Introduction
Thermoregulation is an important tool that enables
mammals to adapt to changes in ambient temperature and also serves
a role in defence against infections. In mammals, there are
mechanisms involving the cytokines interleukin (IL)1β, IL6 and
tumour necrosis factor-α that have been reported to increase body
temperature in infection and initialize the fever response
(1–4).
In a cold climate the body also needs thermogenesis to maintain
body temperature, and in this regard, it is mainly central
sympathetic signalling that induces production of heat (5). In previous studies, it has been
indicated that IL4 and IL13 activate macrophages to secrete
catecholamines, which enhance and sustain the thermogenic response
(6,7).
In mice, a substantial portion of this heat is produced in the
brown adipose tissue (BAT) (8).
Since IL6 is among the factors considered to induce
thermogenesis in infection, the present study aimed to investigate
whether IL6 is also important for cold induced thermogenesis. There
is data to suggest that IL6 serum levels are increased during cold
exposure in mice (9). Furthermore,
IL6 deficient (−/−) mice exposed to 4°C during a relatively short
(8 h) period exhibited 2.5°C lower core body temperature compared
with wild-type (Wt) mice exposed to the same condition (10). Given that the initial phase of
cold-induced thermogenesis originates mainly from shivering and
other adaptations to cold rather than non-shivering thermogenesis
(11), the present study wanted to
investigate if IL6 is involved in adaptation to long-term exposure
to cold, i.e. non-shivering thermogenesis.
It has been reported that IL6−/− mice exposed to 4°C
for 3 days exhibited a lower content of uncoupling protein 1 (Ucp1)
protein in inguinal white adipose tissue (WAT) compared with Wt
mice housed under the same conditions (12), which further implies that the
thermoregulation of IL6−/− mice may also differ from Wt mice when
exposed to cold over a longer time period. The present aim was to
investigate the hypothesis that IL6 is important for maintaining
body temperature during 6 days of cold exposure. A brain-specific
IL-6 knockout mouse model was also used to determine if IL6
signalling in the central nervous system (CNS) is important for the
thermogenesis response. The main findings in the present study
supported the hypothesis that IL6 and IL6Rα are important for body
temperature regulation in cold-exposed mice.
Materials and methods
Animals
Global IL6 deficient (IL6−/−) mice on a C57BL/6
background and C57BL/6 Wt control mice were obtained from Jackson
Laboratories (Bar Harbor, ME, USA). For conditional inactivation of
IL6 receptor α (IL6Rα) in the CNS, transgenic mice expressing the
Cre recombinase under the control of the Nestin promoter and
enhancer (Jackson Laboratories) (13)
and with intact IL6Rα gene (IL6Rα+/+) were bred to
homozygous IL6RαloxP/loxP mice in the animal facility at
the University of Gothenburg (Gothenburg, Sweden). The resulting
heterozygous F1 offspring (IL6Rα+/loxP) were either
positive or negative for Nestin-Cre. From mating of
IL6Rα+/loxP with IL6Rα+/loxP Nestin-Cre mice,
F2 animals were obtained of the desired genotypes
[IL6RαloxP/loxP Nestin-Cre (IL6RαNesCre) and
IL6Rα+/+ Nestin-Cre (WtNesCre)].
WtNesCre mice were used as control littermates for
conditional IL6Rα knockout mice. All animal procedures were
approved by the Committee on the Ethics of Animal Experiments at
the University of Gothenburg, Sweden (permit no. 142-2014), and
were conducted in accordance with the EU Directive 2010/63/EU for
animal experiments (14).
The study was performed in two separate cohorts of
animals (IL6−/− or IL6RαNesCre mice and their
corresponding Wt controls, respectively.) The first cohort
comprised 3-month-old male IL6−/− (n=10) and Wt (n=10) mice
weighing 27.6±0.7 g and 27.7±0.4 g, respectively, and the second
cohort comprised 7-month-old male IL6RαNesCre (n=6) and
WtNesCre (n=10) mice weighing 33.2±1.3 g and 35.2±0.6 g,
respectively. All male mice with the correct genotype were
included, despite there being unequal group sizes. All mice were
housed 4–5 per cage under standard conditions at 20–22°C and ~50%
humidity, with free access to food (Teklad global 16% protein
rodent diet; Envigo, Huntingdon, UK) and water, and under a 12-h
light/dark cycle (lights on from 7 a.m. to 7 p.m.).
Body temperature, oxygen consumption
and locomotor activity
Telemetry devices (G2 E-mitter, MiniMitter, Bend,
OR, USA) were implanted via a 1 cm incision through the skin and
peritoneum. Following implantation the peritoneum was sewed with
Polysorb 5-0 suture (Covidien, Dublin, Ireland) and the skin was
closed with Reflex 7 clips (CellPoint Scientific, Inc.,
Gaithersburg, MD, USA). The surgery was performed under 3%
isoflurane (Baxter, Deerfield, IL, USA) anaesthesia. The mice were
left to recover for 1 week prior to the start of experiments. After
recovery from telemetry implantation the mice were housed
individually and gradually brought from 20°C to 4°C over a period
of 6 h and kept at 4°C for 6 days. Animals had free access to fresh
water and standard food pellets (Tekland Global 16% protein rodent
diet), and were kept under standardized conditions as above. Body
weight and food intake was recorded every day during the cold
exposure experiment and the mice were euthanized by intraperitoneal
injection of a mixture of 150 mg/kg ketamine (Ketaminol; Intervet,
Boxmeer, the Netherlands) and 2 mg/kg medetomidine (Domitor vet.,
Orion Pharma, Espoo, Finland) and confirmed by decapitation
immediately following the cold exposure period.
Oxygen consumption and carbon dioxide production
were measured by indirect calorimetry in an INCA metabolic system
(Somedic, Hörby, Sweden) as previously described (10). The system comprises a sealed chamber
with regulated air flow and temperature. It also includes sensors
for the MiniMitter telemetry system, which are designed to measure
core body temperature with 0.01°C accuracy and three-dimensional
locomotor activities via the implanted G2 E-mitter transponders.
