Introduction
Nanoparticle albumin-bound (nab)-paclitaxel is a
nanoparticle formulation of human serum albumin (HSA) that is 130
nm in size (1-3).
Taxol, another paclitaxel formulation, forms micelles in the blood
after injection, prolonging the time of increased paclitaxel
concentrations in the blood. This leads to high hematological
toxicity (2,3). In contrast, nab-paclitaxel
nanoparticles collapse immediately after injection. Therefore, when
the incorporated paclitaxel is released into the bloodstream, it
binds to HSA (2,3), and thus the increased blood paclitaxel
concentration is not prolonged. Indeed, the recommended therapeutic
dose of nab-paclitaxel is higher than that of Taxol (4).
The intratumoral paclitaxel levels were found to be
elevated in an in vivo xenograft model following
nab-paclitaxel treatment relative to treatment with a
Cremophor-based paclitaxel formulation (1) that is similar to Taxol. Albumin is
transcytosed through endothelial cells via the albumin receptor
gp60, and tumor cells take up albumin as an energy source (5). The higher therapeutic efficacy of
nab-paclitaxel relative to Taxol (4)
is considered to result from the reduced hematological toxicity and
increased accumulation of paclitaxel in tumors mediated by albumin
receptors. Therefore, the binding affinity between blood HSA and
paclitaxel is an important factor for assessing its efficacy.
High pressure is used to manufacture nab-paclitaxel
(2), which may affect the
conformation and oligomerization of nab-paclitaxel HSA (nab HSA),
as HSA oligomers have different biochemical characteristics from
those of monomeric HSA (6-8). If
the manufacturing process of nab-paclitaxel influences oligomer
formation and/or the native HSA structure, the binding affinity of
paclitaxel for nab HSA may differ from that of generic HSA,
possibly reducing the efficacy of nab-paclitaxel in the clinical
setting. As indicated above, nab-paclitaxel has superior
therapeutic efficacy to Taxol (4),
suggesting that nab HSA has similar biochemical characteristics to
generic HSA and that binding affinities of both HSAs for paclitaxel
are comparable.
To confirm the hypothesis that nab HSA is similar to
generic HSA, the binding affinities of paclitaxel for nab HSA and
generic HSA (control HSA) were compared. In addition, the
affinities of docetaxel for nab HSA and control HSA were
determined, as their binding sites are similar (9).
Materials and methods
Chemical reagents and proteins
Docetaxel was purchased from Tokyo Chemical
Industry. Paclitaxel was purchased from IndenaSpA. HSA and
ubiquitin were purchased from Sigma-Aldrich; Merck KGaA. Unless
indicated otherwise, all other chemicals were of analytical reagent
grade and purchased from commercial sources.
Depletion of paclitaxel from
nab-paclitaxel HSA
An aliquot of nab-paclitaxel (7.5 mg; Celgene
Corporation) was dissolved in 2.5 ml PBS (pH 7.4; Thermo Fisher
Scientific, Inc.). The solution was centrifuged at 20,000 x g for
15 min at 4˚C, and the supernatant was collected (HSA fraction).
The treatment was repeated with PD10 (GE Healthcare) three times to
deplete the paclitaxel. The PD10-treated fraction was dialyzed
against PBS three times with a Slide-A-Lyzer dialysis cassette (MW
cutoff, 3 kDa; Thermo Fisher Scientific, Inc.). The protein
concentration in the dialyzed fraction was adjusted to 1 mg/ml with
PBS. The protein concentration was determined using a BCA Protein
assay kit (Thermo Fisher Scientific, Inc.).
Paclitaxel quantification
For paclitaxel quantification,
((-)-(1S,2S,3R,4S,5R,7S,8S,10R,13S)-4,10-diacetoxy-2
-benzoyloxy-5,20-epoxy-1,7-dihydroxy-9-oxotax-11-en-13-yl(2R,3S)-3-benzoylamino-2-hydroxy-3-benzylpropionate)
was used as an internal standard (IS). To each 100 µl sample, 150
µl acetonitrile/ethanol solution (2:1) containing 3.3 µg/ml IS was
added. The mixture was filtered using a GL ChromatoDisc (diameter
0.45 µm, GL Science). The reverse-phase-HPLC system LC-20AB
prominence series (Shimadzu Corp.) was used. Chromatographic
separation was performed using a Wakopak Navi C30 (5 μm particle,
2.0x150 mm; FUJIFILM Wako Pure Chemical) at a column temperature of
40˚C. The mobile phase consisted of 0.1% aqueous phosphoric acid
(A) and acetonitrile (B). Isocratic elution with 47% (B) was
performed for 50 min at a flow rate of 0.25 ml/min whilst
monitoring the UV absorption at 254 nm. Paclitaxel and IS were
eluted at 10.8 and 15.6 min, respectively.
