Introduction
Head and neck cancer (HNC) is the seventh most
common malignancy worldwide in men and also the thirteenth most
common in women (1). HNC may be
subdivided into oral, oropharyngeal, laryngeal and skin cancers
(2). Squamous cell carcinoma of the
head and neck (HNSCC) is the most common histopathological
diagnosis within these subgroups. Alcohol and tobacco consumption
are risk factors for carcinogenesis (3). Sun light exposure also has an impact on
the development of skin cancer in the head and neck area (4). In the last decade, human papilloma virus
(HPV) has also emerged as an aetiological factor in the development
of HNSCC (5).
Oral squamous cell carcinoma (OSCC) has the second
worst prognosis after laryngeal squamous cell carcinoma among HNCs;
laryngeal squamous cell carcinoma has a progression-free 5 year
survival <50%, while the progression-free 5 year survival of
OSCC is <70% (6). Extended tumour
growth and lymph node metastasis are frequently detected at primary
diagnosis (7). Surgical treatment
options to reconstruct the complex anatomy of the head and neck are
available. Adequate functional recovery may be achieved with use of
reconstructive microvascular flaps combined with sufficient
maintenance of health following surgery (8). Nevertheless, recurrence and metastasis
generally occurs in 25% of patients following operative treatment
with curative intention (9).
Treatment possibilities in cases of recurrence or metastasis are
limited and no marked improvement of survival probability has been
achieved with these treatment options to date (10); existing protocols including
radiochemotherapy with cisplatin, or in cases of epidermal growth
factor receptor (EGFR) expression (11), use of EGFR antibodies, have not
provided the required therapeutic progress (12). Therefore, advanced treatment
strategies should be developed. The molecular interactions of
cancer cells are complex and various pathways are involved. This
complexity has been described by Hanahan and Weinberg as
constituting the hallmarks of cancer (13). Cells within a tumour are heterogenous
and have different capacities, and various cell reactions to
existing therapies may occur; in particular, the capability to
invade the surrounding tissue and gain increased metabolism are
among the most important functions (14). An important achievement has been the
detection of cancer stem cells (CSCs). These cells are considered
to be a central element of cancer development, first described in
1997 (15,16). They have typical stem cell
characteristics including self-renewal and differentiation
potential and may be responsible for malignant cancer
characteristics including resistance to therapy, metastasis and
recurrence (17). It has been
reported only when CSCs amount to 10% of cells in a tumour mass
that they negatively influence the prognosis of patients (17). An aim of oncology research is to
analyse CSCs and their characteristics, first for individual risk
stratification, and furthermore to develop targeted therapies.
Aldehyde dehydrogenase 1 (ALDH1) is an enzyme located in the
cytoplasma and mitochondria. It has been identified in various
cancers including glioblastoma and breast cancer, in which it was
determined as a predictive marker of worse prognosis (18,19). High
expression of ALDH1 was preferential to cells with CSC
characteristics (20) and ALDH1 was
detected as a CSC marker (CSCM) in HNSCC cell lines (21). Previous study demonstrated a
correlation between ALDH1 and oropharyngeal cancers (OPSCCs) and
indicated a malignant influence of this CSCM expression on
prognosis (22). However to date, to
the best of our knowledge, no large-scale comparable studies on
OSCC and the expression of ALDH1 have been performed. An aim of the
current study was to evaluate ALDH1 expression in HPV+ and – OSCC.
The expression of p16ink4a, a surrogate marker for HPV
detection, and one of the proteins encoded by the cell cycle
interacting protein cyclin-dependent kinase inhibitor 2A (CDKN2A)
gene (23), was a further molecular
topic of the present study. DNA methylation, for example associated
with smoking, may lead to mutation of the CDKN2A gene and thus
p16ink4a. This tumour suppressor is established to be
mutated in HNSCC and breast and colon cancers (24–26).
Recently, interactions of p16ink4a have been established
to serve functions in regulating CSC and reversing the senescence
of CSC during therapy (27).
Furthermore, the overexpression of p16ink4a is
considered to have negative prognostic influence and promote tumour
recurrence and poor survival (28).
The current study aim was to evaluate the expression of ALDH1 and
p16ink4a in OSCC via immunohistochemistry (IHC) and to
crosslink expression profiles with clinicopathological data of
patients.
Materials and methods
Participants
The present study involved 186 patients with OSCC.
