Introduction
Heart failure (HF) is defined as an abnormal cardiac
function and/or structure that lead to the inability of the heart
to deliver oxygen to the tissues at a rate that compensates for
their metabolic requirements. A major cause of pediatric HF in
developing countries is rheumatic fever (RF) and rheumatic heart
disease (RHD) (1). RF and RHD
present serious problems worldwide according to the Global Burden
of Diseases in 2015(2). There have
been crucial efforts by the World Health Organization (WHO) to
prevent and control RHD (3).
Improving the quality of housing and reducing overcrowding in
combination with providing penicillin for early management of
streptococcal infections are the most important steps needed to
eradicate RHD (4). While there has
been an evident decline in its prevalence in Egypt since the 1950s,
acute rheumatic fever (ARF) and RHD remain national issues, and
efforts by the Egyptian authorities to control ARF and RHD are
ongoing (5).
Despite advancements in diagnostic work-up
techniques, patients with RHD and HF may be diagnosed late or
undiagnosed in developing countries due to the lack of
echocardiography and experienced cardiologists in rural health
centers. Therefore, sensitive and reliable serum markers as a
simple laboratory test in conjunction with echocardiography are
required for early detection of RHD and HF (6). The importance of studying cardiac
biomarkers in the diagnosis and prognostic stratification of
children with HF has been well emphasized; these biomarkers may act
as future therapeutic targets (7).
Similarly, noninvasive tools to monitor patients with HF before the
occurrence of clinical deterioration are also required (8).
Matrix metalloproteinases (MMPs) are zinc-dependent
endopeptidases that belong to the metzincin superfamily. MMPs have
a key role in tissue remodeling in cardiovascular diseases (CVDs).
MMPs are a promising target of therapy, as the administration of
doxycycline ‘a tetracycline antibiotic that acts as a nonspecific
MMP inhibitor’ in myocardial infarction patients improved
ischemia/perfusion injury. Further studies on the implications of
specific MMPs in different CVDs are required to clarify their role
in disease progression and to aid the discovery of new therapeutic
targets (9).
Gelatinases (MMP-2 and MMP-9) are the most
frequently analyzed MMPs in HF. According to the majority of
studies, active myocardium remodeling is associated with increased
activity and concentrations of MMP-2 and MMP-9, which can be useful
markers to identify patients at risk for HF development, and as
indicators of patient outcome (10).
In one study; circulating MMP-9, in contrast to MMP-2, was the only
predictor of late-onset congestive HF (11).
We hypothesized that the serum MMP-9 concentration
may be elevated in pediatric patients with RHD and HF, and its
level can be correlated with the HF severity. Thus, the aim of the
present study was to evaluate the sensitivity and accuracy of the
MMP-9 level to predict HF development in children with RHD and to
determine its effectiveness to indicate the degree of HF as
detected by echocardiography.
Patients and methods
Patients
This prospective study was conducted at the
Pediatric Cardiology Unit, Pediatrics Department, Zagazig
University Hospital, Al Sharqia Governorate, Egypt, from September
2015 to September 2019. The patients eligible for inclusion were
consecutive children with age ≤16 years of either sex and from the
same Egyptian ethnicity. The patients were newly diagnosed with RHD
by clinical presentation and echocardiography based on the World
Heart Federation criteria (12) and
whose parents agreed to provide written informed consent for
participation. Patients with underlying lung pathologies, bronchial
asthma, congenital heart diseases (CHDs), cardiomyopathy, aortic
aneurisms, end organ failure, cancers, or other complications of
RHD such as atrial fibrillation, pulmonary hypertension and
thromboembolic disorders were excluded from the study.
A total of 125 children with newly diagnosed RHD
were enrolled in the study. However, 27 were excluded: 4 patients
had CHDs; 7 had lung disease according to X-ray examinations; 9 had
coexisting complications; 4 had increased serum creatinine levels;
and 3 had inadequate serum samples. Ultimately, this study included
98 patients with a median age (range) of 13.5 (8.5-16) years. A
total of 40 patients (43.2%) had comorbid HF at presentation, while
the remaining 58 (56.8%) had RHD without HF. The control group
included 44 healthy, age- and sex-matched children.