Native PAGE and Coomassie Brilliant
blue (CBB) staining
Native PAGE was performed as described previously
with slight modifications (10).
Briefly, 10 µl 2X concentrated sample buffer (20% glycerol and
0.05% bromophenol blue) was added to 10 µl 1 mg/ml HSA solution.
The mixture was loaded onto a 5-20% SuperSep Ace precast gel
(FUJIFILM Wako Pure Chemical) and electrophoresed in 3.0 g/l
Tris(hydroxymethyl)aminomethane and 14.4 g/l glycine running
buffer. The gel was stained with CBB Stain One Super at 20-25˚C for
60 min (Nacalai Tesque).
Binding affinity measurements
Surface plasmon resonance (SPR) analysis was
performed using a Biacore X100 (GE Healthcare), with binding
affinity determined by multiple-cycle analysis, as described
previously (11,12). Briefly, nab HSA or control HSA was
immobilized on a CM7 sensor chip with an Amine Coupling kit (GE
Healthcare). The target immobilization level was set to 24,000 RU.
During preliminary experiments, it was determined that ubiquitin
immobilization in the reference cells reduced non-specific binding
of paclitaxel (data not shown). Therefore, ubiquitin was
immobilized on a reference cell with the Amine Coupling kit by the
time and flow method (contact time 420 sec). Paclitaxel solutions
at concentrations of 0.625, 1.25, 2.5, 5.0 and 10 µM in 0.5% DMSO
with 4 µg/ml carboxymethyl dextran (MW 10 kDa, Tokyo Chemical
Industry) and buffered using 50 mM Tris-HCl buffer to pH 7.4
(Sigma-Aldrich; Merck KGaA) were prepared. Each paclitaxel solution
was added to the sensor chip at a flow rate of 30 µl/min for 30 sec
followed by addition of washing buffer [0.5% DMSO-4 µg/ml
carboxymethyl dextran (MW 10 kDa)-50 mM Tris-HCl buffer (pH 7.4)]
at a flow rate of 30 µl/min for 60 sec.
For docetaxel analysis, a CM5 sensor chip (GE
Healthcare) was used. The target immobilization levels of nab HSA
and control HSA were set to 10,000 RU. As a reference, ubiquitin
was immobilized with the Amine Coupling kit using a method similar
to that described above. Docetaxel solutions at concentrations of
3.125, 6.25, 12.5, 25, 50 and 100 µM in 0.1% DMSO-0.05%
polyethylene glycol (MW 20 kDa; FUJIFILM Wako Pure Chemical) 50 mM
Tris-HCl buffer (pH 7.4) were prepared. Each docetaxel solution was
added to the sensor chip at a flow rate of 30 µl/min for 30 sec
followed by the addition of washing buffer [0.1% DMSO-0.05%
polyethylene glycol-(MW 20 kDa)-50 mM Tris-HCl buffer (pH 7.4)] at
a flow rate of 30 µl/min for 30 sec.
The SPR sensorgram was globally fitted using a 1:1
binding model. KD values were calculated using Biacore
X100 Evaluation Software version 2.0.1 in affinity mode (GE
Healthcare). All SPR experiments were repeated at least three
times. Data are presented as the mean ± standard deviation.
