Introduction
Multiple sclerosis (MS) is a severe neurological
disorder in which oligodendrocytes are destroyed leading to
disability, physical impairment, reduced quality of life and,
potentially, mortality (1). MS
affects 2.5 million individuals worldwide (2,3). The
majority of individuals with MS are in the 20–50-year age range,
and the disease tends to affect women 2–3 times more than men
(4,5).
The main etiology of MS remains largely unknown, though genetics
and environmental risk factors are considered to serve a major role
in disease pathology (6). The human
leukocyte antigen (HLA) complex has been linked with susceptibility
to a number of neurodegenerative diseases, including MS (7). Although it is apparent that the HLA
region is associated with MS disease risk, the specific genes
responsible have been difficult to determine (7). Over 200 genes associated with MS have
been identified and, of these, 110 non-HLA genetic loci have been
detailed and 13 HLA loci identified (6,8). The
haplotype of the HLA-DR15 antigen,
DRB1*15:01-DQA1*01:02-DQB1*06:02, has been studied and found to be
notably associated with MS, increasing the risk of disease
development by 3-fold (9). In the
current study the aim was to determine the HLA types expressed in
Bahraini patients with MS and compare them with those in control
subjects. Additionally, it was determined whether the DRB1*15:01
allele was associated with MS in the Bahraini population
studied.
Materials and methods
Study subjects
Study subjects included 50 Bahraini patients with MS
undergoing treatment, who were diagnosed at the Department of
Neuroscience of Salmaniya Medical Complex (SMC, Manama, Bahrain).
The clinical diagnosis was relapsing remission (RR) in 43 patients,
primary progressive (PP) in 4 patients, and secondary progressive
(SP) in 3 patients. All MS patients recruited for the study were
assessed independently by a neurologist who diagnosed a clinical
relapse, in the case of the relapsing cohort, or confirmed clinical
remission of the patients in the remission cohort. A magnetic
resonance imaging (MRI) brain scan confirmed MS diagnosis according
to the McDonald criteria (10).
Demographic (age at onset of first symptoms and sex) and clinical
(disease course and disability status) data, and the findings of
all paraclinical tests were recorded for all patients. Disability
was evaluated using the Kurtzke expanded disability status scale
(EDSS) (11). A total of 50 healthy
Bahraini control subjects with no neurological problems or history
of autoimmune and inflammatory disease were also enrolled in the
study as volunteers, from the SMC Blood Bank.
Inclusion and exclusion criteria included the
following: Patients and controls should be of Bahraini origin;
patients should be in relapse stage; and healthy control subjects
should be without any autoimmune diseases or neurological
disorders.
Written informed consent was obtained from all
patients under a protocol that was approved by the Research and
Ethics Committee of the College of Medicine and Medical Sciences,
Arabian Gulf University, Bahrain. Control subjects provided consent
on donation of blood samples. Ethical approval from the Ministry of
Health, Bahrain was also obtained.
Sample collection and DNA
extraction
Blood samples (4 ml) from the MS patients were
collected at the Department of Neuroscience, SMC, while control
subject samples were collected from the SMC Blood Bank. DNA was
extracted from the Buffy coat using a QIAamp® DNA Mini
and Blood Mini kit (Qiagen GmbH, Hilden, Germany). The DNA samples'
purity was measured with a NanoDrop 1000 spectrophotometer (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
HLA typing
Genotyping of the HLA alleles HLA-A, -B and -DR was
performed using polymerase chain reaction sequence-specific primers
(PCR-SSP) from Bio-Rad Medical Diagnostics GmbH (Dreieich,
Germany). PCR was performed with the HLA SSP series (cat. no.
189738) according to the manufacturer's protocol, as follows:
Initial denaturation at 94°C for 2 min; 10 cycles of denaturation
at 94°C for 10 sec and annealing and extension at 65°C for 60 sec;
and 20 cycles of denaturation at 94°C for 10 sec, annealing at 61°C
for 50 sec and extension at 72°C for 30 sec. The PCR products were
identified using 2% agarose gel electrophoresis visualized with
ethidium bromide staining. Following completion of the
electrophoresis, the gel was placed on a UV transilluminator and
photographed for documentation and interpretation.
Statistical analysis
HLA analysis was performed by comparing the dominant
allele frequencies and HLA haplotypes between the MS patients and
control subjects using Arelquin version 3.5 (http://cmpg.unibe.ch/software/arlequin3/). The
high-frequency alleles were analyzed for association with clinical
features, by calculating odds ratios (ORs) with 95% confidence
intervals (CIs). Pearson's χ2 and Fisher' exact
probability tests were performed on the VassarStats website
(http://vassarstats.net/) to analyze differences
between the control and MS groups. P<0.05 was considered to
indicate statistical significance.
Results
Demographics and clinical
characteristics
HLA-ABDR allelic typing was performed in 50 MS
patients (male: female, 19:31; mean age, 33.6±9.6 years), of which
43 were in RR stage, 4 PP stage and 3 SP stage. The analysis also
included 50 control subjects (male: female, 47:3; mean age,
38.5±10.6 years). On evaluating MS progression, the degree of
disability indicated by EDSS score was a median of 3.7, and the
mean disease duration in terms of symptoms was 6.6±5.5 years. The
50 MS patients were undergoing treatment with Avonex, Betaferon,
Gilenya, Rebif or Tysabri (12, 9, 4, 6, 17 and 2 patients,
respectively; Table I).
 | Table I.Demographics of the MS patients and
control subjects. |
Table I.
Demographics of the MS patients and
control subjects.
|
| MS patients n=50 | Controls n=50 |
|---|
| Age, years |
|
Range | 18–56 | 19–62 |
| Mean ±
SD | 33.6±9.6 | 38.5±10.6 |
| Sex |
| Male | 19 | 47 |
|
Female | 31 | 3 |
| EDSS score |
|
Range | 1–8 |
|
|
Median | 3.7±1.4 |
|
| Disease duration,
years |
|
Range | 1–20 |
|
| Mean ±
SD | 6.6±5.5 |
|
| MS type |
| RR | 43 |
|
| PP | 4 |
|
| SP | 3 |
|
| Drug |
|
Avonex | 12 |
|
|
Betaferon | 9 |
|
|
Gilenya | 4 |
|
| Not
available | 6 |
|
|
Rebif | 17 |
|
|
Tysabri | 2 |
|
HLA-ABDR alleles frequency in MS
patients and control subjects
The analysis of HLA-ABDR tissue typing in MS
patients detected 9 of HLA-A loci, 23 of HLA-B loci and 9 of HLA-DR
loci (Table II). A higher frequency,
though not to significant extent, was observed of HLA-A alleles in
MS patients compared with in control subjects, namely of A11, A19,
A2 and A3. The frequency of the HLA-A9 allele was significantly
higher in MS patients compared with in control subjects (20 vs. 8%;
OR, 2.875; CI, 1.201–6.883; P=0.014). However, the HLA-A9 allele
did not exhibit significant difference after applying Bonferroni's
correction.
 | Table II.Frequencies of HLA-ABDR alleles in MS
patients and control subjects. |
Table II.
