Introduction
Cardiovascular diseases (CVDs), also termed
circulatory system diseases, affect the heart and corresponding
blood vessels and are established as a worldwide leading cause of
mortality (1). In 2014, over 1.7
million fatalities occurred worldwide as a result of CVDs, with a
total mortality rate of ~31%, and due to the availability of
healthcare, populations in developing countries are more
susceptible to CVDs (2). By 2030, it
is estimated that the total number of mortalities from CVDs will
exceed 2.3 million. To combat this, the World Health Organization
plans to reduce the rates of non-communicable diseases (due to
living, behavioral and environmental causes) to 25% by 2025,
particularly with regard to CVDs (3).
Vascular endothelial cells (VECs) form a continuous
cell mass layer over the endangium of vessels in the body. They not
only complete the metabolic exchange of blood and interstitial
fluid, but also serve as the largest endocrine gland in organisms
(4). VECs produce and secrete over
ten types of bioactive substances that participate in normal blood
flow and blood coagulation, adjust vessel tensions, and control the
proliferation and functions of smooth muscles (5). In addition, VECs also serve an important
role in adjusting the steady state of the cardiovascular system
(6). The circulatory system,
particularly the blood vessel component, is among the main targets
of oxidative stress. Consequently, VECs serve a physiological
defense function of ‘first line of defense’ against stress injuries
to organisms (7,8). The function of endothelial cells is
associated with the occurrence and development of CVDs (9). Among numerous causes for injury to
endothelial cells, oxidative stress is an important inducer of
vascular endothelial dysfunction (10). Therefore, the apoptosis of endothelial
cells in the cardiovascular system is a major indicator of
oxidative stress injury, and a prelude for the occurrence and
evolution of various CVDs (11,12).
Lycium barbarum polysaccharides (LBPs),
water-soluble protein polysaccharides, are a constituent separated
and extracted from the red medlar (also known as wolfberry) fruit
of L. barbarum, a commonly used medicinal herb in Ningxia,
China (13). LBPs have diverse
functions, including eye-protective, anti-aging, anti-oxidant,
immune adjustment, neuroprotective and anti-tumor effects (14-18).
Notably, LBPs may protect the rat reproductive system through an
anti-oxygenation mechanism; they may also prolong the apoptosis
time of rat epithelial cells, inhibit ultraviolet light-induced
peroxidase and free radical-induced cytochrome C expression, and
reduce the oxidation of fractured DNA in rat testis cells.
Furthermore, LBPs have been reported to markedly promote serum sex
hormone levels and superoxide dismutase (SOD) activity, decrease
malondialdehyde (MDA) level, decrease injury to spermatogenic cells
caused by high temperature and H2O2, and
promote normal development of germ cells of the testis (19). However, there is limited research on
whether LBPs protect against oxidative stress in artery endothelial
cells to prevent and cure CVDs. Therefore, the present study aimed
to investigate the protective effects of LBPs, particularly
regarding anti-apoptosis and anti-oxidation, in injured rat artery
endothelial cells (RAECs), to thus provide experimental and
theoretical foundations for the medicinal use of LBPs.
Materials and methods
Materials and equipment
The materials used in the present study included
RAECs (Jiangsu Chi Scientific Co., Ltd.), complete Dulbecco's
modified Eagle's medium (DMEM) containing FBS (both 3-7202; Jiangsu
Chi Scientific Co., Ltd.), a Cell Counting kit-8 (CCK-8; Dojindo
Molecular Technologies, Inc.), LBPs (Ningxia Qiyuan Pharmaceutical,
Co., Ltd.), anti-B-cell lymphoma 2 (Bcl-2) antibody (cat. no.
ab59348; Abcam), anti-Bcl-2-associated X protein (Bax) antibody
[E63] (cat. no. ab32503; Abcam), and β-actin monoclonal antibody
(cat. no. TA811000), horseradish peroxidase (HRP)-goat anti-rat
immunoglobulin G (IgG) (cat. no. TA130038) and HRP-goat anti-rabbit
IgG (cat. no. TA130023) all from OriGene Technologies, Inc. A total
protein extraction kit (KGP250) and bicinchoninic acid assay (BCA)
kit (KGP902; Nanjing KeyGen Biotech. Co., Ltd.), a SOD assay
kit-WST-1, a Microscale MDA assay kit (thiobarbituric acid method)
and a nitric oxide (NO) assay kit (A012-1-2; Colorimetric; Nanjing
Jiancheng Bio-Engineering Institute Co., Ltd.) were also used. The
major instruments included electrophoresis apparatus, a Trans-Blot
SD Semi-Dry Transfer Cell (Bio-Rad Laboratories, Inc.), a light
microscope (Olympus Corporation), an Amersham Imager 600 (GE
Healthcare Life Sciences and a CO2 incubator
(Heraeus).
Cultivation and experimental
grouping
RAECs cell line were cultivated in complete
Dulbecco's modified Eagle's medium (DMEM) to a confluent state
(80-90%) for passage at 37˚C in the CO2 incubator at 5%
CO2. The cells were then divided into five groups: A
control group, H2O2 injury group
(H2O2 group), H2O2+LBPs
(110 µg/ml) group (low-dose group, LT),
H2O2+LBPs (220 µg/ml) group (medium-dose
group, MT) and H2O2+LBPs (440 µg/ml) group
(high-dose group, HT). The doses of LBPs and
H2O2 were selected on the basis of
preliminary experiments, as follows, in which they were safe and
effective without causing toxicity to the RAECs.
Oxidative injury model induced by
H2O2 analyzed through CCK-8
RAECs, cultured in DMEM supplemented with 10% FBS
were seeded in 96-well plates at 2x104 cells/well,
treated with 0, 50, 100, 200, 300, 400 and 500 µmol/l
H2O2 and incubated at 37˚C with 5%
CO2 for 2 h. Subsequently, cell viability was detected
using CCK-8 as described previously (20) and cells were detected under a
microplate reader at wavelength 450 nm. The optical densities (ODs)
were recorded and used to calculate the cell survival rate at each
H2O2 concentration. The
H2O2 concentration that retained 50% survival
of the cells was selected and used to establish an RAEC oxidative
injury model for subsequent experiments.