Patient groups
Patients were categorized into two groups: RHD
without HF and RHD with HF. This categorization was based on the
presence of clinical symptoms and signs of HF, including dyspnea,
orthopnea, lower limb edema, ascites, and intolerance to exercise
with left systolic dysfunction, which was defined as an ejection
fraction (EF) ≤55% as estimated by transthoracic echocardiography
(13).
Data collection and clinical
examination
All patients reported their full medical histories,
including history of rheumatic fever, and underwent general and
thorough cardiac examinations. Children with recurrent ARF were
diagnosed according to the revised Jones criteria 2015(14).
Ethical consideration
Ethical approval for this study was obtained from
Zagazig University Institutional Research Board (ZU-IRB) (approval
no. 6005-23-6-2019). Written informed consent was obtained from the
parents of the children included in the study. All procedures were
performed according to the Declarations of Helsinki (https://www.who.int/bulletin/archives/79(4)373.pdf).
Sample size calculation
The sample size was calculated by applying the
Buderer N 1996 formula (15) at a
95% confidence interval (CI), 95% power, 98% sensitivity and an
expected prevalence of 34% (16). An
additional 10% was added to the calculated sample size to
compensate for dropout.
Methods
Laboratory investigation
Complete blood counts were determined with Sysmex
KX-21 hematology analyzer (Sysmex Corp.). C reactive protein (CRP)
and antistreptolysin O (ASO) levels were determined using automated
nephelometry BN Prospec (Siemens, UK) and turbidimeteric assay
(Cobas 6,000, Roche Diagnostics, Deutschland), respectively. The
normal range for CRP is up to 5 mg/dl, and that for ASO is up to
200 IU/ml. Liver function tests and kidney function tests were
performed using Cobas Integra 400 Plus (Roche Diagnostics). All
tests were performed at the Hematology and Chemistry Units, Zagazig
University Hospital Laboratories.
Measurement of MMP-9 serum levels
Plain tubes were used to collect blood samples from
patients when they were admitted to the hospital with suspected
RHD. Sera were separated and stored at -80˚C until analysis. MMP-9
was analyzed using a commercial MMP-9 ELISA kit (Sunred Biological
Technology), which applies the double antibody sandwich ELISA
technique (17) according to the
manufacturer's guidelines. The laboratory staff was blinded to the
clinical and echocardiography data of the patients.
Imaging techniques
Plain chest X-ray in the
postro-anterior view
This imaging was performed to detect cardiomegaly
and to exclude other chest diseases.
Echocardiography
Conventional detailed echocardiography was performed
in the Cardiology Unit of the Department of Pediatrics, Zagazig
University Hospital. Ventricular systolic function assessment,
chamber dimensions, wall thickness, left atrial diameter, left
ventricular end diastolic diameter, left ventricular end systolic
diameter, fractional shortening (FS), and EF were all measured
using M-mode echocardiography. Pulsed or continuous wave Doppler
ultrasound was used to assess the pressure gradient across the
stenotic or regurgitated flow through the valves as previously
described (18).
Classification of patients with
HF
Based on the EF, patients were classified as mild
HF, EF 40-54%; moderate HF, 30-39%; or severe HF, EF <30. In
addition, patients were classified by the application of the
modified Ross classification (19).
Statistical analysis
The data were analyzed using SPSS version 20.0 (IBM
Corp.). Shapiro-Wilk test was performed to detect the normality of
the data. Parametric numeric data are expressed as means ± standard
deviation and non-parametric data are expressed as medians and
ranges. The unpaired Student t-test and one way analysis of
variance with Fisher's Least Significant Difference (LSD) test were
used to compare means; the Mann-Whitney U and Kruskal-Wallis tests
with Dunn-Bonferroni post hoc method were used to compare medians.
Categorical variables were compared using the Chi square test and
Fisher's exact test when frequencies were less than five.