Frequencies of HLA-ABDR alleles in MS
patients and control subjects.
| HLA-ABDR
allele | MS group frequency,
%, n=50 | Control group
frequency, %, n=50 | P-value | Odds ratio | 95% confidence
interval | Pc |
|---|
| A1 | 13 | 16 | 0.548 | 1.275 | 0.578–2.811 |
|
| A10 | 8 | 23 | 0.003 | 3.435 | 1.454–8.114 | 0.030 |
| A11 | 3 | 2 | 1 | 1.516 | 0.248–9.271 |
|
| A19 | 26 | 20 | 0.312 | 1.405 | 0.724–2.728 |
|
| A2 | 20 | 17 | 0.583 | 1.221 | 0.597–2.497 |
|
| A28 | 6 | 9 | 0.420 | 1.549 | 0.530–4.528 |
|
| A3 | 3 | 2 | 1 | 1.516 | 0.248–9.271 |
|
| A36 | 1 | 3 | 0.621 | 3.062 | 0.313–29.949 |
|
| A9 | 20 | 8 | 0.014 | 2.875 | 1.201–6.883 | 0.126 |
| B12 | 7 | 6 | 0.777 | 1.179 | 0.382–3.641 |
|
| B13 | 2 | 1 | 1 | 2.020 | 0.180–22.646 |
|
| B14 | 5 | 4 | 1 | 1.263 | 0.329–4.849 |
|
| B15 | 5 | 12 | 0.075 | 2.591 | 0.877–7.651 |
|
| B16 | 2 | 6 | 0.279 | 3.128 | 0.616–15.886 |
|
| B17 | 4 | 5 | 1 | 1.263 | 0.329–4.849 |
|
| B18 | 5 | 5 | 1 | 1 | 0.280–3.567 |
|
| B21 | 7 | 4 | 0.350 | 1.807 | 0.512–6.376 |
|
| B22 | 1 | 2 | 1 | 2.020 | 0.180–22.646 |
|
| B27 | 0 | 4 | 0.121 |
|
|
|
| B35 | 12 | 3 | 0.015 | 4.409 | 1.204–16.141 | 0.345 |
| B37 | 2 | 1 | 1 | 2.020 | 0.180–22.646 |
|
| B40 | 10 | 4 | 0.096 | 2.667 | 0.808–8.807 |
|
| B41 | 2 | 1 | 1 | 2.020 | 0.180–22.646 |
|
| B42 | 1 | 1 | 1 | 1 | 0.0617–16.213 |
|
| B47 | 1 | 1 | 1 | 1 | 0.0617–16.213 |
|
| B5 | 17 | 14 | 0.559 | 1.258 | 0.583–2.715 |
|
| B53 | 2 | 0 | 0.497 |
|
|
|
| B7 | 3 | 6 | 0.497 | 2.064 | 0.502–8.493 |
|
| B73 | 3 | 6 | 0.497 | 2.064 | 0.502–8.493 |
|
| B78 | 5 | 3 | 0.720 | 1.702 | 0.396–7.321 |
|
| B8 | 3 | 11 | 0.026 | 3.996 | 1.079–14.791 | 0.59 |
| B81 | 1 | 0 | 1 |
|
|
|
| DR1 | 10 | 16 | 0.207 | 1.714 | 0.737–3.988 |
|
| DR2 | 20 | 20 | 1 | 1 | 0.500–1.999 |
|
| DR3 | 17 | 16 | 0.841 | 1.075 | 0.509–2.269 |
|
| DR4 | 16 | 11 | 0.300 | 1.511 | 0.676–3.512 |
|
| DR5 | 15 | 11 | 0.399 | 1.428 | 0.621–3.284 |
|
| DR6 | 7 | 12 | 0.228 | 1.812 | 0.682–4.811 |
|
| DR7 | 14 | 11 | 0.521 | 1.317 | 0.567–3.062 |
|
| DR8 | 0 | 2 | 0.497 |
|
|
|
| DR9 | 1 | 1 | 1 | 1 | 0.062–16.213 |
|
A higher frequency, though not significant, of HLA-B
alleles was observed in the MS patients compared with in control
subjects, namely of B12, B13, B14, B21, B37, B40, B41, B5, B53, B78
and B81. The frequency of the HLA-B35 allele was significantly
higher in MS patients compared with in controls (12 vs. 3%; OR,
4.409; CI, 1.204–16.141; P=0.015). However, the HLA-B35 allele did
not show significant difference after applying Bonferroni's
correction. A higher frequency, though not significant, of the
HLA-DR alleles was revealed in MS patients compared with control
subjects, of DR3, DR4, DR5 and DR7.
Conversely, the HLA-A10 allele exhibited
significantly higher frequency in control subjects compared with in
MS patients (28 vs. 8%; OR, 3.435; CI, 1.454–8.114; P=0.003), and
HLA-A1 had marginally higher frequency in control subjects than in
MS patients (16 vs. 13%; OR, 1.275; CI, 0.578–2.811; P=0.548).
Furthermore, HLA-B15 was present at higher frequency
in control subjects than in MS patients (12 vs. 5%; OR, 2.591; CI,
0.877–7.651; P=0.075), and HLA-B8 showed significantly higher
allele frequency in control subjects than in MS patients (11 vs.
3%; OR, 3.996; CI, 1.079–14.791; P=0.026); however, HLA-B8 did not
exhibit significant difference after applying Bonferroni's
correction. Additionally, HLA-DR1 (16 vs. 10%; OR, 1.714; CI,
0.737–3.988; P=0.207) and DR6 (12 vs. 7%; OR, 1.812; CI,
0.682–4.811; P=0.228) showed higher allele frequencies in control
subjects than in MS patients.