The data were collected every minute for all variables except
oxygen consumption, which was measured every second minute, over 24
h. The individual baseline oxygen consumption and core body
temperature was established at 20°C and calculated as mean values
of all time-points when the animal was at rest (no locomotor
activity). The same strategy was used to calculate oxygen
consumption and core body temperature during the first and sixth
day of exposure to 4°C.
Gene expression analysis
The hypothalamus, brain stem, interscapular BAT and
retroperitoneal WAT were dissected, snap frozen in liquid nitrogen
and kept at −80°C until analysis. The tissues were homogenized in
Qiazol (Qiagen GmbH, Hilden, Germany) and mRNA was extracted using
an RNeasy Lipid Tissue Mini kit, according to the manufacturer's
instructions (Qiagen). The mRNA concentration of the samples was
measured by NanoDrop spectrophotometry (NanoDrop Technologies;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA). cDNA was
synthesized from 1 µg mRNA with an iScript cDNA synthesis kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Real time quantitative PCR (qPCR) for BAT and WAT
samples was performed in duplicate using Step-One-Plus (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) under
the following conditions: initial denaturation, 10 min/95°C; 40
cycles, 15 sec/95°C, 60 sec/60°C. The brain stem and hypothalamus
samples were analysed by qPCR using an ABI Prism 7900 Sequence
Detection system (Applied Biosystems) under the conditions: initial
denaturation, 20 sec/95°C; 40 cycles, 1 sec/95°C, 20 sec/60°C. The
brain stem and hypothalamus samples were analysed in duplicate with
Universal Taqman MasterMix containing AmpliTaq Gold® DNA
polymerase (Applied Biosystems; Thermo Fisher Scientific Inc.) and
primer-probe assays for agouti related protein (Agrp;
Mm00475829_g1), brain-derived neurotrophic factor (Bdnf;
Mm04230607_s1), ciliary neurotrophic factor (Cntf; Mm00446373_m1),
preproglucagon (Gcg; Mm01269055_m1), neuropeptide Y (Npy;
Mm00445771_m1), tyrosine hydroxylase (Th; Mm00447557_m1) and Ucp2
(Mm00627599_m1) and were normalized to glucuronidase β
(Mm00446953_m1). The BAT and WAT samples were analysed with Taqman