Univariate and multivariate regression analyses were performed
using the binary logistic regression test to evaluate MMP-9 as an
indicator of HF. The numerical variables were dichotomized
according to their medians or means. A receiver operating
characteristic (ROC) curve was set up to calculate the area under
curve (AUC) at 95% CI. The level of serum MMP-9 with the maximum
accuracy was determined as the best cutoff value. Correlations
between variables were tested using the Spearman correlation
coefficient (r). Results were considered significant when
P<0.05.
Results
Both patient groups were homogenous regarding
demographic features (Table I). A
total of 59 (60.0%) patients provided a past history (one to two
years ago) of RF. The past history of RF, clinical manifestations,
and distribution of valve lesions among patients are represented in
Table SI.
 | Table IDemographic characteristics of the
patient groups. |
Table I
Demographic characteristics of the
patient groups.
| Variable | RHD without HF [n=58
(56.8%)] | RHD with HF [n=40
(43.2%)] | P-value |
|---|
| Age (year) | | | 0.30 |
|
Median
(range) | 13.5 (8.5-16) | 13.5 (10.5-15.5) | |
| Body weight (kg) | | | 0.06 |
|
Median
(range) | 32.5 (21-49) | 35.0 (23-51) | |
| Body mass index | | | 0.30 |
|
Median
(range) | 18.5 (13.9-27.2) | 19.3 (15.8-23.5) | |
| Sex, n (%) | | | 0.09 |
|
Male
(n=45) | 23 (39.7) | 22 (55.0) | |
|
Female
(n=53) | 35 (60.3) | 18 (45.0) | |
| Residence, n (%) | | | 0.50 |
|
Urban
(n=13) | 8 (13.7) | 5 (12.5) | |
|
Rural
(n=85) | 50 (86.3) | 35 (87.5) | |
The mean serum MMP-9 levels showed highly
significant differences between the healthy controls (61.0±22.8
ng/ml) and the children with RHD without HF (356.5±74.7 ng/ml),
