Introduction
Dual antiplatelet therapy (DAPT) is a cornerstone
treatment following percutaneous coronary intervention (PCI) for
acute coronary syndrome (1).
Ticagrelor, a direct-acting potent P2Y12 inhibitor, is superior to
clopidogrel, due its faster onset and more potent antiplatelet
inhibitory effect, and is becoming increasingly used as the initial
medication administered following PCI in current guidelines
(2-4).
Ticagrelor is an active metabolite when absorbed, whereas
clopidogrel requires two steps to become an active metabolite.
However, due to the stronger potency and rapid onset of effects of
ticagrelor, the risk of bleeding is higher: Although the rates of
life threatening bleeding are similar, non-procedure-related
bleeding (Gastrointestinal bleeding) is higher in patients
administered ticagrelor (3,4). In the present report, a case of
spontaneous intermuscular hematoma, which may be due to
administration of ticagrelor is described. Furthermore,
single-nucleotide polymorphisms (SNPs) that may be related to
bleeding in ticagrelor using population-based genome-wide
association studies (GWAS) were also identified and are
discussed.
Case report
A 69-year-old Korean man with a 5-year history of
exertional chest pain, 10-year history of hypertension (on
medication), 10-year history of dyslipidemia, a heavy current
smoker (20 pack-years), and a heavy drinker (one bottle of soju
every other day) visited our outpatient clinic at Nowon Eulji
Medical Center, Eulji University. His chest pain had worsened
during the previous 7 days, and he had diaphoresis with pain
radiating to the left arm during his sleep. His vital signs were:
Blood pressure of 150/80 mmHg, heart rate of 78 bpm, respiratory
rate of 20 breaths/min, and a temperature of 36.8˚C. There were no
abnormalities during physical examination. Laboratory tests showed
no abnormalities, except for elevated triglycerides (410 mg/dl),
and there was no elevation in creatinine kinase myocardial band and
troponin-I levels. The prothrombin time (PT) was 10.6 sec
(reference value, 10-13 sec), PT international normalized ratio
(INR) was 0.91 (reference value, 0.85-1.15) and activated partial
thromboplastin time (aPTT) was 28.6 sec (reference value, 25-36
sec). His electrocardiogram was normal, and a 2D-echocardiography
test was performed to show a normal left ventricle (LV) ejection
fraction of 67%, normal LV cavity size, and normal LV contractility
without regional wall motion abnormality. Due to his aggravating
chest pain history with combined symptoms during the resting
period, he was diagnosed with unstable angina. Upon coronary
angiography, 80% stenosis was found in the middle to distal left
anterior descending (LAD) artery, with a normal right coronary
artery. PCI was initiated, and a 2.5x24 mm Biomatrix stent was
implanted in the middle to distal LAD. After PCI, the patient was
administered 100 mg aspirin once daily, 90 mg ticagrelor twice
daily, 1.25 mg bisoprolol once daily, 10 mg lercanidipine once
daily and 20 mg rosuvastatin once daily. The patient was discharged
on the third day without complications.
