Introduction
Male hypogonadism is a clinical syndrome in which
the diagnosis is dependent on hypogonadal signs or symptoms and
unequivocally low serum testosterone (T) levels (1). T replacement therapy can correct sexual
dysfunction, restore libido, increase lean body mass and reduce fat
mass, and increase bone mineral density and vitality (2). The term primary hypogonadism refers to
testicular disorders, which are characterized by low serum T levels
despite high follicle-stimulating hormone (FSH) and luteinizing
hormone (LH) levels. The causes of primary hypogonadism include the
following: i) Genetic conditions (such as Klinefelter syndrome and
gonadal dysgenesis), ii) anatomical defects, iii) infection, iv)
tumors, v) injuries, and vi) iatrogenic causes such as surgery or
certain drugs, including glucocorticoids (GCs) (3).
The term secondary hypogonadotropic hypogonadism
refers to the insufficient release of gonadotropin-releasing
hormone (GnRH) with low to normal FSH, LH and T levels. The causes
of secondary hypogonadism include the following: i)
Hyperprolactinemia (often secondary to pituitary adenoma), ii) GnRH
deficiency with anosmia (Kallmann syndrome), iii) hypothalamic
lesions or disorders, and iv) pituitary lesions or disorders
(4). The term normogonadotropic
hypogonadism refers to the symptoms or signs of hypogonadism
accompanied with low serum T and normal LH levels (5).
Hypogonadism has several symptoms, including sexual
dysfunction, lethargy, depressed mood, poor concentration and
memory, mild anemia, and a diminished sense of well-being (5). The most common features of hypogonadism
in adulthood that suggest androgen deficiency are decreased libido,
impotence, and oligo- or azoospermia. Other symptoms may include
fatigue, loss of bone and muscle mass, and increased fat mass and
related metabolic disorders, in addition to impairment of cognitive
function. Some elderly men may develop a mild T deficiency and
present with symptoms suggestive of hypogonadism in young men
(6).
GC abuse is another cause of male hypogonadism
(7). GCs are classified as
short-acting, intermediate-acting or long-acting (Table I) based on the duration of
adrenocorticotropic hormone (ACTH) suppression and the relative GCs
and mineralocorticoids effects (a group of hormones, including
aldosterone, which regulate the balance of water and electrolytes
in the body) (8).
 | Table IGlucocorticoid equivalent. |
Table I
Glucocorticoid equivalent.
| | Potency relative to
hydrocortisone | Half-life |
|---|
| Treatments | Equivalent
glucocorticoid, mg |
Anti-inflammatory | MC | Plasma, min | Duration of action,
h |
|---|
| Short acting |
|
Hydrocortisone | 20 | 1 | 1 | 90 | 8-12 |
|
Cortef,
Cortisol | 25 | 0.8 | 0.8 | 30 | 8-12 |
|
Intermediate-acting |
|
Prednisone | 5 | 4 | 0.8 | 60 | 12-36 |
|
Prednisolone | 5 | 4 | 0.8 | 200 | 12-36 |
|
Triamcinolone | 4 | 5 | 0 | 300 | 12-36 |
|
Methylprednisolone | 4 | 5 | 0.5 | 180 | 12-36 |
| Long-acting |
|
Dexamethasone | 0.75 | 30 | 0 | 200 | 36-54 |
|
Betamethasone | 0.6 | 30 | 0 | 300 | 36-54 |
|
Mineralocorticoid | | | | | |
|
Fludrocortisone | 0 | 15 | 150 | 240 | 24-36 |
|
Aldosterone | 0 | 0 | 400+ | 20 | - |
Exogenous GC causes an acute reduction in T levels
in men by directly suppressing gonadal steroid secretion; thus, GC
therapy frequently and significantly decreases the serum T levels.
Furthermore, this effect appears to be mediated by the suppression
of GnRH secretion by the hypothalamus (9,10).
The decrease in the total T (TT) and free T (FT)
levels in men who use GCs may be attributed to GC binding to GC
receptors located in several tissues and organs in the body, such
as the Leydig cells, decreasing T biosynthesis via 11β-HSD1
reductase activity (11). Leydig
cell apoptosis is another mechanism attributed to decreased T
levels (12).
The aim of the present study was to investigate the
effects of exogenous GC use, considered a potent cause of male
hypogonadism, on the function of the hypothalamic-pituitary-gonadal
(HPG) axis, and to determine any secondary effects in male
patients.
Materials and methods
Patients
The present study was approved by the Faiha
Specialized Diabetes, Endocrine and Metabolism Center. Each
participant provided written informed consent. Before providing
consent, patients were provided with a sufficient explanation to
ensure that each patient clearly understood the nature of the
study.