Surgical treatment was performed in the Department of Oral and
Maxillofacial Surgery, Klinikum rechts der Isar, Technical
University of Munich (Munich, Germany) between January 2009 and
December 2012. The surgical treatment included tumour resection,
neck dissection with intraoperative margin control and freezing of
sections, and primary reconstruction with microvascular flaps. All
patients were considered pre- and post-operation at the
interdisciplinary conference of head and neck cancer of the
university hospital (Klinikum rechts der Isar). The clinical data
of all patients was archived, and formalin-fixed and
paraffin-embedded tissue was available for all patients. Follow-up
examinations were provided to all enrolled patients according to
the German S3 guidelines for oral cancer (29). These guidelines recommend that oral
cancer patients should be followed up every three months within the
first 2 years after primary diagnosis, and then every 6 months in
years 3 and 4 of follow-up thereafter. Computer tomographical scans
of the head and neck were provided at all second follow-ups.
According to the guidelines, the cancer aftercare follow-up aimed
to be completed after 5 years of the initial diagnosis. All tumour
operations had the intention of curative treatment and included a
neck dissection. As intraoperative controls, an intraoperative
margin control and frozen sections were also obtained in each
tumour operation. As previously described (30), adjuvant cisplatin-based chemoradiation
was performed in cases of lymph node metastasis, extracapsular
spread, tumour infiltration of the bone, tumours of extended size
(T2-T4) and positive microscopic resection margins (R1) according
to the S3 German guidelines for oral cancer. Patients were excluded
from the study if no curative surgical treatment was performed and
the primary treatment was radiochemotherapy. The methods were
approved by the Ethical Committee of the Technical University of
Munich (approval no. 212108) and in accordance with the Declaration
of Helsinki and all patients agreed in writing to the use of their
samples for the present research purposes.
Tissue microarray construction
(TMA)
The centre and invasive front of tumours of all
study participants were included in the TMA (30,31). The
tissues were formalin-fixed and paraffin-embedded in blocks
(30,31). Two pathologists analysed the areas to
be represented in the TMA. Two tumour cores from the tumour centre
and the invasion front and the corresponding lymph nodes were
assembled into the TMA by using a Tissue Microarrayer (Beecher
Instruments, Inc., Sun Prairie, WI, USA) as described previously
(31).
IHC. p16ink4a was stained on the
2-µm-thick sections from each TMA slide using a p16ink4a
antibody (cat. no. 725–4713; Ventana Medical Systems, Inc.; Roche,
Tucson, AZ, USA) as described previously (30). ALDH1 was also stained on 2-µm-thick
sections from each TMA using an ALDH1 primary antibody (cat. no.
611194; BD Biosciences, San Jose, CA, USA) at a dilution of 1:500.
The staining was performed according to the manufacturer's
recommendations and as described in the literature (19). Negative and positive controls were
stained for both antibodies. For p16ink4a staining,
sections of HPV-negative cervix carcinomas were used as the
negative control and HPV-positive cervix carcinoma sections as the
positive control. For ALDH1 staining, ALDH1-positive glioblastoma
sections were the positive control and urothel carcinoma sections
the negative control for this antibody (30,31). The
use of control samples followed the approved protocol of the
institutional ethics committee and all patients from which control
samples were isolated provided informed written consent.
Scoring of ALDH1 and
p16ink4a
The staining was evaluated under a light microscope
at a magnification of ×200. An international scoring system was
used as described previously (32),
which incorporated the intensity of the staining as well as the
number of stained cells. The staining intensity and cell number
were individually scored between 0 and 4. The intensity score was
scored as follows: No staining was scored as 0, weak staining as 1,
intermediate to weak staining as 2, intermediate staining as 3 and
strong staining as 4. Positive cell proportion was assigned as 0 if
<10%, 1 if 10–25%, 2 if 25–50%, 3 if 50–75% and 4 if >75%, as
previously described (31). For each
section, the staining intensity and cell proportion scores were
then multiplied as described (31).
As a result, the subgroup scores 0, 2, 4, 12 and 16 represented
ALDH1 staining. Furthermore, based on the staining results, final
scores for a positivity cut-off was established for ALDH1. The
subgroup scores 12 and 16 were taken to indicate positivity; while
the subgroups 0, 2 and 4 were considered to indicate negativity for
ALDH1. For p16ink4a, the subgroup scores for positivity
were 12 and 16 and for negativity was 0.
HPV detection
HPV detection was performed on DNA purified from the
formalin-fixed, paraffin-embedded (FFPE) tumour tissue (Qiamp DNA
FFPE tissue kit; Qiagen GmbH, Hilden, Germany) by polymerase chain
reaction, and an HPV Array kit (HPV 3.5; Zytomed Systems, Berlin,
Germany) was used to clarify the HPV subtype, as described
previously by our research group (30).
