Introduction
Osteoporosis is a metabolic bone disease that may be
defined as a systemic skeletal destruction characterized by low
bone mass, high bone friability and micro-architectural decline of
bone tissue (1). Clinical
manifestations are easy occurrence of bone fracture and relatively
high rates of mortality and disability caused by hip, extremity and
vertebral fractures. Osteoporosis has become a major public health
issue and is a burden to patients, their families and society
(2).
The pathophysiological mechanism of osteoporosis is
complex (3). The reconstruction of
bone tissue is a key process in maintaining the microstructure and
morphology of bone (4,5). Imbalances in the bone tissue
reconstruction process can manifest as upregulation in the process
of bone resorption by osteoclasts, and reduced osteoblast formation
of bone tissue (6). This results in
systemic loss of bone mass, increased bone fragility, decreased
bone mineral density (BMD), increased risk of bone destruction and
osteoporosis (7).
In 2004, the World Health Organization recommended
that the diagnosis of osteoporosis be performed based on values of
BMD or bone mineral content (BMC) (8): Normal BMD or BMC is within one standard
deviation (SD) of the average bone density in normal adults; BMD is
usually expressed as a T-value (T-score), where T-value = (measured
value - mean value in normal adults of the same gender)/SD of the
mean BMD of normal adults of the same sex and gender. The World
Health Organization sets the average body mass of adults with
normal BMD as the standard, and defines osteoporosis when BMD is
≥-2.5 SD units in postmenopausal women or men >50 years (normal
population: T-score ≤-1 SD; bone mass loss population: −1 SD <
T-Score <-2.5 SD; osteoporosis population: T-Score ≥-2.5 SD).
Through the study of changes in BMD, numerous factors affecting the
BMD value have been identified, including estrogen,
osteoprotegerin, calcitonin, transforming growth factor, thyroid
hormone and gene polymorphisms (9–11).
Previous studies have demonstrated that genetic
factors may be associated with BMD and serve an important role in
the pathogenesis of osteoporosis (12). Data originating from the study of
twins has indicated genetic factors account for up to 85% of
diversity in bone mass (8). Numerous
research institutions have investigated potential associations
between candidate gene polymorphisms and osteoporosis (3,6,7). The vitamin D receptor (VDR) gene
polymorphism is one of the most widely studied, and has been
indicated to be involved in bone mineral homeostasis, bone
remodeling and bone matrix composition (14). Population-based and case-control
studies have similarly identified polymorphisms in several
candidate genes associated with bone mass or osteoporotic fracture,
including VDR (6,13,15).
VDR is a nuclear transcription factor that mediates
the action of 1,25-dihydroxyvitamin D3 and affects calcium
absorption, bone remodeling and mineralization rate. It is located
on the long arm of 12 chromosome (3q11) and consists of 11 exons,
2–9 of which are actively transcribed (16). Several sites have been associated with
BMD in the VDR gene, including ApaI, BsmI, FokI and TaqI (17). The ApaI and BsmI sites are both in
intron 8 of the VDR gene (18). A
number of studies (16,17,19) have
been undertaken in this field [Morrison et al (19) was the first to document an association
between the VDR genotype and bone mass], but the results have been
controversial and no clear correlation between ApaI gene
polymorphism and osteoporosis has been identified. Vladoiu et
al (20) reported that ApaI
polymorphisms in the VDR gene have different phenotypes, which were
associated with significantly different BMD values, suggesting that
it may be used as a genetic marker to predict the risk of
osteoporosis. Mitra et al (21) identified an association between BsmI
and ApaI polymorphisms and BMD in postmenopausal Indian women,
further suggesting that genetic background serves a role. However,
other reports have observed conflicting results. Castelán-Martínez
et al (22) documented that
there was no clear correlation between BMD and ApaI polymorphism in
postmenopausal women in Mexico. Feskanich et al (23) identified that genetic polymorphisms of
ApaI did not affect the risk of osteoporosis.
Another important factor affecting BMD is estrogen
level. Estrogen uses different pathways to regulate biological
activity through the transduction of specific target cell signals.
Rooney and van der Meulen (24)
identified that estrogen exerted its effects primarily through the
use of non-canonical pathways, to thus induce anti-osteoclast death
or inhibit osteoblast death. Therefore, to minimize the effect of
estrogen on BMD changes, postmenopausal women were selected for the
present study purposes.
To the best of our knowledge, there are no previous
studies of gene polymorphisms in postmenopausal Han women in
Xinjiang. The purpose of the present study was to investigate the
potential association between the commonly studied polymorphism in
the VDR gene, ApaI, and osteoporosis in postmenopausal women of Han
nationality in Xinjiang. In this study, the polymerase chain
reaction (PCR)-restriction fragment length polymorphism (RFLP)
technique was used to verify genotypes of the ApaI locus of the VDR
gene. The different genotypes were compared and BMD values were
used to determine the impact of ApaI gene polymorphisms on
osteoporosis, as in previous study of the association between BMD
and genotype (25). Based on this
line of research, it is hoped that screening and diagnosis of
high-risk populations with osteoporosis may support the prevention
of osteoporosis at the molecular level.
Materials and methods
Study population
Participants included 336 unrelated postmenopausal
women who were recruited voluntarily from January to June 2016. The
group included 90 women (67.2±8.6 years old; T-score ≥-2.5 SD) with
osteoporosis and 246 healthy individuals (65.5±7.6 years old;
T-score <-2.5 SD). Participants were recruited from the First
Affiliated Hospital of Shihezi University (Shihezi, China), the
People's Hospital of Xinjiang Uygur Autonomous Region (Urumqi,
China), the People's Liberation Army 474 Hospital and Xinjiang
Production and Construction Corps Hospital (Urumqi, China).
Patients with secondary osteoporosis diseases (diabetes, Cushing's
syndrome, thyroid, nutrition deficiency, myeloproliferative
diseases, bone tumors, connective tissue diseases and congenital
diseases), hepatic renal dysfunction and incomplete clinical data
were excluded.
