Introduction
Clinical treatment options for post-menopausal
hormone receptor positive, human epidermal growth factor receptor-2
negative breast cancer include the use of selective estrogen
receptor modulators and aromatase inhibitors (1,2). Aromatase
inhibitor based monotherapy is frequently combined with selective
small molecule inhibitors of CDK4/6 and mTOR pathways. Long-term
treatment involving single agent therapy or multi-agent combination
therapy is frequently associated with acquired drug resistance
predominantly due to the emergence of cancer stem cells, thereby
impacting therapeutic efficacy and promoting disease progression
(3-5).
Aromatase CYP19 A1 functions as a critical enzyme
for peripheral and intra-tumoral estrogen bio-synthesis via
conversion of adrenal androstenedione to estrone (E1)
and subsequently to estradiol (E2) thereby providing
growth-promoting estrogens. Pharmacological agents Letrozole (LET)
and Exemestane (EXM) are selective inhibitors of aromatase
(1,2).
These agents exhibit acquired tumor resistance in preclinical
models for aromatase-expressing Luminal A breast cancer, as well as
in estrogen receptor-positive clinical breast cancer (6-12).
Naturally occurring non-toxic substances including
dietary supplements and natural botanicals are widely used in
complementary and alternative medicine. Natural products exhibiting
effective inhibition of aromatase activity may represent potential
testable alternatives to the limitations of clinical aromatase
inhibitors.
Tabebuia avellanedae (TA) is a tree native to
the Amazon rainforest. Drinks from the bark of the TA tree have
been traditionally used by the indigenous population to address a
wide variety of health issues. A non-fractionated powder from the
inner bark of TA, under the name Taheebo, is available from Taheebo
Japan, Co., Ltd.. An aqueous extract of Taheebo has been documented
to exhibit anti-cancer activity in animal models for organ site
cancers (13), as well as growth
inhibitory efficacy in human carcinoma-derived cell culture models
for prostate and breast cancer via multiple mechanisms (14-16).
Growth inhibitory efficacy of TA extract in a model for Luminal A
breast cancer subtypes is associated with the differential
expression of proliferation and apoptosis-specific genes (17). In a model for triple-negative breast
cancer the inhibitory efficacy of TA is attributable to inhibition
of G1 to S phase transition, induction of pro-apoptotic
caspase 3/7 activity and modulation of the RB pathway (18).
Recently, Taheebo Japan invented a proprietary
process capable of producing TA powder that is considerably more
finely ground than the original TA powder. The new product marketed
by Taheebo Japan under the name of Taheebo NFD Marugoto (TNM), is
expected to provide superior results to their original Taheebo due
to its reduced particle size and greater aqueous solubility.
In an effort to evaluate the growth inhibitory
effects and anti-aromatase activity of TNM, the experiments in the
present study were designed to i) examine the growth inhibitory
efficacy of TNM in a model for aromatase-expressing post-menopausal
breast cancer, ii) evaluate the effects of TNM on cellular
aromatase activity, and iii) identify possible molecular mechanisms
responsible for the efficacy of TNM.
Materials and methods
Experimental model
The MCF-7AROM cell line represented the
experimental model for the present study. These
ER+/PR+/HER-2- human mammary
carcinoma-derived cells, stably transfected with the aromatase gene
(6,10), possess the characteristics of
aromatase-expressing, post-menopausal Luminal A molecular subtype
of clinical breast cancer.
Test compounds
Taheebo NFD Marugoto (TNM)
This compound is comprised of finely ground powder
from the inner bark of TA tree containing the bioactive agent
Naphthofurandione (NFD) was provided by Taheebo Japan Co., Ltd..
The non-fractionated aqueous stock solution was prepared following
the protocol provided by the supplier. This stock solution of TNM
contains 200 ng of NFD (Personal Communication: Dr Fukuda, Taheebo
Japan). The stock solution was serially diluted in the culture
medium to obtain final concentrations of TNM for the dose response
experiments, and to identify minimally effective, half maximal
(IC50) and maximally cytostatic (IC90)
concentrations that were used for the mechanistic assays.
Letrozole (LET)
Stock solution of LET (molecular mass: 285 kDa,
Sigma-Aldrich; Merck KGaA) was prepared in DMSO and serially
diluted in the culture medium to obtain the final concentration of
1 µM (285 ng).
Exemestane (EXM)
Stock solution of EXM (molecular mass: 296 kDa,
Sigma-Aldrich; Merck KGaA) was prepared in DMSO and was serially
diluted in the culture medium to obtain the final concentration of
10 µM (2,960 ng).
LET and EXM represent the prototypical aromatase
inhibitors. The concentrations of 1 µM LET and 10 µM EXM are
comparable to the effective concentrations traditionally used in
the cell culture experiments, and represent clinically achievable
effective concentrations. These compounds were used as positive
controls for the present experiments.
Anchorage-independent growth
For this assay the stock solution of agar was
prepared by mixing DNA grade agar (Sigma-Aldrich; Merck KGaA) with
an appropriate volume of 2X RPMI-1640 medium. To prepare the
basement layer, this stock solution was diluted to 0.6%, dispersed
in a 6-well plate and allowed to solidify overnight at 37˚C.
Suspension of MCF-7AROM cells, at a density of
5x105 per ml, was prepared in RPMI-1640 medium
containing 0.33% agar, and this cell suspension was overlaid on the
basement layer in the presence or absence of TNM. The cultures were
incubated at 37˚C in a CO2 incubator for 21 days. The
anchorage-independent (AI) colonies were stained with 0.005%
crystal violet and colony counts were determined at x10
magnification. The data were expressed as number of AI
colonies.
Cell cycle progression
For the cell cycle analysis, 5x105 cells
were seeded in T-25 flasks and treated for 24 h. post-seeding with
1, 5 and 10 µg of TNM for 48 h. The cells were harvested by
trypsinization, pelleted at 500 x g, and washed twice with cold PBS
(Sigma-Aldrich; Merck KGaA). The cells were then fixed with cold
70% ethanol, washed with cold PBS, and stained with propidium
iodide (PI, 50 µg/ml, Sigma-Aldrich; Merck KGaA, in PBS), followed
by the addition of ribonuclease (10 µg/ml, Sigma-Aldrich; Merck
KGaA) and incubation for 4 h in the dark. The cell cycle analysis
was performed at the Core Facility of the University of Texas
Health Sciences Center (San Antonio), using optimized protocols
that included i) sorting of PI-stained cells, ii) use of a 488 nm
excitation filter and a 520 nm band pass filter, and iii) gating of
fluorescent events on forward versus side scatter. Cell cycle
progression was monitored using a Becton Dickinson FACSCAN flow
cytometer (BD Biosciences), and the data were analyzed using FACS
Express software (De Novo Software). The data were expressed as a
percentage of cells in G1, S and G2 phases of
the cell cycle.