Fast Advanced Master Mix containing AmpliTaq® Fast DNA
Polymerase (Applied Biosystems) and primer-probe assays for Ucp1
(Mm01244861_m1), fibroblast growth factor 21 (Fgf21;
Mm00840165_g1), peroxisome proliferative activated receptor γ,
coactivator 1-α (Pgc1a; Mm01208835_m1), as well as for Npy and Th,
and were normalized to TATA box binding protein (Mm00446971_m1).
All primer-probe assays included a TaqMan probe with a fluorescent
dye label (FAM) and were purchased from Applied Biosystems (Thermo
Fisher Scientific, Inc.). The relative mRNA levels were obtained by
using the comparative threshold cycle (Cq) method, and calculated
with the ΔΔCq equation (15).
Statistics
Statistical analyses were performed using IBM SPSS
Statistics v20 (IBM Corp., Armonk, NY, USA). Data were analysed
using Student's t-test, with P<0.05 considered to indicate
statistical significance. All data were presented as the mean ±
standard error of the mean, and the mRNA expression levels in the
Wt mouse groups were set to 100% for relative comparison.
Results
Body weight, food intake, body
temperature, oxygen consumption and locomotor activity in global
IL6−/− mice
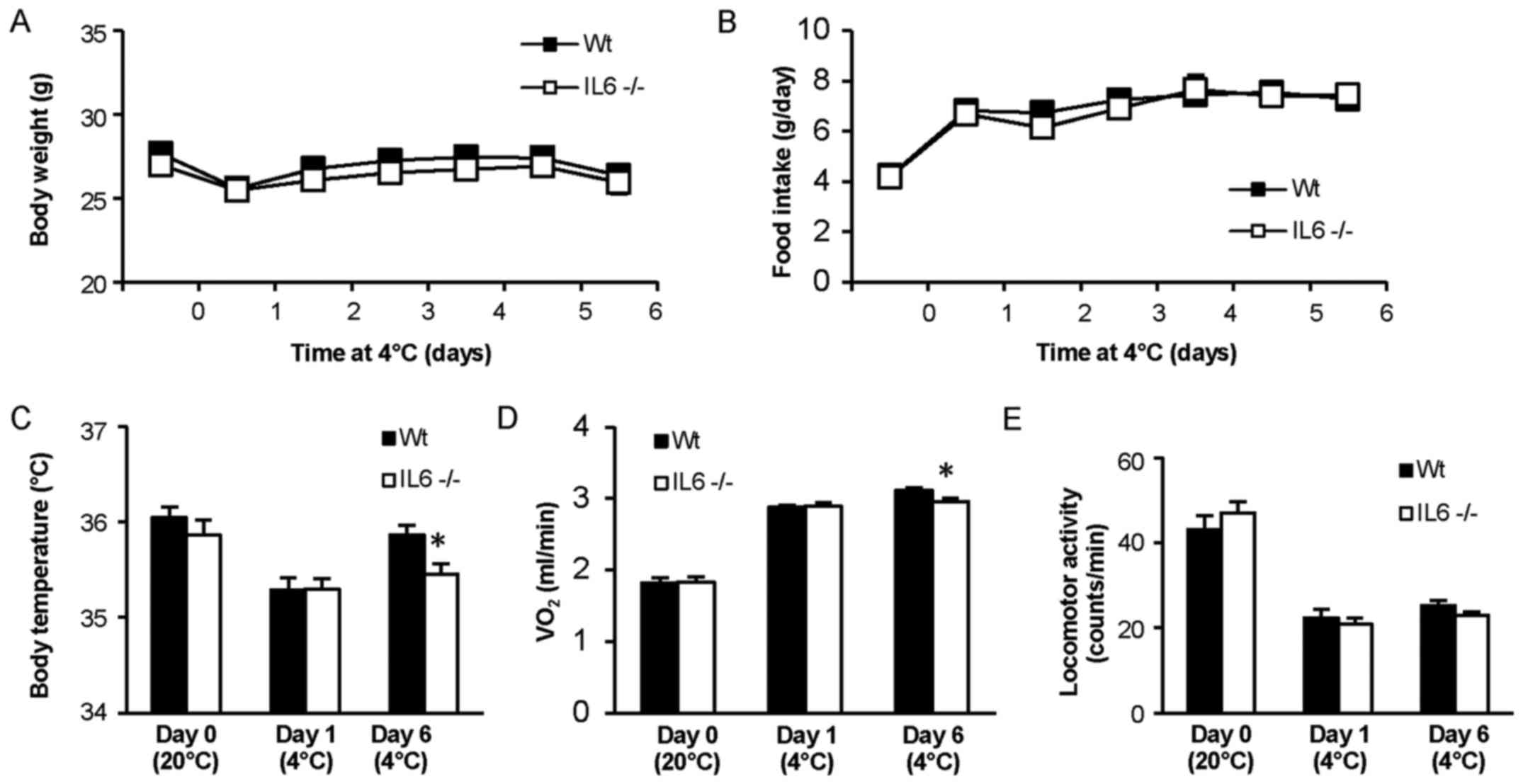
Body weight did not significantly differ between the
IL6−/− and Wt mice at any time-point during the experiment
(Fig. 1A). Food intake was also
similar between the groups and increased markedly in both groups
when exposed to lower ambient temperature (Fig. 1B). Core body temperature did not
differ between IL6−/− and Wt mice at room temperature (20°C) or
during the first day of cold exposure (Fig. 1C), and neither did oxygen consumption
during baseline conditions or the first day at 4°C (Fig. 1D). However, after 6 days in 4°C the
IL6−/− mice exhibited significantly lower body temperature
(P<0.05) and oxygen consumption (P<0.05) compared with Wt
mice. Locomotor activity did not significantly differ between the
groups throughout the measurement period (Fig 1E).
Body weight, food intake, body
temperature, oxygen consumption and locomotor activity in mice with
conditional IL6Rα knockdown in the CNS
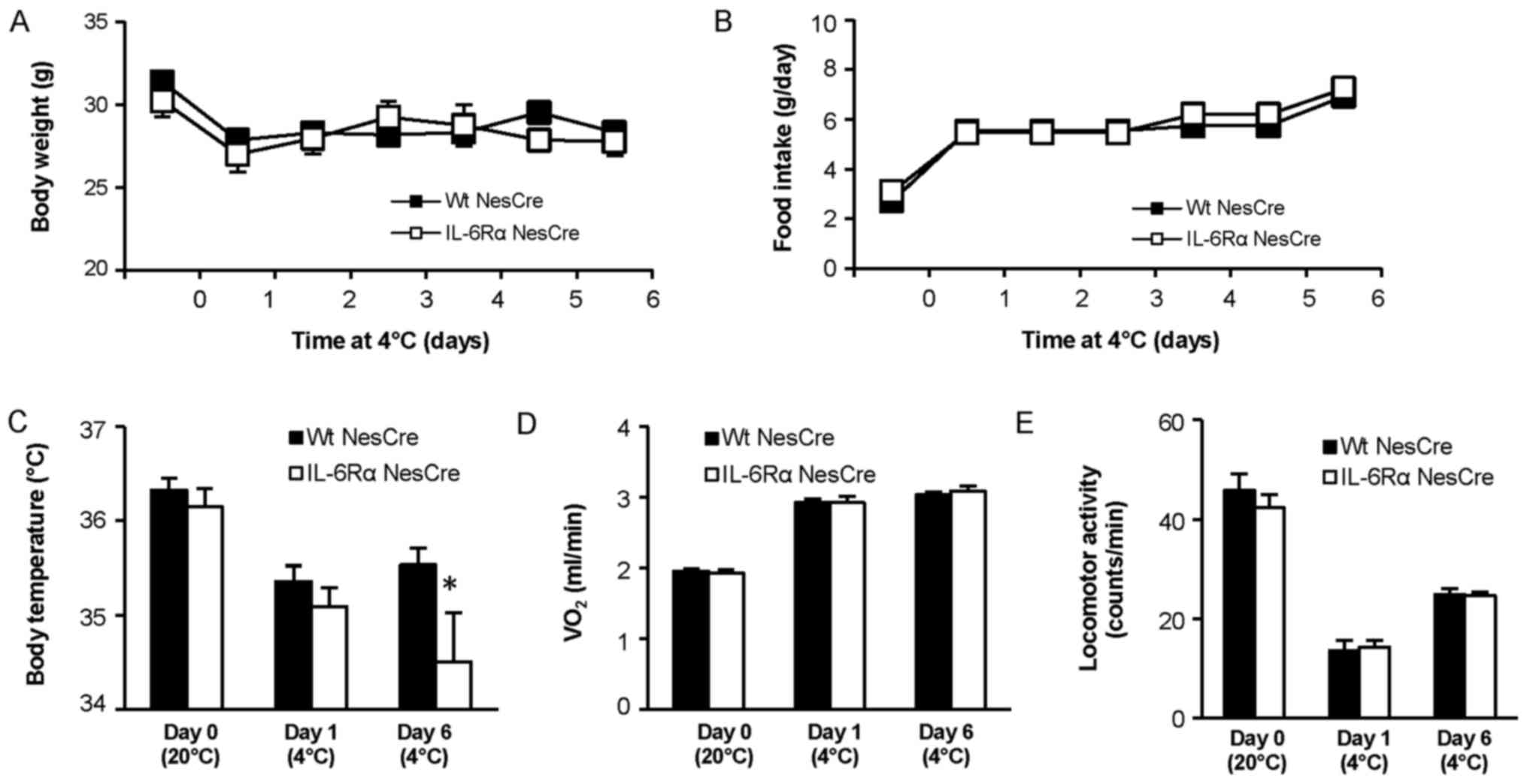
Body weight did not significantly differ between
IL6RαNesCre and WtNesCre mice at baseline or
during the cold exposure (Fig. 2A).