Influence of LBPs on RAEC survival
rate analyzed through CCK-8
Cells were seeded in 96-well plates at
2x104 cells/well and treated with 0.0 (control), 27.5,
55.0, 110.0, 220.0, 440.0, 880.0, 4,400 and 8,800.0 µg/ml LBPs at
37˚C with 5% CO2 for 2 h. The CCK-8 assay was performed
as above. The cells were then detected under a microplate reader at
wavelength 450 nm. The ODs were recorded and used to calculate the
cell survival rate at each LBP concentration. The concentrations
that exerted an inhibitory effect on RAECs were removed.
Determination of LBP effect on
oxidative injury in RAECs
Cells were treated with H2O2
at the 50% inhibitory concentration and the LBP concentrations that
did not result in inhibition of cell viability. Under these
established interventions, cells were seeded in 96-well plates at
2x104 cells/well and cultured at 37˚C with 5%
CO2 for 2 h. The CCK-8 assay was performed as above.
Subsequently, the cells were detected under a microplate reader at
wavelength 450 nm. The ODs were recorded and used to calculate the
cell survival rates of all groups. The optimum protective clinical
concentration of LBPs against oxidative injury in the RAECs was
screened out and used in the following experiments.
Morphological observation of all
groups
The cells in the established treatment groups
(control, H2O2, LT, MT and HT groups) were
placed under an inverted light microscope. Following removal of the
medium, the cell morphologies and quantities were observed at x200
magnification.
Measurement of SOD, MDA and NO
Oxidative stress participates in numerous pathways
associated with pathological changes in endothelial cells (3). To determine whether LBP intervention
could remove reactive oxygen species (ROS) and inhibit ROS-mediated
oxidative stress, the cell culture was collected and centrifuged at
room temperature for 5 min at 3,000 x g, and the commercial kits
were used to detect the levels of SOD activity, MDA and NO by
colorimetric methods, according to the manufacturer's
instructions.
Western blot analysis of Bcl-2 and Bax
expression
Oxidative stress ultimately causes cell apoptosis,
and thus apoptosis factors were studied to determine whether LBPs
could inhibit apoptosis caused by oxidative stress. The typical
index of the Bcl class was selected, including the inhibitor of
apoptosis protein Bcl-2 and the pro-apoptotic protein Bax, which
were used to indirectly assess whether LBPs prevented apoptosis.
Total protein was extracted from cells using the protein extraction
kit, and quantified using the BCA kit, according to the
manufacturer's instructions. Proteins (80 µg) were separated on 12%
SDS-PAGE gels and transferred to polyvinylidene fluoride membranes.
Membranes were then blocked (5% non-fat dry milk in PBS plus 0.1%
Tween-20; room temperature; 2 h), incubated with primary antibody
(4˚C; overnight) and second antibody (room temperature; 2 h) and
then quantified. The primary antibodies used were anti-Bcl-2,
diluted in 1% bovine serum albumin (BSA; Thermo Fisher Scientific,
Inc.) to 1:200, anti-Bax, diluted in 1% BSA to 1:3,000, and
anti-β-actin, diluted in 1% BSA to 1:500; the secondary antibodies
were HRP-goat anti-rat IgG and HRP-goat anti-rabbit IgG, diluted in
1% BSA to 1:5,000. Bands were quantified following detection with
the ECL kit using Image Lab 5.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was conducted on SPSS 16.0
(SPSS, Inc.). Data were expressed as the mean ± standard deviation
of at least three independent replicate experiments. The groups
were compared by one-way analysis of variance followed by Fisher's
least significant difference post hoc analysis, with a significance
level of P<0.05.
Results
Optimal concentration of
H2O2 for the oxidative injury model
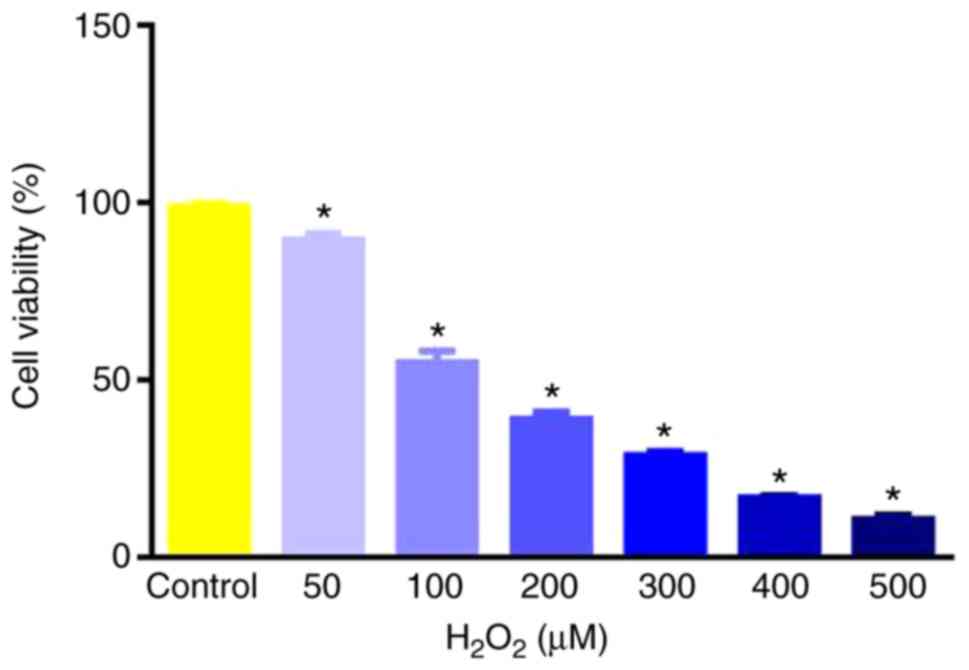
H2O2 injured cells in a
dose-dependent manner. The concentration of
H2O2 that caused ~50% death rate in RAECs was
100 µmol/l (P<0.05 vs. control; Fig.