Caspase activity
Caspase 3/7 activity in the MCF-7AROM
cells was measured using caspase-Glo assay kit (Promega). Briefly,
the cells treated with TNM were homogenized by sonication in
homogenization buffer (25 mmol/l HEPES, pH 7.5, 5 mmol/l
MgCl2, and 1 mmol/l EGTA) and protease inhibitors (all
from Sigma-Aldrich; Merck KGaA). The homogenate was centrifuged at
6,500 x g at 4˚C for 15 min, and the supernatant was collected.
Subsequently, 10 µl of assay reagent was added to 10 µl of
supernatant and the reaction mixture was incubated at room
temperature for 2 h. Resulting luminescence was measured using a
Fluoroskan Luminometer (Thermo Scientific Co.). The data were
expressed as relative luminescent units (RLU).
Gene expression profiling
The effect of TNM on the expressions of apoptosis
regulatory genes BAX and BCL-2, E2
regulatory target genes ESR-1, AROM and PR and
E2 responsive target genes pS2, GRB-2 and
cyclin D1 was examined using reverse transcription
quantitative PCR (RT-qPCR) assay following published protocols
(19). Briefly, RNA from TNM treated
and untreated control cells was isolated using the RNeasy plus kit
(Qiagen Inc.), with a genomic DNA removal step as per the
manufacturer's protocol. Reverse transcription (RT) was carried out
using the Applied Biosystems kit (Applied Biosystems). RT-qPCR and
subsequent analyses were carried out using Smart Mix PCR beads
(Cepheid) with 0.25X SYBR-Green in the Cepheid Smart Cycler to
detect indicated E2 target genes and the housekeeping
gene β-actin transcripts, representing normalization
control. Melt curve analysis was performed after each RT-qPCR cycle
to ascertain PCR product specificity. PCR reactions of the
indicated primer sets for E2 target genes and for
β-actin primer sets gave unique melt peaks, indicative of discrete
amplification products. Reaction mix (25 µl) was prepared
containing MgCl2 (2 mmol/l), 12.5 µl of 2X Taq PCR
Master Mix (Qiagen, Inc.), 0.25X SYBR-Green (Fisher Scientific Co.)
and gene-specific primer sets (0.3 µmol/l each, obtained from the
Core Facility, University of Texas). The PCR reaction was set for
40 cycles, and the data were compared after normalization with
β-actin RNA levels. RT-qPCR assays were performed in duplicate and
repeated at least three times. These data were expressed as ΔΔCq
values for quantification of relative gene expression (20). The primer sequences for the sense (S)
and anti-sense (AS) strands for ESR-1 (gene for ER-α),
AROM, PR, pS2, GRB2, cyclin D1,
BCL-2, BAX and β-actin genes are represented
from 5' to 3' (Table I).
 | Table IPrimer sets used for reverse
transcription-quantitative PCR analysis. |
Table I
Primer sets used for reverse
transcription-quantitative PCR analysis.
| Gene name | Primer
sequence |
|---|
| ESR-1 |
5'-TGTGCAATGACTATGCTTCA-3' (S) |
| |
5'-GCTCTTCCTCCTGTTTTTTA-3' (AS) |
| AROM |
5'-AGCATGCTGTACCAGCCTGT-3' (S) |
| |
5'-TCATCATCACCATGGCCATGT-3' (AS) |
| PR |
5'-ACAGAATTCATGAGCCGGTCCGGG |
| | TGCAAG-3' (S) |
| |
5'ACAAGATCTCCACCCAGAGCCCG |
| | AGGTTT-3' (AS) |
| pS2 |
5'-CATCGACGTCCCTCCAGAAGAG-3' (S) |
| |
5'-CTCTGGGACTAATCACCGTGCTG-3' (AS) |
| GRB2 |
5'-AAATGCTCAGCAAACAGCGG-3' (S) |
| |
5'-TGAAGTGCTGCACATCATTTCC-3' (AS) |
| Cyclin
D1 |
5'-ACGAAGGTCTGCGCGTGTT-3' (S) |
| |
5'-CCGCTGGCCATGAACTACCT-3' (AS) |
| BCL-2 |
5'-CCTGTGGATGACTGAGTACC-3' (S) |
| |
5'-GAGACAGCCAGGAGAAATCA-3' (AS) |
| BAX |
5'-GTTTCATCCAGGATCGAGCAG-3' (S) |
| |
5'-CATCTTCTTCCAGATGGTGA-3' (AS) |
| β-actin |
5'-GACCTCTATGCCAACACAGT-3' (S) |
| |
5'-AGTACTTGCGCTCAGGAGGA-3' (AS) |
Aromatase activity
To measure aromatase enzymatic activity, the
3H2O release assay was used. [3H]
androstenedione represented the substrate and its conversion to
E1 represented a measure for aromatase activity. The
MCF-7AROM cells grown in phenol-free RPMI-1640 medium
supplemented with charcoal-stripped fetal bovine serum
(Sigma-Aldrich; Merck KGaA) were suspended in an assay mixture
containing 0.1% bovine serum albumin, 67 mmol/l KPO4 (pH
7.4), and 2.0 µmol/l progesterone. After sonication, 100 nmol/l of
[3H] androstenedione (25.3 ci/mmol, NET-962;
Perkin-Elmer, Inc.) was added and the mixture was incubated for 10
min at room temperature. NADPH was then added to a final
concentration of 1.2 mmol/l, followed by 37˚C incubation and the
addition of an equal volume of 5% trichloroacetic acid. The
supernatant was collected and extracted with an equal volume of
chloroform. Dextran-coated charcoal was added to the assay mixture,
which was then vortexed and centrifuged at 6,500 x g at 40˚C for 15
min. The supernatant was then added to scintillation fluid and
measured in a scintillation counter (Perkin-Elmer). The data were
expressed as f mole E1 formed, per mg protein, per hour
(21).
Statistical analysis
The experiments for dose response using the
anchorage-independent growth assay were conducted in quadruplicate.
Experiments for cell cycle progression, caspase 3/7 activity,
aromatase activity and gene expression profiling were conducted in
triplicate. The data were expressed as mean ± SD. Statistically
significant differences between the control and treatment groups
were assessed by the two-sample Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Additionally, data from comparisons of multiple treatment groups
were analyzed using analysis of variance (ANOVA) and Dunnett's test
as a post-hoc test with a threshold of α=0.05 (Microsoft Excel 2013
XLSTAT-Base software).