Food intake did not differ between the groups during the
experiment, and an increase in food intake of similar magnitude was
observed for both IL6RαNesCre and WtNesCre
mice when the temperature was reduced to 4°C (Fig. 2B). Core body temperature did not
differ between IL6RαNesCre and WtNesCre mice
at room temperature or during the first day of cold exposure, but
similarly to global IL6−/− mice, core body temperature was lower in
the IL6RαNesCre mice compared with controls after 6 days
at 4°C (P<0.05; Fig 2C). However,
the IL6 knockdown in the CNS did not affect oxygen consumption
(Fig. 2D) or locomotor activity
(Fig. 2E).
Gene expression in the brain stem and
hypothalamus
The mRNA expression of Bdnf, Gcg and Th was
increased 40–50% in the brain stem of IL6−/− mice compared with in
Wt mice after 6 days at 4°C (P<0.05; Fig. 3A). The mRNA expression of Bdnf was
also higher in the brain stem of IL6RαNesCre mice
compared with in WtNesCre mice exposed to 4°C
(P<0.05), but the mRNA levels of Gcg and Th did not differ
between these mice (Fig. 3B). In
contrast to the brainstem, no difference was detected in the mRNA
expression of Bdnf or Th in the hypothalamus of IL6−/− mice or
IL6RαNesCre mice (Fig. 3C and
D). In the hypothalamus from the IL6−/− mice there was a
tendency for increased mRNA expression of Npy compared with Wt
levels (P=0.053; Fig. 3C). Increased
hypothalamic expression of Cntf mRNA (P<0.05) and decreased
hypothalamic expression of Ucp2 mRNA (P<0.05) were identified in
IL6RαNesCre mice compared with WtNesCre
levels (Fig. 3D).
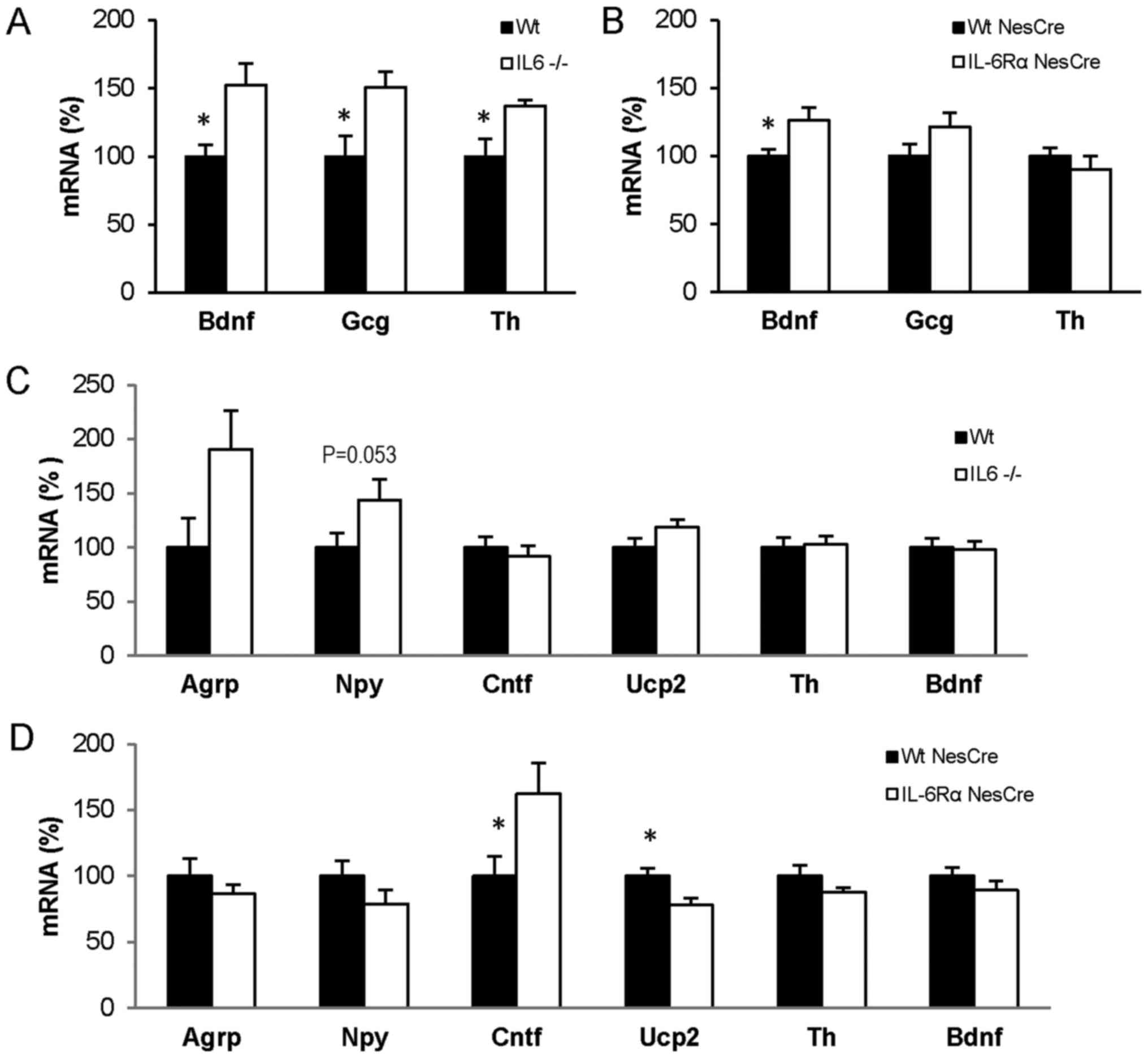 | Figure 3.mRNA expression in brain from mice
exposed to 4°C for 6 days. mRNA expression in (A and B) brain stem
and (C and D) hypothalamus from Wt (n=10) and IL6-deficient
(IL6−/−; n=10) mice and from IL6Rα+/+ Nestin-Cre
(WtNesCre; n=10) and IL6RαloxP/loxP
Nestin-Cre (IL6RαNesCre; n=6) mice. Data are presented
as mean ± standard error of the mean. *P<0.05. IL6,
interleukin-6; IL6Rα, interleukin-6 receptor α; Wt, wild-type;
Bdnf, brain-derived neurotrophic factor; Gcg, preproglucagon; Th,
tyrosine hydroxylase; Agrp, agouti related protein; Npy,
neuropeptide Y; Cntf, ciliary neurotrophic factor; Ucp2, uncoupling
protein 2. |
Gene expression in BAT and WAT
There were no significant differences in the mRNA
levels of Ucp1, Pgc1a, Fgf21, Npy and Th in BAT between IL6−/− and
Wt mice after 6 days at 4°C (Fig.
