Introduction
Laser biostimulation with low and mid-level laser
therapy has been used for a number of years as a well-known method
of healing soft and hard tissues (1-8).
In addition, the anti-inflammatory effects of this therapy have
been described extensively (2,6,9). Low-level laser irradiation (LLLI) is
able to modify different biological pathways, including increases
in mitochondrial respiration, and ATP synthesis proliferation of
mesenchymal stem cells and cardiac stem cells (10,11).
The potential mechanism of the effect of light on
biological structures, in particular vascular smooth muscle, have
been examined previously; experimental studies have suggested that
lasers of power <100 mW cause relaxation of smooth muscle of
blood vessels (12-15).
Two studies (11,13) have demonstrated that LLLI may cause
photorelaxation of blood vessels, including coronary arteries, and
may prevent their restenosis following percutaneous transluminal
coronary angioplasty (11,16). A number of experimental studies
analyzed the effect of LLLI on vascular smooth muscle cells
following pretreatment with α-adrenoceptors agonists (adrenalin and
phenylephrine) and peptides, including vasopressin or endothelin-1
(17,18). To the best of our knowledge, the
effect of non-receptor stimulation has not been analyzed
previously.
Mastoparan-7 is a basic tetradecapeptide isolated
from wasp venom. The mechanism of its action is associated with the
activation of guanine nucleotide-binding proteins (G-proteins). The
peptide has been demonstrated to stimulate phospholipase C (PLC) in
several cellular compartments such as rat mast cells, rat
hepatocytes and human HL-60 leukemia cells. By contrast, inhibition
of PLC by mastoparan has been demonstrated in SH-SY5Y human
neuroblastoma cells and in human astrocytoma cells (19). In vascular smooth cells, it is able to
increase contractility (20-22).
Calcium ions (Ca2+) serve a central role
in general cell processes. According to pathological factors,
Ca2+ concentration changes occur in various cell
compartments, which may induce apoptosis (23-25).
An increase in Ca2+ describes one of final elements of
programmed cell death and physiological concentrations may differ
significantly in cell compartments.
Our previous studies confirmed that mastoparan-7 is
able to increase the calcium load in smooth muscle cytoplasm by
activation of calcium influx from intra and extracellular calcium
stores in an isolated resistant artery model (20,21). In
addition, during ischemia and reperfusion, the pathway associated
with direct stimulation of G-proteins remains active (22).
The primary aim of the present study was to evaluate
the modulatory effect of low and moderate laser irradiation on
vascular smooth muscle contraction induced by direct stimulation of
G-proteins with mastoparan-7.
Materials and methods
Animals
Experiments were performed on isolated and perfused
tail arteries of Wistar rats (10 males; age, 8 weeks; body weight,
250-270 g; Hodowla Zwierząt Laboratoryjnych). Animals were housed
at 20-21˚C with a humidity of 50-60% and 12-h light/dark cycles and
had ad libitum access to food and water. Prior to tail
artery isolation, rats were anesthetized by intraperitoneal
injection of 1,200 mg/kg urethane and euthanized by cervical
dislocation. The study protocol was approved by the Local Ethics
Committee for Experiments on Animals, (Bydgoszcz, Poland). All
studies were performed in accordance with the United States of
America National Institutes of Health guidelines (26).
Drugs and solutions
Mastoparan-7 (G-protein activator), mastoparan-17
(negative control), and Krebs solution containing NaCl (71.8 mM/l),
KCl (4.7 mM/l), CaCl2 (1.7 mM/l) NaHCO3 (28.4
mM/l), MgSO4 (2.4 mM/l), KH2PO4
(1.2 mM/l) and glucose (11.1 mM/l) were purchased from Sigma
Aldrich; Merck KGaA.