Results
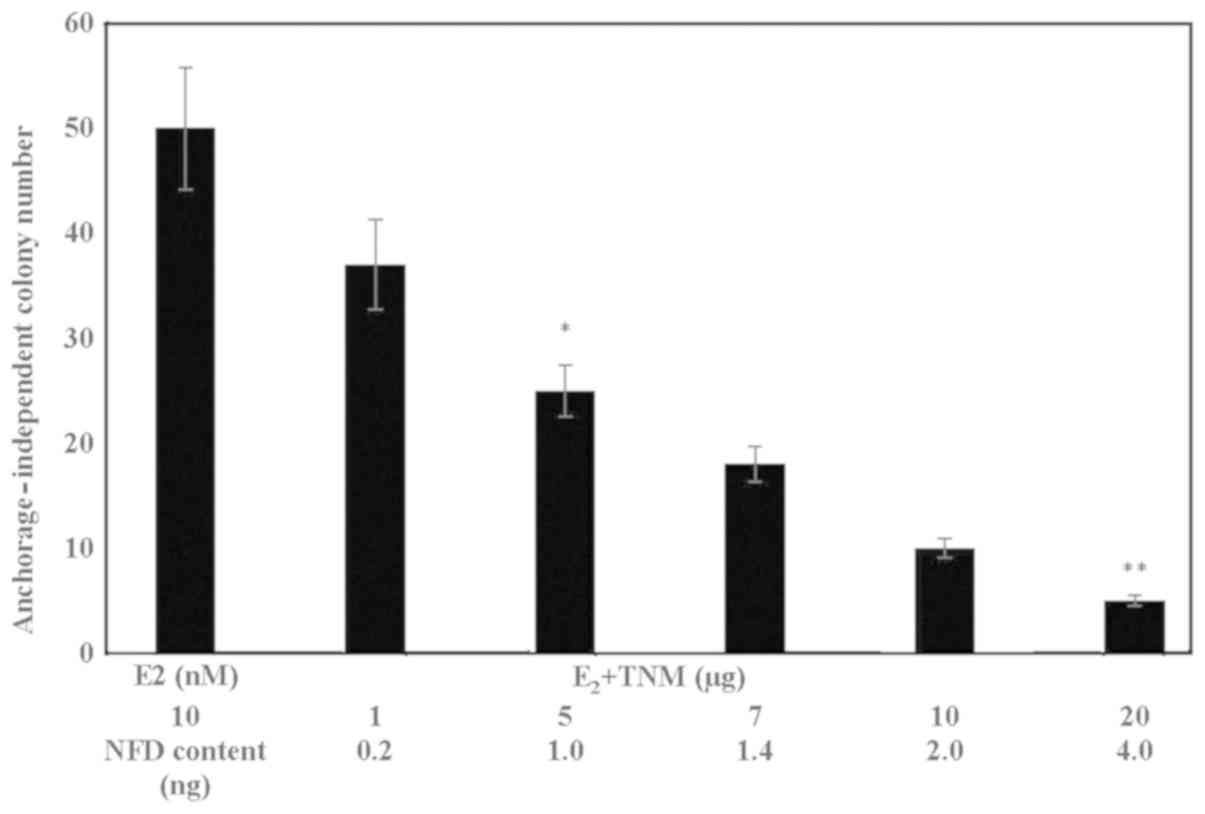
Effect of TNM on anchorage-independent
growth
The data presented in Fig.
1 show a TNM dose-dependent reduction in the number of
E2 promoted anchorage-independent colonies. These data
identified the IC50 as 5 µg TNM (α=0.05), and
IC90 as 20 µg TNM (α=0.05), relative to the
E2-treated controls. The NFD content of TNM at these
concentrations was estimated to be 1.0 and 4.0 ng,
respectively.
Effect of TNM on cell cycle
progression
The data presented in Table II examined the effect of TNM on the
cell cycle progression of MCF-7AROM cells. TNM at the
maximum cytostatic concentration of 10 µg resulted in 62.2% of
cells arrested in the S phase of the cell cycle (P=0.04), relative
to the untreated control. The inhibition in the G1 and
G2 phases were modest and statistically
non-significant.
 | Table IIInhibition of cell cycle progression
by TNM in MCF-7AROM cells. |
Table II
Inhibition of cell cycle progression
by TNM in MCF-7AROM cells.
| | | Cell cycle
phase |
|---|
| Treatment | Concentration
(µg) | %
G1 | % S | %
G2 |
|---|
| Control | - | 63.2±1.5 |
26.2±0.6a | 9.1±0.5 |
| TNM | 10 | 50.2±1.6 |
42.5±0.5b | 6.7±0.4 |
| Δ control | | -20.6% | +63.2% | -26.4% |
Induction of pro-apoptotic activity by
TNM
The induction of cellular apoptosis by treatment
with TNM was examined by monitoring the status of caspase 3/7
activity. In response to treatment with TNM, caspase 3/7 activity
exhibited a dose-dependent increase (Fig.
2A). Thus, relative to the untreated control, TNM at 1.5 µg, 5
µg and 10 µg exhibited a 2.9-fold (α=0.05), an 8.9-fold (α=0.05)
and a 9-fold (α=0.05) increase in caspase 3/7 activity,
respectively.
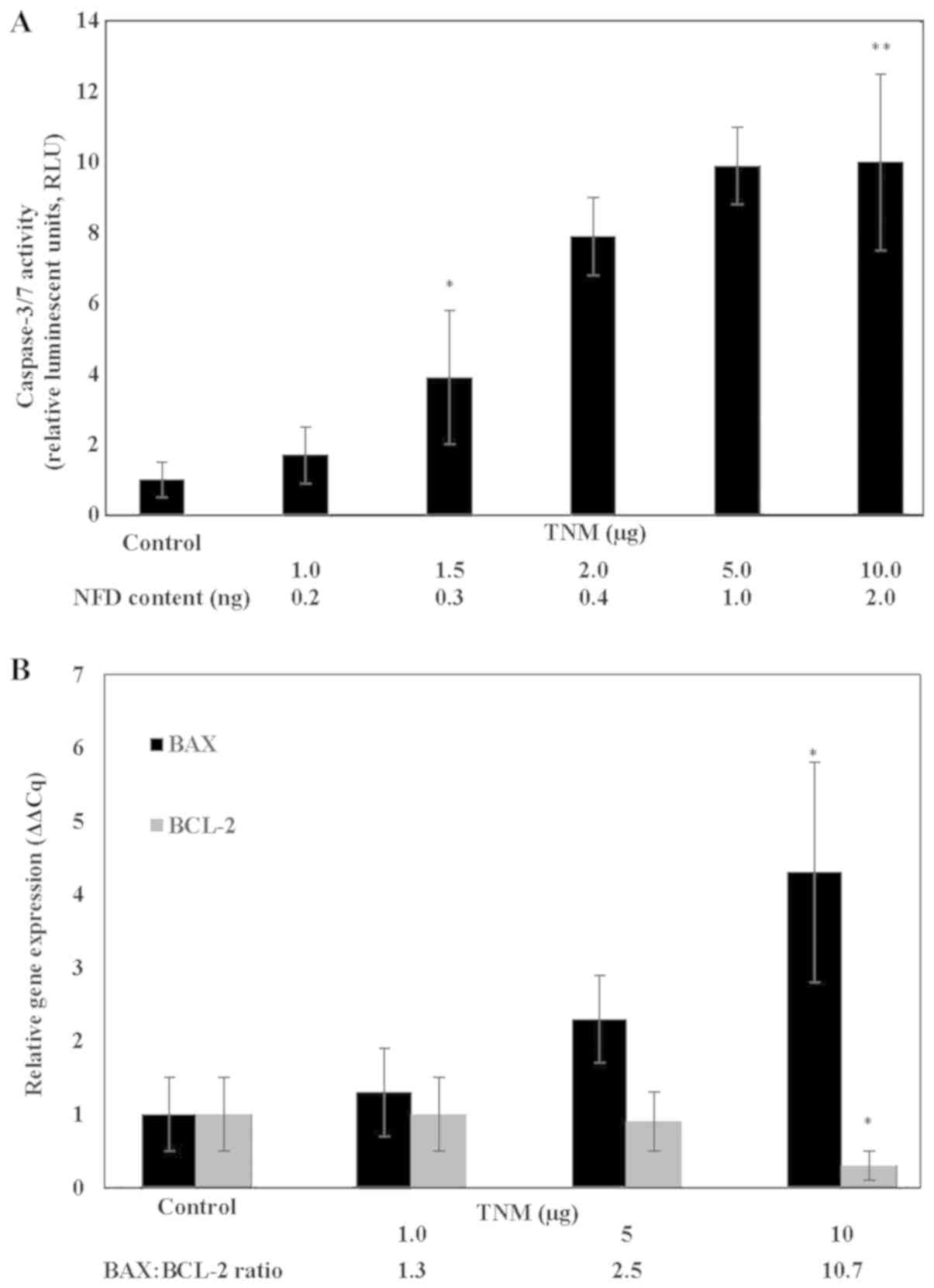 | Figure 2.(A) Induction of caspase activity by
Taheebo NFD Marugoto (TNM) in MCF-7AROM cells. Treatment
with TNM results in a dose-dependent increase in caspase 3/7
activity. Results were presented as RLU mean ± SD, n=3 per
treatment group. Data were analyzed by ANOVA and Dunnett's test.