4A), nor in the same genes in BAT from IL6RαNesCre
mice compared with WtNesCre mice under the same
conditions (Fig. 4B). Similarly, no
differences were identified in the gene expressions of Ucp1, Pgc1a,
Fgf21, Npy and Th in the WAT of IL6−/− mice (Fig. 4C). In the WAT of the
IL6RαNesCre mice, a tendency for lower Pgc1a mRNA levels
(P=0.053) and a significant decrease in Npy levels (P<0.05)
compared with control levels were identified (Fig. 4D).
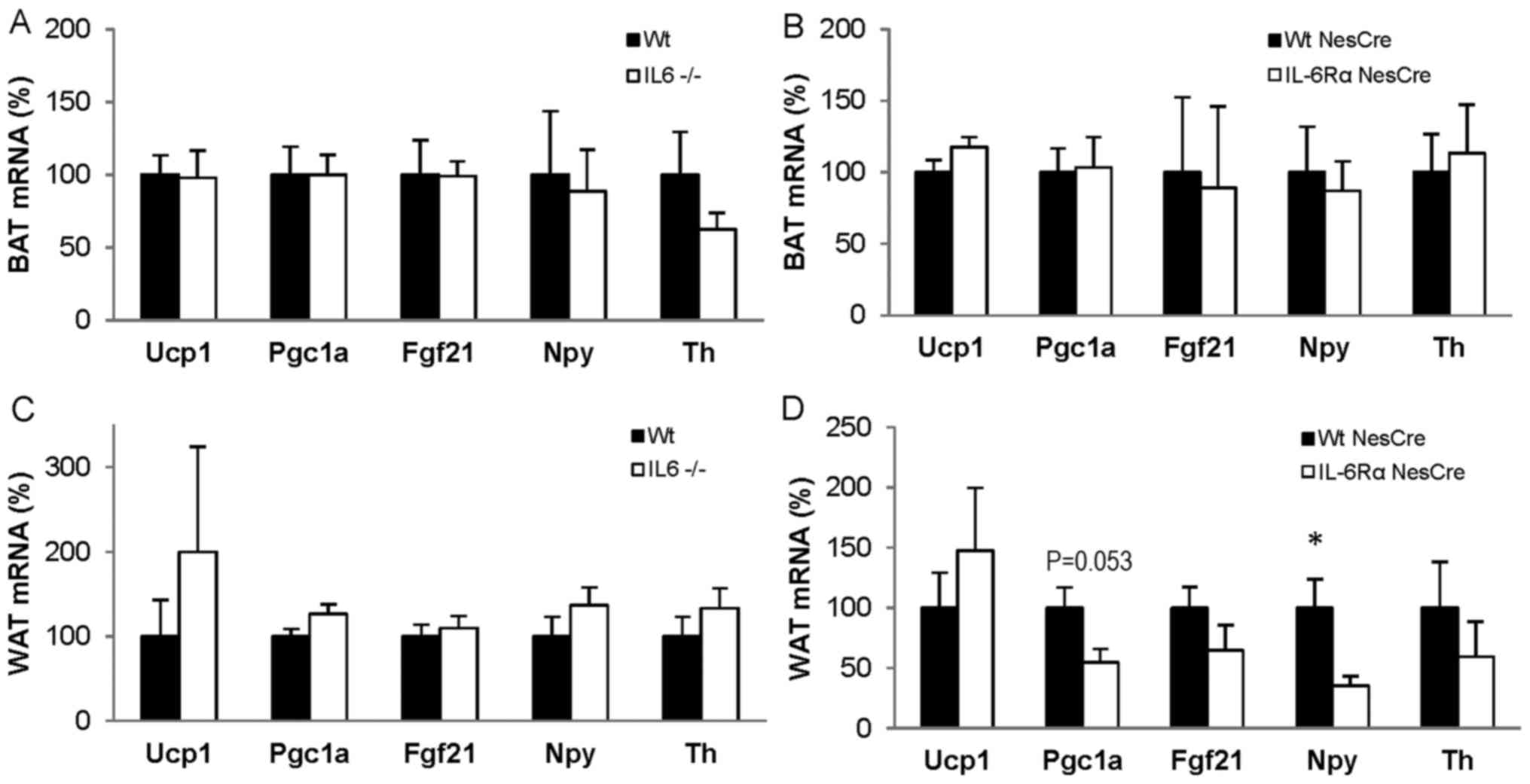 | Figure 4.mRNA expression in adipose tissue
from mice exposed to 4°C for 6 days. mRNA expression in (A and B)
BAT and (C and D) WAT from Wt (n=10) and IL6-deficient (IL6−/−;
n=10) mice and from IL6Rα+/+ Nestin-Cre
(WtNesCre; n=10) and IL6RαloxP/loxP
Nestin-Cre (IL6RαNesCre; n=6) mice. Data are presented
as mean ± standard error of the mean. *P<0.05. IL6,
interleukin-6; IL6Rα, interleukin-6 receptor α; Wt, wild-type; BAT,
brown adipose tissue; WAT, white adipose tissue; Ucp1, uncoupling
protein 1; Pgc1a, peroxisome proliferative activated receptor γ,
coactivator 1-α; Fgf21, fibroblast growth factor 21; Npy,
neuropeptide Y; Th, tyrosine hydroxylase. |
Discussion
Body temperature was decreased in IL6−/− as well as
IL6RαNesCre mice compared with respective Wt controls
after 6 days of cold exposure, indicating a decrease in
thermogenesis. Additionally, oxygen consumption in IL6−/− mice was
lower compared with Wt mice after 6 days at 4°C, indicating lower
energy expenditure in these mice. As mice with conditional IL6Rα
knockdown in the CNS did exhibit lower body temperature after 6
days of cold exposure, this suggests that the IL6 signalling in the
brain may be of importance for thermogenesis during cold
exposure.
