1-p and d=0.05 (margin of error based on 95% confidence interval
and 5% error) (21). Therefore, the
sample size based on the two abovementioned studies ranged between
18 and 142 patients. The present study included 124 participants,
which represented a response rate of 89.21%. Control subjects were
not included due to the difficulty in recruiting a sufficient
control population with normal vitamin D concentration from our
Arab population, due to the high prevalence of vitamin D deficiency
(19,20). This was expected as most individuals
do not expose sufficiently to sunlight because of the traditional
dress of the region that covers most of the body.
Data collection
General information on patient age, sex, smoking
status, vitamin D supplementation, use of statins and history of
chronic renal failure and/or chronic liver disease were obtained
from medical records and through self-reporting. Body weight was
measured in kg using Detecto scales (Detecto Scale, Webb City, MO,
USA) and height was measured in cm using a fixed metric scale in
the clinic. BMI was calculated as body weight (kg)/height
(m)2.
Assessment of musculoskeletal
pain
Musculoskeletal pain was assessed using two
questions from the PainDETECT questionnaire (22). The first question asked participants
to locate sites of usual pain by marking the area of pain on an
illustration of human body. Then, the number of painful sites was
calculated as a continuous variable for pain assessment. In
addition, the number (%) of participants who were complaining of
pain for each body site was reported. The second question asked
participants to indicate average pain intensity over the last month
using a scale from 0–10 (0, no pain; 10, maximum pain).
Assessment of anxiety, depression and
neuropathy
Anxiety and depression were assessed using an Arabic
version of the Hospital Anxiety and Depression Scale (23). Neuropathy was assessed using an Arabic
version of the PainDETECT questionnaire (22). Individuals with 0–12 neuropathy scores
were considered as nociceptive, individuals with 13–18 neuropathy
scores were considered as unclear and individuals with 19–38
neuropathy scores were considered as neuropathic. Both
questionnaires were translated from English to Arabic using a
standard forward-backward translation method.
Blood sampling and laboratory
measurements
Venous blood samples (10 ml) were collected
following overnight fasting to measure fasting blood sugar (FBS),
glycated hemoglobin (HbA1c) and 25-hydroxyvitamin D levels. Within
2 h of collection, serum was prepared by centrifuging the blood
samples for 8 min at 2,100 × g at room temperature using a
high-speed Jouan MR23i centrifuge (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). FBS was measured by the hexokinase method
(24) using a Hitachi 902
auto-analyzer (Roche Diagnostics GmbH, Mannheim, Germany). HbA1c
was measured by turbidimetric inhibition immunoassay (25) using the cobas b 101 system (Roche
Diagnostics GmbH). The concentration of 25-hydroxyvitamin D was
measured by liquid chromatography-tandem mass spectrometry
(LC-MS/MS) using an API-3200 triple quadrupole mass spectrometer
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
guidelines by the National Institute of Standards and Technology
(NIST) as previously reported (26).
Statistical analysis
Statistical analysis was performed using IBM SPSS
version 20 (IBM Corp., Armonk, NY, USA). Non-normally distributed
continuous variables were log-transformed prior to analysis. Data
for continuous variables are expressed as the mean ± standard
deviation or median (25th-75th percentiles). Descriptive data were
expressed as frequency (%). The χ2 and Fisher's exact
tests were used to detect significant differences between
categorical variables. One-way analysis of variance was used to
detect significant differences in continuous variables between
patients with (>30 ng/ml; n=16), insufficient (20–30 ng/ml;
n=34) and deficient (<20 ng/ml; n=74) (26) vitamin D levels followed by Tukey's
post hoc tests for multiple comparisons. The Pearson product-moment
test was used to detect correlations between continuous variables
and multiple linear regression analysis was performed to detect
associations between pain measurements and other variables
including neuropathy and anxiety scores, duration of DM, sex and
statin therapy. All P-values were two-sided and considered
statistically significant at <0.05.