Results
Characterization of nab HSA
To evaluate whether the paclitaxel binding affinity
of nab HSA was similar to that of the control generic albumin, nab
HSA was isolated from nab-paclitaxel. The results of isolation of
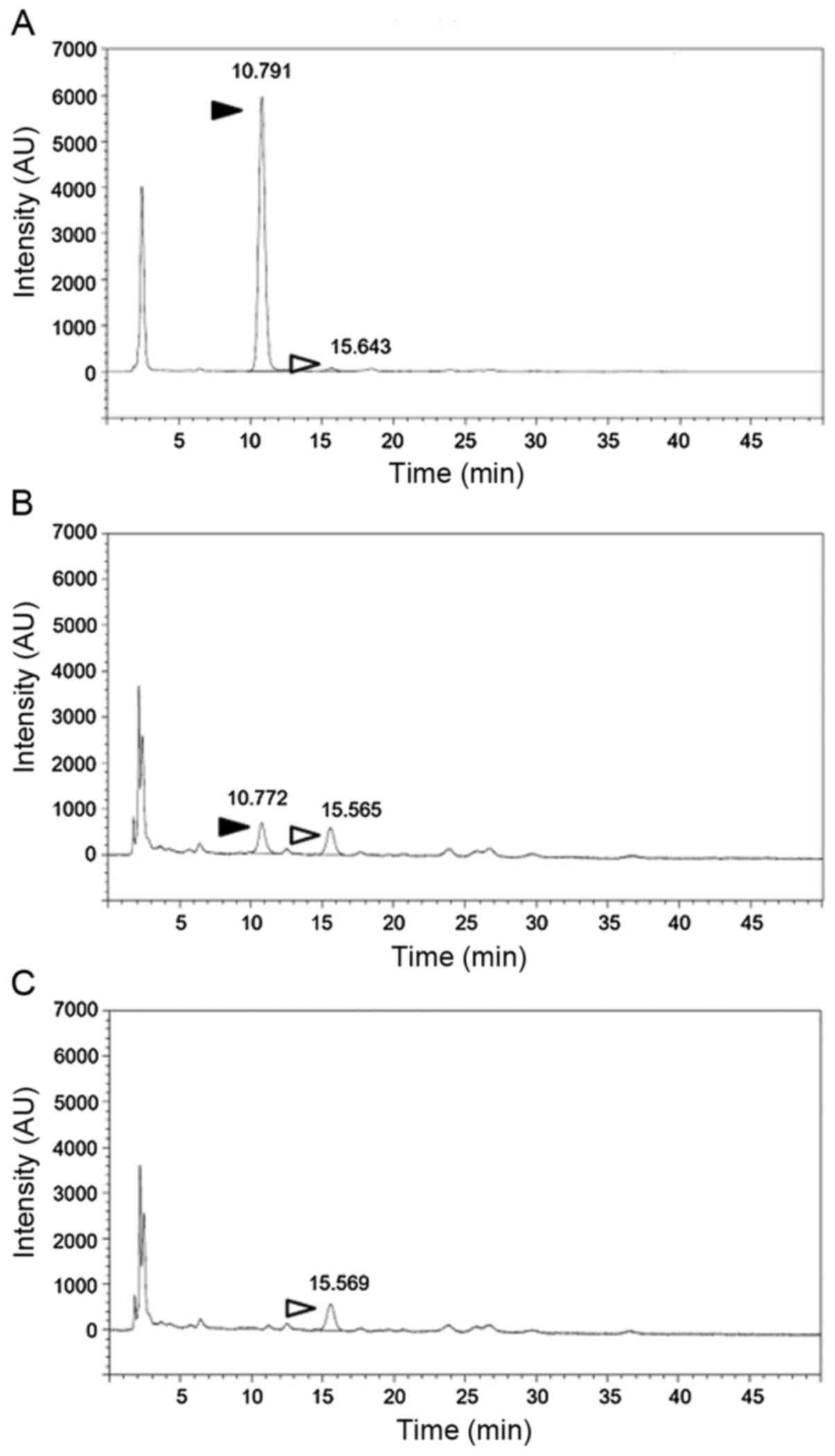
nab HSA and a typical HPLC chromatogram are shown in Table I and Fig.
