Introduction
Allergic rhinitis (AR) is a symptomatic disorder of
the nose induced by an IgE-mediated inflammation following allergen
exposure of the membranes lining the nose (1). Due to its increasing prevalence
worldwide (2,3), including up to 22% in Spain (3,4), and the
burden of symptoms impacting on the general well-being and
health-related quality of life of patients suffering from this
condition, AR represents a serious global health problem (5). AR and asthma frequently coexist, sharing
a number of physiopathologic links and risk factors, including
sensitization to allergens, total IgE levels and a family history
of asthma (6). Between 20 and 50% of
patients with AR have asthma (6,7), and AR
has been demonstrated to occur in ≤80% of patients with asthma
(8). Previous studies have
consequently questioned whether both diseases may represent
different expressions of a same underlying genetic predisposition
(9-13).
Parietaria is a genus of the wind-pollinated
dicotyledonous weeds of the Urticaceae family, which is
predominantly widespread in several areas of southern Europe
(14). Parietaria includes
several subspecies, of which P. judaica and P.
officinalis are the most common. P. judaica grows mainly
on the Mediterranean coasts of Spain, southern France, Italy and
Greece, whereas P. officinalis can be more frequently
located in northern Italy, central France and central and eastern
Europe (14). P. judaica in
the Mediterranean area has a very long pollination period, reaching
peaks in the spring and autumn (15).
P. judaica pollen has been identified to be
the main cause of allergy in the Mediterranean area (16). In a study conducted in Catalonia,
Spain, 47.8% of patients with AR due to P. judaica also
suffered from asthma (11). In
addition, in a retrospective cohort study conducted in Italy,
sensitization to P. judaica significantly increased the risk
of developing asthma in patients with AR, whereas no associations
for other types of pollens were identified (17).
The composition of the allergenic extracts of the
P. judaica pollen has been extensively studied and
molecularly cloned, and the two major allergens (Par j 1 and Par j
2) have been sequenced and characterized (18-21).
Par j 1 and Par j 2 allergens are polypeptides of 14.7 and 11.3
kDa, respectively, that belong to the non-specific lipid transfer
protein (20-22).
The allergens share certain common structural similarities, as well
as the same IgE binding epitopes (21). However, although the Par j 2 allergen
has been recognized as the species-specific allergen marker of
P. judaica sensitization (22), the full allergenic role and
contribution of the two Pars have not been completely
elucidated.
Resemblance and cross-reactivity of the two main
subspecies of Parietaria, P. judaica and P.
officinalis, have been confirmed in several studies (23-26).
Bonura et al (25)
demonstrated that the cross-reactivity between the two subspecies
was due to the presence of the Par j 1 and Par j 2 allergens in the
extracts with a high conserved IgE epitope content; all patients
enrolled in their study exhibited a double-positive skin prick test
(SPT) toward the P. judaica and P. officinalis
extracts with similar IgE concentrations for both subspecies. In
addition, ELISA inhibition experiments were performed to validate
these results, thus demonstrating that the major P. judaica
allergens inhibited most of the P. judaica- and P.
officinalis-specific IgE in similar proportions (25). These results were confirmed in the
study conducted by Patriarca et al (26), demonstrating that P.
officinalis and P. judaica pollen extracts were
completely cross-reactive. In addition, they revealed that one
unique extract of P. officinalis was suitable for the in
vivo diagnosis and immunotherapy of the patients allergic to
Parietaria tested in the study (26).
Considering the above findings, the present study
aimed to confirm that the use of a single P. officinalis
extract in patients allergic to P. judaica and P.
officinalis may be a suitable option for the diagnosis and
treatment of patients sensitized to Parietaria in a high
pollinated area of the Spanish Mediterranean coast.
Materials and methods
Study Design
It was a single centre, single visit, observational,
cross-sectional study of adult patients diagnosed with AR and/or
bronchial asthma sensitized to Parietaria pollen. The
principal objective of the study was to assess the in vitro
cross-reactivity between allergens present in P. judaica and
P. officinalis. The secondary objective was to compare in
vitro reactivities between P. judaica and P.
officinalis in allergic patients sensitized to
Parietaria.
Patients treated at the Hospital of Sagunto in a
highly exposed to Parietaria pollen area of the Spanish
Mediterranean coast (Levante) were included in the study from March
to November 2017. Data were obtained either from the medical record
or from a single visit that matched a routine consultation with a
clinician, without interfering with the routine clinical practice.
No therapeutic and/or diagnostic interventions were applied.
Written informed consent was obtained from all study participants,
and ethical approval was obtained from the Ethics Committee of
Clinical Research at the Hospital of Sagunto (Valencia, Spain).
Patients
Patients eligible for this study had to fulfil all
of the following inclusion criteria: i) Age, ≥18 years; ii)
diagnosed with AR and/or bronchial asthma and sensitized to P.
judaica pollen; and iii) without any previous specific
immunotherapy treatment for allergy to Parietaria. In
addition, the patients underwent at least a 7-day period without of
antihistamines and glucocorticoids prior to the study. The
exclusion criteria were as follows: i) Patients with any clinical
conditions preventing them from understanding the implications and
requirements of the study; ii) patients polysensitized to
profilins; and iii) patients under other simultaneous immunotherapy
treatment during the study follow-up period.