Results
Characteristics of participants
Of 139 potential study subjects with T2DM, 124 (47
men and 77 women) agreed to participate in this study (response
rate, 89.2%). The participants' age ranged from 43 to 79 years,
with a mean age of 59.3±9.5 years. The duration of T2DM ranged from
1 to 17 years, with a mean of 5.0±3.4 years. The mean BMI was
30.9±3.6 kg/m2 and the median values of FBS and HbA1c
were 7.7 (6.1–10.4) mmol/l and 7.5 (6.6–8.8)%, respectively. A
total of 21 (16.9%) participants were current smokers, while 20
(16.1%) and 83 (66.9%) participants were former smokers and
non-smokers, respectively.
Assessment of musculoskeletal
pain
Table I illustrates
the frequency of pain according to body location. The neck (60.5%),
lower back (60.5%), head (56.5%), right knee (49.2%), right
shoulder (39.5%), left knee (39.5%), right lower leg (39.5%), left
lower leg (34.7%) and feet (33.9%) were the most common painful
sites reported by the study participants. The mean number of
painful sites was 5.9±3.0 and the mean pain intensity over the last
month was 3.6±2.4 (data not shown).
 | Table I.Frequency of pain according to body
site. |
Table I.
Frequency of pain according to body
site.
| Site of pain | n (%) |
|---|
| Neck | 75 (60.5) |
| Lower back | 75 (60.5) |
| Head | 70 (56.5) |
| Right knee | 61 (49.2) |
| Right shoulder | 49 (39.5) |
| Left knee | 49 (39.5) |
| Right lower leg | 49 (39.5) |
| Left lower leg | 43 (34.7) |
| Feet | 42 (33.9) |
| Right forearm | 33 (26.6) |
| Left forearm | 32 (25.8) |
| Left shoulder | 29 (23.4) |
| Right hand | 28 (22.6) |
| Left arm | 26 (21.0) |
| Right arm | 25 (20.2) |
| Left hand | 23 (18.5) |
| Right upper leg | 16 (12.9) |
| Left upper leg | 14 (11.3) |
Assessment of anxiety, depression and
neuropathy scores
Abnormal anxiety scores were determined in 25
(20.2%) participants, while 28 (22.6%) and 71 (57.3%) participants
were considered to have borderline and normal anxiety scores,
respectively. Abnormal depression scores were reported in 41
(33.1%) participants, while 37 (29.8%) and 46 (37.1%) participants
were considered to have borderline and normal depression scores,
respectively. Neuropathy was indicated in 38 (30.6%) participants,
while 36 (29.0%) and 50 (40.3%) participants were considered to
have unclear and normal neuropathy scores, respectively (data not
shown).
Determination of study variables
according to vitamin D status
As presented in Table
II, the number of painful sites, pain intensity over the last
month, anxiety, depression and neuropathy scores, BMI and HbA1c
were not significantly associated with vitamin D status (all
P>0.05). In addition, vitamin D status was not associated with
sex or statin therapy, and there was no statistically significant
difference in age, FBS or duration of DM among patients with
sufficient, insufficient and deficient vitamin D levels (all
P>0.05).
 | Table II.Determination of study variable
associations according to vitamin D status. |
Table II.
Determination of study variable
associations according to vitamin D status.