However, a month after discharge, the patient
visited the emergency department (ED) with abrupt pain and bruising
in the right chest wall and axilla area, which caused him to wake
up from his sleep. He denied trauma, particularly in the right
chest area. His electrocardiogram and cardiac enzymes were normal,
and laboratory tests showed no abnormalities except a sudden drop
in hemoglobin levels from 14.6 to 10.0 mg/dl, while his platelet
level was 248,000/mm3, PT level 11.8 sec, PT INR 1.01
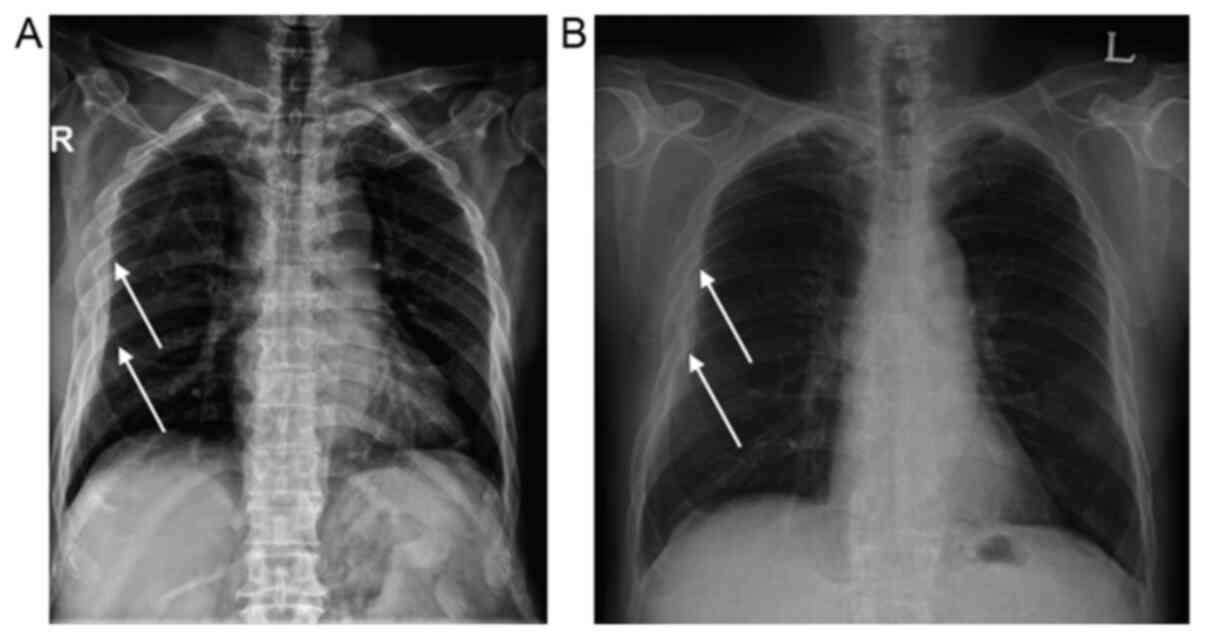
and aPTT 28.6 sec. His chest radiograph (CXR) showed subcutaneous
haziness in the right chest area with swelling (Fig. 1A). An immediate chest computed
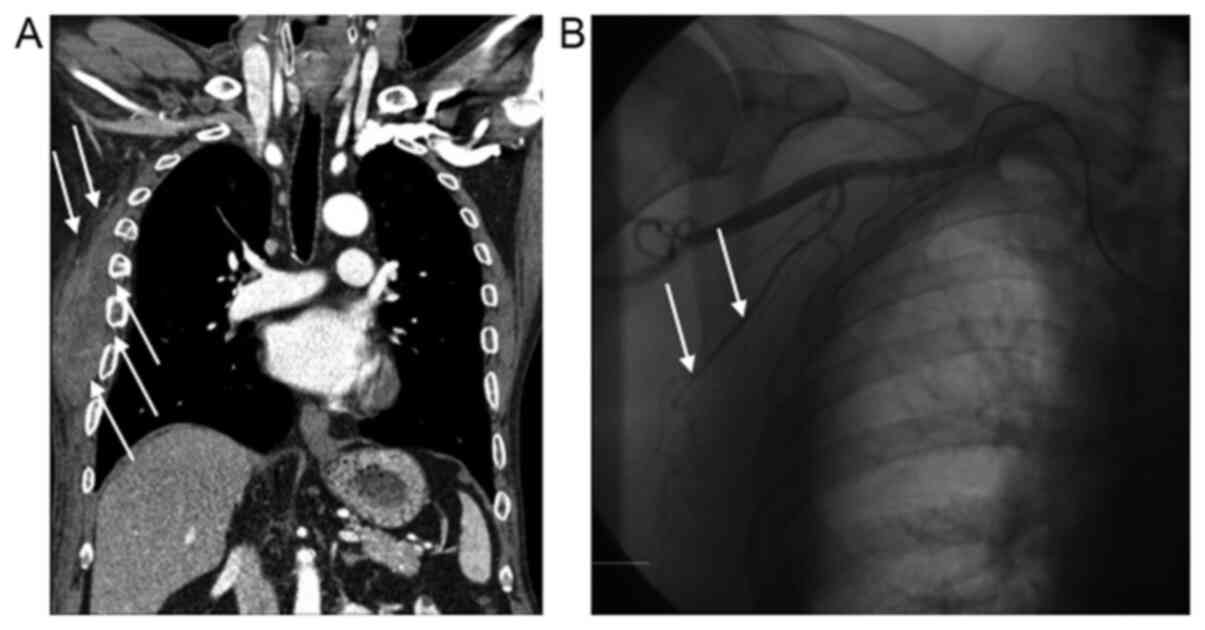
tomography scan showed an intermuscular hematoma in the right
subcutaneous lesion and fracture at the 4th, 5th, 6th and 7th ribs
(Fig. 2B), although the patient
strongly denied a recent history of trauma. An immediate thoracic
angiography was performed on the right lateral thoracic artery and
multiple right intercostal arteries, showing no arterial or venous
bleeding (Fig. 2B). On comparing the
CXR after PCI (Fig. 1B) and the CXR
after visiting the ED (Fig. 1A), a
bone deformity was initially seen in the post-PCI CXR (Fig. 1A). An extensive history of the
patient was obtained, where he admitted that he had right rib
fractures 4 months prior to PCI intervention and 5 months prior to
his ED visit. Therefore, intermuscular hematoma at the right chest
wall was concluded to be caused by spontaneous bleeding from
ticagrelor and aspirin, as there is no evidence of hematoma from
healing bone fractures. After discontinuation of aspirin and
ticagrelor for 1 week, the patient was restarted on DAPT: 100 mg of
aspirin once a day and 75 mg of clopidogrel once a day for 4
months. On the follow-up outpatient clinic visit, there were no
symptoms of ischemia nor bleeding events.
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Discussion
In the present case, the patient was not categorized
as having a high risk of bleeding. According to the hypertension,
abnormal renal/liver function, stroke, bleeding history or
predisposition, labile INR, elderly, drugs/alcohol (HAS-BLED)
scoring system (5). The HAS-BLED