Untreated control < 1.5 µg TNM*, untreated control
<10 µg TNM** (α=0.05). TNM; Taheebo NFD Marugoto,
NFD, naphthofurandione, RLU, relative luminescent units; SD,
standard deviation; ANOVA, analysis of variance. (B) Modulated
expression of apoptosis-specific genes in MCF-7AROM
cells by Taheebo NFD Marugoto (TNM). Treatment with TNM results in
upregulated expression of BAX and downregulated expression
of BCL-2 genes. Results are presented as relative gene
expression (ΔΔCq) mean ± SD, n=3 per treatment group. Data were
analyzed by two-sample Student's t-test. BAX: *P=0.01
vs. untreated control. BCL-2: *P=0.02 vs. untreated
control. TNM; Taheebo NFD Marugoto, BAX; BCL-2 associated X
protein; BCL-2; B cell lymphoma-2; SD, standard deviation. |
At the molecular level, TNM treatment resulted in a
dose-dependent increase in pro-apoptotic BAX and a reciprocal
decrease in anti-apoptotic BCL-2 gene expression. Thus,
treatment with 10 µg TNM resulted in a 3.3-fold increase (P=0.01)
in the pro-apoptotic BAX and a 70% decrease (P=0.02) in the
anti-apoptotic BCL-2 expression, relative to the respective
untreated controls. This reciprocal modulation in the expression of
apoptosis-specific genes resulted in an increased BAX: BCL-2 ratio
(Fig. 2B).
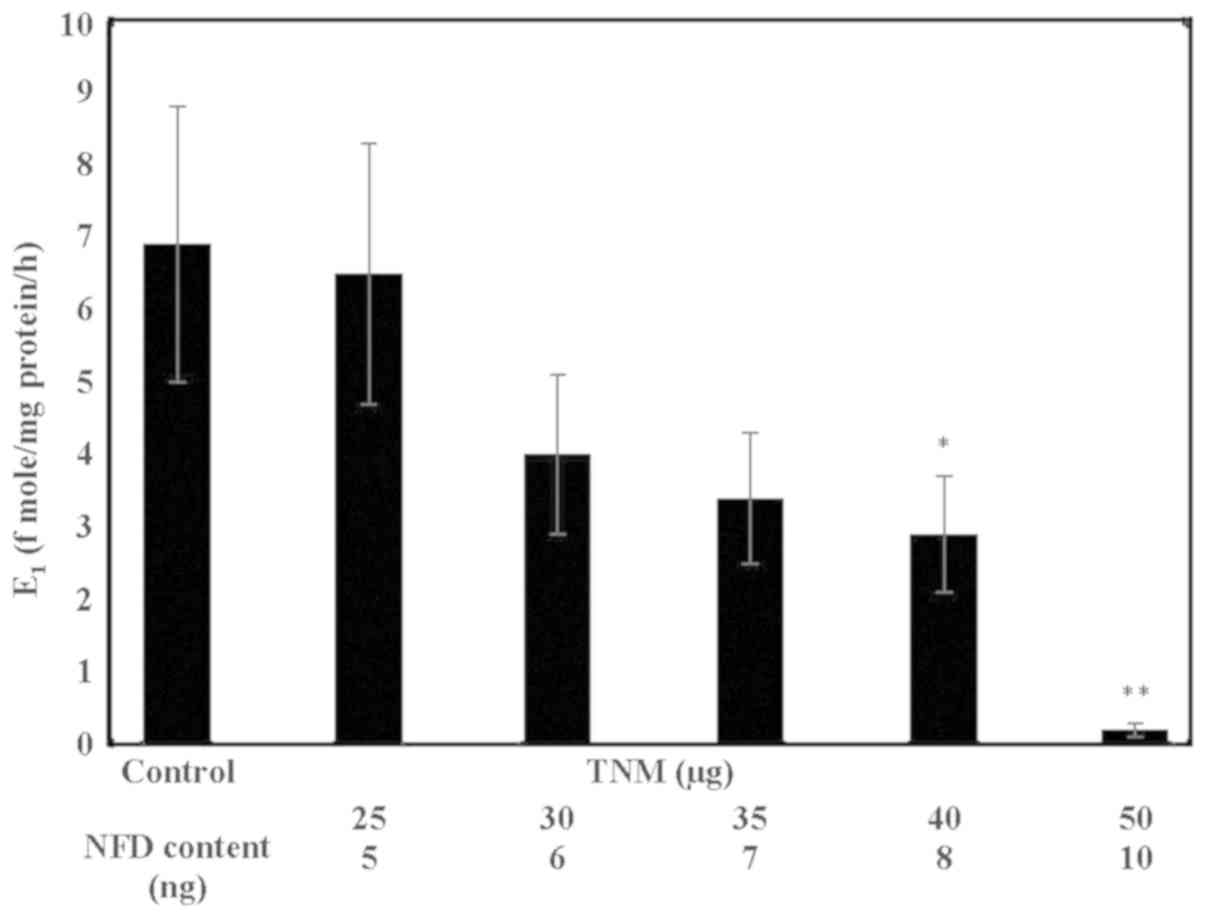
Inhibition of aromatase activity by
TNM
In response to treatment with TNM,
MCF-7AROM cells exhibited dose-dependent inhibition in
aromatase activity as measured by the extent of conversion of
androstenedione to E1. Thus, TNM treatment at 30, 40 and
50 µg resulted in a 42% (α=0.05), a 57.9% (α=0.05) and a 97.1%
(α=0.05) decrease in the aromatase activity, relative to the
untreated control (Fig. 3).