|
| 25-hydroxyvitamin D
status |
|
|---|
|
|
|
|
|---|
| Variable | Sufficient, >30
ng/ml (n=16) | Insufficient, 20–30
ng/ml (n=34) | Deficient, <20
ng/ml (n=74) | P-value |
|---|
| Age, years | 58.0±10.2 | 58.6±8.6 | 59.3±9.5 | 0.76 |
| Sex |
|
|
| 0.20 |
|
Male | 3 (18.8) | 15 (44.1) | 29 (39.2) |
|
|
Female | 13 (81.2) | 19 (55.9) | 45 (60.8) |
|
| BMI (kg/m2) |
|
|
| 0.66 |
| Normal
(18.6–24.9) | 1 (6.2) | 0 (0.0) | 4 (5.4) |
|
|
Overweight (25–29.9) | 7 (43.8) | 15 (44.1) | 28 (37.8) |
|
| Obese
(>30) | 8 (50.0) | 19 (55.9) | 42 (56.8) |
|
| Duration of
diabetes mellitus, years | 4.9±4.1 | 4.7±2.6 | 5.2±3.5 | 0.80 |
| HbA1c |
|
|
| 0.69 |
| <7%
(controlled) | 7 (43.8) | 14 (41.2) | 25 (33.8) |
|
| ≥7%
(uncontrolled) | 9 (56.2) | 20 (58.8) | 49 (66.2) |
|
| Log[fasting blood
sugar (mmol/l)] | 0.9±0.1 | 0.9±0.2 | 0.9±0.2 | 0.35 |
| Smoking status |
|
|
| 0.92 |
| Current
smoker | 2 (12.5) | 6 (17.6) | 13 (17.6) |
|
| Former
smoker | 2 (12.5) | 7 (20.6) | 11 (14.9) |
|
| None
smoker | 12 (75.0) | 21 (61.8) | 50 (67.7) |
|
| Number of painful
sites | 4.69±3.0 | 6.7±3.8 | 5.8±2.6 | 0.09 |
| Pain intensity over
the last month (0–10 scale) | 3.8±2.7 | 4.2±2.6 | 3.4±2.2 | 0.24 |
| Anxiety score |
|
|
| 0.18 |
| Normal
(0–7) | 5 (31.2) | 21 (61.8) | 45 (60.8) |
|
|
Borderline (8–10) | 5 (31.2) | 6 (17.6) | 17 (23.0) |
|
|
Abnormal (11–21) | 6 (37.5) | 7 (20.6) | 12 (16.2) |
|
| Depression
score |
|
|
| 0.40 |
| Normal
(0–7) | 6 (37.5) | 11 (32.4) | 29 (39.2) |
|
|
Borderline (8–10) | 2 (12.5) | 11 (32.4) | 24 (32.4) |
|
|
Abnormal (11–21) | 8 (50.0) | 12 (35.3) | 21 (28.4) |
|
| Neuropathy
score |
|
|
| 0.68 |
|
Nociceptive (0–12) | 6 (37.5) | 18 (38.2) | 31 (41.9) |
|
| Unclear
(13–18) | 4 (25.0) | 18 (38.2) | 19 (25.7) |
|
|
Neuropathic (19–38) | 6 (37.5) | 8 (23.5) | 24 (32.4) |
|
| Statin therapy |
|
|
| 0.15 |
|
Yes | 7 (43.8) | 16 (47.1) | 47 (63.5) |
|
| No | 9 (56.2) | 18 (52.9) | 27 (36.5) |
|
Association between pain measurements
and study variables
As presented in Table
III, both the number of painful sites and pain intensity over
the last month were directly correlated with the duration of DM and
neuropathy score (all P<0.05). Pain intensity over the last
month was also directly correlated with the mean number of painful
sites (P<0.01). The association of the mean number of painful
sites with anxiety score was determined as not statistically
significant (P=0.06). Furthermore, the pain measurements were not
significantly correlated with 25-hydroxyvitamin D concentration,
age, BMI, HbA1c, FBS or depression score (all P>0.05). Further
multiple linear regression analyses (Table IV) identified the number of painful
sites and pain intensity over the last month as dependent
variables, both of which were significantly associated with
neuropathy score (P<0.05). In addition, pain intensity over the
last month was significantly associated with the mean number of
painful sites (P<0.01). Neither the number of painful sites nor
pain intensity over the last month was identified to be
significantly associated with sex or the use of statins (all
P>0.05).
 | Table III.Correlation between pain measurements
and other variables. |
Table III.
Correlation between pain measurements
and other variables.
|
| Number of painful
sites | Pain intensity over
the last month |
|---|
|
|
|
|
|---|
| Variable | r | P-value | r | P-value |
|---|
| Age, years | 0.09 | 0.33 | 0.01 | 0.27 |
| Body mass index,
kg/m2 | 0.14 | 0.13 | 0.14 | 0.13 |
| Log[glycated
hemoglobin (%)] | −0.11 | 0.23 | <-0.01 | 0.99 |
| Log[fasting blood
sugar (mmol/l)] | −0.06 | 0.54 | 0.03 | 0.78 |
| Duration of
diabetes mellitus, years | 0.19 | 0.03 | 0.20 | 0.03 |
|
Log[25-hydroxyvitamin D (ng/ml)] | <-0.01 | 0.96 | 0.05 | 0.61 |
| Anxiety score | 0.17 | 0.06 | 0.15 | 0.11 |
| Depression
score | 0.02 | 0.87 | 0.07 | 0.42 |
| Neuropathy
score | 0.63 | 0.00 | 0.46 | 0.00 |
| Number of painful
sites | – | – | 0.46 | 0.00 |
 | Table IV.Association between pain measurements
and other variables. |
Table IV.