1). Therefore, 100 µmol/l was selected for the oxidative injury
model to be tested in subsequent assays.
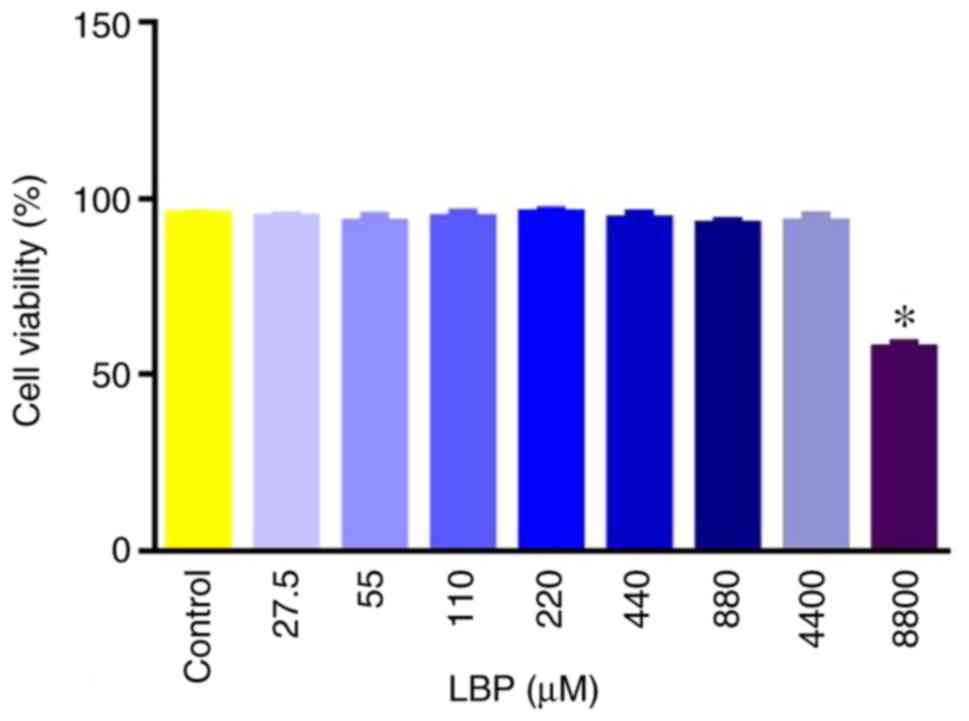
Selection of LBP dosage
The RAECs was not affected by different
concentrations of LBPs except at the concentration of 8,800 µg/ml,
which significantly reduced cell viability compared with the
control (P<0.05; Fig. 2). Thus,
8,800 µg/ml was removed from further experiments.
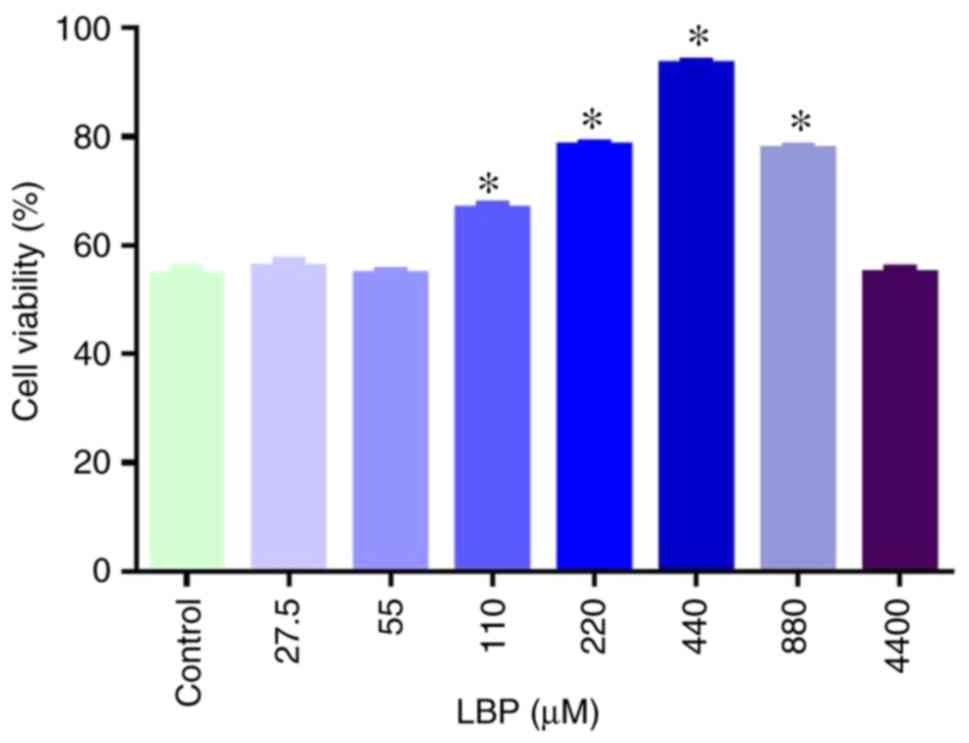
Protective effect of LBPs
Following selection of the safe LBP dosages, the
oxidative injury model was treated with different concentrations of
LBPs to test the effect of the LBPs on cell injury (Fig. 3). LBPs at ≤55 or 4,400 µg/ml did not
exert a protective effect in the oxidative-injured RAECs. By
contrast, LBP concentrations between 110 and 880 µg/ml
significantly protected the RAECs (P<0.05 vs. control), in an
apparent dose-dependent manner between 110 and 440 µg/ml. Thus, the
concentration of 440 µg/ml LBPs exerted the most marked protective
effect, and 110, 220 and 440 µg/ml were used as low, medium and
high intervention dosages of LBPs, respectively, to characterize
their protective effects against oxidative cell injury.