Study design and conduction
Following dissection from the surrounding tissues,
2-3-cm long segments of rat tail arteries were cannulated and
connected to a perfusion device. The distal part was held in place
with 500 mg weight and the tail was placed in a 20 ml container
filled with oxygenated Krebs solution at 37˚C (pH 7.4). The
perfusion pressure was continuously measured. Perfusion solution
flow was gradually increased using a peristaltic pump to 1 ml/min,
until the optimum perfusion pressure of 2-4 kPa was reached
(25). The study utilized a
semiconductor laser (400 mW, wavelength =810 nm), operating in
continuous-wave mode. Subsequent to achieving maximal vasospasm,
the arteries were rinsed and stabilized for a period of 30 min
prior to exposure to laser irradiation. The arteries were placed on
a plate and the laser header was positioned on a tripod ~1 cm from
the irradiated tissue. The irradiation was applied directly on the
blood vessels without utilization of a glass chamber. The laser was
applied in increasing doses of 10 mW (‘L1’; E=1.8 J), 30 mW (‘L2’;
E=5.5 J), 110 mW (‘L3’; E=19.8 J). Time of exposition was 3 min for
each irradiation (17). In
experiments performed on endothelium-denudated arteries, the
endothelium was removed with the compressed air (temperature=37˚C,
pressure = 1.0-1.1 atm). The absence of endothelium was confirmed
with an acetylcholine test (single dose of acetylcholine