The protocol of the present study was approved by
the Institutional Medical Ethics Committee of Shihezi University,
and all participants received and signed informed consent
documents.
Laboratory analysis
Genotyping was performed by researchers who were
blinded to the case/control status of the study subjects. Blood
samples (3 ml) were collected in EDTA tubes and frozen in liquid
nitrogen for 2 h after collection. Genomic DNA was extracted from
peripheral white blood cells using a TIANamp Blood DNA Maxi kit
(Tiangen Biotech Co., Ltd., Beijing, China), according to the
manufacturer's protocol. A 740-bp fragment containing the ApaI
polymorphism was amplified by PCR using specific primers (forward,
5′-CAGAGCATGGACAGGGAGCAA-3′ and reverse,
5′-TCATGGCTGAGGTCTCAAGGG-3′) as described previously (13). The thermocycler conditions were 95°C
for 3 min, followed by 35 cycles of 94°C for 30 sec, 65°C for 30
sec and 72°C for 30 sec, and ending with 72°C for 10 min (Eppendorf
Mastercycler Gradient; Eppendorf, Hamburg, Germany). The 740-bp
product fragment containing the ApaI polymorphism was digested with
ApaI endonuclease (Thermo Fisher Scientific, Inc.). The ApaI
fragments of the VDR gene were separated by 1.5% agarose gel
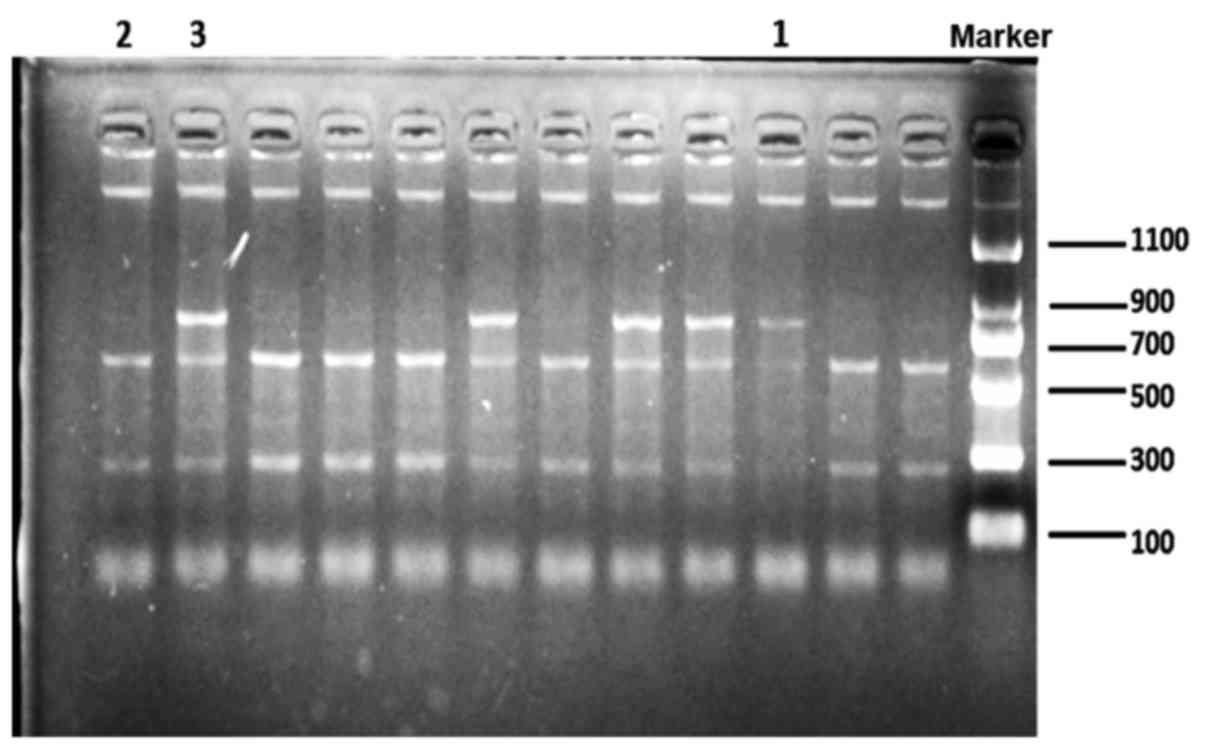
electrophoresis with ethidium bromide staining. The genotypes were
represented as AA (740 bp), Aa (740, 520 and 220 bp) and aa (520
and 220 bp) (26).