Association between pain measurements
and other variables.
| Dependent
variable | R2 | Analysis of
variance | Model | B | β | t-value | P-value |
|---|
| Pain intensity | 0.28 | F=9.17,
P<0.01 | Constant | 0.47 | – | 0.61 | 0.54 |
| over the last
month |
|
| Neuropathy
score | 0.08 | 0.24 | 2.43 | 0.02 |
|
|
|
| Duration of DM | 0.08 | 0.11 | 1.30 | 0.20 |
|
|
|
| Number of painful
sites | 0.22 | 0.28 | 2.90 |
<0.01 |
|
|
|
| Sex | 0.38 | 0.08 | 0.95 | 0.34 |
|
|
|
| Statin therapy | −0.31 | −0.07 | −0.80 | 0.43 |
| Number of | 0.36 | F=13.27,
P<0.01 | Constant | 2.69 | – | 2.72 | 0.01 |
| painful sites |
|
| Neuropathy
score | 0.24 | 0.56 | 6.87 |
<0.01 |
|
|
|
| Duration of DM | 0.07 | 0.08 | 0.97 | 0.33 |
|
|
|
| Anxiety score | 0.03 | 0.04 | 0.56 | 0.58 |
|
|
|
| Sex | −0.04 | −0.01 | −0.09 | 0.93 |
|
|
|
| Statin therapy | −0.47 | −0.08 | −1.00 | 0.32 |
Correlation between vitamin D level
and neuropathy score
As pain measurements were significantly associated
with neuropathy score, further analysis was performed to determine
whether there is an association between neuropathy score and
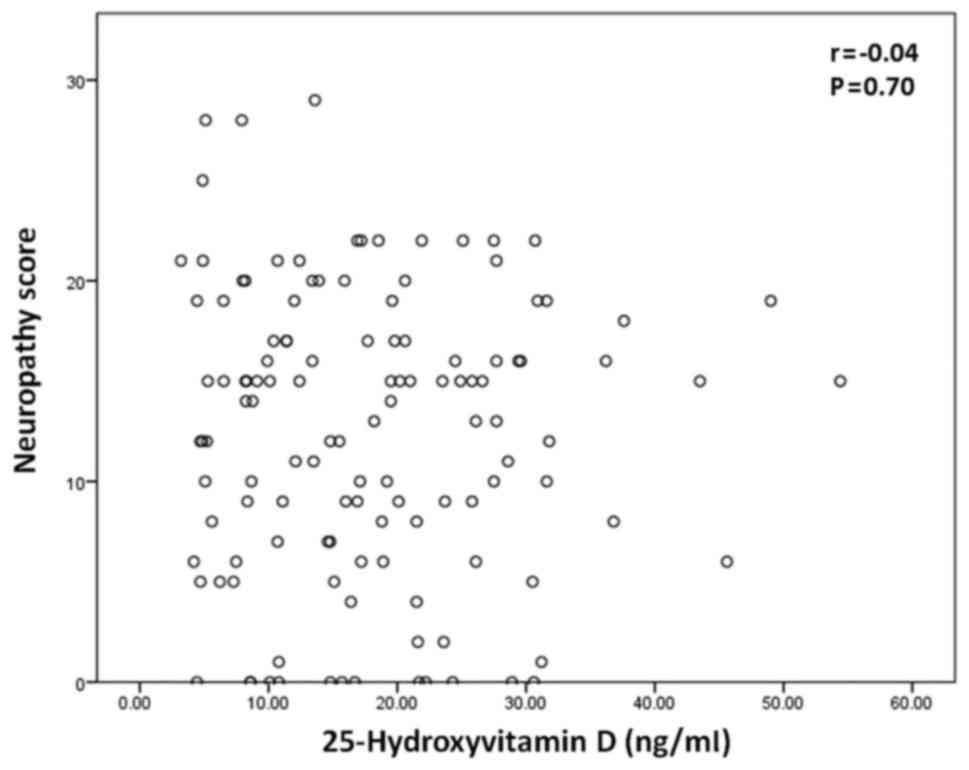
25-hydroxyvitamin D concentration. As depicted in Fig. 1, there was no significant correlation
between 25-hydroxyvitamin D concentration and neuropathy score
(P>0.05).