The present study was a case-controlled study
performed at Faiha Specialized Diabetes, Endocrine and Metabolism
Center (FDEMC) in Basrah, Iraq. The study was performed between
June 2017 and June 2018. All participants were admitted to FDEMC
due to an endocrine disorder. In addition to clinical examination,
age and medical history (for example, smoking history) were
obtained as shown in Table II.
 | Table IIGeneral characteristics of the study
population. |
Table II
General characteristics of the study
population.
| Variables | n, % or mean ±
standard deviation |
|---|
| Median age, years
(range) | 37 (17-50) |
| Smoker | |
|
Yes | 107 (70.40) |
|
No | 45 (29.60) |
| BMI, kg/m² | |
| Total Testosterone,
ng/dl (range) | 391.64±208.38
(246-916) |
| Free Testosterone,
ng/dl | 8.90±5.93 |
| Sex hormone-binding
globulin, nmol/l (range) | 34.42±25.47
(10-60) |
| Follicle stimulating
hormone, mlU/ml (range) | 4.24±2.71
(1-13) |
| Luteinizing
hormone, mlU/ml (range) | 5.82±3.82
(1-9) |
| Prolactin, nmol/l
(range) | 14.55±9.04
(4-30) |
| Estradiol, pg/ml
(range) | 19.11±12.57
(range) |
Using prepared study questionnaires, patients were
asked about the following: i) Type of steroid used
(oral/parenteral/depot injections); ii) method of use
(prescription/nonprescription); iii) estimated total equivalent
cumulative dose; and iv) current or previous GC use.
The inclusion criteria were adult males aged 18-55
years who were currently using or at any time in the previous year
used GCs at a dose of 7.5 mg prednisolone or equivalent for at
least 10 days, regardless of their symptoms or causes of referral;
based on the criteria used by Crawford et al (13). Previous GC users were stopping GC in
the last 30 days and less than this period which mean current users
(14).
The exclusion criteria were any patients with at
least one of the following: History of orchitis; undescended testis
(unilateral or bilateral) and unilateral or bilateral testicular
volume <4 ml; established atherosclerosis or cardiovascular
disease; concomitant use of known erectile dysfunction-induced
medications (such as antidepressants or opioids); history of spine
injury; patients who had previously undergone prostate surgery;
history of radiation therapy to the pelvic area; patients who had
previously undergone surgery to the external genitalia and patients
with an acute illness for the past month; history of critical
illness requiring intensive care unit or coronary care unit
admission in the past 3 months (15); any head injury or trauma in the past
year (16); pituitary adenoma or
history of hypopituitarism; underlying diseases [for example,
asthma, chronic obstructive pulmonary disease, osteoarthritis,
rheumatoid arthritis (RA)]; or use of any anabolic steroid.
Of the 187 participants enrolled in the present
study, 35 were excluded and 100 used different types of GC (median
age, 37 years; range 17-50 years). Of the GC users, 57 patients
(57%) were current GC users (median age, 37 years; range 17-50
years), and 43 patients (43%) were not currently using GCs (median
age, 36 years; range 17-49 years). The control group was comprised
of 52 participants (34.21%) who were considered healthy (median
age, 38 years; range 19-49 years), with 7 participants (13.65%)
being biochemically diagnosed with hypogonadism.
Physical evaluation
Body hair thickness, presence of gynecomastia,
testicular volume, pubic and axillary hair assessment, and
wrinkling around the face were physically examined to assess the
presence of hypogonadism. Signs of steroid use including moon face,
buffalo hump, supraclavicular fat pad, thin skin, abdominal
obesity, striae, proximal muscle weakness, bruises and petechiae
were assessed. Furthermore, a thorough physical examination was
performed.
Anthropometric analysis including body weight and
height measurements was performed with the patient wearing light
clothes and no shoes, using a stadiometer (SECA-763™). Body mass
index was calculated using the following formula: Weight (kg)
divided by height (m)2.
Blood pressure was measured using an electronic
Omron HEM-780 automatic upper arm blood pressure monitor (Omron
Healthcare), with two readings 5 min apart, and the average blood
pressure was taken. Hypertension was diagnosed if the patient was
previously or currently taking medications to treat hypertension
and had a systolic blood pressure ≥140 mmHg and/or diastolic blood
pressure of 90 mmHg.
Laboratory tests
For each patient, 10 ml venous blood was obtained in
the morning (between 8:00 and 9:00 am). Subsequently, the blood
samples were analyzed using a Cobas E411 Analyzer
electrochemiluminescence immunoassay (Roche Diagnostics).