between the healthy controls and children with RHD and HF
(422.3±63.5 ng/ml), and between children with RHD without HF and
children with RHD and HF (P<0.001 for each) (Table SII).
The univariate analysis showed that the serum MMP-9
level was associated with the risk of HF development [odds ratio
(OR), 3.2; 95% CI, 1.3-7.3, P=0.008). Potential confounders from
the univariate analysis (Table II)
including hemoglobin (Hb)%, TLC, and ASO were introduced in a
multivariate model, and the multivariate analysis revealed that
MMP-9 was an independent indicator of HF in children with RHD (OR,
2.6; 95% CI, 1.0-6.7, P=0.04) (Table
II).
 | Table IIUnivariate and multivariate analyses
of the predictors of heart failure. |
Table II
Univariate and multivariate analyses
of the predictors of heart failure.
| | Univariate
analysisb | Multivariate
analysisc |
|---|
|
Factorsa | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Hb ≥11.8 | 0.3 | 0.13-0.73 | 0.008 | 0.3 | 0.1-0.9 | 0.05 |
| TLC ≥13.2 | 3.2 | 1.4-7.5 | 0.006 | 3.3 | 1.3-8.6 | 0.01 |
| ASO ≥178.5 | 3.1 | 1.3-7.3 | 0.008 | 3.1 | 1.2-7.9 | 0.02 |
| MMP-9 ≥383.3 | 3.2 | 1.3-7.3 | 0.008 | 2.6 | 1.0-6.7 | 0.04 |
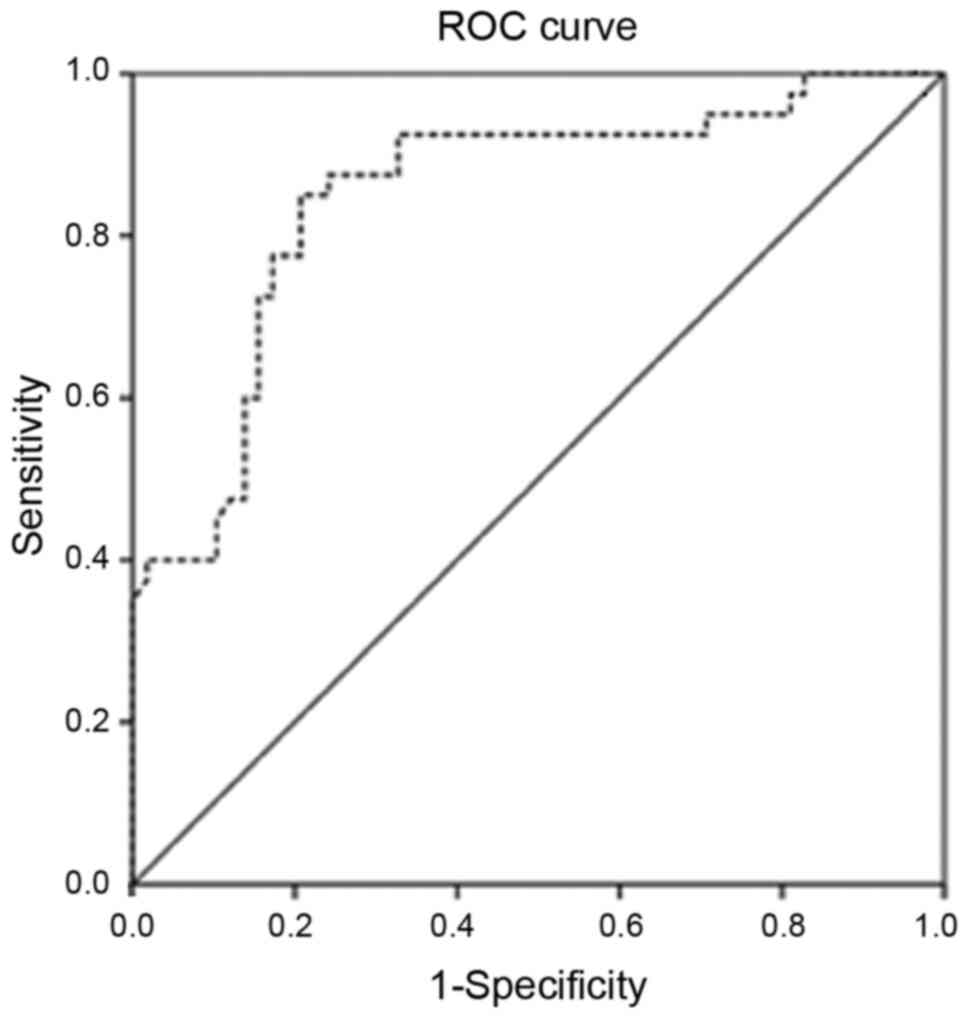
The AUC of MMP-9 in patients was 0.85 (95% CI,
0.76-0.94) (Fig. 1). The MMP-9 level
of 386.9 ng/ml was considered the best cutoff point with the
maximal diagnostic accuracy to predict HF in children with RHD. At
this level, MMP-9 predicted HF with 95% (95% CI, 83.08-99.39)
sensitivity, 74.14% (95% CI, 60.96-84.74) specificity, a positive
predictive value of 71.70% (95% CI, 61.96-79.75), a negative
predictive value of 95.56% (95% CI, 84.67-98.82) and 82.65% (95%
CI, 73.69-89.56) accuracy. Cross tabulation of the patients is
shown in Table SIII.