1A-C, respectively. The results indicated that paclitaxel was
successfully depleted by repeated PD-10 treatment. The lowest
paclitaxel concentration used in our calibration curve for HPLC
analysis was 0.32 µM, and there was no detectable paclitaxel signal
in the fraction treated three times with PD-10 (Fig. 1C), indicating that the remaining
paclitaxel concentration was <0.32 µM. Additionally, it has been
reported that the KD values of paclitaxel-HSA binding
are 0.42-69.9 µM (10-12),
indicating that the remaining paclitaxel concentration shown in
Table I was lower than the lowest
reported KD value. Thus, the isolated nab HSA was
suitable for the binding affinity experiments.
 | Table ISummary of nab HSA purification. |
Table I
Summary of nab HSA purification.
| | HSA | Paclitaxel |
|---|
| Treatment | HSA, mg | Yield, % | Paclitaxel,
µg/ml | Yield, % |
|---|
| Pre treatment | 7.5 | 100 | 179.4 | 100.0 |
| Supernatant | 6.9 | 92.1 | 56.8 | 31.6 |
| Precipitate | 0.6 | 8 | 192.2 | 64.3 |
| PD10, 1st | 7 | 92.8 | 2.3 | 1.8 |
| PD10, 2nd | 5 | 67 | ND | ND |
| PD10, 3rd | 3.8 | 50.8 | ND | ND |
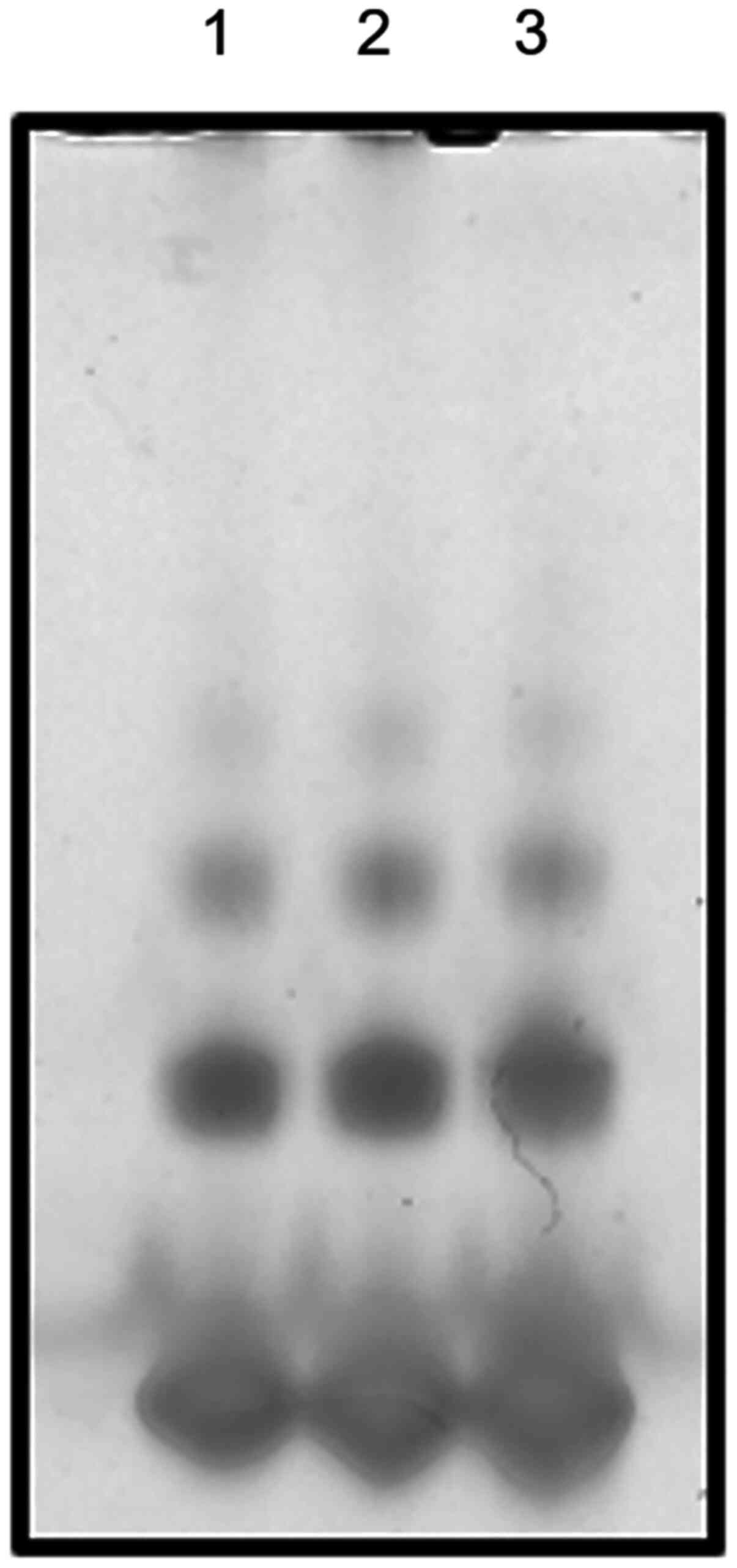
Next, whether the albumin isolation steps affected
oligomer formation was evaluated. Fig.