Statistics
SPSS software for Windows, version 23.0 (IBM Corp.,
Armonk, NY, USA) was used for statistical analysis. The
Mann-Whitney U test and χ2 test were used to analyse the
data. Survival rates were evaluated by the Kaplan-Meier method and
presented as the mean ± standard deviation and confidence interval
(CI). To test for the significance of differences in survival
probabilities the log-rank test was used. Risk regression models as
the Kruskal Wallis test was used to assess the association of CSCM
and p16ink4a expression with tumour size and lymph node
metastasis. The Cox proportional hazards model was used to analyse
multivariate survival rates. P<0.05 was considered to indicate
statistical significance.
Results
Clinical data
Baseline clinical characteristics of the 186
enrolled patients with a diagnosis of OSCC are listed in Table I. Follow-up data revealed the mean
survival time of the cohort to be 44.54±86.81 months (95% CI:
32.06–57.02). Survival rate was significantly influenced in cases
of lymph node recurrence (P=0.020). This occurred in 18 patients of
the total cohort. In these cases, survival time decreased to
36.31±21.39 months (95% CI: 26.43–46.19). Another notable
significant effect on survival rate was observed with regard to
local recurrence (p=0.027), which affected 25 patients, with
survival time decreased to 28.49±23.97 months (95% CI:19.09–37.89).
At primary diagnosis, the following aspects had significant
influence on overall survival rate; patients with Union for
International Cancer Control (UICC) stage III cancer had
significantly reduced survival time compared with the cases of
stages I and II cancer (P=0.003), as did patients with UICC stage
IV cancer (P=0.001); patients with N2 stage cancer had
significantly reduced survival time compared with cases of N0 stage
cancer (P=0.001); and stage 4 grading had significant negative
influence on survival compared with stage 1–3 grading (P=0.001)
(data not shown).
 | Table I.Baseline clinicopathological
characteristics of the OSCC patients (n=186). |
Table I.
Baseline clinicopathological
characteristics of the OSCC patients (n=186).
| Clinical
parameter | Patients |
|---|
| Median age, years
(range) | 58.68
(41.02–85.82) |
| Gender |
|
|
Male/female | 142/44 |
| UICC stage |
|
| I | 38 |
| II | 39 |
|
III | 29 |
|
IVa | 80 |
| Tumour size |
|
| T1 | 48 |
| T2 | 78 |
| T3 | 31 |
|
T4a | 29 |
| N stage (lymph node
metastasis) |
|
| N0 | 73 |
| N1 | 38 |
|
N2a/b/c | 9/37/29 |
| Extracapsular
spread | 10 |
| Grading |
|
| G1 | 10 |
| G2 | 135 |
| G3 | 33 |
| G4 | 8 |
Association of ALDH1 expression with
survival rate
No difference was observed between the different
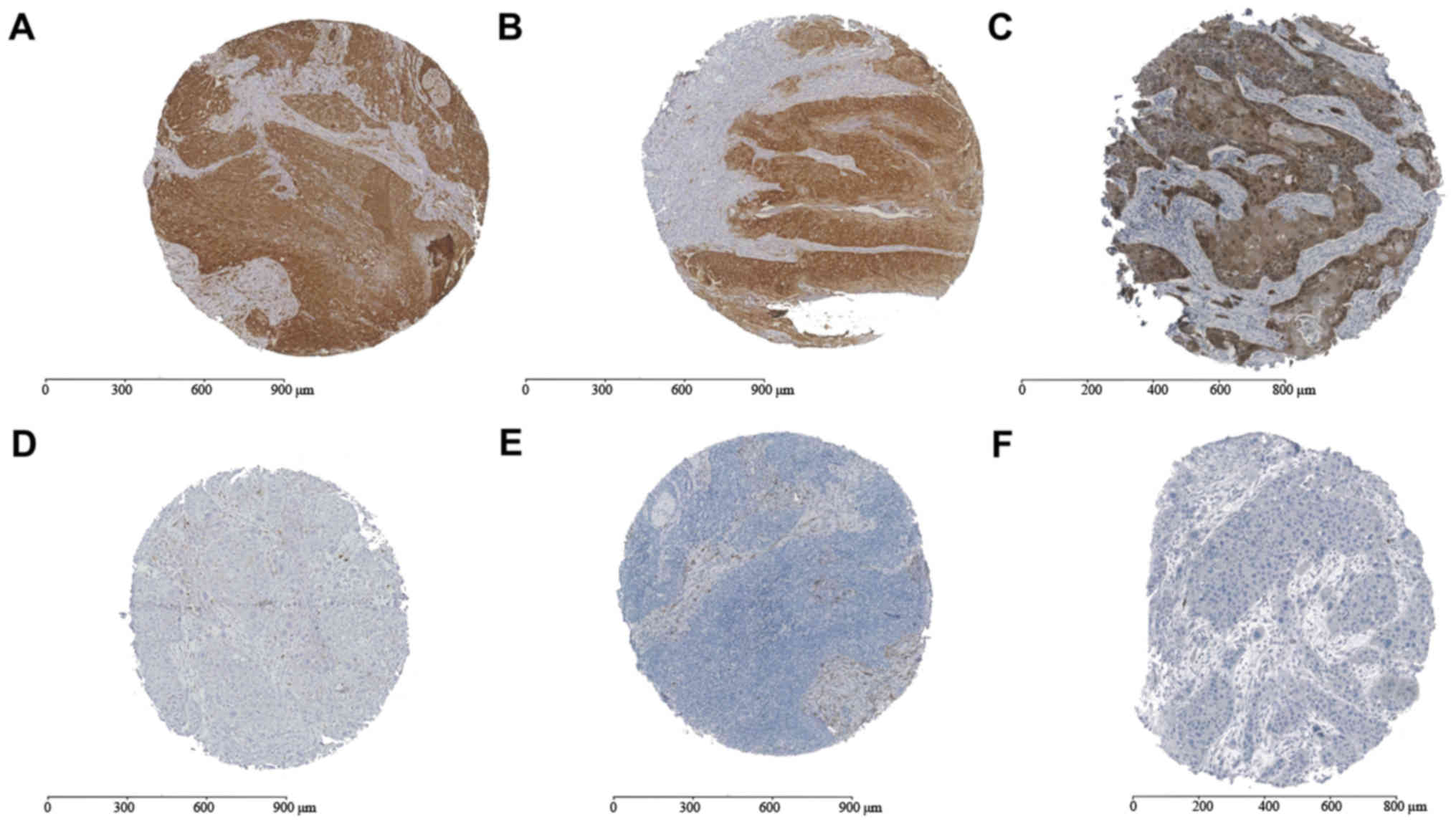
tumour areas regarding positivity for ALDH1 (Fig. 1). Low ALDH1 expression was determined
in 24 patients and high expression in 162 patients. Overall
survival rates did not differ significantly between the
ALDH1-positive cases (44.90±91.40 months; 95% CI: 30.71–58.93) and