Study variables
For the primary objective analysis, in vitro
reactivity of patient sera (allergy-specific IgE levels) to
immunotherapy extracts (P. officinalis) were determined by
ELISA. Two different immunotherapy treatments were tested: 3 ml
(10,000 UT/ml) Allergovit® (Allergopharma; Merck KGaA)
and 2.5 ml (1,000 DPP/ml) Depigoid® (Laboratorios Leti,
S.L.). Protein extracts of the immunotherapy products were obtained
by incubation with 10 mM PBS and 150 mM NaCl for 1 h at 4˚C. The
supernatant was dialyzed against H2O and lyophilized.
Subsequently, protein content was precipitated with 10%
trichloroacetic acid, and the pellet was washed with cold acetone.
The Bradford protein assay was used to quantify the protein content
of the immunotherapy products.
ELISA
The reactivity of the sera was determined by ELISA
assays produced in the laboratory of the Biotechnology and Plants
Genomic Center at Polytechnic University of Madrid. Immunotherapy
products were coated on ELISA plates at 50 µg/ml diluted in PBS for
2 h at 37˚C. Following blocking for 1 h at room temperature with
blocking buffer (Sigma-Aldrich; Merck KGaA), the plates were washed
with PBS-0.05% Tween-20 and incubated with serum samples diluted
1:3 in PBS. The presence of the IgE was detected by incubation with
horseradish peroxidase (HRP)-conjugated anti-IgE antibodies
(1:3,000; cat. no. A9667; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature and revealed with OPD substrate (Thermo Fisher
Scientific, Inc.). Optical density (OD) was measure at 450 nm in a
microplate reader. OD values were counted as positive if they
exceeded the mean OD of the negative controls by >3 standard
deviations (i.e., values >0.132). Positive values corresponded
to reactive sera, whereas negative values corresponded to sera that
were not considered reactive. Subsequently, cross-reactivity
between allergens from P. judaica and P. officinalis
(Allergovit®) pollen extracts was measured by the ELISA
inhibition method.
ELISA inhibition
Sera of the patients exhibiting high reactivity to
the sample (i.e. the highest OD values determined by the ELISA
method) were selected for ELISA inhibition and were used as a
reference of the index of stimulation of the sera without
inhibition. The sera were inhibited by incubation with 30 µg pollen
protein extract from P. judaica. Immunotherapy protein
extracts were coated on ELISA plates (50 µg/ml) and following
blocking as described above, the plates were incubated with serum
samples inhibited with pollen protein extract of P. judaica
and developed with anti IgE-HRP antibodies and OPD subtract. ELISA
experiments were performed at the Biotechnology and Plants Genomic
Center at Polytechnic University of Madrid (Madrid, Spain).
To analyse the protein content of the extracts, the
samples were analysed by SDS-PAGE and Coomassie staining. Briefly,
SDS-PAGE was performed using a 15% polyacrylamide gel and 0.1% SDS
under reducing conditions with 2-mercaptoethanol. Proteins were
visualised by staining with 0.25% Brilliant blue R250 (cat. no.
B0149' Sigma-Aldrich; Merck KGaA), 9% acetic acid and 50% methanol
by agitation on a shaker for 30 min at room temperature. Coomassie
brilliant blue staining was removed using a destaining solution
(40% methanol and 7% acetic acid) with gentle agitation at room
temperature until the protein bands were visible.
For the secondary objective analysis, a SPT was
performed to compare the in vivo reactivities between the
two Parietaria subspecies in allergic patients sensitized to
Parietaria by evaluating the specific mean IgE levels to
P. judaica and P. officinalis and the total mean IgE
levels obtained either from the patient medical records or a
regular visit to the clinician. In addition, associations between
the variables measured by the SPT for P. judaica and P.
officinalis were analysed. Patients underwent SPT with P.
judaica (ALK-Abelló®) and P. officinalis
(Allergopharma®) aeroallergen plus a positive and
negative control placed on the skin ≥2 cm apart. The positive
control was 10 mg/ml histamine dichloride, and the negative control
was glycerinated saline histamine at the same concentration. Test
reading was performed at 15 min.
Statistical analysis
Statistical analyses were performed using SPSS
version 22.0 (IBM Corp.). Sample size was calculated based on the
necessary number of patients to achieve the primary objective
(in vitro cross-reactivity between allergens present in
P. judaica and P. officinalis). The sample size of
the present study was calculated based on the results of a previous
study that identified a significant correlation (r=0.98;
P<0.0001) between patient sera specific IgE levels for P.
judaica and P. officinalis with a sample of 30 patients
(26).
For the overall descriptive analysis, quantitative
variables were presented as the mean ± SD or median with first
quartile (Q1), third quartile (Q3), minimum and maximum.
Qualitative variables were described with absolute and relative
frequencies with the valid percentages (percentages that do not
include missing data) and total percentages (the sum of the valid
responses plus the missing values). In cases where these two
percentages had different values, the valid percentage was
reported. Absent data were left as missing values.