Comparative inhibition of aromatase
activity by TNM, LET and EXM
The comparative efficacy of TNM and LET for
inhibition of aromatase activity revealed that the extent of
inhibition of 63.8% (P=0.04) by 40 µg TNM (NFD content 8 ng) was
essentially similar to 62.3% inhibition (P=0.04) induced by 285 ng
(1 µM) of LET, relative to the untreated control (Table III).
 | Table IIIComparative efficacy for aromatase
inhibition by TNM and LET in MCF-7AROM cells. |
Table III
Comparative efficacy for aromatase
inhibition by TNM and LET in MCF-7AROM cells.
| Treatment | Concentration | Aromatase activity
(E1 fmole/mg protein/h) | Inhibition (%
control) |
|---|
| Control | - |
6.9±0.4a | - |
| TNM (40 µg) | 8 ng NFD |
2.5±0.1b | 63.8 |
| LET (1 µM) | 285 ng |
2.6±0.1b | 62.3 |
The comparative efficacy of TNM and EXM for
inhibition of aromatase activity is presented in Table IV. These data revealed that the
extent of inhibition of 98.5% (P=0.01) by 100 µg TNM (NFD content
20 ng) was essentially similar to 97.1% inhibition (P=0.01) induced
by 2,960 ng (10 µM) of EXM, relative to the untreated control.
 | Table IVComparative efficacy for aromatase
inhibition by TNM and EXM in MCF-7AROM cells. |
Table IV
Comparative efficacy for aromatase
inhibition by TNM and EXM in MCF-7AROM cells.
| Treatment | Concentration | Aromatase Activity
(E1 fmole/mg protein/h) | Inhibition (%
control) |
|---|
| Control | - |
6.9±0.30a | - |
| TNM (100 µg) | 20 ng NFD |
0.1±0.08b | 98.5 |
| EXM (10 µM) | 2,960 ng |
0.2±0.10b | 97.1 |
Inhibition of E2 regulated
target gene expression by TNM
The data obtained from the effect of TNM on the
expression of ESR-1 (gene for ER-α), AROM and PR
genes are presented in Fig. 4A. The
extent of inhibition at 10 µg of TNM for ESR-1 was 90% (P=0.01),
for AROM it was 61% (p=0.04) and for PR it was 61% (P=0.04).
Thus, treatment with TNM resulted in substantial downregulated
expressions of select genes that are regulated by
E2.
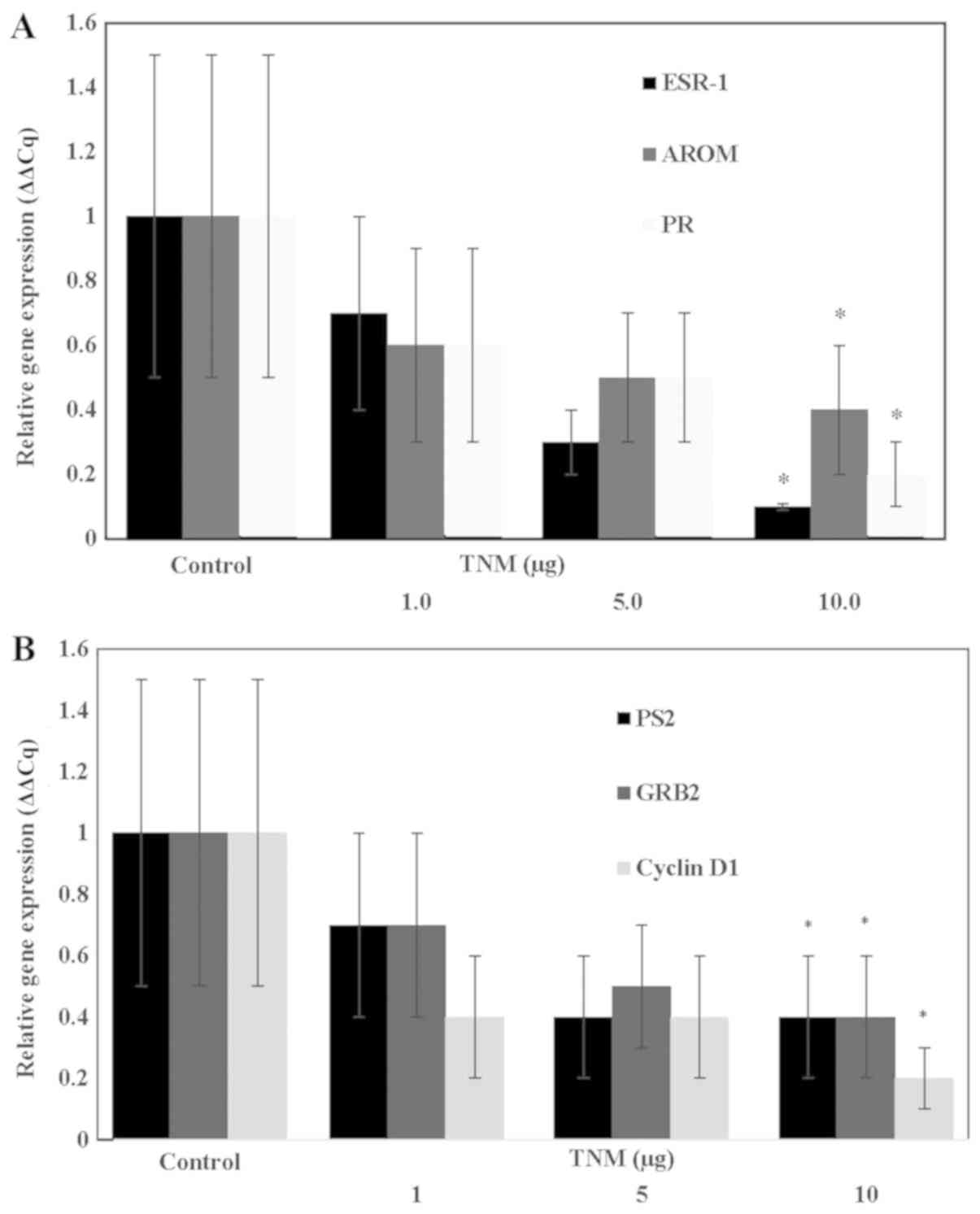 | Figure 4.(A) Inhibition of estrogen regulated
gene expression by Taheebo NFD Marugoto (TNM) in
MCF-7AROM cells. Treatment with TNM results in
down-regulated expression of ESR-1, AROM and PR genes. Results were
expressed as relative gene expression mean (ΔΔCq) ± SD, n=3 per
treatment group. ESR-1: Untreated control >10 µg TNM*
(α=0.05), AROM: untreated control >10 µg TNM*
(α=0.05), PR: untreated control >10 µg TNM* (α=0.05).