2 shows that the amount of oligomer in the isolated nab HSA was
similar to that in the parental pretreatment nab HSA (7,13). Thus,
the isolation steps showed a limited influence on the oligomer
formation of nab HSA.
Paclitaxel and docetaxel binding to
nab HSA and control HSA
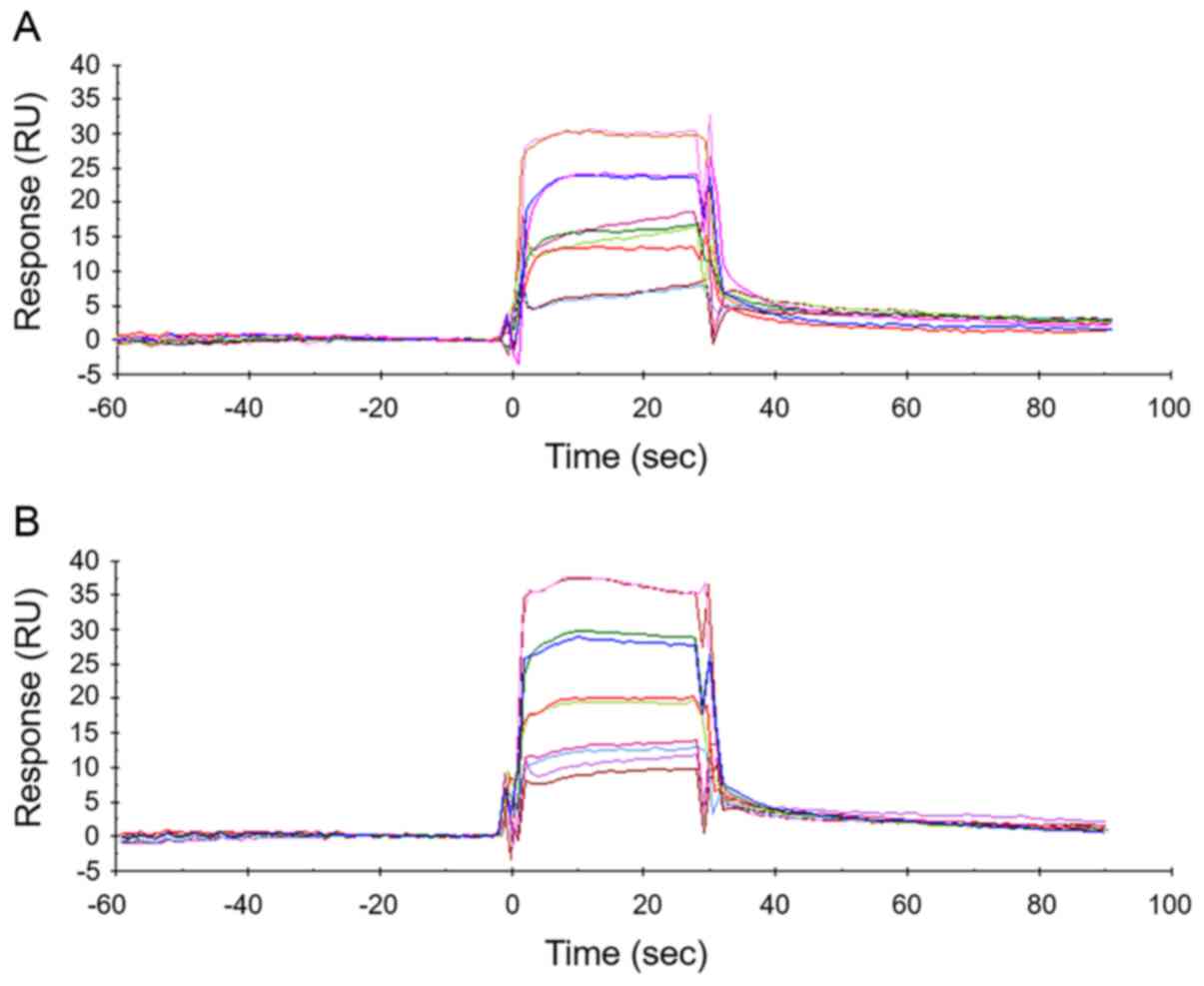
SPR analysis was analyzed to confirm that the
paclitaxel binding affinity of nab HSA was similar to that of nab
HSA. Representative sensorgrams are shown in Fig. 3A and B. The KD values for the binding
of paclitaxel to nab HSA and control HSA were similar (8.93±8.60
and 7.39±5.81 µM, respectively).