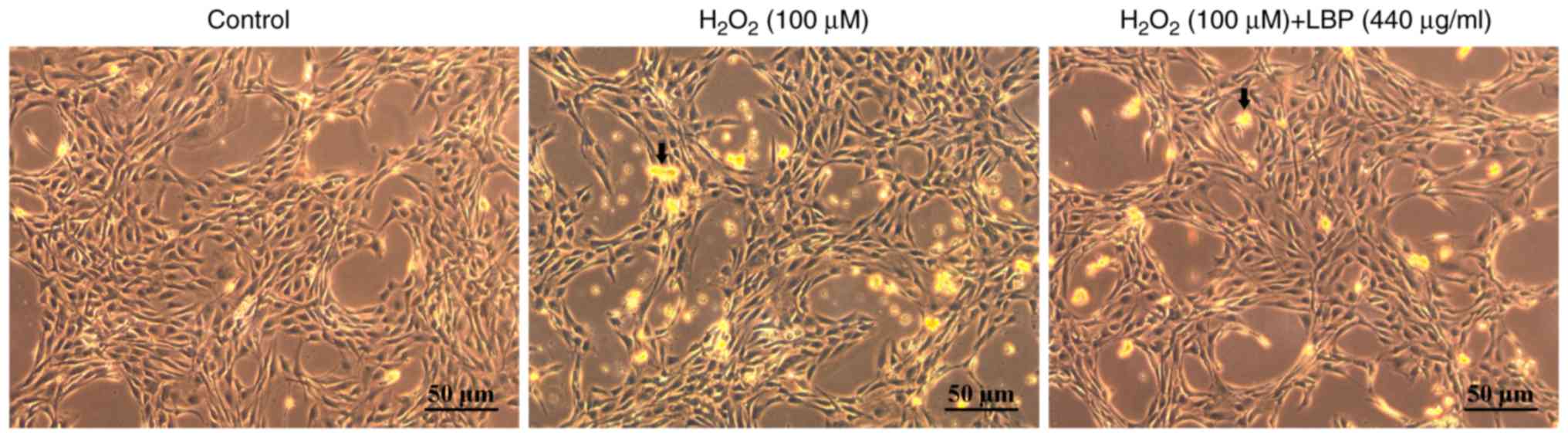
Morphology of the cells
Normal RAECs under the microscope (magnification,
x200) exhibited shuttle-like morphology with round or oval, clear
nuclei and an ordered, paving stone arrangement (Fig. 4). Furthermore, the cell nuclei were of
similar sizes, with evenly distributed chromatin, and were located
centrally in the cells. These cells formed a relatively confluent
monolayer. Conversely, under the stimulation of 100 µmol/l
H2O2, the gaps in the RAEC monolayer were
enlarged and the adherence of cells was lost. Regions of the cell
nuclei were strongly stained, indicating nuclei shrinkage. When the
nuclei shrank, the chromatin became darker, which was matched by
cell enlargement in certain cells. Granular, condensed chromatin
was rarely identified. In the presence of 440 µg/ml LBPs, the
majority of cells exhibited the same morphological characteristics
as the normal control group (Fig.
4).
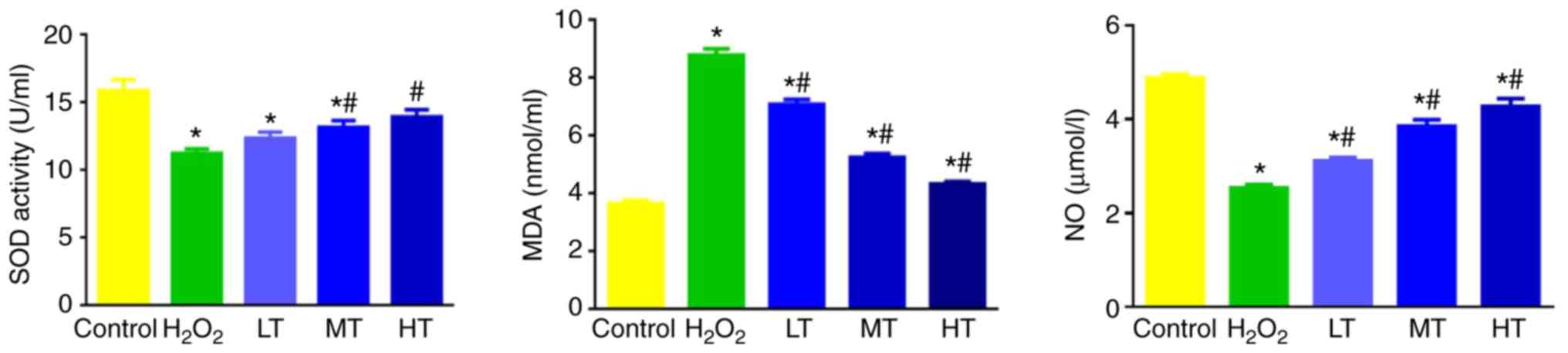
SOD activity, MDA and NO levels in
cell culture supernatant
As depicted in Fig. 5,
SOD activity, NO and MDA contents were altered between the groups.
Notably, SOD activity and NO content significantly decreased while
MDA content significantly increased in the
H2O2 group compared with control group
(P<0.05). In turn, SOD activity in the MT and HT group, and NO
content in all three LBP groups were increased, while MDA content
in the three LBP groups was decreased, compared with the
H2O2 group (all P<0.05); however, the
levels remained significantly altered compared with the control
(P<0.05; Fig. 5).