10-5 M/l). To evaluate constriction associated with the
intracellular calcium, experiments were performed in calcium-free
Krebs solution (phase 1) and after switching to the Krebs solution
analysis of constriction related to calcium influx from
extracellular space was performed (phase 2).
Data analysis and statistical
procedures
Investigations were performed using a TSZ-04 system
(Experimetria Kft.). Perfusion pressure was measured on BPR-01 and
BPR-02 (Experimetria Kft.) devices, and vascular smooth muscle
tension was measured using an FSG-01 transducer connected with a
Graphtec GL820 midi LOGGER digital recorder (Graphtec Corporation).
All transducers used in the experiments were produced by
Experimetria Kft. Concentration-response curves (CRCs) were
calculated according to the van Rossum method (27). Maximum response of tissue
(Emax) was calculated as a proportion of the maximal
response for phenylephrine. The half maximal effective
concentration (EC50) was estimated using classical
pharmacologic methods with the pD2 value, the negative
logarithm of the EC50. The CRC and Emax
values were used in all calculations estimating the statistical
significance. Mastoparan-17 was included in all experiments as the
negative control.
Results are presented as mean ± standard deviation.
Tukey's Honest Significant Difference test was used following
two-way analysis of variance for multiple comparisons of means.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using STATISTICA
12.5 (StatSoft, Inc.).
Results
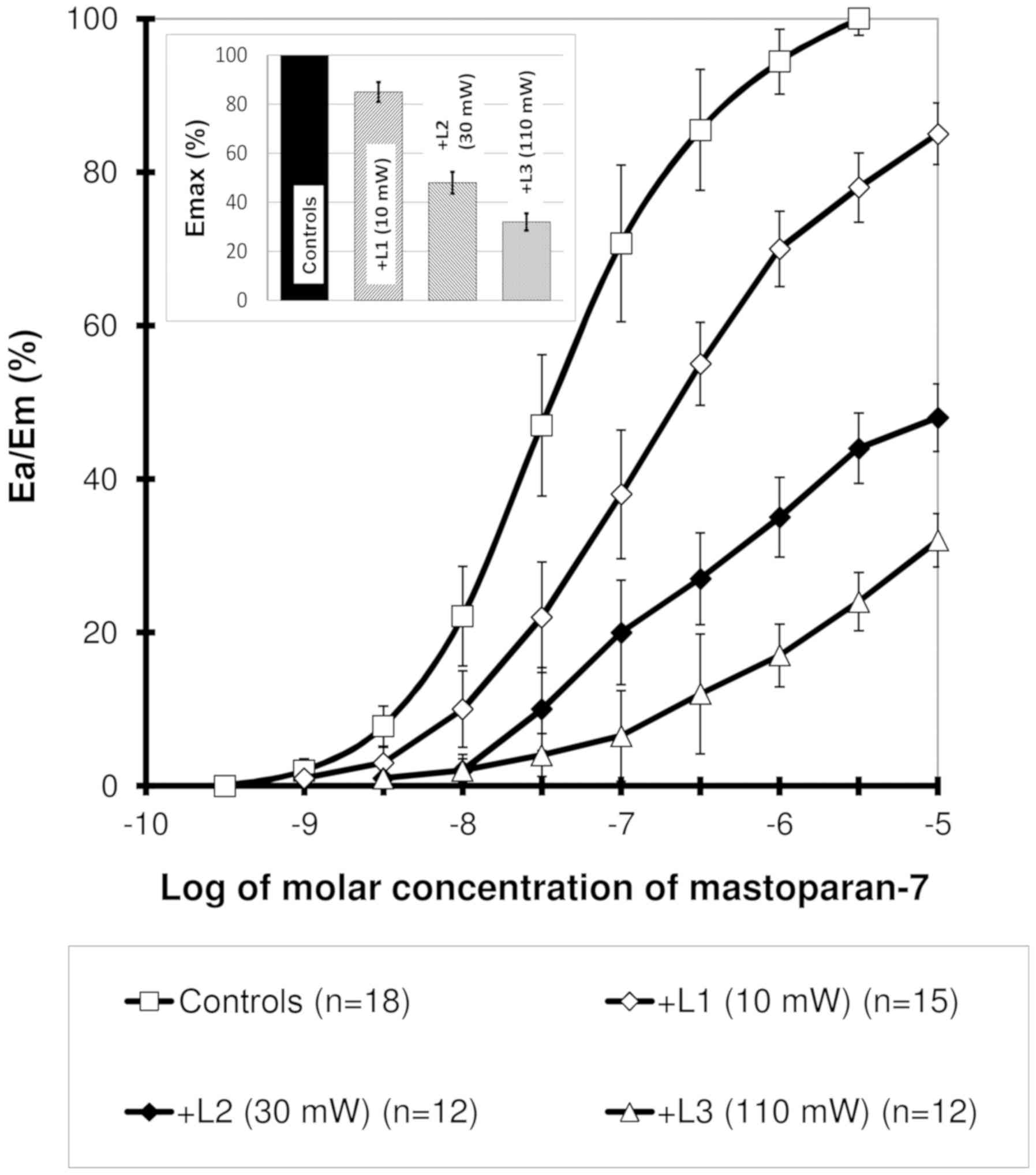
Concentration-response curve obtained
for mastoparan-7 presents a sigmoidal association
In the laser-irradiated arteries, a significant
rightward shift with significant decrease in maximal responses was
observed. This effect was dependent on the power of laser
irradiation (Fig. 1). For all samples
in which the effect was measured as ≥20%, the difference was deemed
statistically significant. The EC50 values were
4.43±2.2x10-8, 2.4±0.56x10-7,
3.2±0.72x10-7 and 7.7±0.3x10-7 M/l in the
control and 10, 30 and 110 mW laser irradiation groups,
respectively. Significant (P<0.001) changes were observed for
all laser power groups in comparison with the control.
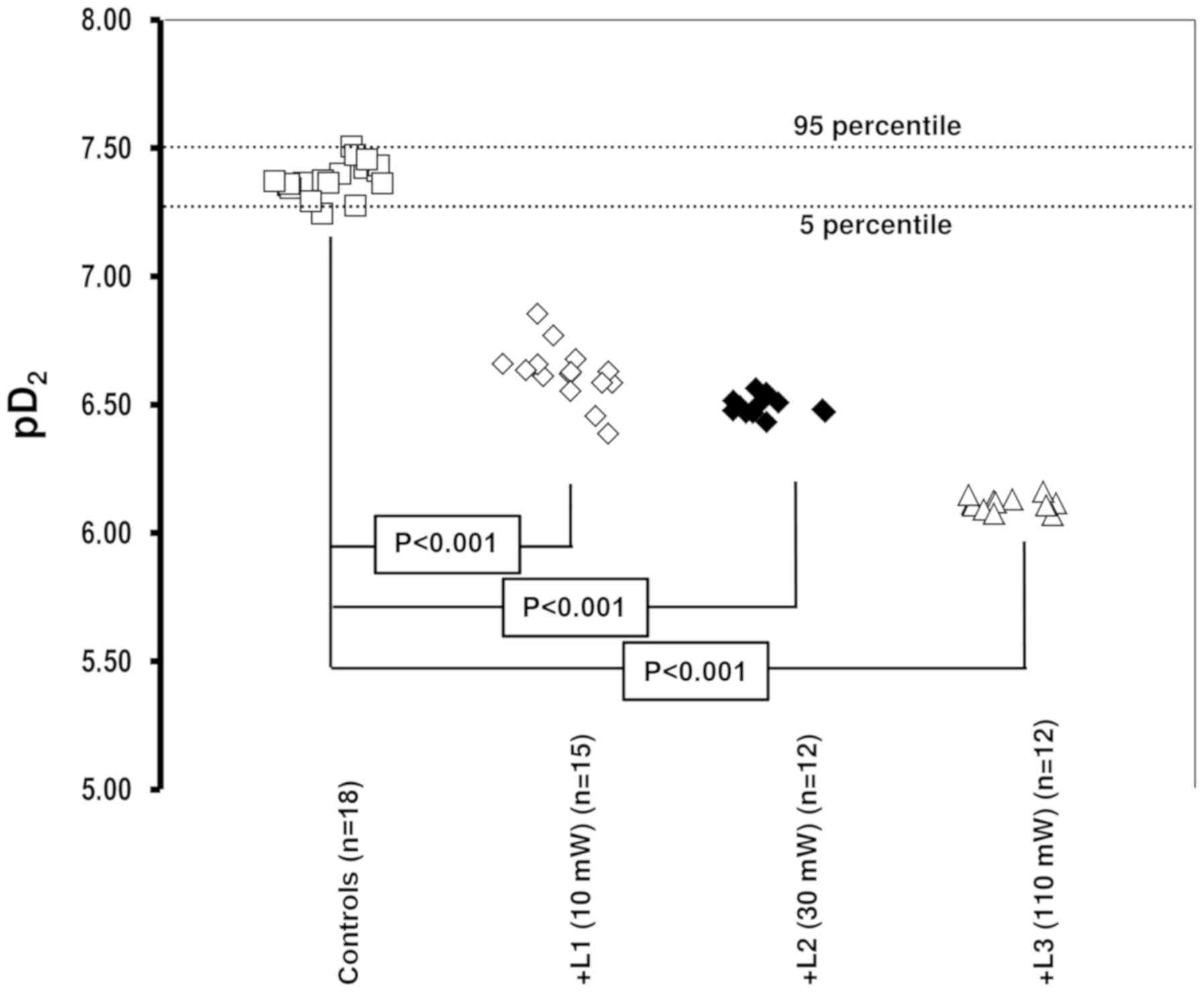
In experiments performed on the
endothelium-denudated arteries, the EC50 values were
2.25±2.5x10-9, 2.73±1.9x10-9,
2.63±2.1x10-9 and 2.15±1.8x10-9 M/l in the
control and 10, 30 and 110 mW laser irradiation groups,
respectively. Values did not differ significantly. All
Emax, EC50 and pD2 values are
presented in Table I. Changes in
trends in pD2 values are presented in Fig. 2.
 | Table IEC50, maximal response and relative
potency for mastoparan-7 in controls and in the 10, 30 and 110 mW
laser irradiation groups for normal and endothelium-denudated
arteries. |
Table I
EC50, maximal response and relative
potency for mastoparan-7 in controls and in the 10, 30 and 110 mW