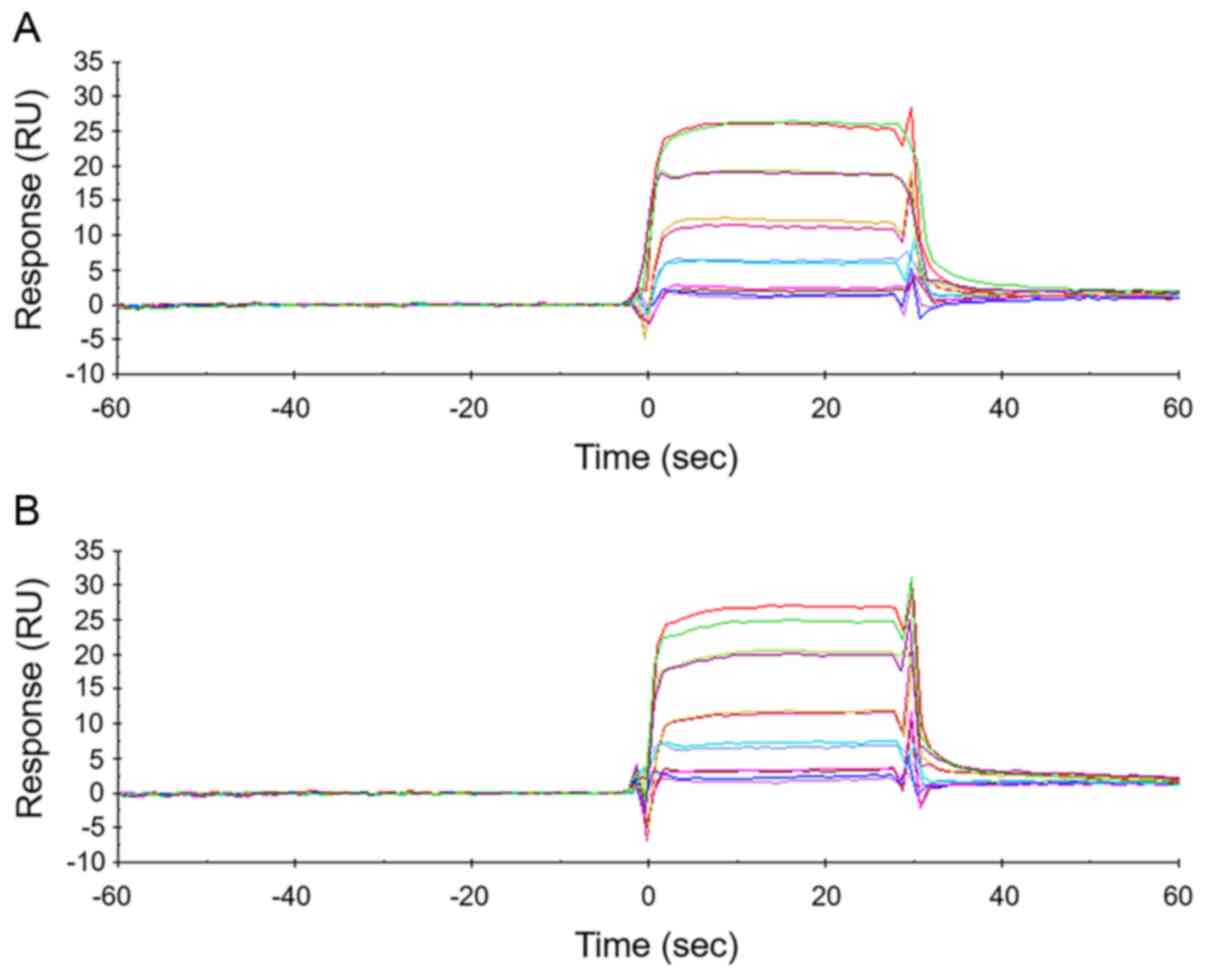
Next, the KDs were determined for the
binding of docetaxel to both HSAs, as its binding site on HSA is
similar to that of paclitaxel (9).