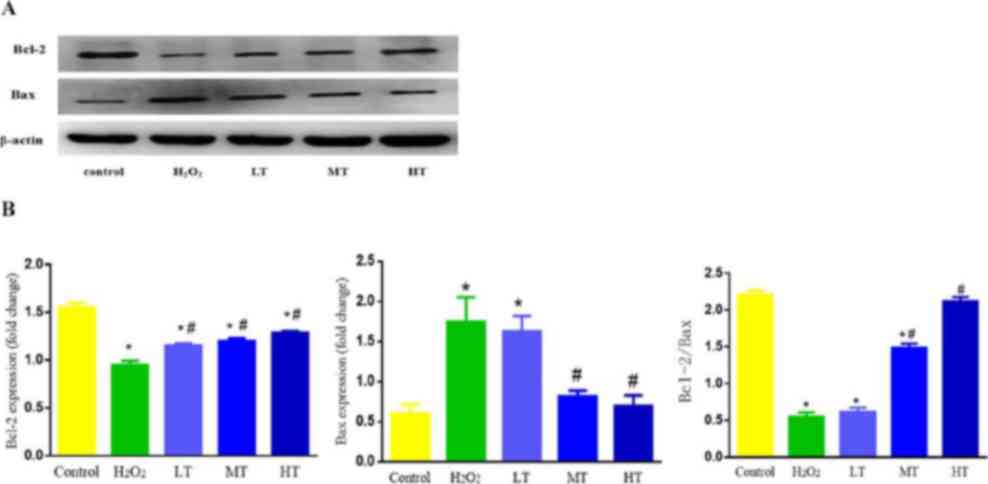
Bcl-2 and Bax protein expression
Under the simulation of H2O2,
the protein expression of apoptosis factors was also altered
(Fig. 6). Notably, Bcl-2 expression
decreased and Bax expression increased in the
H2O2 group compared with control group (both
P<0.05). In turn, Bcl-2 expression in all three LBP groups
increased, while Bax expression in the MT and HT groups decreased
compared with the H2O2 group (all P<0.05),
with these altered Bax levels being statistically similar to those
in the control group (P>0.05; Fig.
6B). The changes observed among the LBP groups appeared
dose-dependent (Fig. 6A and B).
Furthermore, the expression ratio of Bcl-2/Bax
protein in the H2O2 group decreased compared
with the control group (P<0.05). The Bcl-2/Bax ratios in the MT
and HT groups increased compared with the
H2O2 group (both P<0.05), with the HT
group deemed statistically similar to the control group (P>0.05;
Fig. 6B). These changes again
appeared dose-dependent (Fig. 6A and
B).
Discussion
Due to the environmental, diet and lifestyle factors
in modern society, CVDs have become increasingly frequent and tend
to occur at younger ages (21-23).
Thus, there has been increasing focus on the prevention of these
diseases. Red medlar is a tradition medical and food product used
in China (24). In traditional
Chinese medicine, medlar is considered to nourish the liver, kidney
and heart, and balance ‘yin’ and ‘yang’ in the body (25). Therefore, the biological effects of
medlar should be a focus of clinical research. Despite its history
of use, the mechanisms underlying the effects of medlar in body
remain unclear. LBPs, as the effective constituents of medlar, may
directly block the action of toxins in cells and activate
microglial cells in the central nervous system (26). Compared with the potent stimulatory
effects of glossy ganoderma and ginseng reported in the immune
system, LBPs may moderately stimulate the immunoreactivity of the
central nervous system (27). A
number of studies have indicated that LBPs can exert marked
anti-oxidant and anti-aging effects against oxidation-induced cell
injury (28,29). Notably, LBPs could protect lens
epithelial cells from H2O2 stress by
promoting the expression of anti-apoptotic protein Bcl-2 and
upregulating Bcl-2/Bax expression (30). Furthermore, LBPs protected
seminiferous epithelial cells from thermal-induced injuries,
protected DNA from oxidation injury in rat testis cells and
markedly decreased the blood glucose of diabetic rats (31).
Oxidative stress occurs under normal conditions in
the body, and there are a series of adaptive mechanisms that
protect cells from associated injuries. Normally, the production
and elimination of ROS are under dynamic equilibrium, and thus they
exert no lasting damage to cells. However, strong harmful
stimulation may disrupt this balance and generate abundant levels
of ROS that exceed the scavenging activity of the antioxidant
system. As a consequence, the body generates an oxidative stress
response to promote cell apoptosis, though pathological injury may
still result (32). It is established
that the ROS-mediated oxidative stress is a key inducer of cell
apoptosis (33). ROS may also serve
as a messenger in cells that is able to promote the activation of
apoptosis to indirectly cause cell injury (34). The cell injury also induces the
production of more ROS in cells. Taken together, oxidative stress
is an important mechanism of organ injury and participates in the
occurrence and development of various diseases (35).
Previous animal experiments by our group
demonstrated that under exhaustive exercise and heat stress
conditions, LBPs markedly inhibited oxidative stress and liquid
peroxidation, improved compliance of the thoracic aorta, vessel
diastolic function and exercise tolerance, and adjusted oxidase
activities (36); however, the
detailed mechanisms were undetermined. Therefore, the aim of the
present study was to investigate the effect of LBP on
H2O2-induced oxidative stress injury in
RAECs, to thus determine the mechanism of the LBP protective
effects. Initially, various H2O2
concentration concentrations were tested to determine the optimum
dose for an oxidative stress model, and the
H2O2 concentration of 100 µmol/l was selected
based on its induction of ~50% cell survival.
Subsequently the effect of LBPs on the viability of
RAECs was assessed, where it was observed that the cells were not
inhibited by concentrations of LBPs <8,800 µg/ml. However,
following the application of different concentrations of LBPs in
the model cells, it was observed that the LBP concentrations below
55 or higher than 4,400 µg/ml did not exert a protective effect in
the oxidative-injured RAECs; the effective concentration of LBPs
was between 110-880 µg/ml, with the protective effect was most
marked at 440 µg/ml. Therefore, 110-440 µg/ml doses were applied in
subsequent assays to evaluate the mechanism of the LBP protective
effect.
It is established that SOD is a primary scavenger of
free radicals in the body and may also eliminate hazardous
metabolic substances (37,38). Ageing has been associated with the
production and accumulation of oxygen radicals in the body
(39). It has been clinically
demonstrated that SOD is associated with physiological pathology
and the production/development of various diseases in humans
(40,41). SOD is able to adjust blood lipids and,
it may prevent atherosclerosis and CVDs caused by hyperlipidemia
and reduce the content of lipid peroxide (42,43). The
activity level of SOD, as a free-radical scavenger, reflects the
ability of cells clear free radicals in vivo (44). MDA, as a major product of membrane
lipid peroxidation, is also an injury marker that reflects the
extent of cell injury. Additionally, MDA content is an important
index that reflects membrane lipid peroxidation (45). Thus, the present study detected the
change in both SOD activity and MDA content prior to and following
LBP treatment in H2O2-injured cells.