Bivariate analyses with the parametric paired-sample
t-test were performed to assess the secondary objective, assuming a
normal distribution of the sample continuous variables. The 95%
confidence intervals (CI) were estimated when required. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients
A total of 24 patients meeting all the inclusion
criteria entered the study and were included in the analysis. The
mean age of the participants was 40 years (range, 26-67 years), and
37.5% of the patients were female. All patients suffered from AR
and 41.7% (n=10) were diagnosed with bronchial asthma. None of the
patients presented comorbidities, such as diabetes or arterial
hypertension, and 22.7 and 25% were regarded as current smokers or
daily alcohol drinkers, respectively. Table I displays the clinical characteristics
of each patient included in the study.
 | Table IClinical characteristics of all
patients enrolled in the present study. |
Table I
Clinical characteristics of all
patients enrolled in the present study.
| Patient no. | Bronchial asthma
diagnosis | Atopy | SPT: P.
judaica (D+d)/2, mm | SPT: P.
officinalis (D+d)/2, mm |
|---|
| 1 | No | Yes | 8.00 | 6.50 |
| 2 | Yes | Yes | 14.00 | 13.50 |
| 3 | Yes | Yes | 10.00 | 11.00 |
| 4 | No | Yes | 8.50 | 7.50 |
| 5 | No | Yes | 16.00 | 10.00 |
| 6 | No | Yes | 8.50 | 8.50 |
| 7 | No | Yes | 10.00 | 9.00 |
| 8 | Yes | Yes | 7.00 | 6.50 |
| 9 | No | Yes | 13.50 | 12.00 |
| 10 | Yes | Yes | 10.00 | 7.00 |
| 11 | Yes | Yes | 6.50 | 7.50 |
| 12 | Yes | Yes | 11.00 | 10.50 |
| 13 | No | Yes | 8.50 | 9.50 |
| 14 | Yes | Yes | 10.00 | 9.50 |
| 15 | No | Yes | 4.50 | 4.00 |
| 16 | No | Yes | 10.00 | 8.00 |