HLA-B18 and HLA-DR2 exhibited equal frequencies
between the study populations. HLA-DR15 was present at lower
frequency in MS patients than in control subjects (6 vs. 12%; OR,
2.136; CI, 0.769–5.938) but the difference was not statistically
significant (P=0.138; Table VI).
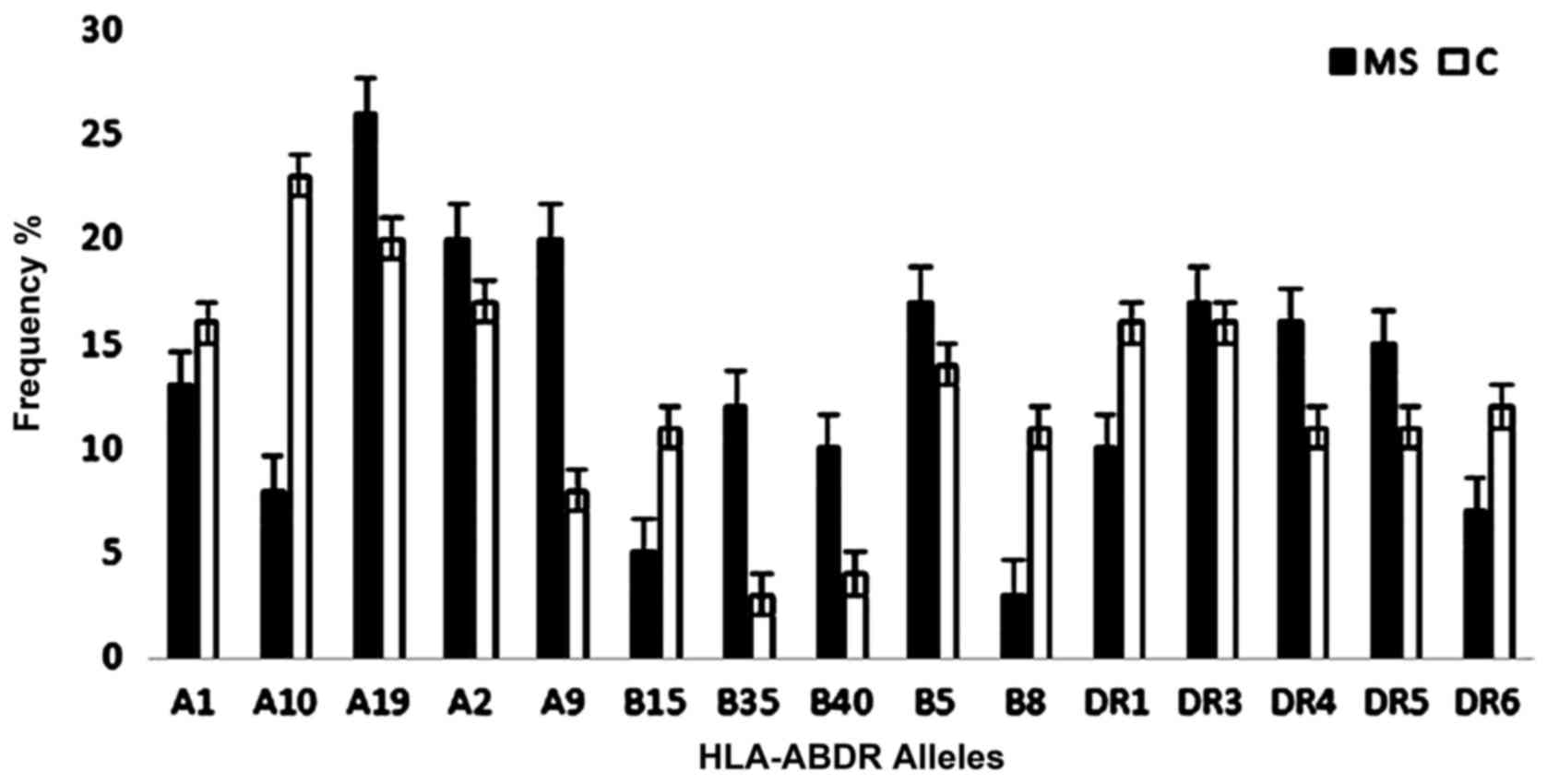
Fig. 1 presents the most frequent
HLA-ABDR alleles detected in MS patients and control subjects.
Figs. 2 and 3 show representative gel electrophoresis
results of PCR products for the HLA loci in an MS patient and a
healthy control subject.
 | Figure 2.Example electrophoresis gel of PCR
products for HLA loci in a patient with multiple sclerosis.