the ALDH1-negative cases (41.81±37.83; 95% CI: 26.01–56.83;
P=0.78). High expression of ALDH1 in tumour-infiltrated lymph nodes
(ALDH1LN) exhibited an association with more advanced
clinicopathological stages compared with low expression of ALDH1:
High expression of ALDH1LN was significantly associated with UICC
stage IV (P=0.044) and T4 stage cancer (P=0.03). High expression of
ALDH1 in the tumour centre and invasive front did not exhibit a
significant association (data not shown).
Association of p16ink4a
with survival rate
p16ink4a positivity was detected in 8
cases. The mean survival rate of p16ink4a-positive
patients was 32.38±11.94 months (95% CI: 24.11–40.65); no
significant difference was determined between the
p16ink4a-positive and -negative groups (P=0.12). A total
of 5 patients of the cohort tested positive for p16ink4a
and HPV subtype 16 (for whom mean survival rate was 37.00±11.35
months; 95% CI: 27.05–46.95); no significant difference was
determined between the p16ink4a and HPV 16-positive and
-negative groups P=0.13). A total of 3 patients tested positive for
p16ink4a and negative for HPV (for whom mean survival
rate was 24.67±12.92 months; 95% CI: 10.05–39.29); these patients
exhibited significantly decreased survival time compared with the
mean survival rate of the total cohort (P=0.048). A total of 178
patients were negative for p16ink4a and HPV and had a
mean survival time of 51.16±41.23 months (CI: 45.12–57.20; compared
with the total cohort, P=0.35) (data not shown).
Association of ALDH1 and
p16ink4a/HPV with survival rate
A total of 5 of the p16ink4a-positive
cases that also tested HPV-positive exhibited positive ALDH1
expression. This group had a mean survival time of 34.85±9.29
months (95% CI: 25.56–44.14). The remaining
p16ink4a-positive cases with negative ALDH 1 expression
(n=3) had a mean survival of 28.26±12.90 months (95%
CI:13.66–42.86). No significant difference was identified between
the survival times of these groups (P=0.57), or in comparison to
the survival times of the whole cohort and the
p16ink4a-positive/ALDH1-positive subgroup (P=0.25) or
the whole cohort and the p16ink4a positive/ALDH1
negative subgroup (P=0.15). A total of 2 of the HPV-positive
patients were ALDH1-negative [mean survival rate, 32.59±13.85
months; 95% CI:13.34–51.84), while 3 exhibited positivity for ALDH1
(mean survival rate, 39.94±8.02 months; 95% CI:30.86–49.02); no
influence was observed on survival rates between these latter
groups (P=0.69). Additionally, no influence was observed on the
survival rates between these latter subgroups and the total cohort
(P=0.54 for ALDH1-negative and HPV-positive patients; P=0.60 for
ALDH1-positive and HPV-positive patients). Lymph node recurrence
and local recurrence occurred in one ALDH1-positive,
p16ink4a-positive and HPV-negative case (survival rate,
17.92 months). Isolated local recurrence was diagnosed in one
ALDH1-positive, p16ink4a-positive and HPV-positive case
(survival rate, 30.57 months) (data not shown).