Thermogenesis during cold exposure is an important
homeostatic mechanism in mammals involving several tissues
including the brain, BAT and skeletal muscle (5,16). A
previous view of the different roles of tissues involved in
thermogenesis was that the skeletal muscle was mainly involved in
shivering thermogenesis while BAT was the main tissue responsible
for non-shivering thermogenesis (17). More recently there has been data to
suggest that skeletal muscle is also important for non-shivering
thermogenesis in mammals (16,18,19).
However, the present study focused on the BAT, WAT and the brain,
but not the skeletal muscle component of thermogenesis. In future
studies it would be interesting to investigate the expression of
mitochondria-associated genes in skeletal muscle following cold
exposure in IL6−/− mice, since IL6 is expressed in skeletal muscle
following muscle contractions (20).
In a recent study, the IL6 levels in serum were
demonstrated to increase in mice following 15 days of exposure to
cold temperature (4°C) (9).
Furthermore, decreased body temperature observed in the present
study in IL6−/− mice housed for 6 days in cold ambient temperature
indicates that the decrease in temperature in cold-exposed IL6−/−
for 8 h (10) is not transient. Thus,
the present results suggest that the previously observed acute
decrease in body temperature in IL6−/− mice may be due to an
incomplete thermogenesis response to the cold environment and not
just a stress effect of sudden decrease in ambient temperature.
The adaption to lower ambient temperature was
evident by the increases in food intake and oxygen consumption in
all mice independent of genotype. Both Wt and IL6−/− mice ate close
to double the quantity of food per day in cold temperature (4°C)
compared with when housed at room temperature (20°C), and since
there was no difference in food intake between mice with different
genotypes, food intake as a cause of differing body temperature
seems unlikely. However, the IL6−/− mice consumed significantly
less oxygen after 6 days at cold ambient temperature compared with
the Wt mice, suggesting that lower energy expenditure may in part
explain the lower body temperature in IL6−/− mice. The lower energy
expenditure in combination with increased food intake in the IL6−/−
mice may in the long-term result in increased fat mass, in line
with previously published results of mature-onset obesity in IL6−/−
mice (21).
The IL6RαNesCre mice also exhibited a
lower body temperature after 6 days of cold exposure compared with
the control mice, but no marked differences in food intake or
oxygen consumption. This may indicate that the mice with
brain-specific loss of IL6Rα are attempting to compensate for the
lower body temperature in a distinct way to the global IL6−/− mice.
For instance, there is a possibility that these mice have decreased
recruitment of BAT thermogenesis, which could initiate
hyper-recruitment of non-shivering thermogenesis in muscle, as
previously reported in Ucp1−/− mice (22) and mice with ablated BAT (19).
The mRNA expressions of Bdnf, Gcg and Th were
increased in the brain stem of IL6−/− mice after 6 days of cold
exposure. Hindbrain injections of Bdnf have previously been
identified to increase core body temperature in rats, indicating
that the brain stem is important for mediating the thermogenesis
response (23). Furthermore, Bdnf in
the hypothalamus, particularly in the medial and posterior
paraventricular regions, may stimulate adaptive thermogenesis in
BAT (24). The present study observed
no difference in Bdnf mRNA levels in the hypothalamus (without
dissecting separate nuclei), but the finding of increased Bdnf in
the brain stem of cold-exposed IL6−/− mice indicates that Bdnf in
the brain stem may be among the mechanistic factors involved in
counteracting the low body temperature in IL6−/− and
IL6RαNesCre mice.
Gcg is a complex gene that via different splicing
variants encodes glucagon, glucagon like peptide (Glp)1 and Glp2.
In the brain stem the Gcg product is spliced into Glp1 and Glp2,
which are produced in equal quantities (25), and therefore the increase in Gcg
detected in the brain stem probably mirrors an increase in Glp1,
which has previously been reported to increase BAT thermogenesis
(26–28). Th is required in the synthesis of
noradrenalin, and noradrenalin is required to activate BAT
thermogenesis (8). Since IL6−/− mice
exhibited a lower body temperature than Wt mice, the increase of
Gcg and Th may be a mechanism attempting to increase thermogenesis
in BAT through the Glp1 and catecholamine pathways.
mRNA expression profiles in the hypothalamus
exhibited an increase in the neurotrophic factor Cntf in the
IL6RαNesCre mice compared with controls. Cntf is a
member of the IL6 family with effects on energy balance (29), and the loss of IL6Rα signalling
specifically in the brain of the IL6RαNesCre mice may
have possibly induced a compensatory increase in Cntf expression in
the hypothalamus of these mice. Overexpression of Ucp2 in specific
neurons in the hypothalamus has been reported to decrease core body
temperature in mice (30), and UCP2
may also co-localize with Agrp/Npy in the hypothalamus and
influence their metabolic regulation (31). The decreased levels of Ucp2 in the
hypothalamus of IL6RαNesCre mice may have been a method
for increasing the body temperature of these mice. In a previous
study by our group an increase in the mRNAs of the orexigenic genes
Agrp and Npy was noted in the hypothalamus of IL6−/− mice compared
with Wt mice after 4 weeks at cold ambient temperature (32). This was not as evident after 6 days of
cold exposure, but was close to reaching significance for Npy in
this present study. NPY signalling from the arcuate nucleus of the
hypothalamus has been identified to decrease Th mRNA in the brain
stem and hypothalamus, resulting in decreased BAT thermogenesis
(33). However, decrease in Th mRNA
in the hypothalamus was not identified presently, and conversely,
an increase of Th mRNA was observed in the brain stem of the IL6−/−
mice. The reason for the tendency towards increased expression of
Npy and Agrp is unclear, given that no significant difference was
determined in food intake at 4°C between the mouse groups.