Data were analyzed by ANOVA and Dunnett's test. TNM, Taheebo NFD
Marugoto; ESR-1, gene for ER-α. (B) Inhibition of estrogen
responsive gene expression by Taheebo NFD Marugoto (TNM) in
MCF-7AROM cells. Treatment with TNM results in
downregulated expression of PS2, GRB2 and cyclin
D1 gene. Results were presented as relative gene expression
(ΔΔCq) mean ± SD, n=3 per treatment group. PS2: Untreated control
>10 µg TNM* (α=0.05), GRB2: Untreated control >10
µg TNM*, cyclin D1: Untreated control > 10 µg
TNM*. Data were analyzed by ANOVA and Dunnett's test.
ESR-1: gene for estrogen receptor-α, AROM, aromatase; PR;
progesterone, SD, standard deviation; TNM, Taheebo NFD Marugoto;
PS2, estrogenresponsive gene; GRB2, growth factor receptor binding
protein 2; ANOVA, analysis of variance; SD, standard deviation. |
Inhibition of E2 responsive
target gene expression by TNM
The data shown in Fig.
4B examined the effect of TNM on the expression of select
E2 responsive genes. The extent of inhibition at 10 µg
of TNM for pS2 was 62% (P=0.04), for GRB2 it was 61% (P=0.04), and
for cyclin D1 it was 82% (P=0.01). Thus, TNM treatment resulted in
a substantial downregulation of E2 responsive gene
expressions.
Discussion
Metastatic breast cancer is a leading cause of
cancer related mortality for women in the USA (22). The ER-α positive, aromatase-expressing
Luminal A subtype of post-menopausal breast cancer responds to
aromatase inhibitors (6,10). However, long-term therapy is
frequently associated with acquired resistance that negatively
impacts efficacy and facilitates disease progression.
In addition to the present MCF-7AROM
model, other cellular models stably transfected with the aromatase
gene have been developed from human mammary carcinoma derived MCF-7
and T47D cell lines. These models have been utilized to examine the
effects of aromatase inhibitors and investigate the mechanisms
responsible for resistance to AI-based endocrine therapy. For
example, MCF-7AROM cells have exhibited resistance to
Fluvestrant and cross resistance to Letrozole, Anastrazole,
Exemestane (9) and
T47DAROM cells have exhibited resistance to Letrozole
and sensitivity to anti-progestin (23). Additionally, AI resistance has been
documented to involve the upregulated expression of HER-2(10). Collectively, these three cellular
models offer valuable experimental approaches to identify
efficacious aromatase inhibitors and also to investigate molecular
mechanisms responsible for acquired resistance to aromatase
nihibitors.
Non-toxic natural nutritional products may represent
testable alternatives for endocrine therapy-resistant
post-menopausal breast cancer (17,18,
28-31), and thereby, may provide treatment options against the
clinical limitations of current aromatase inhibitor-based therapy
(2,3,6,9,10). Two
species belonging to the Tabebuia genus have documented anti-cancer
activity. The anti-cancer effects of T. avellanedae and
T. chrisantha are documented in preclinical xeno-transplant
models (13) and in mice carrying
Ehrilch ascites tumor (24). The use
of TA in traditional medicine is not well documented. However, the
effect of an aqueous extract of TA has been examined on the status
of quality of life in patients with multiple organ site cancers
that are at advanced metastatic stages (25). Additionally, the effects of the TA
quinone NFD have been documented on head and neck cancer and on a
patient with lung metastasis (26).
Experiments in the present study were designed to
examine the growth inhibitory efficacy of a non-fractionated
aqueous extract from TNM on a cellular model for
aromatase-expressing Luminal A subtype of post-menopausal breast
cancer.
The ER-α-positive human mammary carcinoma-derived
MCF-7 cell line is dependent on E2 for
anchorage-independent growth in vitro and tumor development
in vivo. These in vitro and in vivo end points
exhibit a strong positive correlation (27). Thus, the in vitro endpoint that
determines the number of AI colonies is a surrogate end point
biomarker for cancer risk. E2 promoted
anchorage-independent colony number was reduced in response to the
treatment with TNM in MCF-7AROM cells. In this context,
it is noteworthy that several mechanistically distinct nutritional
herbs have demonstrated inhibitory effects on anchorage-independent
colony formation in MCF-7 cells (28-31),
suggesting their potential efficacy for the reduction of breast
cancer risk.
At the mechanistic levels, the anti-proliferative
effects of TNM were evidenced by induction of S-phase arrest and
resultant inhibition of cell cycle progression. The pro-apoptotic
effects of TNM were evidenced by the dose-dependent induction of
caspase 3/7 activity. The pro-apoptotic BAX and
anti-apoptotic BCL-2 genes are critical for the
mitochondrial intrinsic apoptosis (32). TNM treatment resulted in a
dose-dependent increase in the expression of pro-apoptotic
BAX gene and decrease in the expression of anti-apoptotic
BCL-2 gene. These data exhibiting reciprocal modulation of
mRNA expression for these genes provide mechanistic leads that
support the pro-apoptotic effect of TNM in the present
MCF-7AROM model. In the E2-mediated signal
transduction pathways pS2, GRB2 and cyclin D1
represent classical E2 responsive target genes (11,12). Thus,
collectively, the inhibitory effects of TNM on E2
regulated ESR-1 (gene for ER-α), PR and AROM
genes and on E2 responsive pS2, GRB2 and
cyclin D1 genes provide mechanistic leads relevant to
possible molecular targets for the efficacy of TNM via the ER
signal transduction pathways.
Long-term treatment with the pharmacological
inhibitors of aromatase is frequently associated with systemic
toxicity and acquired drug resistance (2,3,6,10) leading
to the emergence of therapy resistant stem cells. By contrast,
naturally occurring TNM may exhibit lower systemic toxicity and may
lack drug resistance that is induced by pharmacological inhibitors
of aromatase activity. Inhibitory effect of TNM on aromatase
activity is evidenced by its ability to reduce the conversion of
androstenedione to E1 in MCF-7AROM cells in a
dose-dependent manner. The specificity of this effect is indicated
by the induction of aromatase inhibition by two selective
pharmacological aromatase inhibitors LET and EXM. In this context,
it is noteworthy that TNM at a concentration of 4 µg (NFD content:
8 ng) exhibits aromatase inhibition that is essentially comparable
to 285 ng of LET. Therefore, based on the NFD content of TNM, it
requires 35.6-fold higher concentration of LET which is indicative
of a 35.6-fold greater potency of TNM. Additionally, TNM at a
concentration of 100 µg (NFD content: 20 ng) exhibits aromatase
inhibition, which is essentially comparable to 2,960 ng of EXM.