Densitometry study
BMD at the lumbar spine (L1-L4), Ward's triangle,
great trochanter and femoral shaft were measured using a
dual-energy X-ray absorptiometry (DXA) scanner (GE Lunar DPX
Prodigy; GE Healthcare, Chicago, IL, USA) (6). BMD was calculated by dividing BMC (g) by
bone area (cm2), and expressed as g/cm2
(8). By comparing with the SD of BMD
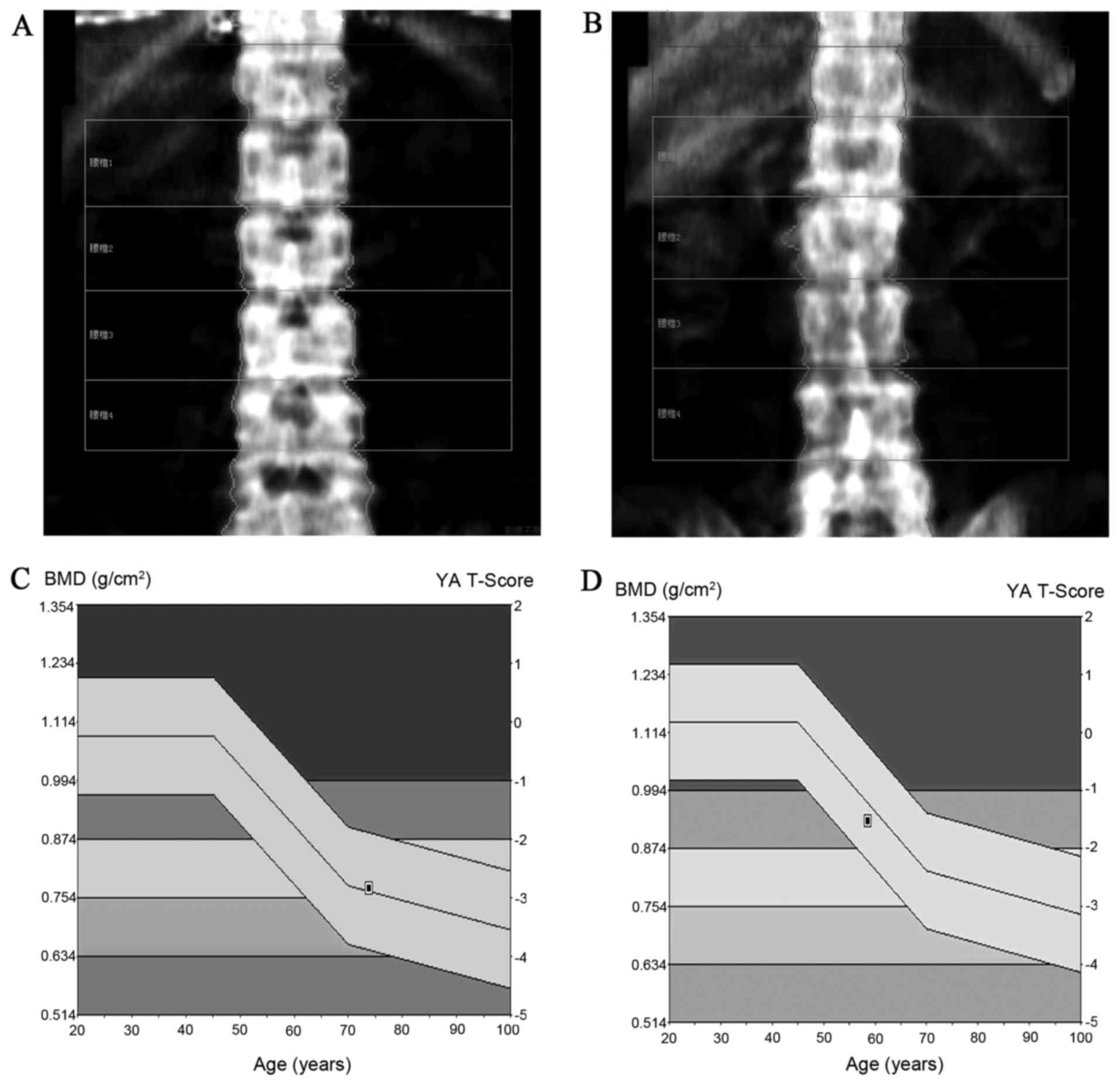
in the normal reference population, the T-score of BMD in
participants was calculated to determine the diagnosis of
osteoporosis (normal population: T-score ≤-1 SD; bone mass loss
population: −1 SD < T-Score <-2.5 SD; osteoporosis
population: T-Score ≥-2.5 SD). Grouping was based on measurement
results. Additionally, body weight and height were measured to
calculate body mass index (BMI).
Statistical analysis
Statistical analysis of the results was performed
with SPSS 20.0 (IBM Corp., Armonk, NY, USA). For analysis of
subject characteristics, quantitative data were presented as means
± standard deviation. The Hardy-Weinberg equilibrium (HWE)
distribution of the ApaI allelic and genotypic frequencies was
assessed with the χ2 test. One-way analysis of variance
was used to compare between different genotypes followed by the
Bonferroni's post hoc test. Analysis of co-variance (ANCOVA) was
used to compare the VDR genotypes adjusted for co-variants age and
BMI. Genotypic frequencies were not normally distributed, and a
bootstrap procedure was applied to the ANCOVA test. In this case,
comparisons were performed with a non-parametric Kruskal Wallis
test. Unconditional multivariable logistic regression models were
used to measure the association of age and BMI with BMD. Age and
BMI as risk factors for osteoporosis were assessed via odds ratios
(ORs) and 95% confidence intervals (CIs). In all analyses,
significance was defined at P<0.05.
Results
Genotypes and alleles
A total of 336 females were included in the study.
The overall ApaI allelic and genotypic frequencies are listed in
Table I. Frequencies of ApaI included
in the analysis were consistent with the HWE (P>0.05). The
enzyme digestion of ApaI was analyzed by agarose gel
electrophoresis to examine band size consistency and confirm
variants of the gene (Fig. 1).
 | Table I.Frequency distribution of ApaI
genotypes and alleles. |
Table I.
Frequency distribution of ApaI
genotypes and alleles.
|
| Frequency of total,
n (%) |
|---|
|
|
|
|---|
|
| Genotype | Allele |
|---|
|
|
|
|
|---|
| Gene | Total, n | AA | Aa | aa | Total, n | A | a |
|---|
| ApaI | 336 | 21 (6.3) | 94 (28.0) | 221 (65.8) | 672 | 136 (20.2) | 536 (79.8) |
Among the 90 patients with osteoporosis, the
frequency distribution of ApaI genotypes was 5 (5.6%) individuals
with AA, 25 (27.8%) with Aa and 60 (66.6%) with aa. Among the 246
participants in the control group, the frequency distribution of
ApaI genotypes was 16 (6.5%) individuals with AA, 69 (28.0%) with
Aa and 161 (65.4%) with aa (Table
II). Data analysis indicated no significant association between
ApaI genotype and osteoporosis (P=0.946).
 | Table II.Frequency distribution of ApaI
genotypes and alleles in the osteoporosis and control groups. |
Table II.