There was no significant correlation in any of the
parameters, including the duration of symptoms, CRP and ASO titer
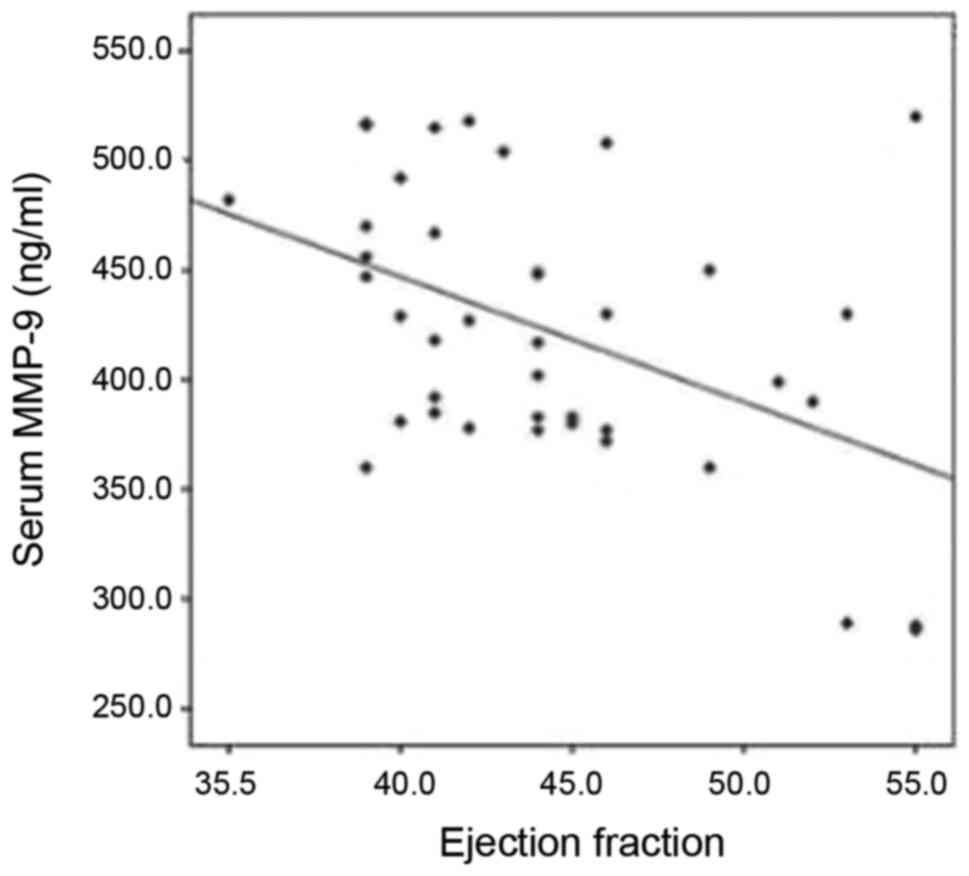
(Table SIV). MMP-9 appeared to
significantly increase with the deterioration of EF and FS (P=0.01
and P=0.02, respectively) (Fig. 2;
data not shown). In this cohort; 33 mild and 7 moderate cases were
included. The mean ± SD MMP-9 was 413±62 and 464±53, respectively
(P=0.055). By the application of the modified Ross classification;
36 patients were in Class II and 4 patients were in Class III. The
mean ± SD MMP-9 was 416±63 and 478±29, respectively (P=0.059).
Discussion
In the present study, serum matrix metalloproteinase
9 (MMP-9) concentrations did not correlate with C reactive protein
(CRP) levels in any group. Previous studies considering MMP-9 in
rheumatic heart disease (RHD) that can support our results are
rare. Previous research has shown that CRP has a positive feedback
on serum MMP-9. For example, Cimmino et al examined the
effect of CRP on MMP-9 gene expression in vitro in vascular
smooth muscle and in vivo in patients with acute coronary
syndrome (ACS) (20). Harfoosh et
al detected a positive correlation between MMP-9 and CRP serum
levels in patients with ACS (21).
Although ACS encompasses myocardial inflammation and remodeling,
the definite pathology could differ from that in RHD and HF. This
can explain the contrast between our results and those of other
studies.
In the present study, the mean serum MMP-9 level in
the control group was lower than that in both the RHD without heart
failure (HF) and RHD with HF groups, indicating that this serum
marker can be a promising screening tool for subclinical RHD among
school-aged children particularly in high risk endemic areas in
conjunction with echocardiography. Although echocardiography has
superiority in detecting subclinical RHD, Dougherty et al
stated that portable echocardiography is expensive, heavy to
transport, with limited battery capacity and functionality; in
addition, it requires well-trained cardiologists or medical staff
members (16).
Serum MMP-9 at a cutoff point 386.9 ng/ml was found
to be a sensitive predictor of HF in children with RHD. Zhao et
al (22) found that MMP-9 is
overexpressed in the myocardium of patients with RHD congestive HF.
Most of the studies concerning MMP-9 in HF, such as studies by
Abou-Raya et al (23) Collier
et al (24) and Morishita
et al (25), suggest that
active myocardial remodeling is associated with increased MMP-9
concentrations (10).