Representative sensorgrams are shown in Fig. 4A and B. The KD for nab HSA was
44.3±9.50 µM, whereas that for control HSA was 55.9±2.28 µM,
indicating that the docetaxel binding affinities of both HSAs are
comparable.
Discussion
In the present study, SPR experiments were used to
confirm the hypothesis that nab HSA is characteristically similar
to control generic HSA. The KDs of nab HSA and control
HSA for paclitaxel were 8.93±8.60 and 7.39±5.81 µM, respectively.
The affinity of paclitaxel-HSA binding has not been examined
previously by SPR, to the best of our knowledge. The KD
values of paclitaxel-HSA binding determined by other methods were
0.42-69.9 µM (10-12),
consistent with the results of the present study. Additionally, the
docetaxel binding affinities for nab HSA and control HSA were
determined as the binding region is similar between paclitaxel and
docetaxel. The KDs of docetaxel for nab HSA and control
HSA were 44.3±9.50 and 55.9±2.28 µM, respectively, whereas the
reported docetaxel KD determined by SPR was 199 µM
(14), showing a discrepancy with
the docetaxel-HSA KDs measured in the present study.
Paal et al (9) reported the
paclitaxel KD calculated in a docking study was 80-fold
lower than the experimentally determined KD. They
concluded that this difference was caused by variations in
experimental conditions, which may also be the case in the present
study.
Fatty acids can bind to HSA and modulate PTX-HSA
binding (15), suggesting that
contamination by fatty acids affects drug-HSA binding affinities.
To address this issue, fatty acid-free and highly purified HSA was
used as the control HSA. Additionally, pharmaceutical grade
nab-paclitaxel purchased from Celgene was used. Regulatory
authorities strictly ensure that pharmaceutical products are free
from impurities in accordance with ICH Q3 (ich.org/page/quality-guidelines). Therefore, the
results of the present study were not influenced by residual
impurities.
There are several water-insoluble anti-cancer
agents, such as rapamycin and docetaxel. It is suggested that this
nab technology is useful for solubilizing these water-insoluble
agents. Of interest, nab-rapamycin is under clinical investigation
for use as a treatment for sarcomas (16), and Khodaei et al reported that
rapamycin may primarily bind to site I on HSA via a probe
displacement study (17). As
indicated above, nab HSA is characteristically similar to control
generic HSA, suggesting that nab HSA does not influence
nab-rapamycin efficacy in the clinical setting.
In conclusion, the binding affinities of paclitaxel
and docetaxel for nab HSA and control HSA were found to be
comparable. Additionally, the manufacturing process did not
influence the paclitaxel binding affinity for nab HSA. It is
well-established that nab-paclitaxel is more effective than Taxol.
This higher therapeutic efficacy of nab-paclitaxel than that of
Taxol is considered to result from the reduced hematological
toxicity and increased accumulation of paclitaxel in tumors
mediated by albumin receptors (4).
Therefore, the results of the present study also suggest that nab
HSA does not affect the clinical effectiveness of
nab-paclitaxel.
Acknowledgements
The authors would like to thank Dr Takeshi Onda, Dr
Shouichi Ohno and Ms. Sayaka Yako (Nippon Kayaku Co., Ltd.) for
providing fruitful discussions and technical support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets and materials used during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KS and MK designed the study. TS, MO, JS and CK
designed the study, performed the experiments and wrote the
manuscript. TS, KS and MK reviewed and edited the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Desai N, Trieu V, Yao Z, Louie L, Ci S,
Yang A, Tao C, De T, Beals B, Dykes D, et al: Increased antitumor
activity, intratumor paclitaxel concentrations, and endothelial
cell transport of cremophor-free, albumin-bound paclitaxel,
ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res.