| 17 | No | Yes | 10.00 | 9.50 |
| 18 | No | Yes | 8.50 | 5.00 |
| 19 | Yes | Yes | 7.50 | 7.00 |
| 20 | No | Yes | 13.50 | 8.50 |
| 21 | Yes | Yes | 11.50 | 9.00 |
| 22 | No | Yes | 12.00 | 11.00 |
| 23 | No | Yes | 12.00 | 9.00 |
| 24 | Yes | Yes | 7.50 | 8.50 |
In vitro cross-reactivity between
allergens present in P. judaica and P. officinalis
The Bradford protein test revealed that
Allergovit® had a protein content of 0.5 µg/µl, whereas
Depigoid® had a protein content of 0.05 µg/µl. The
protein content in Allergovit® (mainly from P.
officinalis extracts) and the absence of protein content in
Depigoid® was confirmed by electrophoresis under
denatured conditions and Coomassie staining.
The in vitro ELISA method performed in
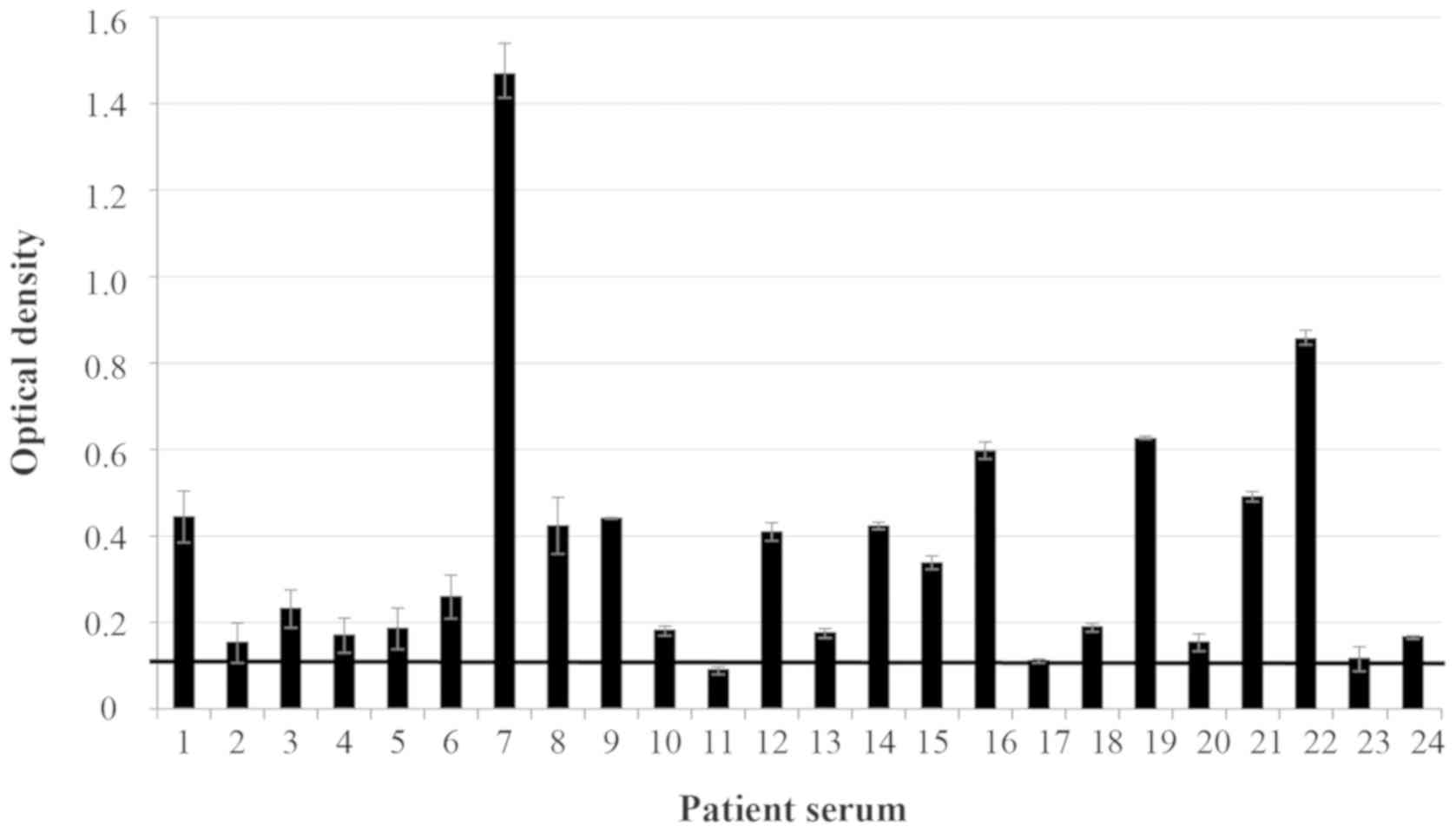
high-binding 96-well plates coated with Allergovit®
demonstrated that 87.5% (n=21) of the patient sera were reactive
(i.e. exhibited high levels of specific IgE to P.
officinalis) when the SD of each sample was not considered
(Fig. 1), and 79.1% (n=19) of the
patient sera were reactive when the OD value (minus SD) was above
the threshold of 0.132 (data not shown). High specific IgE ranged
between 0.091 and 1.47 OD when the SD of each sample was not
considered and between 0.082 and 1.407 OD when SD was included. No
reaction in the patient sera was observed when the ELISA plates
were coated with Depigoid®.
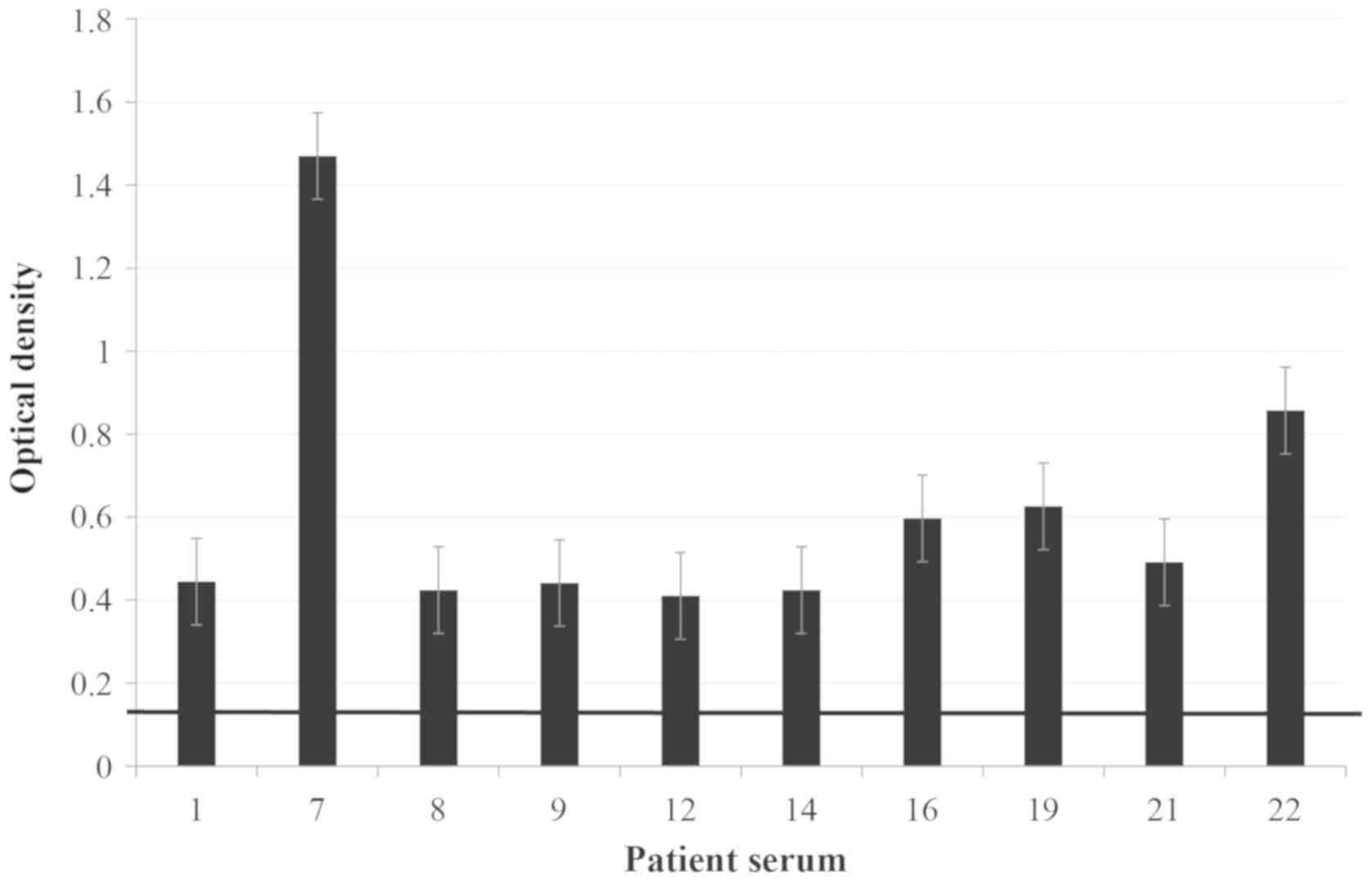
Sera of the 10 patients (1, 7, 8, 9, 12, 14,
16, 19, 21 and
22) with the highest reactivity to
the sample (i.e. highest OD values identified by the ELISA)
(Fig. 2) were selected to test
cross-reactivity between allergens from P. judaica and P.