LH, FSH, dehydroepiandrosterone sulfate, ACTH,
cortisol, TT, sex hormone-binding globulin (SHBG) and prolactin
(PRL) levels were measured.
The serum albumin levels were measured using a Cobas
C311 fully automated chemical analyzer (Roche Diagnostics). FT
levels were calculated based on TT and SHBG levels using an
online-based calculator (issam.ch/freetesto.htm).
The total cumulative GC dose for all participants
currently using GCs was calculated by multiplying the duration of
GC use by the equivalent GC value (Table
I) and subsequently by the daily dosage [for both prednisolone
and dexamethasone per Orem (PO)]. For the depot preparations, the
total cumulative dose was calculated by multiplying the total
received dose by the duration of use.
Statistical analysis
Statistical analysis was performed using SPSS
version 23.0 (IBM, Corp.). Bivariate analysis was used to analyze
continuous variables and frequencies, and percentages for the
categorical variables. A χ2 test was used to compare
categorical variables, with 95% confidence intervals. A two-tailed
P-value of P<0.05 was considered to indicate a statistically
significant difference. Receiver operating characteristic (ROC)
curves were used to compare the predictive value of the different
statistical values, the area under the curve (AUC) and the cutoff
values, with both the sensitivity and specificity values were
obtained.
Results
Characteristics of the cohort
General characteristics of the entire cohort are
presented in Table II. The mean age
was 37 years (range 17-50), and 107 (70.40%) individuals in the
cohort were smokers. The mean BMI of all participants was
26.60±6.68 kg/m². Of the GC users, 3 (3%) exhibited clinical
features of hypogonadism, all of these were current GC users, and
two of these showed stigmata of clinical hypercortisolism.
Based on the hormonal assessments, the mean TT level
was 391.64±208.38 ng/dl, the mean FT level was 8.90±5.93 ng/dl and
the mean SHBG level was 34.42±25.47 nmol/l. The mean FSH level was
4.54±3.58 mlU/ml, the LH level was 5.82±3.82 mlU/ml, the prolactin
level was 14.55±9.04 S and the mean estradiol level was
19.11±12.57.
Laboratory markers of the hypothalamic pituitary
adrenal axis in patients using GCs were as follows: ACTH levels,
33.42±26.71 pg/ml; DHEA-S levels, 129.37±123 µg/dl; and cortisol
levels, 11.90±11.23 µg/dl.
Effect of current GC exposure
The effect of current GC use on the HPG axis is
shown in Table III. There was
clear and significant reduction in both the TT and FT levels
(P<0.001). Current GC use had no significant effect on the SHBG,
FSH, LH, PRL and estradiol (E2) levels.
 | Table IIIEffect of current glucocorticoid use
on the hypothalamic-pituitary-gonadal axis. |
Table III
Effect of current glucocorticoid use
on the hypothalamic-pituitary-gonadal axis.
| Factors | Currently using
glucocorticoidsb | Healthy
controlsb | P-value |
|---|
| Total testosterone,
ng/dl | 318.08±209.61 | 430.40±166.59 |
<0.001a |
| Free testosterone,
ng/dl | 7.05±4.09 | 9.97±5.49 |
<0.001a |
| Sex hormone-binding
globulin, nmol/l | 32.11±27.47 | 35.55±27.35 | 0.492 |
| Follicle
stimulating hormone, mlU/ml | 4.24±2.74 | 3.98±2.19 | 0.569 |
| Luteinizing
hormone, mlU/ml | 5.64±4.99 | 5.88±3.12 | 0.745 |
| Prolactin,
ng/ml | 15.47±11.06 | 14.14±8.16 | 0.509 |
| Estradiol,
pg/ml | 19.83±13.54 | 16.22±12.66 | 0.185 |
Effect of previous GC use
The effects of discontinuing GCs on the HPG axis is
shown in Table IV. There was a
significant increase in E2 level (P=0.022) in patients who
previously used GCs compared with healthy patients, whereas the
levels of all hormones did not differ significantly between the
healthy controls and patients who previously used GCs.
 | Table IVEffect of discontinuation of
glucocorticoid use on the hypothalamic-pituitary-gonadal axis. |
Table IV
Effect of discontinuation of
glucocorticoid use on the hypothalamic-pituitary-gonadal axis.