Negative control (position H1), HLA-A25 (position H2), HLA-A32
(position C3), HLA-A32 (position G3), HLA-B50 (position B7),
HLA-B50 (position C7), HLA-B44 (position H7), HLA-DR4 (position
A10), HLA-DR13 (position B11), HLA-DR13 (position B11), HLA-DR53
(position B12), HLA-DR52 (position C12) and HLA-DR13 (position
H12). HLA, human leukocyte antigen. |
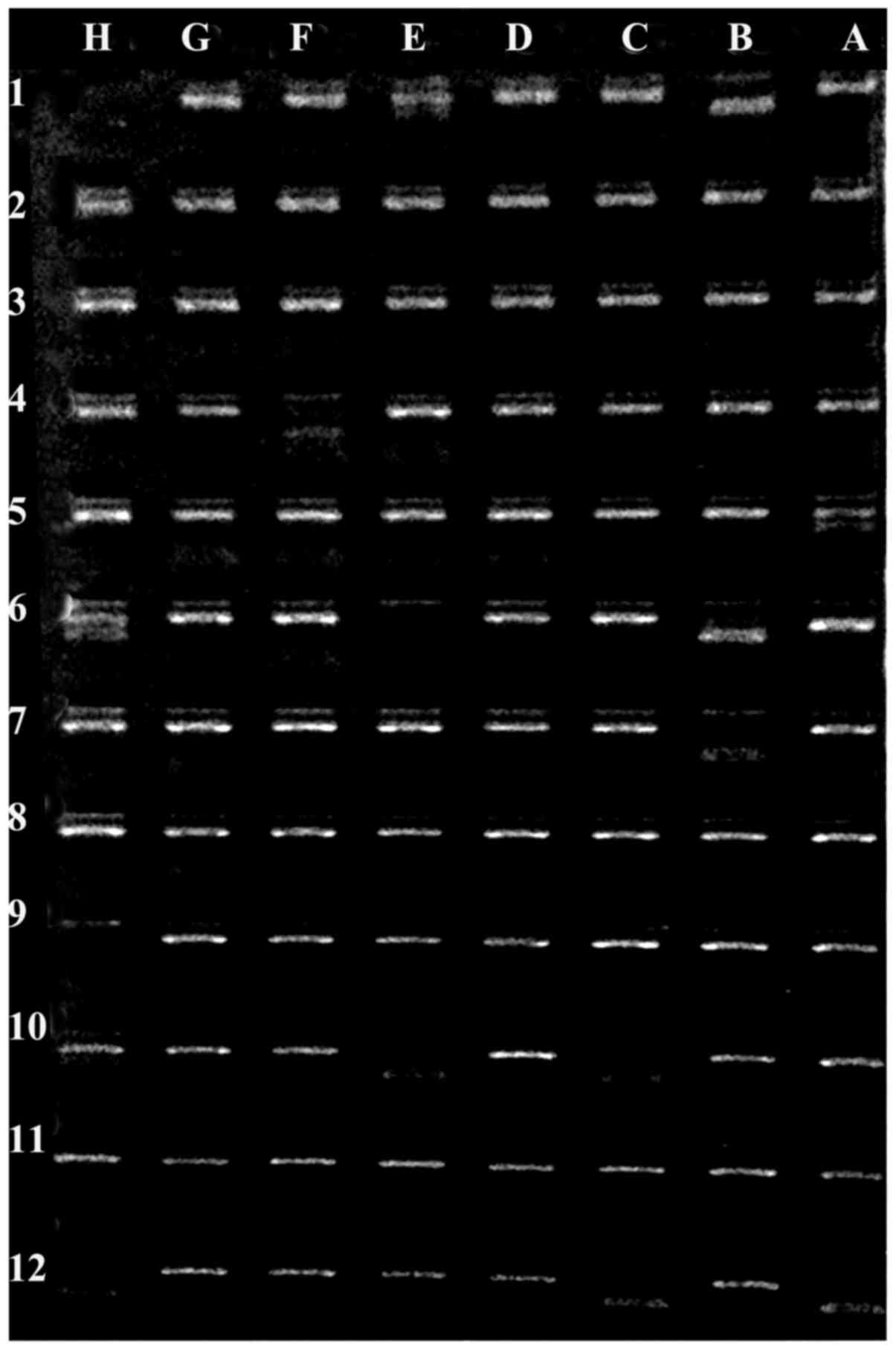 | Figure 3.Example electrophoresis gel of PCR
products for HLA loci in a healthy control subject. Negative
control (position H1), HLA-A24 (position B1), HLA-A3 (position E1),
HLA-B8 (position F4), HLA-B8 (position A5), HLA-B8 (position A6),
HLA-B47 (position B6), HLA-B8 (position H6), HLA-B47 (position B7),
HLA-DR17 (position C10), HLA-DR15 (position E10), HLA-Bw6 (position
H10), HLA-DR51 (position A12), HLA-DR52 (position C12) and HLADR17
(position H12). HLA, human leukocyte antigen. |
 | Table VI.HLA-ABDR alleles of equal frequency
between MS patients and control subjects. |
Table VI.
HLA-ABDR alleles of equal frequency
between MS patients and control subjects.
| HLA-ABDR
allele | MS group frequency,
%, n=50 | Control group
frequency, %, n=50 | P-value | Odds ratio | 95% confidence
interval |
|---|
| B18 | 5 | 5 | 1 | 1 | 0.280–3.567 |
| DR2 | 20 | 20 | 1 | 1 | 0.500–1.999 |
|
DR15 | 6 | 12 | 0.138 | 2.136 | 0.769–5.938 |
|
DR16 | 14 | 8 | 0.174 | 0.534 | 0.214–1.337 |
Association of high-frequency HLA-ABDR
alleles with MS clinical features
HLA alleles A19, A2, A9, B35, B5, B40, DR3 and DR5
were more common in female MS patients, while HLA-DR4 was more
common in male MS patients. HLA-B5 exhibited significantly higher
allele frequency in female patients than in male patients
(P=0.001). Regarding disease progression, these alleles were
notably more frequent in the RR stage of MS. The high-frequency
HLA-ABDR alleles of MS patients also appeared associated with EDSS
scores between 3 and 4, and with disease duration ranging from 4 to
9 years (Table III).
 | Table III.High-frequency HLA-ABDR alleles and
clinical features of MS patients. |
Table III.
High-frequency HLA-ABDR alleles and
clinical features of MS patients.
|
| Sex |
|
| MS type |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| HLA-ABDR
allele | F | M | P-value | Age, mean ± SD | RR | PP | SP | EDSS score, mean ±
SD | Disease duration,
mean ± SD |
|---|
| A19 | 12 | 10 | 0.6315 | 33.8±9.8 | 18 | 2 | 2 | 3.5±0.3 | 7.7±7.0 |
| A2 | 9 | 7 | 0.5838 | 35.0±1.4 | 14 | 1 | 1 | 3.6±1.0 | 7.2±5.6 |
| A9 | 11 | 5 | 0.1016 | 33.0±7.0 | 15 | 1 | 0 | 4.0±1.4 | 4.8±4.8 |
| B35 | 7 | 3 | 0.1821 | 33.6±12.7 | 9 | 1 | 0 | 3.2±1.0 | 6.4±7.0 |
| B5 | 15 | 1 | 0.0001 | 33.1±9.8 | 14 | 1 | 1 | 3.7±0.7 | 5.6±0.7 |
| B40 | 5 | 4 | 1 | 35.6±8.4 | 7 | 1 | 1 | 4.0±0.3 | 8.0±7.0 |
| DR3 | 12 | 5 | 0.0624 | 33.5±7.7 | 15 | 0 | 2 | 3.4±0.7 | 7.0±2.8 |
| DR4 | 6 | 8 | 0.5656 | 36.0±2.8 | 12 | 1 | 1 | 3.7±0.7 | 8.8±4.9 |
| DR5 | 8 | 7 | 0.7772 | 31.0±2.8 | 12 | 2 | 1 | 3.7±1.4 | 6.7±5.3 |
High-frequency allele subtypes of MS patients and
control subjects. Table IV presents
the high-frequency allele subtypes of HLA-ABDR in MS patients
compared with control subjects. Regarding HLA-A genotypes; HLA-A19,
and HLA-A9 were higher in MS patients compared to control subjects.