officinalis by ELISA inhibition and were also used as a
reference of the index of stimulation of the sera without
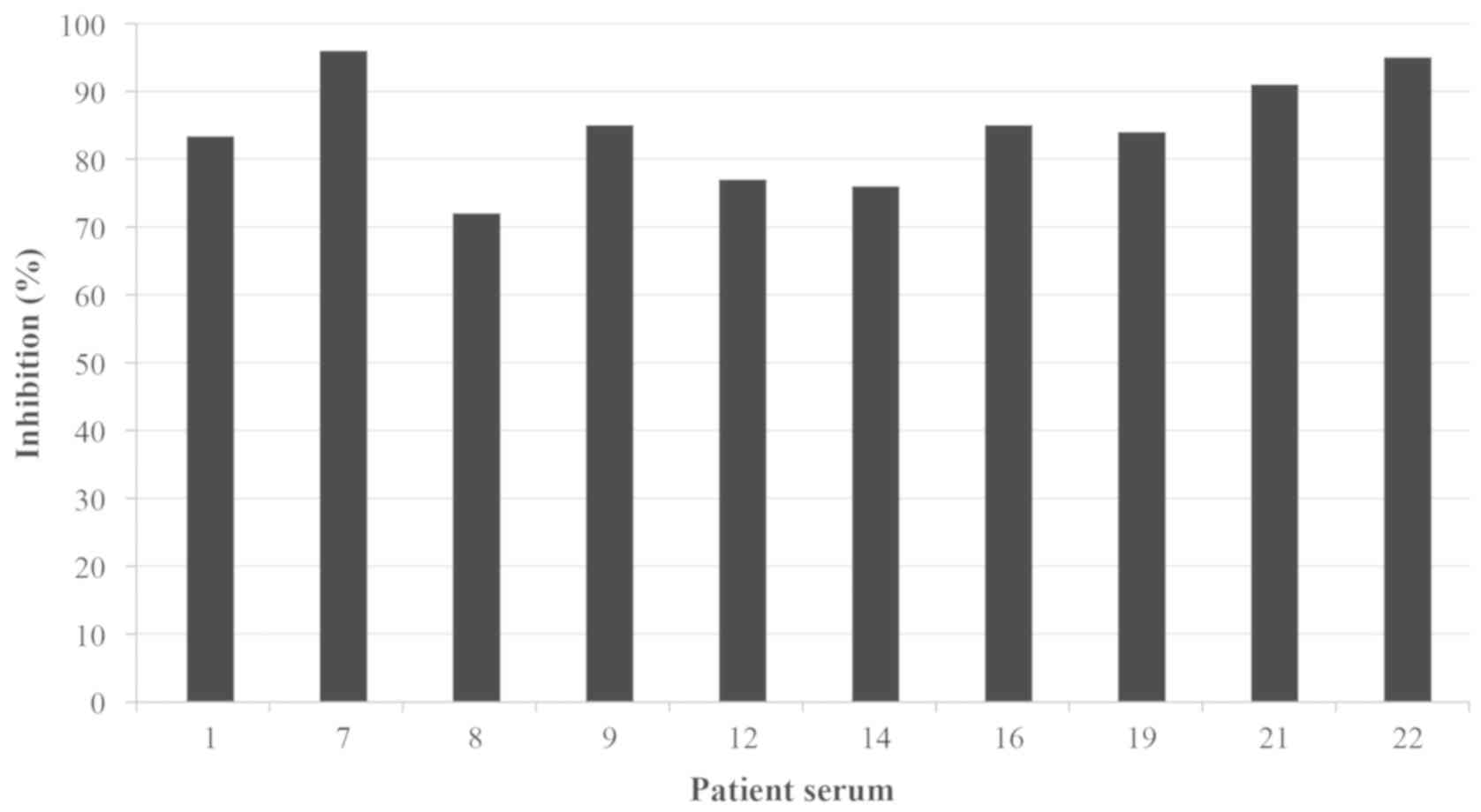
inhibition. ELISA inhibition assay of the IgE binding to P.
officinalis exhibited inhibition values >70% (ranging
between 71.76 and 95.97%) in the sera of the 10 patients (Fig. 3), confirming cross-reactivity between
the two Parietaria subspecies.