Frequency distribution of ApaI
genotypes and alleles in the osteoporosis and control groups.
|
| Control
(n=246) | Osteoporosis
(n=90) |
|---|
|
|
|
|
|---|
|
| n | Frequency, % | n | Frequency, % | P-value |
|---|
| Genotype |
| AA | 16 | 6.5 | 5 | 5.6 |
|
| Aa | 69 | 28.0 | 25 | 27.8 |
|
| aa | 161 | 65.4 | 60 | 66.7 | 0.946 |
| Allele |
| A | 101 | 20.5 | 35 | 19.4 |
|
| a | 391 | 79.5 | 145 | 80.6 |
|
BMD and genotype
The results of the DXA scan were uniformly recorded
in accordance with BMD and T-score standards (Fig. 2). The ApaI genotype of patients and
controls was not associated with BMD at any bone site tested
(Table III). On further BMD
analysis, following adjustment for potential confounding factors,
including age, no significant differences in BMI were observed
between the genotypes (Table
IV).
 | Table III.Comparison of BMD value between
different ApaI genotypes in the osteoporosis and control
groups. |
Table III.
Comparison of BMD value between
different ApaI genotypes in the osteoporosis and control
groups.
|
| BMD,
g/cm3 |
|
|
|
|
| Osteoporosis | Control |
|---|
|
|
|
|
|---|
| Genotype | AA | Aa | aa | P-value | AA | Aa | aa | P-value |
|---|
| Lumbar L2-L4 | 0.830±0.237 | 0.845±0.175 | 0.907±0.164 | 0.383 | 1.087±0.153 | 1.114±0.161 | 1.124±0.140 | 0.334 |
| Wards triangle | 0.497±0.073 | 0.514±0.130 | 0.554±0.145 | 0.291 | 0.813±0.190 | 0.746±0.169 | 0.771±0.178 | 0.153 |
| Great
trochanter | 0.565±0.060 | 0.607±0.099 | 0.624±0.115 | 0.311 | 0.817±0.155 | 0.780±0.141 | 0.784±0.131 | 0.138 |
| Femoral shaft | 0.857±0.094 | 0.913±0.129 | 0.977±0.151 | 0.446 | 1.176±0.166 | 1.125±0.169 | 1.133±0.155 | 0.561 |
 | Table IV.BMD at the lumbar spine L1-L4, Ward's
triangle, great trochanter and femoral shaft according to VDR ApaI
genotype. |
Table IV.