| Factors | Previous
glucocorticoid useb | Healthy
controlsb | P-value |
|---|
| Total testosterone,
ng/dl | 489.81±236.22 | 430.40±166.59 | 0.119 |
| Free testosterone,
ng/dl | 10.08±6.26 | 9.97±5.49 | 0.926 |
| Sex hormone-binding
globulin, nmol/l | 39.37±22.30 | 35.55±27.35 | 0.449 |
| Follicle
stimulating hormone, mlU/ml | 3.68±2.31 | 3.98±2.19 | 0.498 |
| Luteinizing
hormone, mlU/ml | 5.68±2.97 | 5.88±3.12 | 0.743 |
| Prolactin,
ng/ml | 15.13±7.70 | 14.14±8.16 | 0.567 |
| Estradiol,
pg/ml | 22.33±10.70 | 16.22±12.66 | 0.022a |
Patients who were previously using GCs had a mean E2
level of 22.33±10.70 pg/ml, whereas patients who were currently
using GCs had a mean E2 level of 19.83±13.54 pg/ml, and this
difference was significant. The subgroup analysis of these
different GC formulations showed that only prednisolone PO
(tablets), dexamethasone PO, and betamethasone acetate injections
were statistically significantly associated with hypogonadism
(P=0.018, 0.009 and 0.002, respectively).
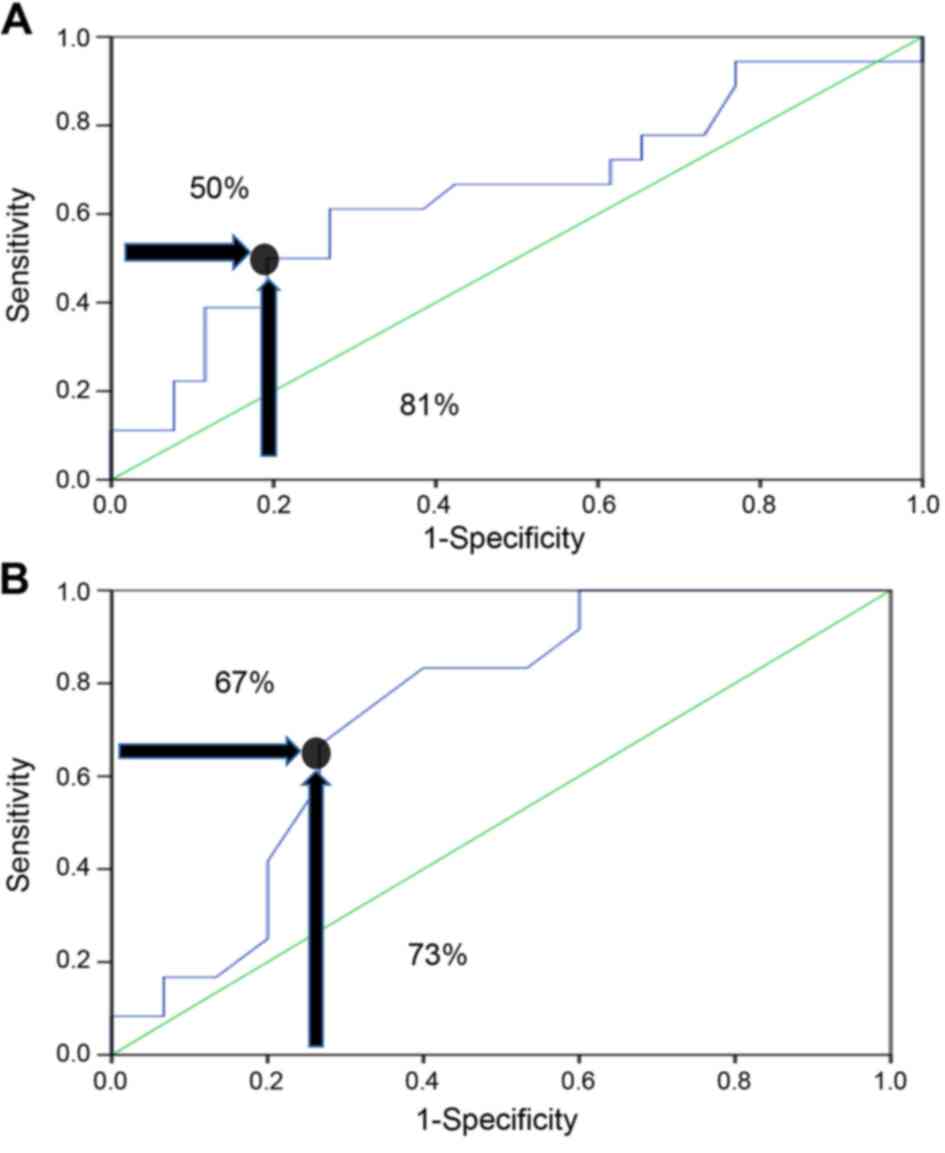
The area under the curve (AUC) based on the receiver
operating characteristics (ROC) demonstrated that there was a
3.7-fold increase in the risk of hypogonadism in patients who used
a total cumulative equivalent dose of >240 mg, and this
increased risk was most likely due to GC use, with a specificity of
81% and a sensitivity of 50% (P=0.038; Fig. 1A).