score of the patient was 2 due to being aged >65-years and his
current use of medication, which is under the high-risk value of
≤3. Thus, when the patient presented to the ED with right back pain
and bruising, it was initially assumed that the large intermuscular
hematoma was due to recent trauma. However, scrutiny of the
post-PCI CXR and the CXR during his ED visit showed that fractures
were initially present, after which the patient admitted that he
had rib fractures 4 months to PCI; however, the possibility of
spontaneous hematoma is very low from healing rib fractures
(6). Furthermore, thoracic
angiography of the right lateral thoracic artery and multiple right
intercostal arteries showed that there was no evidence of recent
trauma leading to arterial or venous rupture. Therefore, by ruling
out other causes of bleeding, it was concluded that the ticagrelor
was the cause of spontaneous bleeding.
According to the Platelet Inhibition and Patient
Outcomes (PLATO) trial, ticagrelor exhibits superior efficacy
compared with clopidogrel in reducing cardiovascular events and
mortality at the expense of increased risk of non-fatal bleeding
(3). However, ethnicity was not
considered in this trial, and Asians are known to have a higher
risk of being susceptible to bleeding complications when
administered antithrombotics or fibrinolytics (7-10).
Kang et al (11) performed a
retrospective analysis of the PLATO trial and showed that there was
no significant differences in the risk of major, fatal or minor
bleeding between Asian and non-Asian patients when they were on
ticagrelor or clopidogrel. Nevertheless, there is a doubt as to
whether the bleeding events of ticagrelor are lower than that of
clopidogrel, particularly in the Asian population. Furthermore,
there are no large-randomized trials that have been performed in
the Asian population to evaluate adverse events, to the best of our
knowledge.
In particular, when searching for other reported
cases of intermuscular hematoma, only one case was documented in
China. Feng et al (12)
reported a similar case in which the patient had a spontaneous
hematoma under his right scapular site in the setting of ticagrelor
and aspirin after PCI without a history of trauma.