BMD at the lumbar spine L1-L4, Ward's
triangle, great trochanter and femoral shaft according to VDR ApaI
genotype.
|
| BMD1,
g/cm2 | BMD2,
g/cm2 |
|---|
|
|
|
|
|---|
| Site | AA (n=21) | Aa (n=94) | aa (n=221) | P-value | AA (n=21) | Aa (n=94) | aa (n=221) | P-value |
|---|
| L1-L4 | 1.026±0.203 | 1.043±0.203 | 1.065±0.175 | 0.454 | 1.011±0.084 | 1.021±0.193 | 1.014±0.139 | 0.422 |
| Wards triangle | 0.738±0.217 | 0.685±0.190 | 0.712±0.195 | 0.387 | 0.702±0.097 | 0.602±0.132 | 0.694±0.204 | 0.331 |
| Great
trochanter | 0.758±0.176 | 0.734±0.151 | 0.741±0.145 | 0.778 | 0.726±0.222 | 0.669±0.142 | 0.712±0.115 | 0.717 |
| Femoral shaft | 1.10±0.204 | 1.068±0.184 | 1.091±0.168 | 0.537 | 0.991±0.102 | 1.011±0.126 | 1.112±0.122 | 0.495 |
Age/BMI and BMD
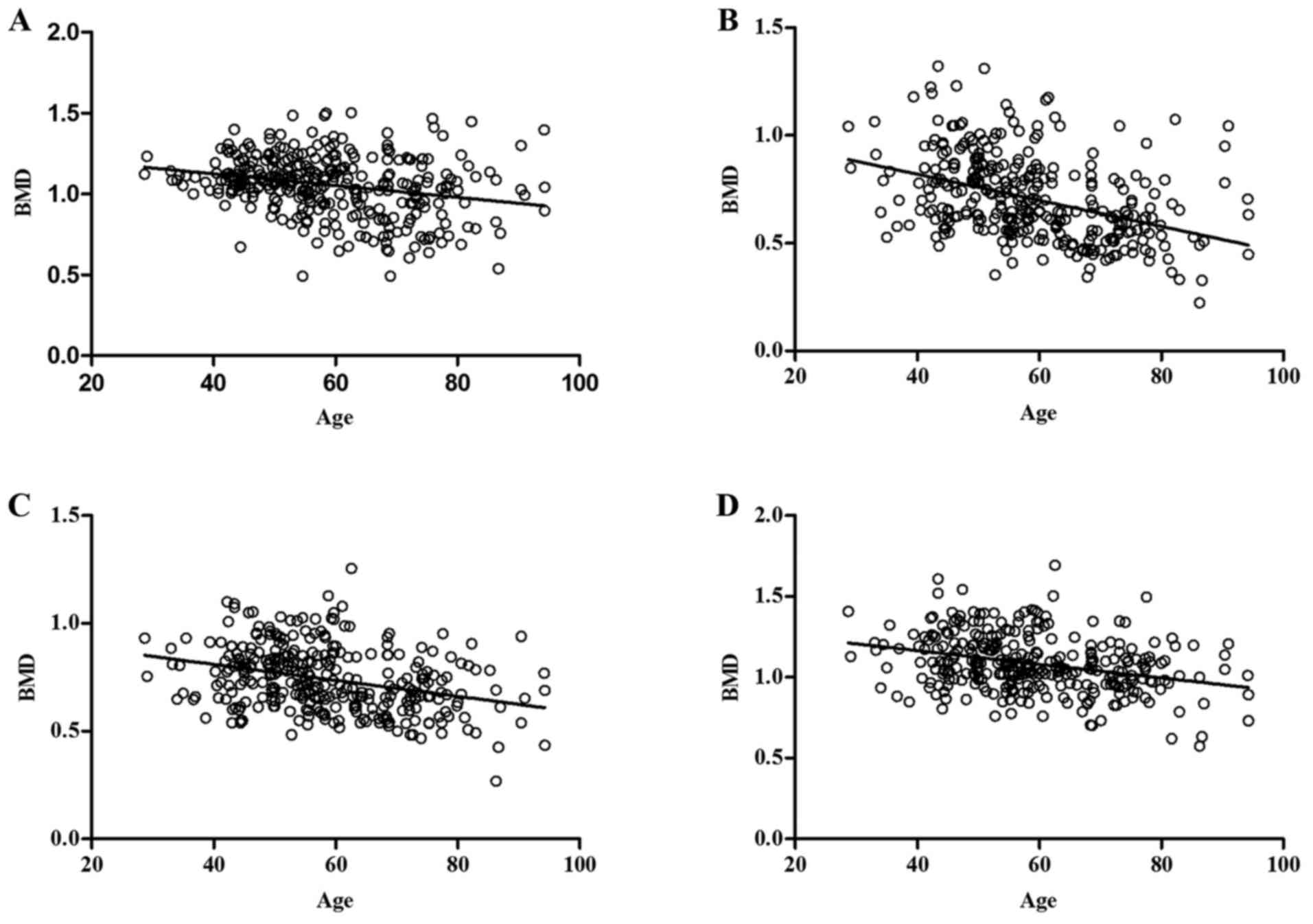
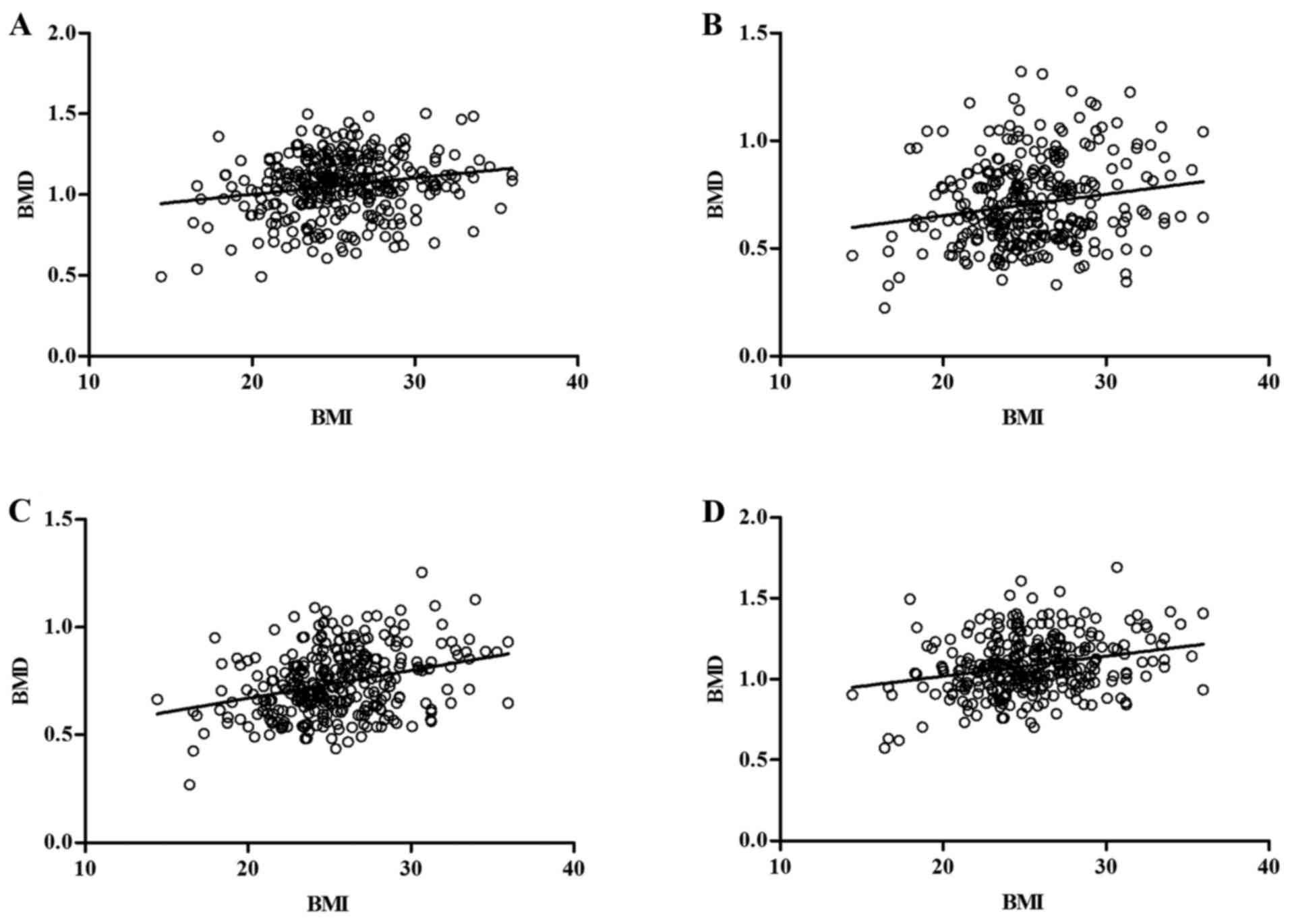
Using ANCOVA to compare age/BMI with BMD, it was
determined that age was negatively associated with BMD, and BMI
positively associated with BMD at all bone sites tested (Table V and Figs.
3 and 4). Analysis of risk
factors indicated that age was a risk factor for osteoporosis (OR:
0.464, 95% CI: 0.372–0.580, P=0.001), and BMI a protective factor
in osteoporosis (OR: 1.502, 95% CI: 1.008–2.240, P=0.032; Table VI).
 | Table V.Correlation analysis of bone mineral
density at different sites with age and BMI. |
Table V.
Correlation analysis of bone mineral
density at different sites with age and BMI.
|
| Age | BMI |
|---|
|
|
|
|
|---|
| Site | r | P-value | r | P-value |
|---|
| Lumbar spine | −0.316 | 0.00018 | 0.195 | 0.00034 |
| Wards triangle | −0.451 | 0.00022 | 0.181 | 0.001 |
| Great
trochanter | −0.373 | 0.00056 | 0.309 | 0.00057 |
| Femoral shaft | −0.356 | 0.00017 | 0.253 | 0.004 |
 | Table VI.Analysis of age and BMI as
osteoporosis risk factors. |
Table VI.