Therefore, based on the NFD content of TNM, it requires 148-fold
higher concentration of EXM which is indicative of a 148-fold
greater potency of TNM. It is also noteworthy that extracts from
natural products such as resveratrol and ellagitannin derivatives
have documented anti-aromatase activity (33,34). In
addition, natural products such as sulforaphane, benzyl
iso-thiocyanate, a vitamin A derivative all-trans retinoic acid and
a terpenoid carnosol have been documented to target cancer stem
cells (35-37).
The present data identified several potential
mechanistic leads responsible for TNM-mediated anti-aromatase
activity. For example, the clinical aromatase inhibitor LET binds
the active site of the aromatase enzyme, and EXM functions as a
substrate analogue for the enzyme (1). TA downregulates ESR-1 and E2
metabolizing enzymes CYP1A1 and CYP1B1(17), and ER-α, and as its ligand
E2 induces aromatase expression (38). Thus, TNM-mediated inhibition of the
ER-α gene ESR1 and of AROM raise the possibility that
TNM may be effective via one or more of the mechanisms discussed
above.
At present, direct evidence to support efficacy of
the active principle in TNM is equivocal. However, published
evidence has demonstrated anti-cancer activity of NFD in animal
models (13). In addition to NFD,
another quinone β-lapachone (β-LAP) has been documented to be
present in trace amounts in the non-fractionated aqueous extracts
of TA and TNM. This minor constituent, at higher pharmacological
concentrations, exhibits anti-cancer activity in preclinical
xeno-transplant models of epithelial organ site cancers via
distinct molecular mechanisms (15,16,39-41).
However, it is important to recognize that at the low
concentrations of TA or TNM used, the levels of β-LAP remain
essentially undetectable (42).
The data from the present study have identified
several potential mechanistic leads for the inhibitory efficacy of
TNM in cellular models of human cancer. These leads include
modulation of the RB signaling pathway, inhibition of
cyclin-dependent kinase, inhibition of Cdc dual phosphatase,
inhibition of cyclo-oxygenase-2, inhibition of telomerase and
downregulated global expression of several genes that are involved
in cell cycle progression, cellular apoptosis and hormone
metabolism (14-18).
Thus, collectively, these lines of evidence provide potential leads
that the efficacy of TNM in the present model is likely due to its
NFD content.
In conclusion, the data presented in this study,
provide evidence for the growth inhibitory activity of TNM in a
cellular model for aromatase-expressing post-menopausal breast
cancer. More importantly, these data identify mechanistic leads as
a proof of concept that TNM may be a superior naturally occurring
substitute for clinical aromatase inhibitors. Overall, the present
study validates an experimental approach for mechanistic evaluation
of additional natural products that exhibit anti-aromatase
activity. In this context, strong mechanistic leads for the
efficacy of TNM in the present cell culture study identify future
experimental approaches that are designed to provide clinically
translatable therapeutic evidence for the use of TNM. These
approaches may include experiments on the MCF-7AROM
transplant model to examine the effects of TNM on tumor progression
and on molecular characteristics of tumors relevant to altered
E2-mediated signal transduction pathways, Additionally,
for clinical translatability of the present preclinical study,
experiments to obtain clinical data on absorption, distribution,
metabolism and excretion (ADME) of TNM, and on data for human
safety, tolerability and efficacy of TNM may provide valuable
information..
Acknowledgements
Not applicable.
Funding
Major funding support for this research was provided
by Taheebo Japan, Co., Ltd. (Osaka, Japan), and by the
philanthropic contributions to the American Foundation for Chinese
Medicine through Randall and Barbara Smith Foundation and the
Sophie Stenbeck Family Foundation.
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
NT conceived the study design, formulated the
experimental protocols, and prepared the manuscript. HBN conducted
all the experiments, organized and analyzed the data and
participated in the preparation of the manuscript. GYCW selected
the test agent and contributed to data interpretation and
preparation of the manuscript. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnston SRD and Dowsett M: Aromatase
inhibitors for breast cancer: Lessons from the laboratory. Nat Rev
Cancer. 3:821–831. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Ali S and Coombes RC: Endocrine-responsive
breast cancer and strategies for combating resistance. Nat Rev
Cancer. 2:101–112. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Ma CX, Reinert T, Chmielewska I and Ellis
MJ: Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer.
15:261–275. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Ma CK, Gao F, Luo J, Northfeld DW, Goetz
M, Forero A, Hoog J, Naughton M, Ademuyiwa F, Suresh R, et al:
NeoPalAna: Neoadjuvant Palbociclib, a cyclin-dependent kinase 4/6
inhibitor, and Anastrozole for clinical stage 2 or 3 estrogen
receptor- positive breast cancer. Clin Cancer Res. 23:4055–4065.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Taglieri L, De Iuliis F, Giuffrida A,
Giantulli S, Silvestri I and Scarpa S: Resistance to the mTOR
inhibitor everolimus is reversed by the downregulation of survivin
in breast cancer cells. Oncol Lett. 14:3832–3838. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brodie A, Jelovac D and Long BJ:
Predictions from a preclinical model: Studies of aromatase
inhibitors and antiestrogens. Clin Cancer Res. 9:455S–459S.
2003.PubMed/NCBI
|
|
7
|
Boér K: Impact of palbociclib combinations
on treatment of advanced estrogen receptor-positive/human epidermal
growth factor 2-negative breast cancer. OncoTargets Ther.
9:6119–6125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alves CL, Elias D, Lyng M, Bak M,
Kirkegaard T, Lykkesfeldt AE and Ditzel HJ: High CDK6 protects
cells from Fulvestrant-mediated apoptosis and is predictor of
resistance to Fulvestrant in estrogen receptor-positive metastatic
breast cancer. Clin Cancer Res. 22:5514–5526. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hole S, Pedersen AM, Hansen SK, Lundqvist
J, Yde CW and Lykkesfeldt AE: New cell culture model for aromatase
inhibitor-resistant breast cancer shows sensitivity to fulvestrant
treatment and cross-resistance between letrozole and exemestane.
Int J Oncol. 46:1481–1490. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sabnis G and Brodie A: Understanding
resistance to endocrine agents: Molecular mechanisms and potential
for intervention. Clin Breast Cancer. 10:E6–E15. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moy B and Goss PE: Estrogen receptor
pathway: Resistance to endocrine therapy and new therapeutic
approaches. Clin Cancer Res. 12:4790–4793. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
O'Hara J, Vareslija D, McBryan J, Bane F,
Tibbitts P, Byrne C, Conroy RM, Hao Y, Gaora PO, Hill ADK, et al:
AIB1:ERα transcriptional activity is selectively enhanced in
aromatase inhibitor-resistant breast cancer cells. Clin Cancer Res.
18:3305–3315. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ebina T: Anti-tumor effect of hot water
extract of Taheebo tea: Comparison with other biological
preparations. Biotherapy. 16:321–327. 2002.
|
|
14
|
Brisson M, Nguyen T, Vogt A, Yalowich J,
Giorgianni A, Tobi D, Bahar I, Stephenson CR, Wipf P and Lazo JS:
Discovery and characterization of novel small molecule inhibitors
of human Cdc25B dual specificity phosphatase. Mol Pharmacol.