Implications of the present study may be limited, as
in the hypothalamus of IL6RαNesCre mice, an increase in
Agrp, Npy or Th mRNA compared with control levels was not detected.
In future studies it would be interesting to expose these mice to a
longer cold exposure, to investigate if the cold ambient condition
would eventually induce upregulation of Npy, Agrp and Th as seen in
the IL6−/− mice.
Npy and Th in BAT have also been suggested to
contribute to BAT-based thermogenesis during cold exposure
(34). In the present study, the mRNA
levels of Npy and Th in BAT were similar between groups, but a
significant decrease of Npy mRNA was identified in the WAT of
IL6RαNesCre mice compared with controls. This may
indicate a role of NPY in the browning of WAT, a process defined as
increased Ucp1 expression in WAT (35), besides the recruitment of BAT
thermogenesis.
Ucp1 mRNA expression in WAT did not differ between
IL6−/− and Wt mice after 6 days of cold exposure, which is in line
with the previous finding of no difference between IL6−/− and Wt
mice after 3 days of exposure to 4°C (12). However, Knudsen et al (12) observed a lower Ucp1 protein content in
inguinal WAT of IL6−/− mice compared with Wt mice, indicating that
IL6 could have an impact on Ucp1 protein content, although the mRNA
levels do not differ. The gene expression of the thermogenic
biomarkers Pgc1a, and Fgf21 was not decreased in the IL6−/− mice,
but there was a near significant decrease of Pgc1a in the WAT of
IL6RαNesCre mice, indicating a lower degree of browning
of WAT in these mice (36). However,
the present study did not perform histology of WAT or BAT, which
may have been informative regarding, for instance, the degree of
browning of the WAT. With regard to Ucp1 in BAT, the mRNA levels
were similar to controls in both global IL6−/− and brain specific
IL6RαNesCre mice after 6 days of cold exposure. Previous
studies of IL6−/− mice at room temperature and after 3 days of cold
exposure identified no difference in Ucp1 protein content between
IL6−/− and controls (10,12). The possible role of IL6 in increasing
the thermogenesis activity in BAT is therefore not obvious.
However, there is one study indicating an association between
chronic elevation of IL6 in the CNS and increased Ucp1 protein
levels in BAT in rats (37). This
elevation in Ucp1 levels was not observed in denervated BAT tissue,
indicating that sympathetic nerve signalling may be involved
(37).
Collectively, the data suggest that IL6 is not only
involved in body temperature regulation during infection, but also
contributes to long-term cold induced thermogenesis. Furthermore,
the results from brain-specific knockdown of IL6Rα indicate that
the impact of IL6 on thermogenesis is mediated by IL6Rα signalling
in the brain.
Acknowledgements
Michael Axelsson and Ola Svensson are gratefully
acknowledged for providing access to the climate chamber at the
Zoology Department, University of Gothenburg, Sweden. The abstract
was presented at the European Obesity Summit (EOS) - Joint Congress
of EASO and IFSO-EC 1–4 June 2016 in Gothenburg, Sweden, and
published as abstract no. PO1.046 in Obes Facts 9 (Suppl 1):
2016.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
EE designed the study, performed the implantation of
telemetry devices and dissected tissues. FA performed the in
vivo experiments and dissected tissues. ES performed the gene
expression analysis of the tissues and interpreted the
corresponding data. VP designed the study, performed the in
vivo experiments, analysed and interpreted the physiological
data, performed the gene expression analysis and wrote the
manuscript. All authors read and approved the final study.
Ethics approval and consent to
participate
All animal procedures were approved by the Committee
on the Ethics of Animal Experiments at the University of
Gothenburg, Sweden (permit no. 142-2014) and were conducted in
accordance with the EU Directive 2010/63/EU for animal
experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luheshi GN: Cytokines and fever.
Mechanisms and sites of action. Ann N Y Acad Sci. 856:83–89. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leon LR: Invited review: cytokine
regulation of fever: studies using gene knockout mice. J Appl
Physiol 1985. 92:2648–2655. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sundgren-Andersson AK, Ostlund P and
Bartfai T: IL-6 is essential in TNF-alpha-induced fever. Am J
Physiol. 275:R2028–R2034. 1998.PubMed/NCBI
|
|
4
|
Zetterström M, Sundgren-Andersson AK,
Ostlund P and Bartfai T: Delineation of the proinflammatory
cytokine cascade in fever induction. Ann N Y Acad Sci. 856:48–52.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morrison SF and Nakamura K: Central neural
pathways for thermoregulation. Front Biosci. 16:74–104. 2011.
View Article : Google Scholar
|
|
6
|
Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J,
David T, Mukundan L, Brombacher F, Locksley RM and Chawla A:
Alternatively activated macrophages produce catecholamines to
sustain adaptive thermogenesis. Nature. 480:104–108. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian
X, Locksley RM, Palmiter RD and Chawla A: Eosinophils and type 2
cytokine signaling in macrophages orchestrate development of
functional beige fat. Cell. 157:1292–1308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cannon B and Nedergaard J: Brown adipose
tissue: Function and physiological significance. Physiol Rev.
84:277–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bal NC, Maurya SK, Pani S, Sethy C,
Banerjee A, Das S, Patnaik S and Kundu CN: Mild cold induced
thermogenesis: Are BAT and skeletal muscle synergistic partners?
Biosci Rep. 37:BSR201710872017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wernstedt I, Edgley A, Berndtsson A, Fäldt
J, Bergström G, Wallenius V and Jansson JO: Reduced stress- and
cold-induced increase in energy expenditure in
interleukin-6-deficient mice. Am J Physiol Regul Integr Comp
Physiol. 291:R551–R557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cannon B and Nedergaard J: Nonshivering
thermogenesis and its adequate measurement in metabolic studies. J
Exp Biol. 214:242–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knudsen JG, Murholm M, Carey AL, Biensø
RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen
JB, et al: Role of IL-6 in exercise training- and cold-induced UCP1
expression in subcutaneous white adipose tissue. PLoS One.