Analysis of age and BMI as
osteoporosis risk factors.
| Variable | β | Standard error | Odds ratio | 95% Confidence
interval | Wald
χ2 | P-value |
|---|
| Age | −0.767 | 0.113 | 0.464 | 0.372–0.580 | 45.967 | 0.001 |
| BMI | 0.407 | 0.204 | 1.502 | 1.008–2.240 | 3.995 | 0.032 |
Discussion
Primary osteoporosis is the subject of ongoing
research. At present it is established as a systemic metabolic
disease affected by numerous factors (27). In general, osteoporosis occurs due to
alterations in the number and activity of cells involved in bone
metabolism, which itself is a process consisting of a balance
between bone formation and bone destruction (28). Changes in bone tissue absorption and
production, resulting in changes in BMD, cause a reduction in bone
strength, fractures and osteoporosis (29). Previous study of osteoporosis has
revealed that it is influenced by genetic factors (30). To the best of our knowledge, this is
the first study to investigate the association between the ApaI
polymorphism of the VDR gene and osteoporosis in postmenopausal Han
females in the Xinjiang region. Through previous research, our
group identified that osteoporosis is a multifactorial disease with
a strong genetic component, and the results of this study are
consistent with those of Zeljic et al (31), but genetic association studies in
osteoporosis have reported conflicting results.
The present study identified that the distribution
of ApaI genotypes and alleles in a population of Han Chinese
postmenopausal women was consistent with HWE. The ApaI aa genotype
was the most abundant, accounting for 65.8% of the total. Kang
et al (32) reported that the
ApaI genotype distributions in a South Korean population were 69.3%
for aa, 25.4% for Aa and 5.3% for AA, which were similar to those
of Han females in the present study. Sassi et al (33) reported that the frequencies of ApaI
genotype distribution in a postmenopausal female Tunisian
population were 15.5% for aa, 45.6% for Aa and 38.9% for AA, which
differs markedly from the present study. The frequency distribution
of the ApaI genotype in the present study was similar to that of
Han women in urban areas including Guangzhou, Beijing and Harbin
(34,35). It is also consistent with the
distribution of genotypes in countries including South Korea and
Japan (20,36). However, the distribution of gene
frequency in populations from the USA and Europe, including Spain
and Portugal (37,38), exhibit significant differences, and
marked difference has been identified in the frequencies of
comparative genes between Uygur and Kazakh populations (39). Therefore, the comparative analyses of
gene frequency distributions in Mongolians and Caucasians have
revealed that ApaI genotype may exhibit racial differences.
In the present study, there was a negative
correlation between age and BMD at different sites. During aging,
the body undergoes senescence. Bone tissue is among of the most
affected parts of the body during the aging process (40). Therefore, the risk of senile
osteoporosis increases with age (41). Additionally, a positive correlation
was identified between BMI and BMD. The effect of BMI on bone
tissue has been associated with the extent of weight-bearing
pressure (37). The increase of
pressure in individuals with high BMI can directly stimulate
baroreceptors in the bone tissue (42). As a result, osteogenic differentiation
may increase, the rate of bone resorption may decrease, ultimately
accelerating bone formation and thereby increasing BMD and
decreasing the risk of developing osteoporosis (43).
The present study identified no direct association
between BMD and specific genotypes of the ApaI polymorphism. This
is consistent with previous studies in different populations
(37,38,44). A
lack of association has also been described by Moran et al
(37) and Yu et al (44). However, there are previous studies
associating the ApaI variant with increased risk of osteoporosis,
including those by Castelán-Martínez et al (22) and Pedrera-Canal et al (45). Therefore to date, studies have
reported controversial results, and the effect of common ApaI
polymorphisms on BMD is yet to be concluded.
It is likely that VDR gene expression is affected by
environmental factors. Stathopoulou et al (16) suggested that calcium homeostasis may
serve a role in this process. However, it should be noted that ApaI
polymorphisms reportedly have no effect on protein expression,
since they are located in the non-coding region of the VDR gene
(45). The current results on ApaI
polymorphisms support the proposal that population variants of a
given genotype may be diverse and multifactorial (46). However, it was also demonstrated that
there was no significant association between VDR ApaI polymorphisms
and BMD in the studied population. Thus, further studies that
investigate gene polymorphism and osteoporosis relevance would be
beneficial to clinicians in diagnosing and preventing
osteoporosis.
There were certain limitations in the present study.
Firstly, patient sample size was relatively small compared with the
total population in Xinjiang, and thus whether and how ApaI
genotype influences bone microarchitecture requires further
investigation in larger cohorts. Secondly, selection of samples was
only conducted in Xinjiang, and therefore ApaI genetic polymorphism
studies should be expanded to multiple regions and races to further
investigate the associations with osteoporosis among different
ethnicities. Thirdly, future studies in larger samples of
post-menopausal women in Xinjiang should focus on multiple
haplotypes, rather than single polymorphisms, to aid clarify the
potential overall effects of common VDR polymorphisms.
In conclusion, no significant association between
the common VDR polymorphism ApaI and BMD was observed at target
skeletal sites in postmenopausal Han Chinese women in the Xinjiang
area. Age was negatively associated with BMD at different sites and
identified as a risk factor; while BMI was positively associated
with BMD and identified as a protective factor.
Acknowledgements
The authors would like to thank Professor Xiaobin
Cui at the Shihezi University School of Medicine (Shehezi, China)
for his suggestions and assistance.
Funding
The present study was supported by the National
Science Foundation of China (grant nos. 81760404, 81772407 and
81660374) and the Xinjiang Bingtuan Special Program (grant nos.
2014CC002 and 2016BC001).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
DM and XD were responsible for conception and design
of the study. HJ, JL, FP, HX and WZ were responsible for the
acquisition of data and analysis and interpretation of the data.
ZC, CS, LP and WW were involved in drafting the manuscript or
revising it critically for important intellectual content. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Institutional Medical Ethics Committee of Shihezi University
(Shehezi, China), and all participants signed informed consent
documents agreeing to their participation.