It was observed that the treatment with LBPs at
moderate and high doses (220 and 440 µg/ml) significantly enhanced
SOD activity in the H2O2-induced oxidative
stress group; notably, SOD activity was improved to a statistically
similar level to that of the control group with 440 µg/ml LBPs.
This may be associated with lycium polysaccharide reducing the
fluidity of the plasma membrane and thus reducing damage by free
radicals. The MDS levels in the oxidative injury group were also
inhibited in a dose-dependent manner by LBPs, probably due to the
LBPs enhancing the activity of anti-oxidase enzymes in free radical
clearance.
The common pathological cause of many CVDs is
endothelial dysfunction. NO is an important vasodilatation factor
produced by VECs. The reduced rate of clearance and excessive
production of ROS are important causes of endothelial dysfunction.
The present data indicate that LBPs may clear high levels of ROS to
thus exert an antioxidant effect, and thereby enhance NO usage rate
and adjust vasodilatation function, which would ultimately improve
the resistance of cells to oxidative stress. Collectively these
results indicate that LBPs may prevent
H2O2-induced cell apoptosis and modulate, at
least in part, the ROS-NO axis.
Cell apoptosis is a structured form of cell death
controlled by gene under certain physiological or pathological
conditions. This programmed cell death involves the activation,
regulation and expression of diverse genes (46). Cell apoptosis is essential in
maintaining the body at steady state and the normal physiological
functions of organs (47). Cell
apoptosis results from stimulation by external factors and
pathological events in cells. It is controlled by specific genes,
and is induced and inhibited by stimulating factors (including
H2O2) in the cell microenvironment (30) Exogenous factors may also exert
stimulatory effects through certain signaling pathways (48). The Bcl-2 gene family serves a
meditative and regulatory role in cell apoptosis (49). Key anti-apoptosis and pro-apoptosis
factors in this family are Bcl-2 and Bax (50). As a proto-oncogene, Bcl-2 is a
mitochondrial membrane protein and is considered the most important
anti-apoptosis gene (51).
Conversely, Bax expression may enhance antagonism of Bcl-2 and
thereby promote cell apoptosis; H2O2 may
activate this Bax expression (52).
The apoptosis precursor proteins, including Bax, may alter
translocation through mitochondrial membranes, but their activation
may be resisted by the anti-apoptotic proteins of mitochondrial
membranes, which form heterodimers and maintain membrane integrity
(53). For instance, the
anti-apoptotic protein Bcl-2 protects mitochondria by inhibiting
Bax activation and subsequent translocation of cytochrome C through
the mitochondrial membrane (54); in
this regard, a lack of Bcl-2 would allow the breakdown of
mitochondrial outer membrane integrity and the release of
cytochrome C (55). Thus, the Bcl-2
family adjusts cell apoptosis mediated by the mitochondrial pathway
through anti-apoptosis and pro-apoptosis factors, as well as
participating in the crosstalk of the mitochondria and death
receptor pathways (56). In cases of
apoptosis mediated by oxidative stress, the expression of
Bcl-2-related proteins may be altered, affecting cell apoptosis.
Notably, the present data indicated that oxidative stress reduced
the ratio of Bcl-2/Bax. This was improved following LBP
intervention. Thus, LBPs may moderately protect cells from
H2O2-induced injury and produce an
apoptosis-inhibitory effect, ultimately attenuating the oxidative
stress condition. Notably, the higher-dose LBP intervention almost
recovered cell condition to a normal state. Overall these results
may aid to develop methods for attenuating or preventing oxidative
stress in endothelial cells for the clinical treatment of CVDs.
In conclusion, H2O2-induced
oxidative stress in RAECs, while LBPs upregulated SOD and NO and
downregulated MDA through an apparent antioxidant defense
mechanism. Furthermore, LBPs increased Bcl-2/Bax expression
potentially through an antiapoptosis system. Therefore, LBPs may
protect VECs from H2O2-induced injury through
anti-oxidation and anti-apoptosis effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560052), the
University-level scientific research project of Ningxia Medical
University (grant no. XM2018002), and the Ningxia High School
First-Class Disciplines (West China First-Class Disciplines Basic
Medical Sciences at Ningxia Medical University; grant no.
NXYLXK2017B07).
Availability of data and materials
All data sets generated or analyzed during the study
are included in the published article.
Authors' contributions
GL conceived and designed the experiments. SX
performed the experiments and prepared the manuscript. XH performed
experiments. LZ and LN analyzed the data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mladěnka P, Applová L, Patočka J, Costa
VM, Remiao F, Pourová J, Mladěnka A, Karlíčková J, Jahodář L,
Vopršalová M, et al: Comprehensive review of cardiovascular
toxicity of drugs and related agents. Med Res Rev. 38:1332–1403.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim KS, Song CG and Kang PM: Targeting
oxidative stress using nanoparticles as atheranostic strategy for
cardiovascular diseases. Antioxid Redox Signal. 30:733–746.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
World Health Organization: Comprehensive
global monitoring framework, including indicators, and a set of
voluntary global targets for the prevention and control of
noncommunicable diseases. World Health Organization, 2012.
|
|
4
|
Zeng Z: Internal secretion of vascular
endothelial cells. Chin J Int Med. 37:77. 1998.(In
Chinese)http://www.cqvip.com/Main/Detail.aspx?id=2964468.
|
|
5
|
Blatter LA: Tissue specificity: SOCE:
Implications for Ca2+ handling in endothelial cells. Adv
Exp Med Biol. 993:343–361. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rajendran P, Rengarajan T, Thangavel J,
Nishigaki Y, Sakthisekaran D, Sethi G and Nishigaki I: The vascular
endothelium and human diseases. Int J Biol Sci. 9:1057–1069.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamada T, Egashira N, Bando A, Nishime Y,
Tonogai Y, Imuta M, Yano T and Oishi R: Activation of p38 MAPK by
oxidative stress underlying epirubicin-induced vascular endothelial
cell injury. Free Radic Boil Med. 52:1285–1293. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Closhen D, Bender B, Luhmann HJ and
Kuhlmann CR: CRP-induced levels of oxidative stress are higher in
brain than aortic endothelial cells. Cytokine. 50:117–120.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Higashi Y: Mechanisms of impairment of
endothelial cell. Nihon Rinsho. 70:1519–1523. 2012.(In Japanese).