Comparison of in vivo reactivities
between two Parietaria subspecies in allergic patients sensitized
to Parietaria
All patients enrolled in the study exhibited double
skin positivity against P. judaica and P. officinalis
extracts, as assessed by the SPT (Table
II). A parametric paired-sample Student's t-test identified
greater wheal sizes for P. judaica compared with those for
P. officinalis when measured by the longest wheal diameter
(D; mean ± SD, 11.8±3.4 mm for P. judaica and 10.4±3.1 mm
for P. officinalis; P=0.030), the longest diameters
perpendicular to D (d; mean ± SD, 7.6±2 mm for P. judaica
and 7±1.8 mm for P. officinalis; P=0.036) and (D+d)/2 (mean
± SD, 10±2.7 mm for P. judaica and 8.7±2.2 mm for P.
officinalis; P=0.002). Positive skin reactivity was also
observed for the positive control (histamine) with a mean ± SD of
8.8±2.4 mm for D, 6.5±1.5 mm for d and 7.7±1.7 mm for (D+d)/2. The
negative control exhibited negative reactions in all patients.
Differences between the levels of IgE specific to P. judaica
and P. officinalis could not be assessed as specific IgE
levels to P. officinalis were not available.
 | Table IISPT of in vivo reactivities
between two Parietaria subspecies in allergic patients
sensitised to Parietaria. |
Table II
SPT of in vivo reactivities
between two Parietaria subspecies in allergic patients
sensitised to Parietaria.
| SPT result | Mean (95% CI) | SD | Median | Minimum | Maximum | Q1 | Q3 | N |
|---|
| Positive control
(histamine) |
|
D, mm | 8.8 (7.8-9.8) | 2.4 | 8.0 | 5.0 | 17.0 | 7.3 | 10.0 | 24 |
|
d, mm | 6.5 (5.8-7.1) | 1.5 | 6.0 | 4.0 | 1 | 5.0 | 7.8 | 24 |
|
(D+d)/2,
mm | 7.7 (7.0-7.1) | 1.7 | 7.0 | 5.0 | 12.5 | 6.6 | 9.0 | 24 |
| Negative
control |
|
D, mm | 0.0 (-) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 24 |
|
d, mm | 0.0 (-) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 24 |
|
(D+d)/2,
mm | 0.0 (-) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 24 |
| Parietaria
Judaica |
|
D, mm | 11.8a (10.4-13.3) | 3.4 | 11.0 | 5.0 | 19.0 | 10.0 | 15.0 | 24 |
|
d, mm | 7.6a (6.7-8.4) | 2.0 | 7.0 | 4.0 | 12.0 | 6.3 | 9.0 | 24 |
|
(D+d)/2,
mm | 10.0a (8.8-11.1) | 2.7 | 10.0 | 4.5 | 16.0 | 8.1 | 11.9 | 24 |
| Parietaria
officinalis |
|
D, mm | 10.4
(9.1-11.7) | 3.1 | 10.0 | 5.0 | 18.0 | 9.0 | 12.0 | 24 |
|
d, mm | 7.0 (6.2-7.7) | 1.8 | 6.0 | 3.0 | 10.0 | 6.0 | 8.8 | 24 |
|
(D+d)/2,
mm | 8.7 (7.8-9.6) | 2.2 | 8.8 | 4.0 | 13.5 | 7.1 | 9.9 | 24 |
Discussion
The identification and characterization of
cross-reactive allergens provides clinicians with useful and
practical guidance to optimize and improve diagnosis and
immunotherapy treatment for patients with AR. The present study was
conducted with the objective of assessing the cross-reactivity
between two subspecies of the genus Parietaria, P.
judaica and P. officinalis, in a sample of 24 patients
diagnosed with AR and/or bronchial asthma due to Parietaria
pollen, from the Levante region of the Spanish Mediterranean coast.
For this purpose, immunotherapy extracts for the treatment of
allergy to Parietaria (Allergovit® containing
P. officinalis) were used. The final sample of patients was
lower compared with the initially calculated number (30 patients);
however, Bonura et al (25)
had previously identified cross-reactivity between the two
Parietaria subspecies by using the rPar j 1 and rPar j 2
allergens in a sample of 25 patients.
The Bradford protein test confirmed that
Allergovit®, in contrast to Depigoid®,
contained proteins of P. officinalis extracts; it is widely
known that a high variability exists in extracts of the same
allergenic grass species produced by different companies, both
qualitatively and quantitatively (27).