Comparison between current GC use and
no previous use
Regarding the 31 patients using dexamethasone PO,
the risk of hypogonadism was 7.5-fold if they were taking a total
dexamethasone cumulative dose of >18.9 mg, with a sensitivity
and a specificity of 67 and 73%, respectively (P=0.015; Fig. 1B). Oral dexamethasone can cause
hypogonadism at lower total cumulative equivalent doses. A ROC
curve was not plotted for patients who were currently using GCs
(prednisolone, dexamethasone injections, methylprednisolone
acetate, and betamethasone acetate) due to the smaller numbers of
patients who were currently using each type of these GCs.
The formulations of dexamethasone injection and
methylprednisolone acetate were not statistically associated with
hypogonadism (Table V).
 | Table VFrequency of hypogonadism among
patients currently using glucocorticoids compared with individuals
who had never used glucocorticoids. |
Table V
Frequency of hypogonadism among
patients currently using glucocorticoids compared with individuals
who had never used glucocorticoids.
| | Hypogonadism, n
(%) | | | |
|---|
| Treatments | Yes | No | Odds ratio | Confidence
interval | P-value |
|---|
| Glucocorticoid | | | 4.34 | 1.67-11.31 |
<0.001c |
|
User | 23 (40.4) | 34 (59.6) | | | |
|
Not
user | 7 (13.5) | 45 (86.5) | | | |
| Prednisolone
PO | | | 8.57 | 1.57-46.71 | 0.018a |
|
User | 4 (57.1) | 3 (42.9) | | | |
|
Not
user | 7 (13.5) | 45 (86.5) | | | |
| Dexamethasone
PO | | | 2.87 | 1.26-6.52 | 0.009b |
|
User | 12 (38.7) | 19 (61.3) | | | |
|
Not
user | 7 (13.5) | 45 (86.5) | | | |
| Dexamethasone PO,
Cumulative dose | | | 7.5 | 1.46-38.28 | 0.015a |
|
≥18.9
mg | 8 (66.7) | 4 (33.3) | | | |
|
<18.9
mg | 4 (21.1) | 15 (78.9) | | | |
| Dexamethasone
injection | | | 1.85 | 0.29-11.6 | 0.47 |
|
User | 1(25) | 3(75) | | | |
|
Not
user | 7 (13.5) | 45 (86.5) | | | |
| Methylprednisolone
acetate injection | | | 3.3 | 1.2-9.01 | 0.047a |
|
User | 4 (44.4) | 5 (55.6) | | | |
|
Not
user | 7 (13.5) | 45 (86.5) | | | |
| Betamethasone
acetate injection | | | 4.33 | 1.87-10.02 | 0.002b |
|
User | 7 (58.3) | 5 (41.7) | | | |
|
Not
user | 7 (13.5) | 45 (86.5) | | | |
| Total cumulative
equivalent dose | | | 3.72 | 1.05-13.22 | 0.038a |
|
≥240 mg | 9 (64.3) | 5 (35.7) | | | |
|
<240
mg | 14 (32.6) | 29 (67.4) | | | |
Comparison between current GC use and
previous GC use
There was a significant association between the
current use of GCs and the state of hypogonadism (23 patients; 40%)
compared with those who previously used GCs, (3 patients; 7%;
P<0.0001). This association was primarily due to use of oral
dexamethasone use in (12 patients; 39%).
Table VI shows the
effects of previous GC use on the HPG axis compared with patients
who currently use GCs. The mean TT level was 318.08±209.61 ng/dl in
patients currently using GCs and 489.81±236.22 ng/dl in patients
who previously used GCs, and this difference was significant
(P<0.0001). The mean FT level was 10.08±6.26 ng/dl for previous
GC users, and 7.05±4.09 ng/dl in current GC users (P=0.004).
 | Table VIFrequency of hypogonadism among
patients currently using glucocorticoids compared with patients who
had previously used glucocorticoids. |
Table VI
Frequency of hypogonadism among
patients currently using glucocorticoids compared with patients who
had previously used glucocorticoids.