Consent for publication
All participants agreed in writing to the
publication of associated data following anonymization.
Competing interests
The authors declare no competing interests.
References
|
1
|
Sabet FA, Raeisi Najafi A, Hamed E and
Jasiuk I: Modelling of bone fracture and strength at different
length scales: A review. Interface Focus. 6:201500552016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melton LJ III: Who has osteoporosis? A
conflict between clinical and public health perspectives. J Bone
Miner Res. 15:2309–2314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seremak-Mrozikiewicz A, Barlik M, Drews K,
Bogacz A, Tatuśko J, Piotrowska A, Spaczyński M, Grześkowiak E and
Mrozikiewicz PM: The genetic variants of RANKL/RANK/OPG signal
trial in postmenopausal women with osteopenia and osteoporosis.
Arch Perinat Med. 17:72–80. 2011.
|
|
4
|
Barrett-Connor E, Siris ES, Wehren LE,
Miller PD, Abbott TA, Berger ML, Santora AC and Sherwood LM:
Osteoporosis and fracture risk in women of different ethnic groups.
J Bone Miner Res. 20:185–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YD, Yim DH, Eom SY, Moon SI, Park CH,
Kim GB, Yu SD, Choi BS, Park JD and Kim H: Differences in the
susceptibility to cadmium-induced renal tubular damage and
osteoporosis according to sex. Environ Toxicol Pharmacol.
38:272–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang G, Yang J, Zheng X, Zhu J, Shi W,
Chen A, Chen G and Zhou F: Association of genetic polymorphisms of
GALNT3 and VDR with osteoporosis in postmenopausal women. Exp Ther
Med. 12:2629–2633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu J, Shang DP, Yang S, Fu DP, Ling HY,
Hou SS and Lu JM: Association between the vitamin D receptor gene
polymorphism and osteoporosis. Biomed Rep. 5:233–236. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura T: WHO diagnostic criteria for
osteoporosis and trends in Europe and USA. Nihon Rinsho. 62 (Suppl
2):235–239. 2004.(In Japanese). PubMed/NCBI
|
|
9
|
Pocock NA, Eisman JA, Hopper JL, Yeates
MG, Sambrook PN and Eberl S: Genetic determinants of bone mass in
adults. A twin study. J Clin Invest. 80:706–710. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guéguen R, Jouanny P, Guillemin F, Kuntz
C, Pourel J and Siest G: Segregation analysis and variance
components analysis of bone mineral density in healthy families. J
Bone Miner Res. 10:2017–2022. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krall EA and Dawson-Hughes B: Heritable
and life-style determinants of bone mineral density. J Bone Miner
Res. 8:1–9. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao L, Liu WL, Liu Y and Zhao YX:
Vascular endothelial dysfunction may be a common initial factor of
development of vascular calcification and osteoporosis. Negative.
2017, http://en.cnki.com.cn/Article_en/CJFDTotal-DSJY201701007.htm
|
|
13
|
Liu L, Zhu Q, Wang J, Xi Q, Zhu H and Gu
M: Gene expression changes in human mesenchymal stem cells from
patients with osteoporosis. Mol Med Rep. 12:981–987. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen XE, Chen P, Chen SS, Lu J, Ma T, Shi
G, Zhou Y, Li J and Sheng L: A population association study of
vitamin D receptor gene polymorphisms and haplotypes with the risk
of systemic lupus erythematosus in a Chinese population. Immunol
Res. 65:750–756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DO, Kim JH, Yoo BC and Yoo JH: Is
osteoporosis a risk factor for ankle fracture? Comparison of bone
mineral density between ankle fracture and control groups.
Osteoporos Sarcopenia. 3:192–194. 2017. View Article : Google Scholar
|
|
16
|
Stathopoulou MG, Dedoussis GV, Trovas G,
Theodoraki EV, Katsalira A, Dontas IA, Hammond N, Deloukas P and
Lyritis GP: The role of vitamin D receptor gene polymorphisms in
the bone mineral density of Greek postmenopausal women with low
calcium intake. J Nutr Biochem. 22:752–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massidda M, Corrias L, Bachis V, Cugia P,
Piras F, Scorcu M and Calò CM: Vitamin D receptor gene
polymorphisms and musculoskeletal injuries in professional football
players. Exp Ther Med. 9:1974–1978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colombini A, Brayda-Bruno M, Lombardi G,
Croiset SJ, Ceriani C, Buligan C, Barbina M, Banfi G and Cauci S:
BsmI, ApaI and TaqI polymorphisms in the vitamin D receptor gene
(VDR) and association with lumbar spine pathologies: An Italian
Case-Control Study. PLoS One. 11:e01550042016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morrison NA, Qi JC, Tokita A, Kelly PJ,
Crofts L, Nguyen TV, Sambrook PN and Eisman JA: Prediction of bone
density from vitamin D receptor alleles. Nature. 367:284–287. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vladoiu S, Oros S, Manda D, Rosca R and
Ianas O: Vitamin D receptor, BsmI, FokI, ApaI, TaqI and estrogen
receptor alpha, PvuII and XbaI, gene polymorphisms in women with
osteoporosis. Endocrine Abstracts. 32:P1042013.
|
|
21
|
Mitra S, Desai M and Ikram Khatkhatay M:
Vitamin D receptor gene polymorphisms and bone mineral density in
postmenopausal Indian women. Maturitas. 55:27–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castelán-Martínez OD, Vivanco-Muñoz N,
Falcón-Ramírez E, Valdés-Flores M and Clark P: Apa1 VDR
polymorphism and osteoporosis risk in postmenopausal Mexican women.
Gac Med Mex. 151:472–476. 2015.(In Spanish). PubMed/NCBI
|
|
23
|
Feskanich D, Hunter DJ, Willett WC,
Hankinson SE, Hollis BW, Hough HL, Kelsey KT and Colditz GA:
Vitamin D receptor genotype and the risk of bone fractures in
women. Epidemiology. 9:535–539. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rooney AM and van der Meulen MCH: Mouse
models to evaluate the role of estrogen receptor α in skeletal
maintenance and adaptation. Ann NY Acad Sci. 1410:85–92. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horst-Sikorska W, Dytfeld J, Wawrzyniak A,
Marcinkowska M, Michalak M, Franek E, Napiórkowska L, Drwęska N and
Słomski R: Vitamin D receptor gene polymorphisms, bone mineral
density and fractures in postmenopausal women with osteoporosis.