laser irradiation groups for normal and endothelium-denudated
arteries.
| A, Normal
vessels |
|---|
| Groups | Na |
%Emaxb | EC50
(M/l) | pD2 | RPc | P-value |
|---|
| Control | 18 | 100 |
4.43±2.2x10-8 | 7.35±0.21 | 1.000 | - |
| Laser treatment,
mW | | | | | | |
|
+L1(10) | 15 | 85±8 |
2.4±0.56x10-7 | 6.62±0.13 | 0.185 | <0.0001 |
|
+L2(30) | 12 | 48±8 |
3.2±0.72x10-7 | 6.49±0.09 | 0.138 | <0.0001 |
|
+L3(110) | 12 | 32±7 |
7.7±0.3x10-7 | 6.11±0.20 | 0.058 | <0.0001 |
| B,
Endothelium-denudated artery |
| Groups | Na |
%Emaxb | EC50
(M/l) | pD2 | RPc | P-value |
| Control | 18 | 100 |
2.25±2.5x10-9 | 8.65±0.24 | 1.000 | - |
| Laser treatment,
mW | | | | | | |
|
+L1(10) | 15 | 85±8 |
2.73±1.9x10-9 | 8.56±0.21 | 1.213 | ns |
|
+L2(30) | 12 | 48±8 |
2.63±2.1x10-9 | 8.58±0.09 | 1.169 | ns |
|
+L3(110) | 12 | 32±7 |
2.15±1.8x10-9 | 8.67±0.20 | 0.956 | ns |
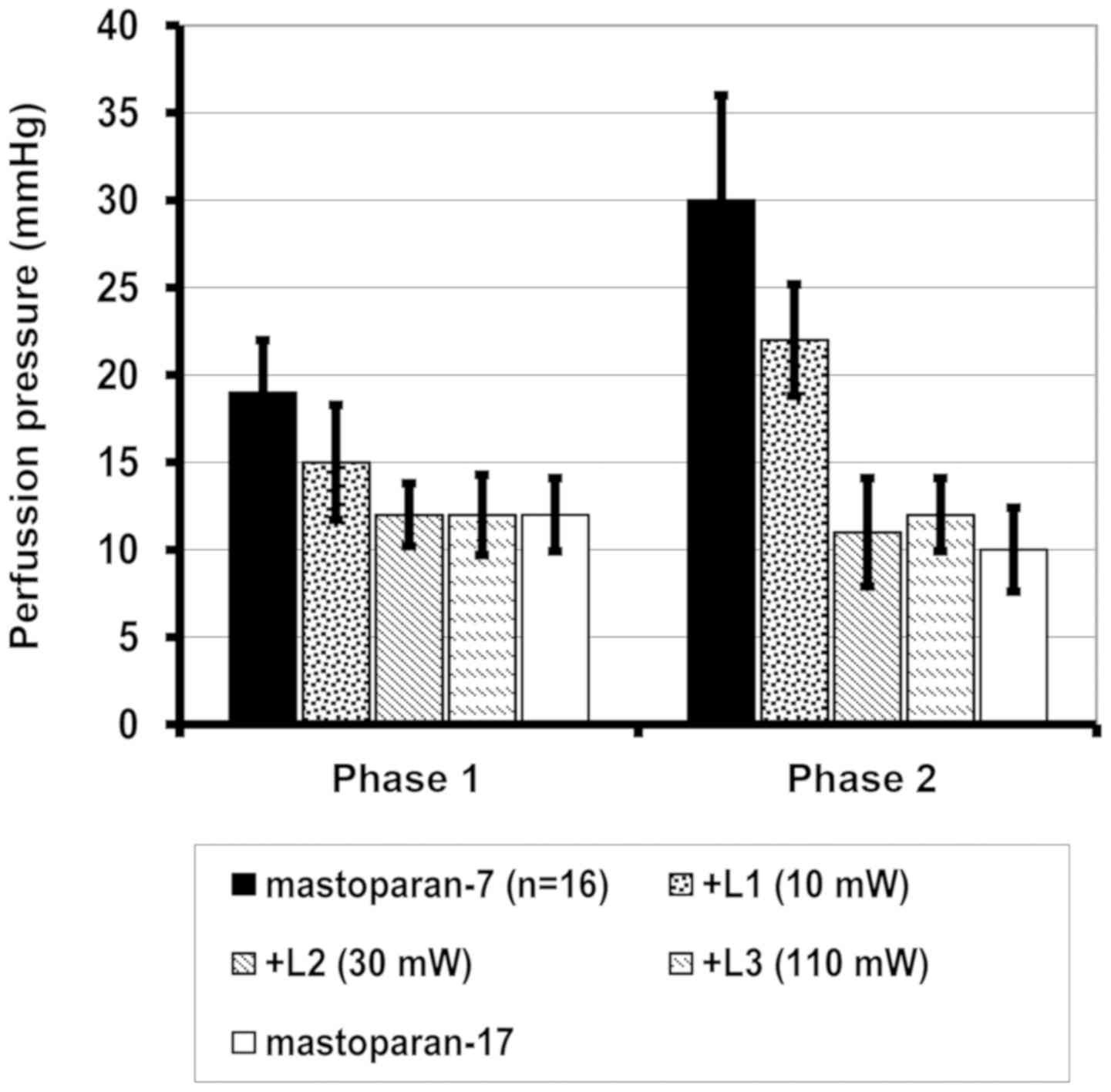
When analyzing the changes in perfusion pressure as
a result of intracellular Ca2+ influx during
mastoparan-7-induced contraction in the control group, a
significant increase was observed in comparison with the negative
control, mastoparan-17 (P<0.001). Following laser irradiation at
10, 30 and 110 mW, a significant decrease in vascular reaction was
observed following influx of extracellular Ca2+ into the
cytoplasm, whereas in intracellular Ca2+, a significant
decrease was only identified at 30 and 110 mW (Table II; Fig.