The limitation of the present report is the lack of
bleeding time (BT) and platelet function tests (PFT) when the
patient visited the ED. BT and PFT are not routine laboratory tests
performed in Korea as these tests are difficult to perform in acute
settings, such as in the ED or before PCI. BT requires skilled
staff from the department of laboratory medicine, and they can be
hard to request on-call. PFT is reported the next day, providing
limited information in an acute setting of bleeding. Furthermore,
as these tests are not reimbursed by the Health and Welfare
Ministry of Korea, patients need to pay at their own expense, which
is hard to explain and request in an acute setting. However,
comparing BT and PFT before and after ticagrelor may have provided
additional information regarding the tendency of bleeding in our
patient, as PT and aPTT time before and after ticagrelor and
aspirin were both in the normal range. Considering that genetics
may have some role in bleeding, we searched the GWAS catalog
(13) and found that Varenhorst
et al (14) performed GWAS on
the PLATO trial to determine whether genetic variations may cause
variations in ticagrelor plasma levels and clinical outcomes. Their
study showed that ticagrelor pharmacokinetics were associated with
SLCO1B1, UGT2B7 and CYP3A4; however, these
associations did not translate into any detectable effect on
efficacy or safety correlating to ticagrelor treatment (14). Li et al (15) showed that SCLO1B1 and
CYP3A4/5 polymorphisms did not affect the pharmacokinetics
and pharmacodynamics of ticagrelor treatment in healthy Chinese
male subjects. Holmberg et al (16), also showed that although
CYP3A4*22 (rs35599367 G>A) impairs elimination, it has no
effect on the bioactivation of clopidogrel, and population-based
genomics show that there is almost no variation in the frequency of
CYP3A4*22 based on populations of diverse ancestry, and thus
cannot explain the increased risk of bleeding in Asians.
Tatarunas et al (17) demonstrated that the CYP4F2
rs3093135 TT allele has a higher antiplatelet effect of ticagrelor
and more frequent nonprocedural bleeding during ticagrelor therapy,
as compared with AA and AT variant carriers. Additionally,
CYP2C19*1 (rs4244285 G allele) has a higher antiplatelet
effect than CYP2C19*2 (rs4244285 A allele). However, when
reviewing the frequency of SNPs related to bleeding in the
Korean/East Asian population, the mentioned SNPs could not explain
the higher risk of bleeding in Asians.
Li et al (18)
documented two SNP locations in the PEAR1 genes that were
related to increased antiplatelet activity: rs12041311 (AA
homozygotes) and rs4661012 (GG homozygotes). In terms of frequency,
the AA frequency for rs12041311 is 16% in Koreans and 21% in East
Asians, whereas it was 10% for the global population. In addition,
the GG frequency of rs4661012 is 25% in Koreans and 32% in East
Asians, whereas globally it is 20%. Hence, a detailed
pharmacogenetics study of rs12041311 and rs4661012 is required to
improve our understanding of the pharmacokinetics and
pharmacodynamics of these gene variants to evaluate the proper dose
and adverse events of ticagrelor in the East Asian population. The
SNPs evaluated from the GWAS are shown in Table I.
 | Table IFrequency of SNPs related to
excretion/elimination of ticagrelor and its metabolites based on
race. |
Table I
Frequency of SNPs related to
excretion/elimination of ticagrelor and its metabolites based on
race.