Discussion
The present study demonstrated that musculoskeletal
pain was highly prevalent among patients with T2DM. Over 50% of the
study participants suffered from chronic neck pain, lower back pain
or headache. Similarly, Molsted et al (7) reported that 60 and 52% of their study
participants with T2DM were suffering from lower back and neck
pain, respectively. The pain frequency in other body sites was
lower, and these results are comparable with those reported by
other studies, including that by Kidwai et al (27), in which the pain frequencies in the
hands, shoulders and upper limbs were 20.5, 19.5 and 32.9%,
respectively. In the present study, pain in the lower extremities,
including the knees, lower legs and feet, was more common compared
with pain in the upper extremities (the arms, forearms, hands, left
shoulder and upper legs). This suggests that the lower extremities,
which bear the weight of the body, are more susceptible to
musculoskeletal pain, particularly when the subjects have increased
BMI (96% of the current study participants were overweight or
obese). This was also confirmed by Viester et al (8), who reported that increased BMI was
associated with the development of musculoskeletal pain in the
lower extremities.
The major goal of the present study was to
investigate whether musculoskeletal pain in patients with T2DM is
associated with serum 25-hydroxyvitamin D levels. The results
identified no significant difference in the number of painful sites
or pain intensity over the last month among patients with
sufficient, insufficient or deficient 25-hydroxyvitamin D levels.
In addition, there was no significant correlation between serum
25-hydroxyvitamin D concentration and the number of painful sites
or pain intensity over the last month. Accordingly, measures of
musculoskeletal pain in T2DM were expected to have no association
with serum 25-hydroxyvitamin D levels. However, the results of the
present study support the findings of Shipton and Shipton (28), who reported that the scientific
evidence for using vitamin D to treat chronic pain is limited due
to the lack of supporting randomized controlled trials. As
musculoskeletal pain may result from anxiety and depression
(15), which may also be caused by
vitamin D deficiency (13,14), it was investigated whether there was
any correlation of pain measurements with anxiety and depression
scores. However, the results revealed no significant correlation
between pain measurements and anxiety or depression scores.
Furthermore, anxiety and depression scores were not significantly
correlated with serum 25-hydroxyvitamin D levels. These results
indicate that musculoskeletal pain in T2DM patients was not
significantly associated with anxiety or depression scores.
By contrast, both the number of painful sites and
pain intensity over the last month were directly correlated with
the duration of T2DM and neuropathy score, indicating that
musculoskeletal pain increases with prolonged duration of T2DM and
increase in neuropathy score. Further multiple linear regression
analyses demonstrated that the number of painful sites and pain
intensity over the last month may be predicted from the neuropathy
score, suggesting that these pain measurements are associated with
the neuropathy score. Further correlation analysis revealed no
statistically significant correlation between neuropathy score and
vitamin D level. Meanwhile, pain intensity over the last month was
associated with the number of painful sites, indicating that pain
intensity may also be predicted from the number of painful
sites.
Musculoskeletal pain may be caused by statin therapy
as an adverse outcome that affects 5–18% of patients treated with
these lipid-lowering agents (29).
Morioka et al (30) reported
that statin users with vitamin D deficiency complained of
musculoskeletal pain at twice the frequency of individuals who were
not taking statins. In addition, they concluded that vitamin D
deficiency modifies the risk of musculoskeletal pain in statin
users (30). In the present study, it
was also investigated whether there are associations between statin
therapy use, vitamin D deficiency and the development of
musculoskeletal pain in patients with T2DM. Statin therapy was
reported in 43.5% of the participants. Vitamin D status was not
significantly correlated with the use of statins; pain
measurements, including both pain intensity over the last month and
number of painful sites, were also not significantly associated
with statin therapy.