3). No additional benefit in increasing the laser power from 30
to 110 mW was observed.
 | Table IIMaximal perfusion pressure for
mastoparan-7 induced contraction activated by Ca2+
influx from intracellular (phase 1) and extracellular
Ca2+ stores (phase 2) in the control and laser
irradiated (10, 30 and 110 mW) groups. |
Table II
Maximal perfusion pressure for
mastoparan-7 induced contraction activated by Ca2+
influx from intracellular (phase 1) and extracellular
Ca2+ stores (phase 2) in the control and laser
irradiated (10, 30 and 110 mW) groups.
| | Phase 1 | Phase 2 |
|---|
| Groups | n | Perfusion pressure,
mmHg | n | Perfusion pressure,
mmHg |
|---|
| Control | 16 | 19.1±3.2 | 16 | 31.2±5.9 |
| Laser treatment,
mW | | | | |
|
+L1(10) | 12 |
15.2±1.7f | 12 |
21.9±3.1a |
|
+L2(30) | 12 |
12.4±3.5a,c | 12 |
11.2±3.8b,d |
|
+L3(110) | 12 |
11.8±5.6a,e | 12 |
12.3±5.7b,d |
| Mastoparan-17 | 10 |
12.2±5.4a | 10 |
10.3±4.7b |
Discussion
Arterial smooth muscle function has been
investigated in a number of different studies, involving typical
pharmacological stimulation with the use of receptor agonists
including α1-adrenoceptors, vasopressin type 1 receptors
or the L-type calcium channel (20-25,28,29).
Direct stimulation of G-proteins with mastoparan-7 was described in
certain studies (20-22,29,30).
The present study investigated the possibility of the induction of
photorelaxation in laser-pretreated arteries. Results from our
previous studies confirmed the occurrence of photorelaxation in
arteries stimulated with receptor agonists (17,18,29,30).
Constriction of vascular smooth muscle cells in the presence of
mastoparan-7 was demonstrated by Kanagy and Webb (29), and Birnbaumer (30) described experiments on spiral cutting
fragments of the common carotid artery.
In the present study, laser irradiation
significantly inhibited mastoparan-7-induced constriction of
vascular smooth muscle cells. In addition, proportional to the
laser power, a decrease in perfusion pressure caused by intra- and
extracellular Ca2+ influx inhibition was observed.
Mastoparan-7 leads to degranulation of mast cells by activation of
phospholipase A2 at a concentration of 5x10-5
M/l (31).
Previous studies performed on vessels suggested that
laser photorelaxation in vessels stimulated pharmacologically is
partially endothelium-dependent process (17,18),
therefore degradation of the vascular endothelium may able to
eliminate the inhibitory effect of laser irradiation on vessels
reactivity. In the experiments performed in the present study,
removal of the vascular endothelium prevented the inhibitory effect
of laser irradiation on vessel walls stimulated by G-protein with
mastoparan-7; consequently, we concluded that laser vasodilation in
mastoparan-7-induced arteries may be an endothelium-dependent
process. Similar effects were observed for endothelin-1 and
phenylephrine stimulation of entothelin-1 and
α1-adrenoceptors, respectively (17).
Similar effects of LLLI-induced vasorelaxation were
described by Gal et al (12)
and Steg et al (13). Results
of their studies indicated that laser irradiation at a power
<100 mW was responsible for constant smooth muscle relaxation,
whereas continuous wave laser at a power <1 W induced
vasoconstriction (14). By contrast,
the opposite effect of endothelium removal was described by Gal
et al (12) and Steg et
al (13), who postulated that the
vasodilatory effect of laser treatment was not
endothelium-dependent. These studies were able to induce smooth
muscle dilation in endothelium-denudated arteries using in
vitro and in vivo experiments. In addition, Steg et
al (13) observed relaxation of
smooth muscles induced by low level pulse lasers, even if the
muscles had not been previously contracted (13). Maegawa et al (32) also described vasodilation of
arterioles in a study investigating the effect of LLLI smooth
muscle reactivity. This study suggested that the observed effects
were partially due to NO release from the vascular endothelium,
particularly in the initial phase, whereas in the late phase LLLI
induced a decrease of Ca2+ in microvascular smooth
muscles. Generally, the process of LLLI-induced vasodilation
appears to be a result of processes occurring in the large and
small vessels, pre-capillary sphincteromas and
microcirculation.