Discussion
CSCs are a major research topic in the field of
oncology. These cells may serve as crucial elements in the growth
of a tumour mass by providing self-renewal capacity and temporary
limited senescence during therapy, recurrence and metastasis
(33). With regard to these aspects,
patient survival rate may be associated with the expression of
CSCMs. Previous literature has reported worse survival rates in
cases presenting with high expression of CSCMs, independent of
cancer localisation (34).
Furthermore, the molecular characteristics of the tumour are
considered to have crucial impact on survival (35). The TNM/UICC stage is not always a
predictable of outcome at initial diagnosis, since TNM/UICC
classification is only based on probabilities and does not take
into account cellular features (36).
The first step is thus to identify CSCs and subsequently to study
the mechanisms that CSCs employ in order to overcome treatment, to
supply chemoresistance and to support aggressive tumour behaviour
(37;38). Various molecular pathways have been studied in CSC
subpopulations. Certain markers on the cell surface have been
indicated to be important for migration and
epithelial-mesenchymal-transition (EMT) (39). Cluster of differentiation (CD)133 and
CD44 have been revealed to be active CSC targets in the process of
EMT and have also been identified to be present in HNSCC (40). A further specific CSC molecule is
ALDH1 (41), which has demonstrated
enriched expression in CSCs of various cancers, and serves a role
as an aldehyde-catalysing cytosolic enzyme (42,43).
ALDH1-positive CSCs have previously exhibited tumorigenic capacity
in HNSCC, which is an important aspect in EMT and linked to
metastasis (44). In OPSCC, an
association has also been detected between the expression of ALDH1
and lymph node metastasis (22). In
the ALDH1-positive group in the present study, increased lymph node
metastasis was identified, as well as an association with advanced
tumour stage compared with the ALDH1-negative group. This
highlights the potential aggressiveness of ALDH1 expression in
cancer, as suggested previously in the literature (45), and supports the significant
association of ALDH1 with lymph node metastasis as reported
previously (46). Furthermore,
advanced T stages have been reported in ALDH1-positive OSCC cases
(47). In particular, an increased
number of nodal metastases may be an important indication that CSCs
frequently undergo EMT. A negative survival outcome is often
discussed in the literature as being associated with enriched ALDH1
cases (48,49). The current study included a limited
number of ALDH1-negative cases among the total population, but
these exhibited worse survival time than the ALDH1-positive group.
However, a significant influence on survival rate in OSCC was not
determined from the current results. The lack of a negative
survival influence conferred by ALDH1 positivity has also been
reported in other studies (50,51).
However, with regard to the current study, the lack of a negative
survival influence may be attributable to the small number of
ALDH1-negative cases and thus to the two groups of ALDH1-positive
and -negative cases being comparable only to a minor degree. The
scoring of the staining, summarized as positive and negative
expression of ALDH1 in the present study, was comparable with that
of other studies (49,52). The use of antibody staining for ALDH1
offered clear cut-off values of positivity. Additionally, clear
results were also obtained following the staining of
p16ink4a. The role of p16ink4a expression in
cancer is controversial. It has been argued that reduced expression
of p16ink4a, which is considered largely as a tumour
suppressor, leads to worse survival (53); However, the role of over- or
underexpression of p16ink4a appears to be dependent on
cancer subtype (54). Some authors
have described enhanced cell migration ability in cases of
p16ink4a overexpression (55). This may increase the potential for
lymph node metastasis. The current identified relatively few cases
of p16ink4a-positive OSCC. These cases had significant
worse survival in the case of HPV negativity, although no specific
clinicopathological features were observed regarding lymph node
metastasis and advanced tumour stage. Due to the small number of
positive cases for p16ink4a, the following conclusions
can only be considered as preliminary. Nevertheless,
p16ink4a interacts in the cell cycle and it may
therefore be suggested that this protein is of importance in OSCC.
No association between the expression of ALDH1 and
p16ink4a was identified in the present study, indicating
the absence of interactions between these two markers. Similar to
our previous study (30), the current
investigation has indicated that HPV has no influence on survival
or cancer stage in OSCC; furthermore, the expression of
p16ink4a has no apparent association with HPV positivity
in OSCC.