| First author,
year | SNP | Genes | Chr | Position | Ref/Alt | Ref/Alt
frequency | East Asian | South Asian | European | American | African | Functional
change | (Refs.) |
|---|
| Tatarunas, 2017 | rs4244285 | CYP2C19*2 | 10 | 94781859 | G/A | 0.78/0.22 | 0.69/0.31 | 0.64/0.36 | 0.86/0.15 | 0.89/0.11 | 0.83/0.17 | Exonic | (17) |
| Holmberg, 2019 | rs35599367 | CYP34A*22 | 7 | 99768693 | G/A | 1.00/0.00 | 1.00/0.00 | 0.99/0.01 | 0.95/0.05 | 0.97/0.03 | 1.00/0.00 | Intronic | (16) |
| Holmberg, 2019 | rs776746 | CYP3A5*3 | 7 | 99672916 | T/C | 0.38/0.62 | 0.29/0.71 | 0.33/0.67 | 0.06/0.94 | 0.20/0.80 | 0.82/0.18 | Splicing | (16) |
| Tatarunas, 2017 | rs3093135 | CYP4F2 | 19 | 15893561 | A/Ta | 0.88/0.12 | 0.94/0.06 | 0.86/0.14 | 0.82/0.18 | 0.88/0.12 | 0.89/0.11 | Intronic | (17) |
| Li, 2017 | rs12566888 | PEAR1 | 1 | 156899255 | G/Ta | 0.61/0.39 | 0.51/0.49 | 0.62/0.38 | 0.91/0.09 | 0.78/0.22 | 0.35/0.65 | Intronic | (15) |
| Li, 2017 | rs4661012 | PEAR1 | 1 | 156915699 | T/Ga | 0.56/0.44 | 0.43/0.57 | 0.42/0.58 | 0.64/0.36 | 0.50/0.50 | 0.71/0.29 | UTR3 | (15) |
| Li, 2017 | rs12041331 | PEAR1 | 1 | 156899922 | G/Aa | 0.67/0.33 | 0.54/0.46 | 0.63/0.37 | 0.91/0.09 | 0.80/0.20 | 0.53/0.47 | Intronic | (15) |
| Li, 2015 | rs6785930 | P2RY12 | 3 | 151338828 | G/Aa | 0.76/0.24 | 0.79/0.21 | 0.73/0.27 | 0.68/0.32 | 0.72/0.28 | 0.83/0.17 | Exonic | (18) |
| Varenhorst, 2015 | rs12371604 | SLCO1B1 | 12 | 21391336 | T/C | 0.62/0.38 | 0.27/0.73 | 0.65/0.35 | 0.75/0.25 | 0.70/0.30 | 0.75/0.25 | Intronic | (14) |
| Varenhorst, 2015 | rs4149056 | SLCO1B1 | 12 | 21331549 | T/Ca | 0.91/0.09 | 0.88/0.12 | 0.96/0.04 | 0.84/0.16 | 0.87/0.13 | 0.99/0.01 | Exonic | (14) |
| Varenhorst, 2015 | rs113681054 | SLCO1B1 | 12 | 21402979 | T/Ca | 0.78/0.22 | 0.55/0.45 | 0.91/0.09 | 0.81/0.19 | 0.83/0.17 | 0.82/0.18 | Intergenic | (14) |
In conclusion, the present case report showed that
ticagrelor, a potent antiplatelet inhibitor, may cause spontaneous
intermuscular bleeding. As there is a concern that Asians may have
a higher risk of bleeding, physicians must be aware of this risk
when prescribing ticagrelor in the Asian population. However,
further research is required to validate the association of the
potential SNPs, particularly the PEAR1 gene and its relation
to risk of bleeding risks amongst individuals of different
races.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BWY contributed to the diagnosis, data curation and
writing of the manuscript. JYH contributed to the conceptualization
of the report and reviewed the manuscript. BWY and JYH confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare no competing interests.
References
|
1
|
Kurz DJ and Eberli FR: Medical therapy of
coronary artery disease after percutaneous intervention. Curr Opin
Pharmacol. 13:287–293. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Amsterdam EA, Wenger NK, Brindis RG, Casey
DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF,
Kontos MC, et al: 2014 AHA/ACC Guideline for the Management of
Patients with Non-ST-Elevation Acute Coronary Syndromes: A report
of the American College of Cardiology/American Heart Association
Task Force on Practice Guidelines. J Am Coll Cardiol. 64:e139–e228.
2014.PubMed/NCBI View Article : Google Scholar : Erratum in: J Am
Coll Cardiol 64: 2713-2714, 2014.