The HLA-A30 allele was more frequent in MS patients compared with
in control subjects (8 vs. 3%; P=0.120). HLA-A24 was significantly
higher in MS patients than in controls even following Bonferroni
correction (16 vs. 5%; P=0.02). HLA-DR11 showed higher frequency in
MS patients than in control subjects (15 vs. 11%; P=0.399).
 | Table IV.High-frequency allele subtypes of MS
patients vs. control subjects. |
Table IV.
High-frequency allele subtypes of MS
patients vs. control subjects.
| HLA-ABDR
allele | MS group frequency,
%, n=50 | Control group
frequency, %, n=50 | P-value | Odds ratio | 95% confidence
interval | Pc |
|---|
| A19 |
|
A29 | 4 | 6 | 0.516 | 1.532 | 0.419–5.603 |
|
|
A30 | 8 | 3 | 0.120 | 2.812 | 0.724–10.924 |
|
|
A31 | 2 | 0 | 0.490 |
|
|
|
|
A32 | 9 | 9 | 1 | 1 | 0.379–2.634 |
|
|
A33 | 3 | 2 | 1 | 1.516 | 0.248–9.271 |
|
| A2 | 20 | 17 | 0.583 | 1.221 | 0.597–2.497 |
|
| A9 |
|
A23 | 4 | 3 | 0.999 | 1.347 | 0.294–6.180 |
|
|
A24 | 16 | 5 | 0.011 | 3.619 | 1.271–10.303 | 0.022 |
| B35 | 12 | 3 | 0.015 | 4.409 | 1.204–16.141 | 0.345 |
| B40 | 7 | 3 | 0.195 | 2.434 | 0.611–9.694 |
|
|
B60 | 1 | 0 | 1 |
|
|
|
|
B61 | 2 | 1 | 1 | 2.020 | 0.180–22.646 |
|
| B5 |
|
B51 | 14 | 7 | 0.106 | 2.163 | 0.834–5.612 |
|
|
B52 | 3 | 7 | 0.194 | 2.434 | 0.611–9.694 |
|
| DR3 |
|
DR17 | 15 | 15 | 1 | 1 | 0.460–2.173 |
|
|
DR18 | 2 | 1 | 1 | 2.020 | 0.180–22.646 |
|
| DR4 | 16 | 11 | 0.300 | 1.541 | 0.676–3.512 |
|
| DR5 |
|
DR11 | 15 | 11 | 0.399 | 1.428 | 0.621–3.284 |
|
Table V reports the
high-frequency allele subtypes of HLA-ABDR in control subjects
compared with MS patients. HLA-A26 exhibited higher allele
frequency in control subjects compared with in MS patients (12 vs.
5%; P=0.075), as well as HLA-DR13 (10 vs. 5%; P=0.161).
 | Table V.High-frequency allele subtypes of
control subjects vs. MS patients. |
Table V.
High-frequency allele subtypes of
control subjects vs. MS patients.
| HLA-ABDR
allele | Control group
frequency, %, n=50 | MS group frequency,
%, n=50 | P-value | Odds ratio | 95% confidence
interval | Pc |
|---|
| A1 | 16 | 13 | 0.548 | 1.275 | 0.578–0.811 |
|
| A10 | 7 | 2 | 0.169 | 3.688 | 0.746–18.211 |
|
|
A25 | 0 | 1 | 1 |
|
|
|
|
A26 | 12 | 5 | 0.075 | 2.591 | 0.877–7.651 |
|
|
A34 | 1 | 0 | 0.999 |
|
|
|
|
A43 | 2 | 0 | 0.490 |
|
|
|
|
A66 | 1 | 0 | 0.999 |
|
|
|
| B15 | 12 | 5 | 0.075 | 2.591 | 0.877–7.651 |
|
|
B62 | 0 | 0 | 0 |
|
|
|
|
B63 | 0 | 0 | 0 |
|
|
|
|
B70 | 0 | 0 | 0 |
|
|
|
|
B71 | 0 | 0 | 0 |
|
|
|
|
B72 | 0 | 0 | 0 |
|
|
|
|
B75 | 0 | 0 | 0 |
|
|
|
|
B76 | 0 | 0 | 0 |
|
|
|
|
B77 | 0 | 0 | 0 |
|
|
|
| B8 | 11 | 3 | 0.026 | 3.996 | 1.079–14.791 | 0.590 |
| DR1 | 16 | 10 | 0.207 | 1.714 | 0.737–3.988 |
|
| DR6 | 12 | 7 | 0.228 | 1.812 | 0.68–4.811 |
|
|
DR13 | 10 | 5 | 0.161 | 2.250 | 0.709–7.141 |
|
|
DR14 | 2 | 2 | 1 | 1 | 0.135–7.392 |
|
Table VI depicts the
subtypes of alleles with equal frequency between the study
populations.
Comparison between HLA-ABDR haplotypes
of MS patients and control subjects
HLA-ABDR haplotypes in patients and controls were
analyzed. Only haplotypes present in at least 2% of the sample were
considered. This identified 90 prevalent haplotypes (data not
shown) in MS patients and control subjects. The haplotype
HLA-A2-B40-DR2 exhibited significantly higher frequency in MS
patients compared with in controls (5 vs. 0%; P=0.030). Meanwhile,
4 haplotypes were common between MS patients and control subjects:
HLA-A1-B5-DR3, HLA-A10-B12-DR4, HLA-A19-B15-DR7 and HLA-A9-B5-DR3.
Among these, 2 showed the same frequency, HLA-A1-B5-DR3 and
HLA-A19-B15-DR7 (2%); the other 2 (HLA-A10-B12-DR4 and
HLA-A9-B5-DR3) were slightly higher in MS patients (3 vs. 2%).