In conclusion, to the best of our knowledge, the
present study is the first to evaluate ALDH1 expression in a large
cohort of OSCC patients, and the interaction of p16ink4a
and HPV status. The results demonstrate that ALDH1 may serve as a
predictive marker for advanced tumour stages, and that it may lead
to a tendency for lymph node metastasis in OSCC. This may be of
notable clinical interest since there remains to be no accurate
method of predicting lymph node metastasis from radiological
findings throughout the clinical disease course. It was further
confirmed that HPV status does not have a significant influence on
survival rate in OSCC, as previously noted by our group. The
expression of p16ink4a was revealed to be associated
with worse survival and to serve as a potential predictive marker
of survival. As p16ink4a exhibits distinct interactions
in the cell cycle, further studies on this protein should be
performed to assess the various phases of the cell cycle in OSCC.
While the current study has some limitations, it may be considered
as a successful preliminary study in providing a basis for future
studies into the underlying mechanisms involved. Future studies
should focus on the evaluation of colony formation among various
OSCC cell lines, particularly in the context of the tumour invasive
front, to determine the involvement of EMT factors. Detection of
ALDH1 at the genomic level may be also important for understanding
the role of ALDH1 as a CSCM. The present study nevertheless has
identified encouraging preliminary results with regard to the value
of ALDH1 as a predictive CSCM for advanced tumour stages in OSCC.
The overexpression of p16ink4a in OSCC is a promising
future topic for ongoing studies involving evaluation of migratory
potential.
Acknowledgements
The authors are thankful to Dr Melanie Boxberg at
the Institute of Pathology, Klinikum rechts der Isar Hospital,
Technical University Munich, for providing the tissue microarray,
and Daniela Hellmann at the Departement for Obstetrics, Klinikum
rechts der Isar, for the technical support. The authors also thank
Professor Manfred Schmitt in memoriam, for providing laboratory
facilities (Department of Obstetrics, Klinikum rechts der
Isar).
Funding
Not applicable.
Availability of data and materials
The datasets analysed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
CG, ED, KDW, OB, CN and AK were responsible for
study design. CG and AK conducted the study. CG collected the data.
CG, OB and CN analysed the data. OB, CG, ED and CN interpreted the
data. OB, CG, CG, ED, KDW and AK drafted the manuscript. OB, CG,
CG, ED, KDW and AK provided manuscript content. CG, KDW and AK
reviewed the integrity of the data analysis. All authors approved
the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
the Technical University of Munich, Munich, Germany (approval no.
212108) and all patients provided written informed consent for the
use of relevant samples.
Consent for publication
Consent for publication was agreed upon in the
written consent forms signed by the patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
La Torre G, Kirch W, Bes-Rastrollo M,
Ramos RM, Czaplicki M, Gualano MR, Thümmler K, Ricciardi W and
Boccia A; GHPSS, ; Collaborative Group, : Tobacco use among medical
students in Europe: Results of a multicentre study using the Global
Health Professions Student Survey. Public Health. 126:159–164.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arantes LM, de Carvalho AC, Melendez ME,
Carvalho AL and Goloni-Bertollo EM: Methylation as a biomarker for
head and neck cancer. Oral Oncol. 50:587–592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar B, Cordell KG, Lee JS, Worden FP,
Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, et al:
EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as
indicators of response to therapy and survival in oropharyngeal
cancer. J Clin Oncol. 26:3128–3137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolk A, Wermker K, Bier H, Götz C and
Eckert AW: Current surgical and adjuvant therapy concepts of
malignant tumors of the facial skin and the pinna.
Laryngorhinootologie. 94:77–85. 2015.(In German). PubMed/NCBI
|
|
5
|
Fakhry C, Sugar E, D'Souza G and Gillison
M: Two-week versus six-month sampling interval in a short-term
natural history study of oral HPV infection in an HIV-positive
cohort. PLoS One. 5:e119182010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagan JV and Scully C: Recent advances in
Oral Oncology 2007: Epidemiology, aetiopathogenesis, diagnosis and
prognostication. Oral Oncol. 44:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lindenblatt RC, Martinez GL, Silva LE,
Faria PS, Camisasca DR and Lourenço SQ: Oral squamous cell
carcinoma grading systems - analysis of the best survival
predictor. J Oral Pathol Med. 41:34–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mücke T, Koschinski J, Wolff KD, Kanatas
A, Mitchell DA, Loeffelbein DJ, Deppe H and Rau A: Quality of life
after different oncologic interventions in head and neck cancer
patients. J Craniomaxillofac Surg. 43:1895–1898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
González-García R, Naval-Gías L,
Román-Romero L, Sastre-Pérez J and Rodríguez-Campo FJ: Local
recurrences and second primary tumors from squamous cell carcinoma
of the oral cavity: A retrospective analytic study of 500 patients.
Head Neck. 31:1168–1180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park MJ, Roh JL, Kim SB, Choi SH, Nam SY
and Kim SY: Prognostic value of circulating biomarker score in
advanced-stage head and neck squamous cell carcinoma. Eur J Cancer.