Lee et al reported that serum MMP-9
concentrations were significantly higher in patients with RHD
>30 years of age, when compared to the controls, and in a
patient group of <30 years there was no significant difference.
These results are contradictory against ours, and can be explained
by the small sample size in that group which included 10 patients
and 10 age-matched controls (26).
Kantor and Rusconi reported that HF in childhood and
young adolescence often presents at an advanced stage; thus,
biomarkers that can support traditional diagnostic tools are
valuable. These authors mentioned MMPs as possible markers but the
existing data were not sufficient (27).
Similarly, de Couto et al adopted the term
‘pre-heart failure’ as the process of cardiac remodeling indicated
by the drop of ejection fraction (EF) which precedes the appearance
of symptoms by years, gains from the early interruption and
reversal of cardiac remodeling is greater than just treating
symptoms (28). Therefore, the
follow-up of children with RHD by MMP-9 can predict the decline in
left ventricular systolic dysfunction early before symptoms
enabling the early intervention before overt irreversible failure
occurs.
In the present study, serum levels of MMP-9 were
negatively correlated with EF and fractional shortening (FS). These
results were also demonstrated by Yan et al (29) who reported that elevated MMP-9 levels
were connected with the deterioration in left ventricular (LF)
function. According to Yoshihisa et al (30); reduced EF is an independent predictor
of increased cardiac event rates. The authors stated that the
initial assessment of LVEF is important for deciding treatment and
predicting prognosis. Based on this; we assumed that the serum
MMP-9 level may be associated with the prognostic status of
patients. Radosinska et al (10) proposed that the MMP-9 level may not
only be a useful marker to identify patients at risk for HF
development but also, it can serve as an indicator of patient
outcome. According to Kantor and Rusconi, the most challenging
point in pediatric HF is the choice of treatments. This may be an
additional value of biomarkers, which may guide decisions of how to
manage and predict patient outcomes (27).
To the best of our knowledge; MMP-9 has not been
previously studied in pediatric patients with RHD either in or
outside Egypt, at the time of writing this manuscript. Therefore,
this study adds to our general understading regarding the
contribution of MMP-9 to the pathogenesis of RHD and HF in children
and draws attention to a new marker and target of therapy for these
patients.
On limitation of the present study was that it was
conducted on symptomatic patients in a single center; the sample
size was also relatively small. Thus, further multicenter studies
with larger cohorts involving other types of MMPs are required to
confirm these results. Future studies concerning MMP-9 in the
screening of school-aged children for subclinical RHD in comparison
with echocardiography, and screening of children with known RHD for
asymptomatic cardiac dysfunction to allow early and effective
intervention before the development of overt HF are highly
recommended. Further investigations including the correlation of
MMPs with other cardiac biomarkers such as natriuretic peptides
(atrial or brain subtypes) or high sensitivity CRP, the severity of
symptoms and valve lesions, prospective follow-up of children with
HF to study the correlation between MMPs and the degree of
deterioration in cardiac function to examine their role in
monitoring these patients, and clinical trials to investigate the
effects of doxycycline in children suffering from RHD and HF are
also recommended.
In conclusion, the MMP-9 serum level can be
considered as an independent sensitive indicator with which to
identify children with RHD who are at risk of HF events. MMP-9 can
be used to assess patients with HF; increased levels may indicate
the need to intensify patient medical therapy. MMP-9 was higher in
children with RHD either with or without HF than in healthy
controls; therefore, MMP-9 is a promising diagnostic marker of
subclinical RHD in school-aged children, although further studies
are required.
Supplementary Material
Table SI. Comparison of the medical
history, clinical manifestations and valve lesions of children with
RHD with and without HF.
Table SII. Comparison of the
laboratory investigations and echocardiography findings of the
patient and control groups.
Table SIII. Cross tabulation of the
patients in regards to MMP.9 results.
Table SIV. Correlation between the
MMP.9 level and all variables in the studied groups.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant
from funding agencies in the public, commercial, or not-for-profit
sectors. All the authors have indicated they have no financial
relationships relevant to this article to disclose.