|
|
3
|
Wallentin L, Becker RC, Budaj A, Cannon
CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et
al: PLATO Investigators: Ticagrelor versus clopidogrel in patients
with acute coronary syndromes. N Engl J Med. 361:1045–1057.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Husted S and van Giezen JJ: Ticagrelor:
The first reversibly binding oral P2Y12 receptor
antagonist. Cardiovasc Ther. 27:259–274. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pisters R, Lane DA, Nieuwlaat R, de Vos
CB, Crijns HJ and Lip GY: A novel user-friendly score (HAS-BLED) to
assess 1-year risk of major bleeding in patients with atrial
fibrillation: The Euro Heart Survey. Chest. 138:1093–1100.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sato N, Sekiguchi H, Hirose Y and Yoshida
S: Delayed chest wall hematoma caused by progressive displacement
of rib fractures after blunt trauma. Trauma Case Rep. 4:1–4.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ross AM, Gao R, Coyne KS, Chen J, Yao K,
Yang Y, Qin X, Qiao S and Yao M: TUCC Investigators. A randomized
trial confirming the efficacy of reduced dose recombinant tissue
plasminogen activator in a Chinese myocardial infarction population
and demonstrating superiority to usual dose urokinase: The TUCC
trial. Am Heart J. 142:244–247. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bhatt DL, Paré G, Eikelboom JW, Simonsen
KL, Emison ES, Fox KA, Steg PG, Montalescot G, Bhakta N, Hacke W,
et al: CHARISMA Investigators: The relationship between
CYP2C19 polymorphisms and ischaemic and bleeding outcomes in
stable outpatients: The CHARISMA genetics study. Eur Heart J.
33:2143–2150. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang TY, Chen AY, Roe MT, Alexander KP,
Newby LK, Smith SC Jr, Bangalore S, Gibler WB, Ohman EM and
Peterson ED: Comparison of baseline characteristics, treatment
patterns, and in-hospital outcomes of Asian versus non-Asian white
Americans with non-ST-segment elevation acute coronary syndromes
from the CRUSADE quality improvement initiative. Am J Cardiol.
100:391–396. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dang MT, Hambleton J and Kayser SR: The
influence of ethnicity on warfarin dosage requirement. Ann
Pharmacother. 39:1008–1012. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kang HJ, Clare RM, Gao R, Held C,
Himmelmann A, James SK, Lim ST, Santoso A, Yu CM, Wallentin L, et
al: PLATO Investigators: Ticagrelor versus clopidogrel in Asian
patients with acute coronary syndrome: A retrospective analysis
from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am
Heart J. 169:899–905.e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng C and Wang L and Wang L: Spontaneous
hematoma in the setting of dual anti-platelet therapy with
ticagrelor: A case report. Oncol Lett. 12:144–146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Buniello A, MacArthur JAL, Cerezo M,
Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy
E, Sollis E, et al: The NHGRI-EBI GWAS Catalog of published
genome-wide association studies, targeted arrays and summary
statistics 2019. Nucleic Acids Res. 47 (D1):D1005–D1012.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Varenhorst C, Eriksson N, Johansson Å,
Barratt BJ, Hagström E, Åkerblom A, Syvänen AC, Becker RC, James
SK, Katus HA, et al: PLATO Investigators. Effect of genetic
variations on ticagrelor plasma levels and clinical outcomes. Eur
Heart J. 36:1901–1912. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li M, Hu Y, Li H, Wen Z, Hu X, Zhang D,
Zhang Y, Xiao J, Tang J and Chen X: No effect of SLCO1B1 and
CYP3A4/5 polymorphisms on the pharmacokinetics and
pharmacodynamics of ticagrelor in healthy chinese male subjects.
Biol Pharm Bull. 40:88–96. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Holmberg MT, Tornio A, Paile-Hyvärinen M,
Tarkiainen EK, Neuvonen M, Neuvonen PJ, Backman JT and Niemi M:
CYP3A4*22 impairs the elimination of ticagrelor,
but has no significant effect on the bioactivation of clopidogrel
or prasugrel. Clin Pharmacol Ther. 105:448–457. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Tatarunas V, Kupstyte N, Zaliunas R,
Giedraitiene A and Lesauskaite V: The impact of clinical and
genetic factors on ticagrelor and clopidogrel antiplatelet therapy.
Pharmacogenomics. 18:969–979. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li MP, Tang J, Wen ZP, Zhang YJ, Zhang W,
Zhou HH, Zhang ZL and Chen XP: Influence of P2Y12
polymorphisms on platelet activity but not ex-vivo antiplatelet
effect of ticagrelor in healthy Chinese male subjects. Blood Coagul
Fibrinolysis. 26:874–881. 2015.PubMed/NCBI View Article : Google Scholar
|