| | Hypogonadism, n
(%) | | | |
|---|
| Treatments | Yes, n=26 | No, n=74 | Odds ratio | Confidence
interval | P-value |
| Glucocorticoid | | | 0.11 | 0.03-04 |
<0.0001a |
|
Current
user | 23 (40.40) | 34 (59.60) | | | |
|
Previous
user | 3 (7.00) | 40 (93.00) | | | |
| Prednisolone
PO | | | 0.12 | 0.01-1.67 | 0.133 |
|
Current
user | 4 (57.10) | 3 (42.90) | | | |
|
Previous
user | 1 (14.30) | 6 (85.70) | | | |
| Dexamethasone
PO | | | 0.05 | 0.01-0.42 |
<0.0001a |
|
Current
user | 12 (38.70) | 19 (61.30) | | | |
|
Previous
user | 1 (3.10) | 31 (96.90) | | | |
| Dexamethasone
injection | | | 1.33 | 0.75-2.34 | 0.444 |
|
Current
user | 1 (25.00) | 3 (75.00) | | | |
|
Previous
user | 0 (0.00) | 5 (100.00) | | | |
| Methylprednisolone
acetate injection | | | 0.45 | 1.29-1.56 | 0.208 |
|
Current
user | 4 (44.40) | 5 (55.60) | | | |
|
Previous
user | 3 (20.00) | 12 (80.00) | | | |
| Betamethasone
acetate injection | | | 2.40 | 1.22-4.68 | 0.069 |
|
Current
user | 7 (58.30) | 5 (41.70) | | | |
|
Previous
user | 0 (0.00) | 4 (100.00) | | | |
Discussion
In the present study, in patients currently using
GC, hypogonadism manifested as low TT levels in <50% of the
study sample. These results are comparable to a cross-sectional
study by Morrison et al (17)
in 1994 at the University of Wales, who studied 61 male patients
with chronic respiratory diseases, 12 of whom were long-term users
of systemic GCs, 31% of which had low TT levels.
Furthermore, TT and FT levels in patients currently
using GC with different formulations were significantly decreased,
similar to a cross-sectional study by Contreras et al
(18) in 1996 at Jose de San Martin
Hospital, which included 17 patients with bronchial asthma and
uveitis on long-acting GCs (methylprednisolone acetate injections).
This significant reduction in FT levels was also consistent with
the results of a case-controlled study conducted in Karolinska
Institute at Huddinge University Hospital in 2002, which enrolled
104 patients with Rheumatoid Arthritis (RA) who received oral
prednisolone and other disease-modifying antirheumatic drugs
(19).
One of the significant findings in the present study
was the increase in E2 levels in patients with a history of
previous GC use, whilst the other HPG axis functions were normal.
The mechanism underlying the increased E2 levels in patients who
previously used GC is unclear, but may be associated with the
activation of the aromatase enzyme after the TT levels return to
normal; as proposed by a retrospective multicenter study by Tan
et al (20) between
2009-2014, which evaluated the electronic health records and
medical chart review of 34,016 patients in 35 geographically
diverse cities in the USA. Other explanations for the increased E2
levels are possibly attributed to the severe loss of libido and
hypogonadism (21), or to the
antagonistic effect of GC on estrogen (22).
The TT and FT levels returned to their normal
reference ranges in patients who previously used GC compared with
those currently using GC. This is likely due to the recovery of the
HPG axis following drug withdrawal, consolidating the idea of
causality of low TT levels in GC users.
There was a 4-fold increased risk of hypogonadism
(low TT levels) in patients currently using GC compared with
GC-naive patients. A similar study assessing the risk of causality
of hypogonadism in patients using GCs has not been performed, to
the best of our knowledge.
Regarding hypogonadism, according to the type of GC
administered/used, >50% of the patients on oral prednisolone
developed hypogonadism compared with those who were not using GCs.
Morrison et al (17) showed
that TT levels decreased to 33% in patients who used
prednisolone.
Regarding other types of GC, in the present study,
it was observed that oral dexamethasone resulted in less
hypogonadism compared with injectable betamethasone and the control
group who never used GCs, warranting further studies on the
differences of different GC formulations in hypogonadism.
An extensive study assessing the effects of
different types of GC on the HPG axis similar to our study has not
been conducted yet, to the best of our knowledge. Most studies use
one or two types of GC only such as the case-control study by
MacAdams et al (9) in 1986,
in which oral prednisolone or methylprednisolone acetate injections
were used by the patients. A clinical trial conducted by Martens
et al (23) in August 1994 in
Seattle, USA, studied the effect of long-standing low-dose oral
prednisolone on 36 men with RA compared with 71 aged-matched
control men with RA not taking prednisolone, and found partial
gonadal failure caused by the decrease in TT levels and slight
increase in FSH and LH levels in the patients taking prednisolone
compared with the control group, in which there was marked increase
in FSH and LH levels with normal TT level.