In conclusion, the results of the present study
suggest that contraction induced by direct activation of G-protein
with mastoparan-7 may by effectively inhibited by laser
irradiation, and may be an endothelium-dependent process. In
addition, this effect was laser power-dependent, and associated
with the inhibition of Ca2+influx from both intra and
extracellular calcium stores.
Acknowledgements
Not applicable.
Funding
The study was funded through departmental sources
(grant no. WN757/2019; Nicolaus Copernicus University, Toruń,
Poland).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article excluding raw data, which are
available from the corresponding author on reasonable request.
Authors' contributions
EG, MMM, HMN, WH and GG conceived and designed the
study. EG, MW and GG performed the experiments. EG, MMM, HMN, MW
and GG collected the data. EG, MMM, HMN, IB, MK, AK, WH and GG
analyzed the data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Local Ethics
Committee for Experiments on Animals, Bydgoszcz, Poland. All
studies were performed in accordance with the United States of
America National Institutes of Health guidelines.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Conlan MJ, Rapley JW and Cobb CM:
Biostimulation of wound healing by low-energy laser irradiation a
review. J Clin Peridontol. 23:492–496. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pourzarandian A, Watanabe H, Ruwanpura SM,
Aoki A, Noguchi K and Ishikawa I: Er:YAG laser irradiation
increases prostaglandin E production via the induction of
cyclooxygenase-2 mRNA in human gingival fibroblasts. J Peridontal
Res. 40:182–186. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Byrnes KR, Waynant RW, Ilev IK, Wu X,
Barna L, Smith K, Heckert R, Gerst H and Anders JJ: Light promotes
regeneration and functional recovery and alters the immune response
after spinal cord injury. Lasers Surg Med. 36:171–185.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chow RT, Johnson MI, Lopes-Martins RA and
Bjordal JM: Efficacy of low-level laser therapy in the management
of neck pain: A systematic review and meta-analysis of randomized
placebo or active-treatment controlled trials. Lancet.
374:1897–1908. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hirakawa S, Fujii S, Kajiya K, Yano K and
Dietmar M: Vascular endothelial growth factor promotes sensitivity
to ultraviolet B-induced cutaneous photodamage. Blood.
105:2392–2435. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ribeiro DA and Matsumooto MA: Low-level
laser therapy improves bone repair in rats treated with
anti-inflammatory drugs. J Oral Rehabil. 35:925–933.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Basford JR: Low intensity laser therapy;
Still not an established clinical tool. Lasers Surg Med.
16:331–342. 1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tumilty S, Mani R and Baxter GD:
Photobiomodulation and eccentric exercise for achilles
tendinopathy: A randomized controlled trial. Lasers Med Sci.
31:127–135. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu JY, Chen CH, Wang CZ, Ho ML, Yeh ML and
Wang YH: Low-power laser irradiation suppresses inflammatory
response of human adipose-derived stem cells by modulating
intracellular cyclic AMP level and NF-κB activity. PLoS One.
8(e54067)2013. View Article : Google Scholar
|
|
10
|
Tuby H, Hertzberg E, Maltz L and Oron U:
Long-term safety of low-level laser therapy at different power
densities and single or multiple applications to the bone marrow in
mice. Photomed Laser Surg. 31:269–273. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Plass CA, Wieselhaler GM, Podesser BK and
Prusa AM: Low-level laser irradiation induces photorelaxation in
coronary arteries and overcomes vasospasm of internal thoracic
arteries. Lasers Surg Med. 44:705–711. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gal D, Chokshi SK, Mosseri M, Clark RH and
Isner JM: Percutaneous delivery of low-level laser energy reverses
histamine-induced spasm in atherosclerotic Yucatan microswine.
Circulation. 82:756–768. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Steg PG, Rongione AJ, Gal D, Dejesus ST,
Clarke RH and Isner JM: Pulsed ultraviolet laser irradiation
produces endothelium-independent relaxation of vascular smooth
muscle. Circuation. 80:189–197. 1989.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gal D, Steg PG, Rongione AJ, Dejesus ST,
Clarke RH and Isner JM: Vascular spasm complicates continuous wave
but not pulse laser irradiation. Am Heart J. 118:934–941.