PubMed/NCBI
|
|
10
|
Bei Y, Das S, Rodosthenous RS, Holvoet P,
Vanhaverbeke M, Monteiro MC, Monteiro VVS, Radosinska J, Bartekova
M, Jansen F, et al: Extracellular vesicles in cardiovascular
theranostics. Theranostics. 7:4168–4182. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rodrigo R, Libuy M, Feliú F and Hasson D:
Oxidative stress-related biomarkers in essential hypertension and
ischemia-reperfusion myocardial damage. Dis Markers. 35:773–790.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu J and Li Y: Effects of salidroside on
exhaustive exercise-induced oxidative stress in rats. Mol Med Rep.
6:1195–1198. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Luo Q, Li Z, Huang X, Yan J, Zhang S and
Cai YZ: Lycium barbarum polysaccharides: Protective effects
against heat-induced damage of rat testes and
H2O2-induced DNA damage in mouse testicular
cells and beneficial effect on sexual behavior and reproductive
function of hemicastrated rats. Life Sci. 79:613–621.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ho YS, Yu MS, Yik SY, So KF, Yuen WH and
Chang RC: Polysaccharides from wolfberry antagonizes glutamate
excitotoxicity in rat cortical neurons. Cell Mol Neurobiol.
29:1233–1244. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu HT, He XJ, Hong YK, Ma T, Xu YP and Li
HH: Chemical characterization of Lycium barbarum polysaccharides
and its inhibition against liver oxidative injury of high-fat mice.
Int J Biol Macromol. 46:540–543. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang WM, Chan E, Kwok CY, Lee YK, Wu JH,
Wan CW, Chan RY, Yu PH and Chan SW: A review of the anticancer and
immunomodulatory effects of Lycium barbarum fruit.
Inflammopharmacology. 20:307–314. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chang HM But PPH (eds): Pharmacology and
Applications of Chinese Materia Medica, Vol. II. World Scientific
Publishing Company Incorporated, Singapore, 1986. https://www.worldscientific.com/worldscibooks/10.1142/0377.
|
|
18
|
Potterat O: Goji (Lycium barbarum
and L. chinense): Phytochemistry, pharmacology and safety in
the perspective of traditional uses and recent popularity. Planta
Med. 76:7–19. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu N, Song N, Liu CY and Yang GL: The
estrogen-like protective effect of Lycium barbarum
polysaccharides in reducing oxidative stress on myocardial cells
from ovariectomized rats. Molecular Medicine Reports. 19:2271–2278.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li J, Zou Y, Ge J, Zhang D, Guan A, Wu J
and Li L: The effects of G-CSF on proliferation of mouse myocardial
microvascular endothelial cells. Int J Mol Sci. 12:1306–1315.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Younus A, Aneni EC, Spatz ES, Osondu CU,
Roberson L, Ogunmoroti O, Malik R, Ali SS, Aziz M, Feldman T, et
al: A systematic review of the prevalence and outcomes of ideal
cardiovascular health in US and Non-US populations. Mayo Clin Proc.
91:649–670. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fang N, Jiang M and Fan Y: Ideal
cardiovascular health metrics and risk of cardiovascular disease or
mortality: A meta-analysis. Int J Cardiol. 214:279–283.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo L and Zhang S: Association between
ideal cardiovascular health metrics and risk of cardiovascular
events or mortality: A meta-analysis of prospective studies. Clin
Cardiol. 40:1339–1346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang D, Li SY, Yeung CM, Chang RC, So KF,
Wong D and Lo AC: Lycium barbarum extracts protect the brain
from blood-brain barrier disruption and cerebral edema in
experimental stroke. PLoS One. 7(e33596)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shan X, Zhou J, Ma T and Chai Q: Lycium
barbarum polysaccharides reduce exercise-induced oxidative stress.
Int J Mol Sci. 12:1081–1088. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Teng P, Li Y, Cheng W, Zhou L, Shen Y and
Wang Y: Neuro-protective effects of lycium barbarum polysaccharides
in lipopolysaccharide-induced BV2 microglial cells. Mol Med Rep.
7:1977–1981. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang RC and So KF: Use of anti-aging
herbal medicine, Lycium barbarum, against aging-associated
diseases. What do we know so far? Cell Mol Neurobiol. 28:643–652.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu Y and Zhang Y: Lycium barbarum
polysaccharides alleviate hydrogen peroxide-induced injury by
up-regulation of miR-4295 in human trabecular meshwork cells. Exp
Mol Pathol. 106:109–115. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Niu T, Jin L, Niu S, Gong C and Wang H:
Lycium barbarum polysaccharides alleviates oxidative damage induced
by H2O2 through down-regulating MicroRNA-194
in PC-12 and SH-SY5Y cells. Cell Physiol Biochem. 50:460–472.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G,
Wang G and Zhong J: Lycium barbarum polysaccharides protect
human lens epithelial cells against oxidative stress-induced
apoptosis and senescence. PLoS One. 9(e110275)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schulz C, Farkas L, Wolf K, Kratzel K,
Eissner G and Pfeifer M: Differences in LPS-induced activation of
bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes
(A-549). Scand J Immunol. 56:294–302. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao W, Pan X, Li T, Zhang C and Shi N:
Lycium barbarum polysaccharides protect against trimethyltin
chloride-induced apoptosis via sonic hedgehog and PI3K/Akt
signaling pathways in mouse neuro-2a cells. Oxid Med Cell Longev.