12:1317–1324. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yardley DA: nab-Paclitaxel mechanisms of
action and delivery. J Control Release. 170:365–372.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen N, Brachmann C, Liu X, Pierce DW, Dey
J, Kerwin WS, Li Y, Zhou S, Hou S, Carleton M, et al: Albumin-bound
nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue
distribution and tumor penetration. Cancer Chemother Pharmacol.
76:699–712. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Untch M, Jackisch C, Schneeweiss A, Conrad
B, Aktas B, Denkert C, Eidtmann H, Wiebringhaus H, Kümmel S,
Hilfrich J, et al: German Breast Group (GBG); Arbeitsgemeinschaft
Gynäkologische Onkologie-Breast (AGO-B) Investigators:
Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant
chemotherapy for early breast cancer (GeparSepto-GBG 69): A
randomised, phase 3 trial. Lancet Oncol. 17:345–356.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Merlot AM, Kalinowski DS and Richardson
DR: Unraveling the mysteries of serum albumin-more than just a
serum protein. Front Physiol. 5(299)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sollenne NP, Wu HL and Means GE:
Disruption of the tryptophan binding site in the human serum
albumin dimer. Arch Biochem Biophys. 207:264–269. 1981.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nakano NI, Shimamori Y and Yamaguchi S:
Binding capacities of human serum albumin monomer and dimer by
continuous frontal affinity chromatography. J Chromatogr A.
237:225–232. 1982.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Matsushita S, Chuang VT, Kanazawa M,
Tanase S, Kawai K, Maruyama T, Suenaga A and Otagiri M: Recombinant
human serum albumin dimer has high blood circulation activity and
low vascular permeability in comparison with native human serum
albumin. Pharm Res. 23:882–891. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Paal K, Shkarupin A and Beckford L:
Paclitaxel binding to human serum albumin - automated docking
studies. Bioorg Med Chem. 15:1323–1329. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Paál K, Müller J and Hegedûs L: High
affinity binding of paclitaxel to human serum albumin. Eur J
Biochem. 268:2187–2191. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Purcell M, Neault JF and Tajmir-Riahi HA:
Interaction of taxol with human serum albumin. Biochim Biophys
Acta. 1478:61–68. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Amani N, Saberi MR and Chamani JK:
Investigation by fluorescence spectroscopy, resonance rayleigh
scattering and zeta potential approaches of the separate and
simultaneous binding effect of Paclitaxel and estradiol with human
serum albumin. Protein Pept Lett. 18:935–951. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Atmeh RF and Shabsoug B: Detection and
semiquantitation of albumin forms in fresh human plasma separated
on gradient polyacrylamide gel by means of electroblotting on
agarose gel matrix. Electrophoresis. 18:2055–2058. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cimitan S, Lindgren MT, Bertucci C and
Danielson UH: Early absorption and distribution analysis of
antitumor and anti-AIDS drugs: Lipid membrane and plasma protein
interactions. J Med Chem. 48:3536–3546. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Paal K and Shkarupin A: Paclitaxel binding
to the fatty acid-induced conformation of human serum albumin -
automated docking studies. Bioorg Med Chem. 15:7568–7575.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gordon EM, Chua-Alcala VS, Kim K, Baby R,
Angel N, Quon D, Wong S and Chawla SP: A phase I/II investigation
of nivolumab and ABI-009 (nab-sirolimus) in advanced
undifferentiated pleomorphic sarcoma (UPS), liposarcoma (LPS),
chondrosarcoma (CS), osteosarcoma (OS), and Ewing sarcoma:
Preliminary efficacy and safety results. J Clin Oncol. 37 (Suppl
15)(11057)2019.
|
|
17
|
Khodaei A, Bolandnazar S, Valizadeh H,
Hasani L and Zakeri-Milani P: Interactions between sirolimus and
anti-inflammatory drugs: Competitive binding for human serum
albumin. Adv Pharm Bull. 6:227–233. 2016.PubMed/NCBI View Article : Google Scholar
|