Compared with the 240-mg total cumulative equivalent
cutoff dose of prednisolone in the present study causing
hypogonadism, the effects of GCs on gonadal function were confirmed
by Morrison et al (17), in
which patients using long-term oral prednisolone (5-20 mg daily),
high-dose inhaled beclomethasone dipropionate (1,500-2,250 µg
daily) or low-dose beclomethasone inhaler (200-800 µg daily). They
compared all groups with 19 patients not receiving GCs, and found
that the TT levels among these patients taking GCs were decreased
by 31%.
Contreras et al (18) studied gonadal function in 17 patients
with bronchial asthma and uveitis who were treated with
methylprednisolone acetate injections. The patients were
administered 10 mg methylprednisolone daily with a total cumulative
dose ranging between 1,200-45,500 mg. The mean cumulative dose was
4,080±367 mg, which resulted in a decrease in TT levels (18).
In MacAdams et al (9) were mainly dependent on the duration of
GC use regardless of the dose and route of administration (either
prednisolone tablets or methylprednisolone acetate injections), and
there was no significant difference between each group (daily or
alternative daily use) in the reduction of TT levels. Arnaud et
al (7) studied the effects of
oral prednisolone use, but did not assess the effects of dose or
duration of prednisolone use.
Oral dexamethasone use results in hypogonadism at
lower total cumulative doses when compared with other types of GC,
and this may be associated with the longer half-life of
dexamethasone, reaching up to 3 days, compared with other GC types
(24) or associated with the potency
of dexamethasone (25).
Hypogonadism affected half of study sample using
GCs, and may be reversible. Based on the results of the present
study, there was marked hypogonadism in patients currently using GC
with a total cumulative dose of 240 mg (equivalent to prednisolone
as shown in Table I) with no
feedback effect on LH and FSH levels. Oral dexamethasone caused
hypogonadism at a lower total cumulative dose.
An increase in E2 levels was observed in patients
previously using GCs, whilst all the other gonadal hormones, and in
particular TT, were within the normal ranges.
The present study has some limitations. Firstly, the
present study was a cross-sectional case-controlled study, with
limited external validity, and thus the observations are not
generalizable. The present study was performed using a relatively
homogenous high-risk population, and as with all observational data
analyses, it was not possible to establish causality from an
association. Additionally, the sample size was small, and a
single-center study. Finally, due to the short duration of the
study, it was not possible to follow up the patients for assessment
of future complications.
A prospective longitudinal study is required to
evaluate patients who withdraw the use of GCs to assess the
reversibility and the temporal relation of the recovery of the HPG
axis.
An awareness campaign is required to reduce the use
of GCs among the general population, which are considered as
dangerous drugs with several side effects, including hypogonadism
as a serious complication.
If GCs are prescribed for any condition, it should
be advised to avoid oral dexamethasone as it may result in
hypogonadism with even low doses compared with other types of
GC.
Acknowledgements
The authors would like to thank Dr Nassar Taha
Yassin, Dr Haider Ayad and Mr Ali Hamza (FDEMC) for providing
medical writing support.
Funding
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AGM and JHA were responsible for the preparation and
drafting of the Introduction and Discussion. AGM and AAM were
responsible for the preparation and drafting of the Materials and
methods. AGM performed the statistical analysis and was responsible
for the preparation and drafting of the Results and the final
manuscript. AGM, AAM and JHA critically revised the manuscript for
important intellectual content. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Faiha
Specialized Diabetes, Endocrine and Metabolism Center (Basrah,
Iraq). Each participant provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pantalone KM and Faiman C: Male
hypogonadism: More than just a low testosterone. Cleve Clin J Med.
79:717–725. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang C and Swerdloff R: Definition of male
hypogonadism. 2011.
|
|
3
|
Melman A and Gingell JC: The epidemiology
and pathophysiology of erectile dysfunction. J Urol. 161:5–11.
1999.PubMed/NCBI
|
|
4
|
Hijazi RA and Cunningham GR: Andropause:
Is androgen replacement therapy indicated for the aging male? Annu
Rev Med. 56:117–137. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bhasin S, Cunningham GR, Hayes FJ,
Matsumoto AM, Snyder PJ, Swerdloff RS and Montori VM: Testosterone
therapy in adult men with androgen deficiency syndromes: An
endocrine society clinical practice guideline. J Clin Endocrinol
Metab. 91:1995–2010. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rey RA, Grinspon R, Gottlieb S, Pasqualini
T, Knoblovits P, Aszpis S, Pacenza N, Stewart Usher J, Bergadá I
and Campo SM: Male hypogonadism: An extended classification based
on a developmental, endocrine physiology-based approach. Andrology.