2016(9826726)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Y, Wang G, Wang T, Cao W, Zhang L
and Chen X: Nrf2-Keap1 pathway-mediated effects of resveratrol on
oxidative stress and apoptosis in hydrogen peroxide-treated
rheumatoid arthritis fibroblast-like synoviocytes. Ann N Y Acad
Sci. 6:2019.https://doi.org/10.1111/nyas.14196.
PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maritim AC, Sanders RA and Watkins JB III:
Diabetes, oxidative stress and antioxidants: A review. J Biochem
Mol Toxicol. 17:24–38. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Z, Su G, Zhang Z, Dong H, Wang Y,
Zhao H, Zhao Y and Sun Q: 25-Hydroxyl-protopanaxatriol protects
against H2O2-induced H9c2 cardiomyocytes
injury via PI3K/Akt pathway and apoptotic protein down-regulation.
Biomed Pharmacother. 99:33–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao Z, Luo Y, Li G, Zhu L, Wang Y and
Zhang X: Thoracic aorta vasoreactivity in rats under exhaustive
exercise: Effects of Lycium barbarum polysaccharides
supplementation. J Int Soc Sports Nutr. 10(47)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang CH, Xu JH, Ren QC, Duan T, Mo F and
Zhang W: Melatonin promotes secondary hair follicle development of
early post-natal cashmere goat and improves cashmere quantity and
quality by enhancing antioxidant capacity and suppressing
apoptosis. J Pineal Res. 67(e12569)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Majumdar A and Kar RK: Orchestration of
Cu-Zn SOD and class III peroxidase with upstream interplay between
NADPH oxidase and PM H+-ATPase mediates root growth in
Vigna radiata (L.) Wilczek. J Plant Physiol. 232:248–256.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liochev SI: Reactive oxygen species and
the free radical theory of aging. Free Radic Biol Med. 60:1–4.
2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kumar M, Chand R and Shah K: Evidences for
growth-promoting and fungicidal effects of low doses of
tricyclazole in barley. Plant Physiol Biochem. 103:176–182.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel
ED, French JP, Fassett JT, Oury TD, Bache RJ and Chen Y:
Extracellular superoxide dismutase deficiency exacerbates pressure
overload-induced left ventricular hypertrophy and dysfunction.
Hypertension. 51:19–25. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Song QQ, Niu JP, Zhang SY, Liang TT, Zhou
J and Feng SS: Effects of simulated heat wave and ozone on high fat
diet ApoE deficient mice. Biomed Environ Sci. 31:757–768.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shatoor AS, Al Humayed S, Alkhateeb MA,
Shatoor KA, Aldera H, Alassiri M and Shati AA: Crataegus Aronia
protects and reverses vascular inflammation in a high fat diet rat
model by an antioxidant mechanism and modulating serum levels of
oxidized low-density lipoprotein. Pharm Biol. 57:38–48.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Saran M, Michel C and Bors W: Radical
functions in vivo: A critical review of current concepts and
hypotheses. Z Naturforsch C. 53:210–227. 1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bai YX, Fang F, Jiang JL and Xu F:
Extrinsic calcitonin gene-related peptide inhibits
hyperoxia-induced alveolar epithelial type II cells apoptosis,
oxidative stress, and reactive oxygen species (ROS) production by
enhancing notch 1 and homocysteine-induced endoplasmic reticulum
protein (HERP) expression. Med Sci Monit. 23:5774–5782.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Omer FAA, Hashim NBM, Ibrahim MY, Dehghan
F, Yahayu M, Karimian H, Salim LZA and Mohan S: Beta-mangostin from
Cratoxylum arborescens activates the intrinsic apoptosis
pathway through reactive oxygen species with downregulation of the
HSP70 gene in the HL60 cells associated with a
G0/G1 cell-cycle arrest. Tumour Biol.
39(1010428317731451)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Radović N, Cucić S and Altarac S:
Molecular aspects of apoptosis. Acta Med Croatica. 62:249–256.
2008.(In Croatian). PubMed/NCBI
|
|
48
|
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G,
Wang G and Zhong J: Lycium barbarum polysaccharides protect
human lens epithelial cells against oxidative stress-induced
apoptosis and senescence. PLoS One. 9(10)(e110275)2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Xi H, Fan X, Zhang Z, Liang Y, Li Q and He
J: Bax and Bcl-2 are involved in the apoptosis induced by local
testicular heating in the boar testis. Reprod Domest Anim.
52:359–365. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gross A: BCL-2 family proteins as
regulators of mitochondria metabolism. Biochim Biophys Acta.
1857:1243–1246. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang X, Li Z, Bai J, Song W and Zhang F:
miR-17-5p regulates the proliferation and apoptosis of human
trabecular meshwork cells by targeting phosphatase and tensin
homolog. Mol Med Rep. 19:3132–3138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Goping IS, Gross A, Lavoie JN, Nguyen M,
Jemmerson R, Roth K, Korsmeyer SJ and Shore GC: Regulated targeting
of BAX to mitochondria. J Cell Biol. 143:207–215. 1998.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cheng EH, Wei MC, Weiler S, Flavell RA,
Mak TW, Lindsten T and Korsmeyer SJ: Bcl-2, Bcl-XL
sequester BH3 domain-only molecules preventing Bax-and Bak-mediated
mitochondrial apoptosis. Mol Cell. 8:705–711. 2001.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome C from mitochondria blocked. Science.
275:1129–1132. 1997.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hsu HC, Liu YS, Tseng KC, Tan BC Chen SJ
and Chen HC: Lgr5 regulates survival through mitochondria-mediated
apoptosis and by targeting the Wnt/β-catenin signaling pathway in
colorectal cancer cells. Cellular Signalling. 26:2333–2342.
2014.PubMed/NCBI View Article : Google Scholar
|