The results of the SPT in the present study revealed
that all patients primarily sensitized to P. judaica pollen,
as evidenced by significant skin reactivity and detected specific
IgE levels, also exhibited significant reactivity to P.
officinalis pollen. The in vitro ELISA test demonstrated
that 87.5% of the patients were reactive to P. officinalis
pollen, as confirmed by an OD ratio between 0.091 and 1.47, when
the SD of the samples was not regarded; 79.1% of the patients still
exhibited a significant OD ratio, between 0.082 and 1.407, when the
SD of the individual samples was considered. In addition, 10
selected patients with the highest reactivity to the pollen extract
(Allergovit®) exhibited ELISA inhibition values of IgE
binding to P. officinalis >70%, confirming the
cross-reactivity between the two Parietaria subspecies.
Additionally, 2 patients reached values >95%. ELISA inhibition
method instead of a direct binding test was selected in our study
for the cross-reactive assessment of polyclonal antibodies, as this
method is considered to be more reliable and to better discriminate
between cross-reacting and non-cross-reacting IgE levels (28).
These results are comparable to the findings
obtained by Patriarca et al (26) in a sample of 30 Italian patients
allergic to Parietaria pollen, and provide further evidence
to confirm the complete cross-reactivity of P. officinalis
and P. judaica extracts, as well as support the use of P.
officinalis pollen extract in the diagnosis and immunotherapy
of Parietaria allergy. In addition, compared with other
cross-reactivity assessment studies, such as those associated with
sensitization to pollen and vegetable foods presented by Aalberse
(29) in their review, the results of
the present study yielded higher cross-reactivity values.
The present study had certain limitations inherent
to its retrospective design. Only patient data from the medical
records as part of their routine medical care were collected, and
total IgE levels, as well as specific IgE levels for P.
judaica, were thus only available for a limited number of
patients. In addition, quantification assays performed for
detecting the levels of IgE specific for P. judaica and
P. officinalis were not comparable, as they used different
methods and detection ranges.
Another limitation was the use of a single
Parietaria antigen concentration for the IgE binding
inhibition assay to determine the subspecies cross-reactivity, as
it was not possible to test inhibition of IgE binding variations
using pollen proteins at different concentrations.
Finally, the results of the present study can only
be generalized to the population of patients allergic to
Parietaria from the Spanish Levante Coast area. Further
similar studies implemented in other geographical zones should be
conducted to verify these results.
In conclusion, the present study demonstrated that
P. judaica and P. officinalis pollen extracts were
highly cross-reactive, and that a unique P. officinalis
pollen extract (Allergovit®) may be used for the
diagnosis and immunotherapy of patients allergic to
Parietaria.
Acknowledgements
Not applicable.
Funding
This study was funded by MERCK SLU.
Availably of data materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NC, II, AA and EEM substantially contributed to the
acquisition, analysis and interpretation of data, drafted the
manuscript, critically revised the manuscript for important
intellectual content, and gave final approval of the version to be
published. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all study
participants and ethics approval for this study was obtained from
the Ethics Committee of Clinical Research at Hospital of Sagunto
(Valencia, Spain).
Patient consent for publication
Not applicable.
Competing interests
Nataly Cancelliere and Ángel Ayuga are employees of
MERCK SLU. The other the authors declare that they have no
competing interests.
References
|
1
|
Bousquet J, Van Cauwenberge P and Khaltaev
N: Aria Workshop Group World Health Organization. Allergic rhinitis
and its impact on asthma. J Allergy Clin Immunol. 108 (Suppl
5):S147–S334. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bousquet J, Khaltaev N, Cruz AA, Denburg
J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica
GW, van Weel C, et al: Allergic rhinitis and its impact on asthma
(ARIA) 2008 update (in collaboration with the World Health
Organization, GA(2)LEN and AllerGen). Allergy. 63 (Suppl
86):S8–S160. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bauchau V and Durham SR: Prevalence and
rate of diagnosis of allergic rhinitis in Europe. Eur Respir J.
24:758–764. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mullol J, Valero A, Alobid I, Bartra J,
Navarro AM, Chivato T, Khaltaev N and Bousquet J: Allergic rhinitis
and its impact on asthma update (ARIA 2008). The perspective from
Spain. J Investig Allergol Clin Immunol. 18:327–334.
2008.PubMed/NCBI
|
|
5
|
Canonica GW, Bousquet J, Mullol J,
Scadding GK and Virchow JC: A survey of the burden of allergic
rhinitis in Europe. Allergy. 62 (Suppl 85):S17–S25. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bousquet J, Vignola AM and Demoly P: Links
between rhinitis and asthma. Allergy. 58:691–706. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pereira C, Valero A, Loureiro C, Dávila I,
Martinez-Cócera C, Murio C, Rico P and Palomino R: Iberian study of
aeroallergens sensitisation in allergic rhinitis. Eur Ann Allergy
Clin Immunol. 38:186–194. 2006.PubMed/NCBI
|
|
8
|
Navarro A, Valero A, Julià B and Quirce S:
Coexistence of Asthma and Allergic Rhinitis in Adult Patients
Attending Allergy Clinics: ONEAIR Study. J Investig Allergol Clin
Immunol. 18:233–238. 2008.PubMed/NCBI
|
|
9
|
Leynaert B, Neukirch C, Kony S, Guénégou
A, Bousquet J, Aubier M and Neukirch F: Association between asthma
and rhinitis according to a topic sensitization in a
population-based study. J Allergy Clin Immunol. 113:86–93.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kanani AS, Broder I, Greene JM and Tarlo
SM: Correlation between nasal symptoms and asthma severity in
patients with atopic and nonatopic asthma. Ann Allergy Asthma
Immunol. 94:341–347. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sala-Cunill A, Bartra J, Dalmau G, Tella
R, Botey E, Raga E and Valero A: Comitè de Allergia Respiratòria de
Catalunya, Spa in:. Prevalence of asthma and severity of allergic
rhinitis comparing 2 perennial allergens: House dust mites and
Parietaria judaica pollen. J Investig Allergol Clin Immunol.