Availability of data and materials
Data are available upon reasonable request from the
corresponding author.
Authors' contributions
The requirements for authorship have been met by all
authors. All authors contributed to the study conception and
design. The original idea was conceived by AAE and MS. AAE and MS
also revised the manuscript before the publication. AMS contributed
to the patient selection, history taking, data collection and blood
sample withdrawal; under the supervision of AAE and MS. MMS
contributed to the data analysis, manuscript writing, and
plagiarism checking. AAA contributed to sample processing, results
collection, data analysis, manuscript writing and submission for
publication. All authors certify that personally wrote at least 90%
of the manuscript. Finally, the manuscript was read and approved by
all the authors. All authors are responsible for the reported
research.
Ethics approval and consent to
participate
Ethics approval for this study was obtained from
Zagazig University Institutional Research Board (ZU-IRB) (approval
no. 6005-23-6-2019). All procedures were performed according to the
Declaration of Helsinki. Signed informed consent was obtained from
the parents of the involved children.
Patient consent for publication
Not applicable.
Competing interests
All the authors have no competing interest to
declare.
Authors' information
Alshymaa A. Ahmed: ORCID ID:
0000-0002-8695-4037.
References
|
1
|
Chaturvedi V and Saxena A: Heart failure
in children: Clinical aspect and management. Indian J Pediatr.
76:195–205. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Watkins DA, Johnson CO, Colquhoun SM,
Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT,
Mayosi BM, Mensah GA, et al: Global, regional, and national burden
of rheumatic heart disease, 1990-2015. N Engl J Med. 377:713–722.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dougherty S, Beaton A, Nascimento BR,
Zühlke LJ, Khorsandi M and Wilson N: Prevention and control of
rheumatic heart disease: Overcoming core challenges in
resource-poor environments. Ann Pediatr Cardiol. 11:68–78.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carapetis JR, Beaton A, Cunningham MW,
Guilherme L, Karthikeyan G, Mayosi BM, Sable C, Steer A, Wilson N,
Wyber R and Zühlke L: Acute rheumatic fever and rheumatic heart
disease. Nat Rev Dis Primers. 2(15084)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sorour KA: Rheumatic heart disease in
Egypt: Gloomy past and promising future. Egypt Heart J. 66:139–142.
2014.
|
|
6
|
Sarkar S, Rastogi M, Chaudhary P, Kumar R,
Arora P, Sagar V, Sahni IS, Shethi S, Thakur K, Ailawadhi S, et al:
Association of rheumatic fever & rheumatic heart disease with
plausible early & late-stage disease markers. Indian J Med Res.
145:758–766. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fernandes BA, Maher KO and Deshpande SR:
Cardiac biomarkers in pediatric heart disease: A state of art
review. World J Cardiol. 8:719–727. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nadar SK and Shaikh MM: Biomarkers in
routine heart failure clinical care. Card Fail Rev. 5:50–56.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Azevedo A, Prado AF, Antonio RC, Issa JP
and Gerlach RF: Matrix metalloproteinases are involved in
cardiovascular diseases. Basic Clin Pharmacol Toxicol. 115:301–314.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Radosinska J, Barancik M and Vrbjar N:
Heart failure and role of circulating MMP-2 and MMP-9. Panminerva
Med. 59:241–253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wagner DR, Delagardelle C, Ernens I, Rouy
D, Vaillant M and Beissel J: Matrix metalloproteinase-9 is a marker
of heart failure after acute myocardial infarction. J Card Fail.
12:66–72. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Reményi B, Wilson N, Steer A, Ferreira B,
Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, et
al: World heart federation criteria for echocardiographic diagnosis
of rheumatic heart disease-an evidence-based guideline. Nat Rev
Cardiol. 9:297–309. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jayaprasad N: Heart failure in children.
Heart views. 17:92–99. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Szczygielska I, Hernik E, Kołodziejczyk B,
Gazda A, Maślińska M and Gietka P: Rheumatic fever-new diagnostic
criteria. Reumatologia. 56:37–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Juneja A and Sharma S: Issues of sample
size in sensitivity and specificity analysis with special reference
to oncology. J Cancer Res Ther. 11:482–484. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dougherty S, Khorsandi M and Herbst P:
Rheumatic heart disease screening: Current concepts and challenges.