1:3–16. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Arnaud L, Nordin A, Lundholm H,
Svenungsson E, Hellbacher E, Wikner J, Zickert A and Gunnarsson I:
Effect of corticosteroids and cyclophosphamide on sex hormone
profiles in male patients with systemic lupus erythematosus or
systemic sclerosis. Arthritis Rheumatol. 69:1272–1279.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mager DE, Lin SX, Blum RA, Lates CD and
Jusko WJ: Dose equivalency evaluation of major corticosteroids:
Pharmacokinetics and cell trafficking and cortisol dynamics. J Clin
Pharmacol. 43:1216–1227. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
MacAdams MR, White RH and Chipps BE:
Reduction of serum testosterone levels during chronic
glucocorticoid therapy. Ann Intern Med. 104:648–651.
1986.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Moghadam-Kia S and Werth VP: Prevention
and treatment of systemic glucocorticoid side effects. Int J
Dermatol. 49:239–248. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Whirledge S and Cidlowski JA:
Glucocorticoids, stress, and fertility. Minerva Endocrinol.
35:109–125. 2010.PubMed/NCBI
|
|
12
|
Yazawa H, Sasagawa I and Nakada T:
Apoptosis of testicular germ cells induced by exogenous
glucocorticoid in rats. Hum Reprod. 15:1917–1920. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crawford BA, Liu PY, Kean MT, Bleasel JF
and Handelsman DJ: Randomized placebo-controlled trial of androgen
effects on muscle and bone in men requiring long-term systemic
glucocorticoid treatment. J Clin Endocrinol Metab. 88:3167–3176.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Costello R, Patel R, Humphreys J, McBeth J
and Dixon WG: Timing of glucocorticoid administration: A
cross-sectional survey of glucocorticoid users in an online social
network for health. Rheumatology (Oxford, England). 56:494–495.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Woolf PD, Hamill RW, Mcdonald JV, Lee LA
and Kelly M: Transient hypogonadotropic hypogonadism caused by
critical illness. J Clin Endocrinol Metab. 60:444–450.
1985.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Woolf P, Hamill R, McDonald J, Lee LA and
Kelly M: Transient hypogonadotrophic hypogonadism after head
trauma: Effects on steroid precursors and correlation with
sympathetic nervous system activity. Clin Endocrinol (Oxf).
25:265–274. 1986.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Morrison D, Capewell S, Reynolds S, Thomas
J, Ali N, Read G, Henley R and Riad-Fahmy D: Testosterone levels
during systemic and inhaled corticosteroid therapy. Respir Med.
88:659–663. 1994.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Contreras LN, Masini AM, Danna MM, Kral M,
Bruno OD, Rossi MA and Andrada JA: Glucocorticoids: Their role on
gonadal function and LH secretion. Minerva Endocrinol. 43–46.
1996.PubMed/NCBI
|
|
19
|
Tengstrand B, Carlström K and Hafström I:
Bioavailable testosterone in men with rheumatoid arthritis-high
frequency of hypogonadism. Rheumatology (Oxford). 41:285–289.
2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tan RS, Cook KR and Reilly WG: High
estrogen in men after injectable testosterone therapy: The low T
experience. Am J Mens Health. 9:229–234. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee JK and Imperato-McGinley J: Estrogen
and the male. 2004.
|
|
22
|
Gong H, Jarzynka MJ, Cole TJ, Lee JH, Wada
T, Zhang B, Gao J, Song WC, DeFranco DB, Cheng SY and Xie W:
Glucocorticoids antagonize estrogens by glucocorticoid
receptor-mediated activation of estrogen sulfotransferase. Cancer
Res. 68:7386–7393. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Martens H, Sheets P, Tenover J, Dugowson
C, Bremner W and Starkebaum G: Decreased testosterone levels in men
with rheumatoid arthritis: Effect of low dose prednisone therapy. J
Rheumatol. 21:1427–1431. 1994.PubMed/NCBI
|
|
24
|
He Y, Yi W, Suino-Powell K, Zhou XE,
Tolbert WD, Tang X, Yang J, Yang H, Shi J, Hou L, et al: Structures
and mechanism for the design of highly potent glucocorticoids. Cell
Res. 24:713–726. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Steven K: Adrenal cortical steroids. Drug
facts and comparisons 5th ed St Louis: Facts and Comparisons, Inc.
pp.122-128, 1997.
|