23:145–151. 2013.PubMed/NCBI
|
|
12
|
Passalacqua G and Canonica GW: Impact of
rhinitis on airway inflammation: Biological and therapeutic
implications. Respir Res. 2:320–323. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
de Benedictis FM, del Guidice MM, Severine
S and Bonifazi F: Rhinitis, sinusitis and asthma: One linked airway
disease. Pediatr Respir Rev. 2:358–364. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
D'Amato G, Cecchi L, Bonini S, Nunes C,
Annesi-Maessano I, Behrehdt H, Liccardi G, Popov T and van
Cauwenberge P: Allergenic pollen and pollen allergy in Europe.
Allergy. 62:976–990. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ariano R, Cecchi L, Voltolini S, Quercia
O, Scopano E and Ciprandi G: AAIITO Study group on Pollen Allergy.
Parietaria pollination duration: Myth or fact? Eur Ann
Allergy Clin Immunol. 49:6–10. 2017.PubMed/NCBI
|
|
16
|
Colombo P, Duro G, Costa MA, Izzo V,
Mirisola M, Locorotondo G, Cocchiara R and Geraci D: An update on
allergens Parietaria pollen allergens. Allergy. 53:917–921.
1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Polosa R, Al-Delaimy WK, Russo C, Piccillo
G and Sarvà M: Greater risk of incident asthma cases in adults with
allergic rhinitis and effect of allergen immunotherapy: A
retrospective cohort study. Respir Res. 6(153)2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Geraci D, Billesbolle KB, Cocchiara R,
Løwenstein H and Ipsen H: Immunochemical characterization of
antigens of Parietaria judaica pollen. Identification of
allergens by means of crossed radio immunoelectrophoresis. Int Arch
Allergy Appl Immunol. 78:421–428. 1985.PubMed/NCBI
|
|
19
|
Ford SA, Baldo BA, Geraci D and Bass D:
Identification of Parietaria judaica pollen allergens. Int
Arch Allergy Appl Immunol. 79:120–126. 1986.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Costa MA, Colombo P, Izzo V, Kennedy H,
Venturella S, Cocchiara R, Mistrello G, Falagiani P and Geraci D:
cDNA cloning, expression and primary structure of Par jI, a major
allergen of Parietaria judaica pollen. FEBS Lett.
341:182–186. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Duro G, Colombo P, Assunta Costa M, Izzo
V, Porcasi R, Di Fiore R, Locorotondo G, Cocchiara R and Geraci D:
Isolation and characterization of two cDNA clones coding for
isoforms of the Parietaria judaica major allergen Par j
1.0101. Int. Arch. Allergy Appl Immunol. 112:348–355.
1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Longo V, Costa MA, Cibella F, Cuttitta G,
La Grutta S and Colombo P: Multiple IgE recognition on the major
allergen of the Parietaria pollen Par j 2. Mol Immunol.
63:412–419. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Corbi AL, Pelaez A, Errigo E and Carreira
J: Cross-reactivity between Parietaria judaica and
Parietaria officinalis. Ann Allergy. 54:142–147.
1985.PubMed/NCBI
|
|
24
|
Dreborg S, Basomba A and Einarsson R:
Sensitivity to Parietaria officinalis and Parietaria
judaica pollen allergens in a Spanish population. Allergol
Immunopathol (Madr). 14:499–508. 1986.PubMed/NCBI
|
|
25
|
Bonura A, Artale A, Marino M, Amoroso S,
Marcucci F, Geraci D and Colombo P: Cross-reactivity between
Parietaria species using the major rParj1 and rParj2
allergens. Allergy Asthma Proc. 27:378–382. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Patriarca G, Marino G, De Pasquale T,
Broutin O, Viatte A, Jain K, Hrabina M, Gouyon B, Moingeon P, Frati
F, et al: Parietaria officinalis pollen extract can replace
Parietaria judaica in the diagnosis and immunotherapy of
Parietaria allergy. Allergy. 65 (Suppl 92):S209–S682.
2010.
|
|
27
|
Sander I, Fleischer C, Meurer U, Brüning T
and Raulf-Heimsoth M: Allergen content of grass pollen preparations
for skin prick testing and sublingual immunotherapy. Allergy.
64:1486–1492. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aalberse RC: Assessment of allergen
cross-reactivity. Clin Mol Allergy. 5(2)2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aalberse RC, Akkerdaas J and van Ree R:
Cross-reactivity of IgE antibodies to allergens. Allergy.
56:478–490. 2001.PubMed/NCBI View Article : Google Scholar
|