Overall a total of 10 haplotypes exhibited higher frequencies in MS
patients than in controls. Meanwhile, 3 haplotypes (HLA-A2-B15-DR3,
HLA-A2-B15-DR7 and HLA-A19-B15-DR1) were present only in control
subjects (Table VII).
 | Table VII.HLA haplotypes of MS patients and
control subjects. |
Table VII.
HLA haplotypes of MS patients and
control subjects.
|
| HLA haplotype |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| HLA-ABDR
allele | A | B | DR | MS group frequency,
% n=50 | Control group
frequency, % n=50 | P-value | Odds ratio | 95% confidence
interval |
|---|
| 1 | A1 | B5 | DR3 | 2 | 2 | 1 | 0.993 | 0.139–7.098 |
| 2 | A19 | B15 | DR7 | 2 | 2 | 1 | 0.993 | 0.139–7.098 |
| 3 | A9 | B5 | DR3 | 3 | 2 | 0.686 | 0.660 | 0.109–3.979 |
| 4 | A10 | B12 | DR4 | 3 | 2 | 0.686 | 0.660 | 0.109–3.979 |
| 5 | A1 | B5 | DR2 | 3 | 0 | 0.123 |
|
|
| 6 | A19 | B35 | DR2 | 3 | 0 | 0.123 |
|
|
| 7 | A19 | B40 | DR2 | 3 | 0 | 0.123 |
|
|
| 8 | A19 | B5 | DR4 | 3 | 0 | 0.123 |
|
|
| 9 | A19 | B14 | DR7 | 3 | 0 | 0.123 |
|
|
| 10 | A2 | B35 | DR2 | 3 | 0 | 0.123 |
|
|
| 13 | A9 | B18 | DR5 | 3 | 0 | 0.123 |
|
|
| 14 | A19 | B5 | DR3 | 4 | 0 | 0.061 |
|
|
| 15 | A2 | B5 | DR4 | 4 | 0 | 0.061 |
|
|
| 16 | A2 | B40 | DR2 | 5 | 0 | 0.030 |
|
|
| 17 | A2 | B15 | DR3 | 0 | 3 | 0.249 |
|
|
| 18 | A2 | B15 | DR7 | 0 | 3 | 0.249 |
|
|
| 19 | A19 | B15 | DR1 | 0 | 4 | 0.124 |
|
|
Discussion
MS is a complex multifactorial disease caused by
interaction between environmental and genetics risk factors
(12). There have been numerous
reports of increased prevalence of MS worldwide in the last 5
years, with the Arabian Gulf area exhibiting a moderate-to-high
prevalence (31–55 MS cases per 100,000 individuals) (13). Over 400 MS cases were identified in
the neuroscience department at SMC (unpublished data). The present
study aimed to assess the genetic risk factors among HLA loci that
may be involved in the pathogenesis of MS in Bahraini patients.
The importance of HLA class I and II in the risk of
developing MS has been widely studied (14). Several studies from the United States
(15) have confirmed the association
of the HLA class II alleles DRB1*15:01, DQA1*01:02 and DQB1*06:02
with MS, while other studies mapped HLA class II DRB5*01:01,
DRB1*15:01, DQA1*01:02 and DQB1*06:02 alleles in the North European
population (16,17). Other alleles may contribute to MS in
European populations, in particular HLA-DQA1*05:01, HLA-B*44:02 and
HLA-C*05 (18). In Sardinian MS
patients, disease pathology has been associated with the
DRB1*03:01, DQA1*05:01, DQB1*02:01 and DRB1*04:05, DQA1*05:01 and
DQB1*03:01 alleles (17,19). In African-Brazilian MS patients, the
strongest association was observed with DQB1*06:02 (19). In Canadian MS families, the DRB1*15:01
allele appeared to determine susceptibility to MS (19).
In the present study, the relative frequencies of
HLA class I and II alleles and haplotypes were assessed in 50
Bahraini patients with MS. Overall, current observations are not in
line with a previous reports (20),
which has concluded the highest risk HLA allele associated with MS
to be HLA-DRB1*15:0, and that for HLA haplotype to be
HLA-DRB1*15:01-HLA-DQA1*01:02-HLA-DQB1*06:02-HLA-DRB5*01:01
(20).
The present results suggested other susceptibility
alleles of HLA-ABDR genotypes, in particular HLA-A9 and B35. In
addition, HLA-ABDR allele subtypes A24 was higher in MS patients
compared with in controls. This finding is similar to results of a
study in Kuwaiti patients with MS disease (21). Additionally, the current results on
HLA-A9 and A19 were similar to findings reported by Chao et
al (22). Furthermore, the higher
frequency observed of HLA-A2 in MS patients is similar to previous
reports across populations from The Netherlands, Switzerland, USA
and Scandinavia (20,23). Our result on HLA-A24 is further in the
line with an Iranian study (24).
Moreover, the findings on HLA-DR3 are similar to those reported in
a Russian Altai territory population (25). Regarding HLA-B5 and DR4, the present
results were similar to an Iraqi study that reported association of
these alleles with MS; however the same study indicated the
protective alleles of HLA-B35 and DR2, which differs from present
findings (26). Previous studies have
suggested that the HLA-A2 allele has a protective effect in MS
(27,28). The current study further indicated a
positive association and therefore protective effect of HLA-A10
allele in healthy control subjects compared with MS patients,
similar to a study by Amirzargar et al (29). By contrast, Al-Shammri et al
(21) and Chao et al (22) found that HLA-A10 was higher in MS
patients. The finding that HLA-B8 and B15 may serve as protective
alleles also differs to previous results of Chao et al
(22) and Jilek et al
(28). Regarding HLA-DR6 as a
protective allele, the current data is in line with the Kuwaiti
study while differing from findings in Japan and Mexico (21,30). The
indication of HLA-DR13 as a protective allele is similar to a
Spanish study (31) but dissimilar to
an Italian study (32).
The current result on HLA-DR15 is dissimilar to
findings in the Russian Altai study, which indicated association
with a high risk of MS (25). HLA-DR2
exhibited an equal allele frequency between the current study
populations, similar to a US study (33).