Mol Biol Rep. 40:383–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dilmec F, Uzer E, Akkafa F, Kose E and van
Kuilenburg AB: Detection of VDR gene ApaI and TaqI polymorphisms in
patients with type 2 diabetes mellitus using PCR-RFLP method in a
Turkish population. J Diabetes Complications. 24:186–191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Becker W, Hujoel PP, Becker BE and
Willingham H: Osteoporosis and implant failure: An exploratory
case-control study. J Periodontol. 71:625–631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noronha-Matos JB and Correia-de-Sá P:
Mesenchymal stem cells ageing: Targeting the ‘purinome’ to promote
osteogenic differentiation and bone repair. J Cell Physiol.
231:1852–1861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khaled EG, Saleh M, Hindocha S, Griffin M
and Khan WS: Tissue engineering for bone production- stem cells,
gene therapy and scaffolds. Open Orthop J. 5 Suppl 2:289–295. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Özbaş H, Tutgun Onrat S and Özdamar K:
Genetic and environmental factors in human osteoporosis. Mol Biol
Rep. 39:11289–11296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeljic K, Elkilany A, Supic G, Surbatovic
M, Djordjevic D, Magic Z and Bozic B: Vitamin D receptor gene
polymorphisms association with the risk of sepsis and mortality.
Int J Immunogenet. 44:129–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang TJ, Jin SH, Yeum CE, Lee SB, Kim CH,
Lee SH, Kim KH, Shin ES and Chae GT: Vitamin D receptor gene TaqI,
BsmI and FokI polymorphisms in Korean patients with tuberculosis.
Immune Netw. 11:253–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sassi R, Sahli H, Souissi C, El Mahmoudi
H, Zouari B, Ben Ammar ElGaaied A, Sellami S and Ferrari SL:
Association of LRP5 genotypes with osteoporosis in Tunisian
post-menopausal women. BMC Musculoskelet Disord. 15:1442014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang P, Ma LH, Wang HY, Zhang W, Tian Q,
Cao DN, Zheng GX and Sun YL: Association between polymorphisms of
vitamin D receptor gene ApaI, BsmI and TaqI and muscular strength
in young Chinese women. Int J Sports Med. 27:182–186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zintzaras E, Rodopoulou P and Koukoulis
GN: BsmI, TaqI, ApaI and FokI polymorphisms in the vitamin D
receptor (VDR) gene and the risk of osteoporosis: a meta-analysis.
Dis Markers. 22:317–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saito M, Eiraku N, Usuku K, Nobuhara Y,
Matsumoto W, Kodama D, Sabouri AH, Izumo S, Arimura K and Osame M:
ApaI polymorphism of vitamin D receptor gene is associated with
susceptibility to HTLV-1-associated myelopathy/tropical spastic
paraparesis in HTLV-1 infected individuals. J Neurol Sci.
232:29–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moran JM, Pedrera-Canal M,
Rodriguez-Velasco FJ, Vera V, Lavado-Garcia JM, Fernandez P and
Pedrera-Zamorano JD: Lack of association of vitamin D receptor BsmI
gene polymorphism with bone mineral density in Spanish
postmenopausal women. PeerJ. 3 Suppl 3:e9532015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rocha O, Lunet N, Costa L and Barros H:
Osteoporosis treatment in Portugal: Trends and geographical
variation. Acta Med Port. 19:373–380. 2006.(In Portuguese).
PubMed/NCBI
|
|
39
|
Pei FH, Wang YJ, Gao SL, Liu BR, Du YJ,
Liu W, Yu HY, Zhao LX and Chi BR: Vitamin D receptor gene
polymorphism and ulcerative colitis susceptibility in Han Chinese.
J Dig Dis. 12:90–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alonso N and Ralston SH: Unveiling the
mysteries of the genetics of osteoporosis. J Endocrinol Invest.
37:925–934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marshall LM, Lang TF, Lambert LC, Zmuda
JM, Ensrud KE and Orwoll ES; Osteoporotic Fractures in Men (MrOS)
Research Group, . Dimensions and volumetric BMD of the proximal
femur and their relation to age among older U.S. men. J Bone Miner
Res. 21:1197–1206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Palermo A, Tuccinardi D, Defeudis G,
Watanabe M, D'Onofrio L, Lauria Pantano A, Napoli N, Pozzilli P and
Manfrini S: BMI and BMD: The potential interplay between obesity
and bone fragility. Int J Environ Res Public Health. 13:E5442016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bijelic R, Balaban J and Milicevic S:
Correlation of the lipid profile, BMI and bone mineral density in
postmenopausal women. Mater Sociomed. 28:412–415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu M, Chen GQ and Yu F: Lack of
association between vitamin D receptor polymorphisms ApaI
(rs7975232) and BsmI (rs1544410) and osteoporosis among the Han
Chinese population: A meta-analysis. Kaohsiung J Med Sci.
32:599–606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pedrera-Canal M, Moran JM, Vera V,
Roncero-Martin R, Lavado-Garcia JM, Aliaga I and Pedrera-Zamorano
JD: Common allelic variants of the vitamin receptor D gene
rs7975232 (ApaI) do not influence bone mineral density figures in
postmenopausal osteoporotic women. Int J Clin Exp Med. 8:8173–8177.
2015.PubMed/NCBI
|
|
46
|
Arji N, Busson M, Iraqi G, Bourkadi JE,
Benjouad A, Bouayad A, Mariaselvam C, Salah S, Fortier C, Amokrane
K, et al: Genetic diversity of TLR2, TLR4, and VDR loci and
pulmonary tuberculosis in Moroccan patients. J Infect Dev Ctries.
8:430–440. 2014. View Article : Google Scholar : PubMed/NCBI
|