1989.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schwengel RH, Gregory KW, Hearne SE, Scott
HJ, Beauman GJ, Mergner WJ, Caplin JL and Ziskind AA:
Characterization of pulsed-dye laser-mediated vasodilatation in a
rabbit femoral artery model of vasoconstriction. Lasers Surg Med.
13:284–295. 1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kipshidze N, Nikolaychik V, Keelan MH,
Shankar LR, Khanna A, Kornowski R, Leon M and Moses J: Low-power
helium: Neon laser irradiation enhances production of vascular
endothelial growth factor and promotes growth of endothelial cells
in vitro. Lasers Surg Med. 28:355–364. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Mackiewicz-Milewska M, Grześk E,
Kroszczyński AC, Cisowska-Adamiak M, Mackiewicz-Nartowicz H, Baran
L, Szymkuć-Bukowska I, Wiciński M, Hagner W and Grześk G: The
influence of low level laser irradiation on vascular reactivity.
Adv Med Sci. 63:64–67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Grześk G, Mackiewicz-Milewska M, Talar J,
Szadujkis-Szadurski L and Bułatowicz I: Rola antagonizmu
fizjologicznego angiotensyny II i biostymulacji laserowej w
modulowaniu reaktywności mięśniówki gładkiej tętnic. Fizjoter Pol.
4:143–150. 2004.
|
|
19
|
Kling TP, Jim SY and Wittkowski KM:
Inflammatory role of two venom components of yellow jackets
(Vespula vulgaris): A mast cell degranulating peptide mastoparan
and phospholipase a1. Int Arch Allergy Immunol. 131:25–32.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Grześk G, Malinowski B, Grześk E, Wiciński
M and Szadujkis-Szadurska K: Direct regulation of vascular smooth
muscle contraction by mastoparan-7. Biomed Rep. 2:34–38.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Grześk E, Tejza B, Wiciński M, Malinowski
B, Szadujkis-Szadurska K, Baran L, Kowal E and Grześk G: Effect of
pertussis toxin on calcium influx in three contraction models.
Biomed Rep. 2:584–588. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grześk E, Darwish N, Stolarek W, Wiciński
M, Malinowski B, Burdziński I and Grześk G: Effect of reperfusion
on vascular smooth muscle reactivity in three contraction models.
Microvasc Res. 121:24–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hajnoczky G, Davies E and Madesh M:
Calcium signaling and apoptosis. Biochem Biophys Res Commun.
304:445–454. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: Releasing power for life and unleashing the
machineries of death. Cell. 112:481–390. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grześk G, Wiciński M, Malinowski B, Grześk
E, Manysiak S, Odrowąż-Sypniewska G, Darvish N and Bierwagen M:
Calcium blockers inhibit cyclosporine A-induced hyperreactivity of
vascular smooth muscle cells. Mol Med Rep. 5:1469–1474.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guide for the Care and Use of Laboratory
Animals. NIH Publication No. 85-23. Division of Research Resources,
National Institutes of Health, Bethesda, MD, 1985.
|
|
27
|
Van Rossum JM: Cumulative dose-response
curves. II. Technique for the making of dose-response curves in
isolated organs and the evaluation of drug parameters. Arch Int
Pharmacodyn Ther. 143:299–330. 1963.PubMed/NCBI
|
|
28
|
Grześk G and Szadujkis-Szadurski L:
Physiological antagonism of angiotensin II and lipopolysaccharides
in early endotoxemia: Pharmacometric analysis. Pol J Pharmacol.
55:753–762. 2003.PubMed/NCBI
|
|
29
|
Kanagy NL and Webb RC: Enhanced vascular
reactivity to mastoparan, a G protein activator, in genetically
hypertensive rats. Hypertension. 23:946–950. 1994.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Birnbaumer L: The discovery of signal
transduction by G-proteins. A personal account and an overview of
the initial findings and contributions that led to our present
understanding. Biochim Biophys Acta. 1768:756–771. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Argiolas A and Pisano JJ: Facilitation of
phospholipase A2 activity by mastoparan, a new class of
mast cell degranulating peptides from wasp venom. J Biol Chem.
258:13697–13702. 1983.PubMed/NCBI
|
|
32
|
Maegawa Y, Itoh T, Hosokawa T, Yaegashi K
and Nishi N: Effects of near-infrared low-level laser irradiation
on microcirculation. Lasers Surg Med. 27:427–437. 2000.PubMed/NCBI View Article : Google Scholar
|