The current study further indicated HLA-A19, A2, A9,
B35, B5, B40, DR5 and DR3 were found to be more common in female MS
patients, in RR stage, in MS patients, in cases with EDSS scores
between 3 and 4, and in cases with disease duration between 4 and 9
years. Notably, HLA-B5 was common and significantly higher
(P=0.0001) in female compared with male patients, whereas HLA-DR4
was more common in male patients in RR stage, and was observed in
patients above 30 years old, similar to a study performed in Qatar
(13). The results on HLA-DR2 and DR4
were similar to the Kuwaiti study. In contrast, a US study and
others suggested have that DR2 is apparent in several forms of MS
(34). Although the HLA-DRB1*15
haplotype may represent the main disease risk factor in populations
of North European origin, several dissimilar allelic associations
have been identified in Southern European populations, Israel
(35) and other countries (30). The comparison of HLA-ABDR haplotypes
between MS and control subjects determined 10 haplotypes with
higher frequency in MS and absence in control subjects. A total of
5 haplotypes carried HLA-A19, 4 carried HLA-B5 and 5 carried
HLA-DR2, indicating susceptibility alleles. A significant positive
association of HLA-A2-B40-DR2 was detected in the Bahraini MS
patients (P=0.030). Therefore, the most potent genetic effects
appear to be conferred by A2 and DR2, which is similar to findings
in several previous studies (9,19,23).
Interestingly, HLA-A9 and B35 were indicated as risk
factor alleles for MS disease, whereas HLA-A10 and B8 were
determined as protective alleles. An obvious association of a
specific haplotype with a particular autoimmune disease in an
ethnic group may not hold in another ethnic group, where haplotypes
associations may be differ entirely. Nevertheless, the present
results indicate that HLA-A2-B40-DR2 haplotype is significantly
higher in Bahraini MS patients when compared with healthy
individuals.
In conclusion, observations of different allele and
haplotypes associations with MS between various populations
emphasizes that there may be a population-specific genetic
susceptibility or protection conferred by HLA alleles for MS in
different populations reported. HLA-DRB1*1501 has been consistently
associated with MS susceptibility in genetic studies. The current
study did not find evidence of association between HLA-DRB1*1501
and MS in the Bahraini patients studied. Certain HLA-ABDR antigens
(A19, A2, A9, B35, B40, B5, DR3, DR4 and DR5) were present at
higher frequencies in the MS Bahraini patients compared with
controls; while HLA-A10 was indicated as a protective allele, being
detected at higher frequency in the control subjects. The current
indications of HLA allele associations with MS differ from
observations in Caucasians and certain Arab populations, though are
similar to findings in Kuwaiti patients.
Acknowledgements
The authors acknowledge the Bahrain Multiple
Sclerosis Patients Society for their assistance in recruiting
patients to the study.
Funding
The present study was supported by a grant (no. 76)
from the Research Committee of the College of Medicine and Medical
Sciences, Arabian Gulf University, Manama, Bahrain.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAN performed the experiments. ST contributed to the
acquisition, analysis and interpretation of data. AHS contributed
to the statistical analysis and writing of the paper. IA performed
the clinical aspects of the study. MB is the principle investigator
of the study and wrote the paper.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the College of Medicine and Medical Sciences, Arabian Gulf
University and the Ethical Committee of the Ministry of Health,
Bahrain. Written informed consent from all subjects agreeing to
their participation and use of samples in the current study was
obtained.
Patient consent for publication
Consent was obtained from the participants agreeing
to the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhead B, Bäärnhielm M, Gianfrancesco M,
Mok A, Shao X, Quach H, Shen L, Schaefer C, Link J, Gyllenberg A,
et al: Mendelian randomization shows a causal effect of low vitamin
D on multiple sclerosis risk. Neurol Genet. 2:e972016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sahraian MA, Sahebkar M, Dehghani R,
Derakhshan-Jazari M, Kazami-Moghaddam V and Kouchaki E: Multiple
sclerosis-A disease on a dramatically rising trend in Iran: Review
of possible reasons. Iran J Neurol. 16:34–40. 2017.PubMed/NCBI
|
|
3
|
Dargahi N, Katsara M, Tselios T,
Androutsou ME, de Courten M, Matsoukas J and Apostolopoulos V:
Multiple sclerosis: Immunopathology and treatment update. Brain
Sci. 7:E782017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harbo HF, Gold R and Tintoré M: Sex and
gender issues in multiple sclerosis. Ther Adv Neurol Disorder.
6:237–248. 2013. View Article : Google Scholar
|
|
5
|
Johnson KM, Zhou H, Lin F, Ko JJ and
Herrera V: Real-world adherence and persistence to oral
disease-modifying therapies in multiple sclerosis patients over 1
year. J Manag Care Spec Pharm. 23:844–852. 2017.PubMed/NCBI
|
|
6
|
Mescheriakova JY, Broer L, Wahedi S,
Uitterlinden AG, van Duijn CM and Hintzen RQ: Burden of genetic
risk variants in multiple sclerosis families in the Netherlands.
Mult Scler J Exp Transl Clin. 2:20552173166487212016.PubMed/NCBI
|
|
7
|
Fernando MM, Stevens CR, Walsh EC, De
Jager PL, Goyette P, Plenge RM, Vyse TJ and Rioux JD: Defining the
role of the MHC in autoimmunity: A review and pooled analysis. PLoS
Genet. 4:e10000242008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moutsianas L, Jostins L, Beecham AH,
Dilthey AT, Xifara DK, Ban M, Shah TS, Patsopoulos NA, Alfredsson
L, Anderson CA, et al; International IBD Genetics Consortium
(IIBDGC), . Class II HLA interactions modulate genetic risk for
multiple sclerosis. Nat Genet. 47:1107–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parnell GP and Booth DR: The multiple
sclerosis (MS) genetic risk factors indicate both acquired and
innate immune cell subsets contribute to MS pathogenesis and
identify novel therapeutic opportunities. Front Immunol. 8:4252017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polman CH, Reingold SC, Banwell B, Clanet
M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M,
Kappos L, et al: Diagnostic criteria for multiple sclerosis: 2010
revisions to the McDonald criteria. Ann Neurol. 69:292–302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Institute of Medicine (US) Committee on
Multiple Sclerosis: Current Status and Strategies for the Future.