Collectively, the findings of the present study
demonstrated that musculoskeletal pain was prevalent among patients
with T2DM, and that pain measurements were not associated with
serum 25-hydroxyvitamin D levels, despite accumulating evidence
supports vitamin D supplementation as a treatment option for such
manifestations (17,31,32).
Measures of musculoskeletal pain were significantly correlated with
the duration of T2DM and neuropathy score, and these measures may
be predicted from the neuropathy score. Thus, preventing and
treating diabetic neuropathy should be prioritized as a treatment
option for managing musculoskeletal pain in patients with T2DM.
However, the significance of these findings may be limited by the
cross-sectional design of the present study. A follow-up study is
required to assess whether vitamin D supplementation or neuropathy
treatment may improve musculoskeletal pain in patients with T2DM.
Another limitation is that self-reporting was used to assess pain,
neuropathy, anxiety and depression among patients. Funding
limitations prevented consultation of a neurology clinic to
definitively diagnose neuropathy, anxiety and depression according
to the clinical guidelines; however, the questionnaires (22,23) that
were used to assess these variables are well-validated and
considered reasonably reliable for research purposes. In addition,
funding limitations prevented enrollment of a larger sample size,
which may affect applications of the findings to a general
population. However, despite the limitations of the present study,
the current findings may draw attention toward the assessment of
musculoskeletal manifestations in patients with T2DM, as well as
encourage further investigations to assess the etiology of
musculoskeletal pain in patients with T2DM and determine whether
vitamin D supplementation and management of neuropathy may be used
for pain relief in such patients.
Acknowledgements
Not applicable.
References
|
1
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 33 Suppl
1:62–69. 2010. View Article : Google Scholar
|
|
2
|
No authors listed: Pharmacologic
management of type 2 diabetes: 2016 interim update. Can J Diabetes.
40:193–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu H, Zhu Y and Leung SW: Is
self-monitoring of blood glucose effective in improving glycaemic
control in type 2 diabetes without insulin treatment: A
meta-analysis of randomised controlled trials. BMJ Open.
6:e0105242016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stolar M: Glycemic control and
complications in type 2 diabetes mellitus. Am J Med. 123
Suppl:S3–S11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh VP, Bali A, Singh N and Jaggi AS:
Advanced glycation end products and diabetic complications. Korean
J Physiol Pharmacol. 18:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pai LW, Hung CT, Li SF, Chen LL, Chung Y
and Liu HL: Musculoskeletal pain in people with and without type 2
diabetes in Taiwan: A population-based, retrospective cohort study.
BMC Musculoskelet Disord. 16:3642015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molsted S, Tribler J and Snorgaard O:
Musculoskeletal pain in patients with type 2 diabetes. Diabetes Res
Clin Pract. 96:135–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Viester L, Verhagen EA, Hengel Oude KM,
Koppes LL, van der Beek AJ and Bongers PM: The relation between
body mass index and musculoskeletal symptoms in the working
population. BMC Musculoskeletal Disorders. 14:2382013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchettini P, Lacerenza M, Mauri E and
Marangoni C: Painful peripheral neuropathies. Curr Neuropharmacol.
4:175–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Straube S, Moore Andrew R, Derry S and
McQuay HJ: Vitamin D and chronic pain. Pain. 141:10–13. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wasserman RH: Intestinal absorption of
calcium and phosphorus. Fed Proc. 40:68–72. 1981.PubMed/NCBI
|
|
12
|
Lips P and van Schoor NM: The effect of
vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol
Metab. 25:585–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoang MT, Defina LF, Willis BL, Leonard
DS, Weiner MF and Brown ES: Association between low serum
25-hydroxyvitamin D and depression in a large sample of healthy
adults: The Cooper Center longitudinal study. Mayo Clin Proc.
86:1050–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Armstrong DJ, Meenagh GK, Bickle I, Lee
AS, Curran ES and Finch MB: Vitamin D deficiency is associated with
anxiety and depression in fibromyalgia. Clin Rheumatol. 26:551–554.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Heer EW, Gerrits MM, Beekman AT, Dekker
J, van Marwijk HW, de Waal MW, Spinhoven P, Penninx BW and van der
Feltz-Cornelis CM: The association of depression and anxiety with
pain: A study from NESDA. PLoS One. 9:e1069072014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bell DS: Reversal of the symptoms of
diabetic neuropathy through correction of vitamin D deficiency in a
type 1 diabetic patient. Case Rep Endocrinol.