92:69–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas SM, Bhola NE, Zhang Q, Contrucci
SC, Wentzel AL, Freilino ML, Gooding WE, Siegfried JM, Chan DC and
Grandis JR: Cross-talk between G protein-coupled receptor and
epidermal growth factor receptor signaling pathways contributes to
growth and invasion of head and neck squamous cell carcinoma.
Cancer Res. 66:11831–11839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fouad YA and Aanei C: Revisiting the
hallmarks of cancer. Am J Cancer Res. 7:1016–1036. 2017.PubMed/NCBI
|
|
14
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Knudsen ES, Dervishaj O, Kleer CG, Pajak
T, Schwartz GF and Witkiewicz AK: EZH2 and ALDH1 expression in
ductal carcinoma in situ: Complex association with recurrence and
progression to invasive breast cancer. Cell Cycle. 12:2042–2050.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schäfer A, Teufel J, Ringel F, Bettstetter
M, Hoepner I, Rasper M, Gempt J, Koeritzer J, Schmidt-Graf F, Meyer
B, et al: Aldehyde dehydrogenase 1A1 - a new mediator of resistance
to temozolomide in glioblastoma. Neuro-oncol. 14:1452–1464. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reid P, Wilson P, Li Y, Marcu LG,
Staudacher AH, Brown MP and Bezak E: In vitro investigation of head
and neck cancer stem cell proportions and their changes following
X-ray irradiation as a function of HPV status. PLoS One.
12:e01861862017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campos MS, Neiva KG, Meyers KA,
Krishnamurthy S and Nör JE: Endothelial derived factors inhibit
anoikis of head and neck cancer stem cells. Oral Oncol. 48:26–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian X, Wagner S, Ma C, Klussmann JP,
Hummel M, Kaufmann AM and Albers AE: ALDH1-positive cancer
stem-like cells are enriched in nodal metastases of oropharyngeal
squamous cell carcinoma independent of HPV status. Oncol Rep.
29:1777–1784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sritippho T, Pongsiriwet S,
Lertprasertsuke N, Buddhachat K, Sastraruji T and Iamaroon A: p16 -
a Possible Surrogate Marker for High-Risk Human Papillomaviruses in
Oral Cancer? Asian Pac J Cancer Prev. 17:4049–4057. 2016.PubMed/NCBI
|
|
24
|
Hoesli R, Birkeland AC, Rosko AJ, Issa M,
Chow KL, Michmerhuizen NL, Mann JE, Chinn SB, Shuman AG, Prince ME,
et al: Proportion of CD4 and CD8 tumor infiltrating lymphocytes
predicts survival in persistent/recurrent laryngeal squamous cell
carcinoma. Oral Oncol. 77:83–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alipoor FJ, Asadi MH and Torkzadeh-Mahani
M: MIAT lncRNA is overexpressed in breast cancer and its inhibition
triggers senescence and G1 arrest in MCF7 cell line. J Cell
Biochem. 18–Jan;2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao P, Hu YC and Talbot IC: Expressing
patterns of p16 and CDK4 correlated to prognosis in colorectal
carcinoma. World J Gastroenterol. 9:2202–2206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milanovic M, Fan DNY, Belenki D, Däbritz
JHM, Zhao Z, Yu Y, Dörr JR, Dimitrova L, Lenze D, Monteiro Barbosa
IA, et al: Senescence-associated reprogramming promotes cancer
stemness. Nature. 553:96–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okuma A, Hanyu A, Watanabe S and Hara E:
p16Ink4a and p21Cip1/Waf1 promote tumour growth by
enhancing myeloid-derived suppressor cells chemotaxis. Nat Commun.
8:20502017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wolff KD, Follmann M and Nast A: The
diagnosis and treatment of oral cavity cancer. Dtsch Arztebl Int.
109:829–835. 2012.PubMed/NCBI
|
|
30
|
Götz C, Drecoll E, Straub M, Bissinger O,
Wolff KD and Kolk A: Impact of HPV infection on oral squamous cell
carcinoma. Oncotarget. 7:76704–76712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bissinger O, Kolk A, Drecoll E, Straub M,
Lutz C, Wolff KD and Götz C: EGFR and Cortactin: Markers for
potential double target therapy in oral squamous cell carcinoma.