Multiple Sclerosis. Joy JE and Johnston RB Jr: National Academies
Press; Washington, DC: 2001
|
|
12
|
Moosazadeh M, Esmaeili R, Mehdi Nasehi M,
Abedi G, Afshari M, Farshidi F and Kheradmand M: Prevalence of
familial multiple sclerosis in Iran: A systematic review and
meta-analysis. Iran J Neurol. 16:90–95. 2017.PubMed/NCBI
|
|
13
|
Deleu D, Mir D, Al Tabouki A, Mesraoua R,
Mesraoua B, Akhtar N, Al Hail H, D'souza A, Melikyan G, Imam YZ, et
al: Prevalence, demographics and clinical characteristics of
multiple sclerosis in Qatar. Mult Scler. 19:816–819. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Link J, Lundkvist Ryner M, Fink K,
Hermanrud C, Lima I, Brynedal B, Kockum I, Hillert J and
Fogdell-Hahn A: Human leukocyte antigen genes and interferon beta
preparations influence risk of developing neutralizing anti-drug
antibodies in multiple sclerosis. PLoS One. 9:e904792014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hebbring SJ, Schrodi SJ, Ye Z, Zhou Z,
Page D and Brilliant MH: A PheWAS approach in studying
HLA-DRB1*1501. Genes Immun. 14:187–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anagnostouli MC, Acquaviva T, Artemiadis
AK, Rentzos M, Karandreas N, Davaki P and Stamboulis E: HLA-DRB1*
alleles genotyping in chronic inflammatory demyelinating
polyneuropathy in Greek patients. J Peripher Nerv Syst. 19:187–189.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cocco E, Sardu C, Pieroni E, Valentini M,
Murru R, Costa G, Tranquilli S, Frau J, Coghe G, Carboni N, et al:
HLA-DRB1-DQB1 haplotypes confer susceptibility and resistance to
multiple sclerosis in Sardinia. PLoS One. 7:e339722012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Healy BC, Liguori M, Tran D, Chitnis T,
Glanz B, Wolfish C, Gauthier S, Buckle G, Houtchens M, Stazzone L,
et al: HLA B*44: Protective effects in MS susceptibility and MRI
outcome measures. Neurology. 75:634–640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alcina A, Abad-Grau MM, Fedetz M,
Izquierdo G, Lucas M, Fernández O, Ndagire D, Catalá-Rabasa A, Ruiz
A, Gayán J, et al: Multiple sclerosis risk variant HLA-DRB1*1501
associates with high expression of DRB1 gene in different human
populations. PLoS One. 7:e298192012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patsopoulos NA, Barcellos LF, Hintzen RQ,
Schaefer C, van Duijn CM, Noble JA, Raj T, Gourraud PA, Stranger
BE, Oksenberg J, et al; IMSGC; ANZgene, . Fine-mapping the genetic
association of the major histocompatibility complex in multiple
sclerosis: HLA and non-HLA effects. PLoS Genet. 9:e10039262013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Al-Shammri S, Nelson RF, Al-Muzairi I and
Akanji AO: HLA determinants of susceptibility to multiple sclerosis
in an Arabian Gulf population. Mult Scler. 10:381–386. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chao MJ, Barnardo MC, Lincoln MR,
Ramagopalan SV, Herrera BM, Dyment DA, Montpetit A, Sadovnick AD,
Knight JC and Ebers GC: HLA class I alleles tag HLA-DRB1*1501
haplotypes for differential risk in multiple sclerosis
susceptibility. Proc Natl Acad Sci USA. 105:13069–13074. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Link J, Kockum I, Lorentzen AR, Lie BA,
Celius EG, Westerlind H, Schaffer M, Alfredsson L, Olsson T,
Brynedal B, et al: Importance of human leukocyte antigen (HLA)
class I and II alleles on the risk of multiple sclerosis. PLoS One.
7:e367792012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalanie H, Kamgooyan M, Sadeghian H and
Kalanie AR: Histocompatibility antigen (HLA) associations with
multiple sclerosis in Iran. Mult Scler. 6:317–319. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smagina IV, El'chaninova SA, Zolovkina AG,
Ignatova IuN and Kudriavtseva EA: Genetic risk factors for multiple
sclerosis in the population of Altay. Zh Nevrol Psikhiatr Im S S
Korsakova. 111:42–45. 2011.(In Russian). PubMed/NCBI
|
|
26
|
Saleem MA, Mukhelif HF, Moussawi KM and
Al-Khafaji JT: Human leukocyte antigen typing in Iraqi multiple
sclerosis patients. Neurosciences (Riyadh). 12:127–132.
2007.PubMed/NCBI
|
|
27
|
Gough SC and Simmonds MJ: The HLA region
and autoimmune disease: Associations and mechanisms of action. Curr
Genomics. 8:453–465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jilek S, Schluep M, Harari A, Canales M,
Lysandropoulos A, Zekeridou A, Pantaleo G and Du Pasquier RA:
HLA-B7-restricted EBV-specific CD8+ T cells are
dysregulated in multiple sclerosis. J Immunol. 188:4671–4680. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amirzargar AA, Tabasi A, Khosravi F,
Kheradvar A, Rezaei N, Naroueynejad M, Ansaripour B, Moradi B and
Nikbin B: Optic neuritis, multiple sclerosis and human leukocyte
antigen: Results of a 4-year follow-up study. Eur J Neurol.
12:25–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hillert J: Human leukocyte antigen studies
in multiple sclerosis. Ann Neurol. 36 Suppl 1:S15–S17. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uría DF: HLA and multiple sclerosis.
Studies of a spanish population. Rev Neurol. 31:1066–1070.
2000.PubMed/NCBI
|
|
32
|
Perini P, Tagliaferri C, Belloni M, Biasi
G and Gallo P: The HLA-DR13 haplotype is associated with ‘benign’
multiple sclerosis in northeast Italy. Neurology. 57:158–159. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barcellos LF, Oksenberg JR, Begovich AB,
Martin ER, Schmidt S, Vittinghoff E, Goodin DS, Pelletier D,
Lincoln RR, Bucher P, et al; Multiple Sclerosis Genetics Group, .
HLA-DR2 dose effect on susceptibility to multiple sclerosis and
influence on disease course. Am J Hum Genet. 72:710–716. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vasconcelos CC, Fernández O, Leyva L,
Thuler LC and Alvarenga RM: Does the DRB1*1501 allele confer more
severe and faster progression in primary progressive multiple
sclerosis patients? HLA in primary progressive multiple sclerosis.
J Neuroimmunol. 214:101–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmidt H, Williamson D and Ashley-Koch A:
HLA-DR15 haplotype and multiple sclerosis: A HuGE review. Am J
Epidemiol. 165:1097–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|