66:824–833. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Choi YH, Kang HS and Yoo MA: Suppression
of human prostate cancer cell growth by β-lapachone via
down-regulation of pRB phosphorylation and induction of Cdk
inhibitor p21 (WAF1/CIP1). J Biochem Mol Biol. 36:223–229.
2003.PubMed/NCBI
|
|
16
|
Lee JH, Cheong J, Park YM and Choi YH:
Down-regulation of cyclooxygenase-2 and telomerase activity by
β-lapachone in human prostate carcinoma cells. Pharmacol Res.
51:553–560. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mukherjee B, Telang N and Wong GYC: Growth
inhibition of estrogen receptor positive human breast cancer cells
by Taheebo from the inner bark of Tabebuia avellanedae tree.
Int J Mol Med. 24:253–260. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Telang NT, Nair HB and Wong GYC: Efficacy
of Tabebuia avellanedae extract on a cell culture model for
triple negative breast cancer. Cancer Res. 74 (Suppl):SABCS,
P5-14-02. 2014.
|
|
19
|
Liu YG, Tekmal RR, Binkley PA, Nair HB,
Schenken RS and Kirma NB: Induction of endometrial epithelial cell
invasion and c-fms expression by transforming growth factor β. Mol
Hum Reprod. 15:665–673. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nair HB, Luthra R, Kirma N, Liu YG,
Flowers L, Evans D and Tekmal RR: Induction of aromatase expression
in cervical carcinomas: Effects of endogenous estrogen on cervical
cancer cell proliferation. Cancer Res. 65:11164–11173.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
American Cancer Society: Cancer facts and
figures. American Cancer Society, Atlanta, GA, 2018.
|
|
23
|
Gupta A, Mehta R, Alimirah F, Peng X,
Murillo G, Wiehle R and Mehta RG: Efficacy and mechanism of action
of Proellex, an antiprogestin in aromatase overexpressing and
Letrozole resistant T47D breast cancer cells. J Steroid Biochem Mol
Biol. 133:30–42. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Panda SP, Panigrahy UP, Panda S and Jena
BR: Stem extract of Tabebuia chrysantha induces apoptosis by
targeting sEGFR in Ehrlich Ascites Carcinoma. J Ethnopharmacol.
235:219–226. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bacowsky H: Investigations on effects of
Taheebo extract on various blood parameters and quality of life in
12 patients suffering from different form of cancer in different
stages. J New Rem Clin. 55:48–55. 2006.
|
|
26
|
Hirata S: An examination of supplement
dose dependence and safety in integrative medicine for cancer:
Based on the experience of Tabebuia avellanedae, a South
American medicinal plant commonly known as Taheebo. Int J Integr
Med. 2:140–144. 2010.
|
|
27
|
Lippman ME, Osborne CK, Knazek R and Young
N: In vitro model systems for the study of hormone-dependent human
breast cancer. N Engl J Med. 296:154–159. 1977.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Telang NT, Li G, Sepkovic DW, Bradlow HL
and Wong GYC: Anti-proliferative effects of Chinese herb Cornus
officinalis in a cell culture model for estrogen
receptor-positive clinical breast cancer. Mol Med Rep. 5:22–28.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Telang N, Li G, Sepkovic D, Bradlow HL and
Wong GYC: Comparative efficacy of extracts from Lycium
barbarum bark and fruit on estrogen receptor positive human
mammary carcinoma MCF-7 cells. Nutr Cancer. 66:278–284.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Telang N, Li G, Katdare M, Sepkovic D,
Bradlow L and Wong G: Inhibitory effects of Chinese nutritional
herbs in isogenic breast carcinoma cells with modulated estrogen
receptor function. Oncol Lett. 12:3949–3957. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Telang NT, Li G, Katdare M, Sepkovic DW,
Bradlow HL and Wong GYC: The nutritional herb Epimedium
grandiflorum inhibits the growth in a model for the Luminal A
molecular subtype of breast cancer. Oncol Lett. 13:2477–2482.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chottanapund S, Van Duursen MB, Navasumrit
P, Hunsonti P, Timtavorn S, Ruchirawat M and Van den Berg M:
Anti-aromatase effect of resveratrol and melatonin on hormonal
positive breast cancer cells co-cultured with breast adipose
fibroblasts. Toxicol In Vitro. 28:1215–1221. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Adams LS, Zhang Y, Seeram NP, Heber D and
Chen S: Pomegranate ellagitannin-derived compounds exhibit
antiproliferative and antiaromatase activity in breast cancer cells
in vitro. Cancer Prev Res (Phila). 3:108–113. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Castro NP, Rangel MC, Merchant AS,
MacKinnon G, Cuttitta F, Salomon DS and Kim YS: Sulforphane
suppresses the growth of triple negative breast cancer stem-like
cells in vitro and in vivo. Cancer Prev Res (Phila). 12:147–158.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim SH and Singh SV: Role of Krüppel-like
factor4-p21CIP1 axis in breast cancer stem-like cell
inhibition by benzyl isothiocyanate. Cancer Prev Res (Phila).
12:125–134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Telang N: Targeting drug resistant stem
cells in a human epidermal growth factor receptor-2-enriched breast
cancer model. World Acad. Sci. J. 1:86–91. 2019. View Article : Google Scholar
|
|
38
|
Kinoshita Y and Chen S: Induction of
aromatase (CYP19) expression in breast cancer cells through a
nongenomic action of estrogen receptor α. Cancer Res. 63:3546–3555.
2003.PubMed/NCBI
|
|
39
|
Woo HJ and Choi YH: Growth inhibition of
A549 human lung carcinoma cells by β-lapachone through induction of
apoptosis and inhibition of telomerase activity. Int J Oncol.
26:1017–1023. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jeon YJ, Bang W, Shin JC, Park SM, Cho JJ,
Choi YH, Seo KS, Choi NJ, Shim JH and Chae JI: Downregulation of
Sp1 is involved in β-lapachone-induced cell cycle arrest and
apoptosis in oral squamous cell carcinoma. Int J Oncol.
46:2606–2612. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bang W, Jeon YJ, Cho JH, Lee RH, Park SM,
Shin JC, Choi NJ, Choi YH, Cho JJ, Seo JM, et al: Shim JH ad Chae
JI: β-lapachone suppresses the proliferation of human malignant
melanoma by targeting specificity protein 1. Oncol Rep.
35:1109–1116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Queiroz ML, Valadares MC, Torello CO,
Ramos AL, Oliveira AB, Rocha FD, Arruda VA and Accorci WR:
Comparative studies of the effects of Tabebuia avellanedae
barh extract and β-lapachone on hematopoietic response of tumor
bearing mice. J Ethnopharmacol. 117:228–235. 2008.PubMed/NCBI View Article : Google Scholar
|