Ann Pediatr Cardiol. 10:39–49. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Isik A, Gursul C, Peker K, Aydın M, Fırat
D and Yılmaz İ: Metalloproteinases and their inhibitors in patients
with inguinal hernia. World J Surg. 41:1259–1266. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koestenberger M, Friedberg MK, Nestaas E,
Michel-Behnke I and Hansmann G: Transthoracic echocardiography in
the evaluation of pediatric pulmonary hypertension and ventricular
dysfunction. Pulm Circ. 6:15–29. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Ross RD: The ross classification for heart
failure in children after 25 years: A review and an age-stratified
revision. Pediatr Cardiol. 33:1295–1300. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cimmino G, Ragni M, Cirillo P, Petrillo G,
Loffredo F, Chiariello M, Gresele P, Falcinelli E and Golino P:
C-reactive protein induces expression of matrix
metalloproteinase-9: A possible link between inflammation and
plaque rupture. Int J Cardiol. 168:981–986. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Harfoosh AK, Elaziz AFA, Mahmoud E and
Saleh A: Matrix metalloproteinase-9 as a potential biomarker for
inflammatory events in acute coronary syndrome, and its relation to
diabetes mellitus in these patients. Al-Azhar Assiut Med J. 13
(Suppl 1):S71–S80. 2015.
|
|
22
|
Zhao Y, Zhou X, Liao X and Yang Z:
Expression and significance of matrix metalloproteinase-1,9, tissue
inhibitor of metalloproteinase-4 and extracellular matrix
metalloproteinase inducer in the myocardium of congestive heart
failure in patients with rheumatic heart diseases. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 34:790–795. 2009.(In Chinese). PubMed/NCBI
|
|
23
|
Abou-Raya S, Naim A and Marzouk S: Cardiac
matrix remodelling in congestive heart failure: The role of matrix
metalloproteinases. Clin Invest Med. 27:93–100. 2004.PubMed/NCBI
|
|
24
|
Collier P, Watson CJ, Voon V, Phelan D,
Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT and McDonald KM: Can
emerging biomarkers of myocardial remodelling identify asymptomatic
hypertensive patients at risk for diastolic dysfunction and
diastolic heart failure? Eur J Heart Fail. 13:1087–1095.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Morishita T, Uzui H, Mitsuke Y, Amaya N,
Kaseno K, Ishida K, Fukuoka Y, Ikeda H, Tama N and Yamazaki T:
Association between matrix metalloproteinase-9 and worsening heart
failure events in patients with chronic heart failure. ESC Heart
Fail. 4:321–330. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee SD, Chen LM, Kuo WW, Shu WT, Kuo WH,
Huang EJ, Tsai CC, Li PC, Liu JY, Chen TH and Huang CY: Serum
insulin-like growth factor-axis and matrix metalloproteinases in
patients with rheumatic arthritis or rheumatic heart disease. Clin
Chim Acta. 367:62–68. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kantor PF and Rusconi P: Biomarkers in
pediatric heart failure: Their role in diagnosis and evaluating
disease progression. Prog Pediatr Cardiol. 31:53–57. 2011.
|
|
28
|
de Couto G, Ouzounian M and Liu PP: Early
detection of myocardial dysfunction and heart failure. Nat Rev
Cardiol. 7:334–44. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yan AT, Yan RT, Spinale FG, Afzal R,
Gunasinghe HR, Arnold M, Demers C, McKelvie RS and Liu PP: Plasma
matrix metalloproteinase-9 level is correlated with left
ventricular volumes and ejection fraction in patients with heart
failure. J Card Fail. 12:514–519. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yoshihisa A, Sato Y, Kanno Y, Takiguchi M,
Yokokawa T, Abe S, Misaka T, Sato T, Oikawa M, Kobayashi A, et al:
Prognostic impacts of changes in left ventricular ejection fraction
in heart failure patients with preserved left ventricular ejection
fraction. Open Heart. 7(e001112)2020.PubMed/NCBI View Article : Google Scholar
|