9:e849102014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reichardt HM, Kellendonk C, Tronche F and
Schütz G: The Cre/loxP system-a versatile tool to study
glucocorticoid signalling in mice. Biochem Soc Trans. 27:78–83.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Directive 2010/63/EU of the European
Parliament and of the Council of 22 September 2010 on the
protection of animals used for scientific purposes. OJ L.
276:33–79. 2010.
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rowland LA, Bal NC and Periasamy M: The
role of skeletal-muscle-based thermogenic mechanisms in vertebrate
endothermy. Biol Rev Camb Philos Soc. 90:1279–1297. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morrison SF, Nakamura K and Madden CJ:
Central control of thermogenesis in mammals. Exp Physiol.
93:773–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pant M, Bal NC and Periasamy M:
Sarcolipin: A key thermogenic and metabolic regulator in skeletal
muscle. Trends Endocrinol Metab. 27:881–892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bal NC, Maurya SK, Singh S, Wehrens XH and
Periasamy M: Increased reliance on muscle-based thermogenesis upon
acute minimization of brown adipose tissue function. J Biol Chem.
291:17247–17257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jonsdottir IH, Schjerling P, Ostrowski K,
Asp S, Richter EA and Pedersen BK: Muscle contractions induce
interleukin-6 mRNA production in rat skeletal muscles. J Physiol.
528:157–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wallenius V, Wallenius K, Ahrén B, Rudling
M, Carlsten H, Dickson SL, Ohlsson C and Jansson JO:
Interleukin-6-deficient mice develop mature-onset obesity. Nat Med.
8:75–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rowland LA, Bal NC, Kozak LP and Periasamy
M: Uncoupling protein 1 and sarcolipin are required to maintain
optimal thermogenesis, and loss of both systems compromises
survival of mice under cold stress. J Biol Chem. 290:12282–12289.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spaeth AM, Kanoski SE, Hayes MR and Grill
HJ: TrkB receptor signaling in the nucleus tractus solitarius
mediates the food intake-suppressive effects of hindbrain BDNF and
leptin. Am J Physiol Endocrinol Metab. 302:E1252–E1260. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An JJ, Liao GY, Kinney CE, Sahibzada N and
Xu B: Discrete BDNF neurons in the paraventricular hypothalamus
control feeding and energy expenditure. Cell Metab. 22:175–188.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Larsen PJ, Tang-Christensen M, Holst JJ
and Orskov C: Distribution of glucagon-like peptide-1 and other
preproglucagon-derived peptides in the rat hypothalamus and
brainstem. Neuroscience. 77:257–270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beiroa D, Imbernon M, Gallego R, Senra A,
Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada
J, et al: GLP-1 agonism stimulates brown adipose tissue
thermogenesis and browning through hypothalamic AMPK. Diabetes.
63:3346–3358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lockie SH, Heppner KM, Chaudhary N,
Chabenne JR, Morgan DA, Veyrat-Durebex C, Ananthakrishnan G,
Rohner-Jeanrenaud F, Drucker DJ, DiMarchi R, et al: Direct control
of brown adipose tissue thermogenesis by central nervous system
glucagon-like peptide-1 receptor signaling. Diabetes. 61:2753–2762.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kooijman S, Wang Y, Parlevliet ET, Boon
MR, Edelschaap D, Snaterse G, Pijl H, Romijn JA and Rensen PC:
Central GLP-1 receptor signalling accelerates plasma clearance of
triacylglycerol and glucose by activating brown adipose tissue in
mice. Diabetologia. 58:2637–2646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stefater MA, MacLennan AJ, Lee N,
Patterson CM, Haller A, Sorrell J, Myers M, Woods SC and Seeley RJ:
The anorectic effect of CNTF does not require action in
leptin-responsive neurons. Endocrinology. 153:2647–2654. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Conti B, Sanchez-Alavez M, Winsky-Sommerer
R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S,
Henriksen S, Zorrilla EP, et al: Transgenic mice with a reduced
core body temperature have an increased life span. Science.
314:825–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coppola A, Liu ZW, Andrews ZB, Paradis E,
Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, et
al: A central thermogenic-like mechanism in feeding regulation: An
interplay between arcuate nucleus T3 and UCP2. Cell Metab. 5:21–33.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schéle E, Benrick A, Grahnemo L, Egecioglu
E, Anesten F, Pálsdóttir V and Jansson JO: Inter-relation between
interleukin (IL)-1, IL-6 and body fat regulating circuits of the
hypothalamic arcuate nucleus. J Neuroendocrinol. 25:580–589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi YC, Lau J, Lin Z, Zhang H, Zhai L,
Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, et al: Arcuate
NPY controls sympathetic output and BAT function via a relay of
tyrosine hydroxylase neurons in the PVN. Cell Metab. 17:236–248.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bal NC, Singh S, Reis FCG, Maurya SK, Pani
S, Rowland LA and Periasamy M: Both brown adipose tissue and
skeletal muscle thermogenesis processes are activated during mild
to severe cold adaptation in mice. J Biol Chem. 292:16616–16625.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nedergaard J and Cannon B: The browning of
white adipose tissue: Some burning issues. Cell Metab. 20:396–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jankovic A, Golic I, Markelic M, Stancic
A, Otasevic V, Buzadzic B, Korac A and Korac B: Two key temporally
distinguishable molecular and cellular components of white adipose
tissue browning during cold acclimation. J Physiol. 593:3267–3280.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li G, Klein RL, Matheny M, King MA, Meyer
EM and Scarpace PJ: Induction of uncoupling protein 1 by central
interleukin-6 gene delivery is dependent on sympathetic innervation
of brown adipose tissue and underlies one mechanism of body weight
reduction in rats. Neuroscience. 115:879–889. 2002. View Article : Google Scholar : PubMed/NCBI
|