Exp Ther Med. 14:4620–4626. 2017.PubMed/NCBI
|
|
32
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.PubMed/NCBI
|
|
33
|
La Porta CA, Zapperi S and Sethna JP:
Senescent cells in growing tumors: Population dynamics and cancer
stem cells. PLOS Comput Biol. 8:e10023162012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Solomon I, Voiculescu VM, Caruntu C, Lupu
M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine Factors and Head and Neck Squamous Cell
Carcinoma: An Affair to Remember. Dis Markers. 2018:97878312018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burke HB: Outcome prediction and the
future of the TNM staging system. J Natl Cancer Inst. 96:1408–1409.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Magni M, Shammah S, Schiró R, Mellado W,
Dalla-Favera R and Gianni AM: Induction of
cyclophosphamide-resistance by aldehyde-dehydrogenase gene
transfer. Blood. 87:1097–1103. 1996.PubMed/NCBI
|
|
39
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boxberg M, Jesinghaus M, Dorfner C, Mogler
C, Drecoll E, Warth A, Steiger K, Bollwein C, Meyer P, Wolff KD, et
al: Tumour budding activity and cell nest size determine patient
outcome in oral squamous cell carcinoma: Proposal for an adjusted
grading system. Histopathology. 70:1125–1137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu J, Mu Q, Thiviyanathan V, Annapragada A
and Vigneswaran N: Cancer stem cells are enriched in Fanconi anemia
head and neck squamous cell carcinomas. Int J Oncol. 45:2365–2372.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ikawa M, Impraim CC, Wang G and Yoshida A:
Isolation and characterization of aldehyde dehydrogenase isozymes
from usual and atypical human livers. J Biol Chem. 258:6282–6287.
1983.PubMed/NCBI
|
|
43
|
Resetkova E, Reis-Filho JS, Jain RK, Mehta
R, Thorat MA, Nakshatri H and Badve S: Prognostic impact of ALDH1
in breast cancer: A story of stem cells and tumor microenvironment.
Breast Cancer Res Treat. 123:97–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Clay MR, Tabor M, Owen JH, Carey TE,
Bradford CR, Wolf GT, Wicha MS and Prince ME: Single-marker
identification of head and neck squamous cell carcinoma cancer stem
cells with aldehyde dehydrogenase. Head Neck. 32:1195–1201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu J, Müller S, Nannapaneni S, Pan L, Wang
Y, Peng X, Wang D, Tighiouart M, Chen Z, Saba NF, et al: Comparison
of quantum dot technology with conventional immunohistochemistry in
examining aldehyde dehydrogenase 1A1 as a potential biomarker for
lymph node metastasis of head and neck cancer. Eur J Cancer.
48:1682–1691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He KF, Zhang L, Huang CF, Ma SR, Wang YF,
Wang WM, Zhao ZL, Liu B, Zhao YF, Zhang WF, et al: CD163+
tumor-associated macrophages correlated with poor prognosis and
cancer stem cells in oral squamous cell carcinoma. BioMed Res Int.
2014:8386322014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rasper M, Schäfer A, Piontek G, Teufel J,
Brockhoff G, Ringel F, Heindl S, Zimmer C and Schlegel J: Aldehyde
dehydrogenase 1 positive glioblastoma cells show brain tumor stem
cell capacity. Neuro-oncol. 12:1024–1033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bednarz-Knoll N, Nastały P, Żaczek A,
Stoupiec MG, Riethdorf S, Wikman H, Müller V, Skokowski J, Szade J,
Sejda A, et al: Stromal expression of ALDH1 in human breast
carcinomas indicates reduced tumor progression. Oncotarget.
6:26789–26803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Morimoto K, Kim SJ, Tanei T, Shimazu K,
Tanji Y, Taguchi T, Tamaki Y, Terada N and Noguchi S: Stem cell
marker aldehyde dehydrogenase 1-positive breast cancers are
characterized by negative estrogen receptor, positive human
epidermal growth factor receptor type 2, and high Ki67 expression.
Cancer Sci. 100:1062–1068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alamgeer M, Ganju V, Kumar B, Fox J, Hart
S, White M, Harris M, Stuckey J, Prodanovic Z, Schneider-Kolsky ME,
et al: Changes in aldehyde dehydrogenase-1 expression during
neoadjuvant chemotherapy predict outcome in locally advanced breast
cancer. Breast Cancer Res. 16:R442014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sharpless NE: INK4a/ARF: A multifunctional
tumor suppressor locus. Mutat Res. 576:22–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Emig R, Magener A, Ehemann V, Meyer A,
Stilgenbauer F, Volkmann M, Wallwiener D and Sinn HP: Aberrant
cytoplasmic expression of the p16 protein in breast cancer is
associated with accelerated tumour proliferation. Br J Cancer.
78:1661–1668. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen YW, Chu HC, Ze-Shiang Lin, Shiah WJ,
Chou CP, Klimstra DS and Lewis BC: p16 Stimulates CDC42-dependent
migration of hepatocellular carcinoma cells. PLoS One.
8:e693892013. View Article : Google Scholar : PubMed/NCBI
|