2012:1650562012.PubMed/NCBI
|
|
17
|
Kragstrup TW: Vitamin D supplementation
for patients with chronic pain. Scand J Prim Health Care. 29:4–5.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palacios C and Gonzalez L: Is vitamin D
deficiency a major global public health problem? J Steroid Biochem
Mol Biol. 144:Pt A. 138–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alhumaidi M, Agha A and Dewish M: Vitamin
D deficiency in patients with type-2 diabetes mellitus in southern
region of saudi arabia. Maedica (Buchar). 8:231–236.
2013.PubMed/NCBI
|
|
20
|
Bayani MA, Akbari R, Banasaz B and Saeedi
F: Status of vitamin-D in diabetic patients. Caspian J Intern Med.
5:40–42. 2014.PubMed/NCBI
|
|
21
|
Higgins JEBWKC: Organizational research:
Determining appropriate sample size in survey research. Inf Technol
Learn Perform J. 19:43–50. 2001.
|
|
22
|
Freynhagen R, Baron R, Gockel U and Tölle
TR: painDETECT: A new screening questionnaire to identify
neuropathic components in patients with back pain. Curr Med Res
Opin. 22:1911–1920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zigmond AS and Snaith RP: The hospital
anxiety and depression scale. Acta Psychiatr Scand. 67:361–370.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt FH: Blood glucose levels in
capilary blood of adults assessed by the hexokinase method
(author's transl). Klin Wochenschr. 51:520–522. 1973.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Genc S, Omer B, Aycan-Ustyol E, Ince N,
Bal F and Gurdol F: Evaluation of turbidimetric inhibition
immunoassay (TINIA) and HPLC methods for glycated haemoglobin
determination. J Clin Lab Anal. 26:481–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sadat-Ali M, Al-Elq AH, Al-Shaikh IH,
Al-Turki HA, Al-Ali AK and Al-Othman AA: Assessment of low vitamin
D among Saudi Arabians. Did we overshoot the runway? Saudi Med J.
35:1243–1249. 2014.PubMed/NCBI
|
|
27
|
Kidwai SS, Wahid L, Siddiqi SA, Khan RM,
Ghauri I and Sheikh I: Upper limb musculoskeletal abnormalities in
type 2 diabetic patients in low socioeconomic strata in Pakistan.
BMC Res Notes. 6:162013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shipton EE and Shipton EA: Vitamin D
deficiency and pain: Clinical evidence of low levels of vitamin D
and supplementation in chronic pain states. Pain Ther. 4:67–87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Stasi SL, MacLeod TD, Winters JD and
Binder-Macleod SA: Effects of statins on skeletal muscle: A
perspective for physical therapists. Phys Ther. 90:1530–1542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morioka TY, Lee AJ, Bertisch S and
Buettner C: Vitamin D status modifies the association between
statin use and musculoskeletal pain: a population based study.
Atherosclerosis. 238:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le Goaziou MF, Bodier E, Souweine G,
Moreau A, Perdrix C, Flori M and Dupraz C: Musculoskeletal chronic
pains and vitamin D deficiency. Study before after in general
practice. Presse Med. 42:(4 Pt 1). e106–113. 2013.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gendelman O, Itzhaki D, Makarov S, Bennun
M and Amital H: A randomized double-blind placebo-controlled study
adding high dose vitamin D to analgesic regimens in patients with
musculoskeletal pain. Lupus. 24:483–489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nuttall FQ: Body mass index: Obesity, BMI,
and health: A critical review. Nutr Today. 50:117–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Esposito K, Chiodini P, Bellastella G,
Maiorino MI and Giugliano D: Proportion of patients at HbA1c target
<7% with eight classes of antidiabetic drugs in type 2 diabetes:
Systematic review of 218 randomized controlled trials with 78 945
patients. Diabetes Obes Metab. 14:228–233. 2012. View Article : Google Scholar : PubMed/NCBI
|