Introduction
Attention deficit/hyperactivity disorder (ADHD) is a
neurodevelopmental disorder characterized by inattention and/or
hyperactive-impulsivity that interferes with brain functioning or
development. While contradictory, the existing data demonstrate
that the prevalence of ADHD may be as high as 5.3% and 2.5% in
children/adolescents and adults, respectively (1). The effects of ADHD have a significant
impact on the social life of patients throughout the entirety of
their lives, starting from disruptive behavior, resulting in poor
performance in standardized tests and impacted social interactions,
which may lead to criminal behavior, substance abuse, lack of
motivation, school exclusion and subsequent effects on professional
development in adulthood (2). In
addition, ADHD has also been found to be associated with lower a
health-related quality of life (3).
Taken together, these impairments result in high ADHD-associated
economic costs (4).
ADHD is characterized by complex alterations in the
neurobiology and neurochemistry, with impaired dopamine and
norepinephrine signaling being the most prominent (5). Previously, it was also demonstrated
that altered glutamatergic neurotransmission is involved in ADHD
pathogenesis (6). Therefore,
unraveling the potential underlying mechanisms implicated in ADHD
pathogenesis is essential for improving our understanding of the
disorder and further development of management strategies (5).
Amino acids serve a significant role in brain
development and functioning (7). In
particular, certain amino acids or their precursors, including
glutamine, glutamate and γ aminobutyric acid, are well-established
to be involved in neuronal signaling as neurotransmitters (8). Correspondingly, disruption of amino
acid metabolism results in significant neurological disorders,
particularly in early ontogenesis (9).
Given the role of an altered neurochemistry in ADHD
pathogenesis as well as the role of amino acids in
neurodevelopment, it is posited that dysregulated amino acid
metabolism may significantly interfere with ADHD. However, the
existing data is rather contradictory. An earlier study by
Bornstein et al (10) found
significantly lower plasma levels of phenylalanine (Phe), tyrosine,
tryptophan (Trp), His and isoleucine in patients with ADD compared
with the healthy controls. Zavala et al (11) showed there was a significant decrease
in plasma Phe and glutamine (Gln) levels, whereas plasma glycine
(Gly) levels were found to be elevated in patients with ADHD.
Improvement in ADHD symptoms was positively associated with
tyrosine, Phe and Trp levels (12).
At the same time, no significant alterations in blood and urinary
levels of Trp, tyrosine and Phe levels were observed in children
with ADHD (13). In view of these
inconsistencies, as well as the use of amino acid supplementation
in ADHD management, precise analyses of amino acid profiles in ADHD
is required and may assist in reconciling these contrasting
results. Thus, the objective of the present study was to evaluate
the levels of circulating serum amino acid levels in children with
ADHD.
Materials and methods
The present study was performed in accordance with
the ethical principles of the Declaration of Helsinki and its
amendments (14). The protocol of
the present study was considered and approved by the Local Ethics
Committee of Yaroslavl State University (Yaroslavl, Russia).
Parents or legal representatives signed an informed consent forms
for participation of their children in the present study prior to
investigation. Examination and blood sample collection was
performed only in the presence of the parents/guardians.
A total of 71 children (54 boys, 17 girls) diagnosed
with ADHD aged 7-14 years old (8.4±2.6 years old) were enrolled in
the present study. The diagnosis of ADHD (ICD-10: F90.0) and other
neuropsychiatric disorders (exclusion criteria) were based on the
clinical records of the outpatient department. ADHD was defined
according to ICD-10 criteria, including inattention, hyperactivity
and impulsivity (≥3 symptoms of each) (15). Only patients that did not take any
specific treatments for ADHD were included in the study, in order
to avoid the confounding effects of any potential side effects of
pharmacological treatment on amino acid metabolism.
In addition, 31 age (8.0±2.9 years old; age range
7-14 years old) and sex-matched neurotypical children (24 boys, 7
girls) were also examined, and used as the control group. The
absence of neuropsychiatric disorders was confirmed during annual
medical examinations. No significant group differences in age
(P=0.183) or sex (P=0.885) were observed between the groups.
Children and their parents were invited to
participate in the study during the annual medical examinations. A
total of 35% of all contacted subjects refused to take part in the
study (36 out of 102 children and parents).
The parents of all the examined children completed
the ADHD Rating Scale-IV for additional verification of the ADHD
diagnosis (16). Total ADHD Rating
Scale-IV scores in the ADHD patients exceeded those in neurotypical
children by a factor of >2 (14.9±9.4 vs. 7.0±5.4,
P<0.001).
Whole blood samples were collected in the morning
and after overnight fasting by cubital vein venipuncture using 9-ml
Vacuette® tubes (Greiner Bio-One International AG). The
samples were subsequently centrifuged for 10 min at 1,600 x g at
room temperature to obtain blood serum that was stored in Eppendorf
tubes at -18˚C until required for analysis.
Evaluation of serum levels of alanine, arginine
(Arg), asparagine, Asp, citrulline, glutamine (Gln), glutamate
(Glu), Gly, histidine (His), hydroxyproline (Hypro), isoleucine,
leucine, lysine (Lys), methionine, ornithine, Phe, proline (Pro),
serine, taurine, threonine, Trp, and Val was performed by
high-performance liquid chromatography (HPLC) with UV-detection at
PerkinElmer S200 (PerkinElmer, Inc.) using a reverse phase Pico Tag
Column for Free Amino Acid Analysis (3.9x300 mm) C18 (EMD
Millipore).
Precolumn derivatization with phenylisothiocyanate
reagent containing 7:1:1:1 (v/v)
methanol:triethylamine:water:phenylisothicyanate was performed
prior to the analysis. Analysis was performed with aqueous sodium
acetate and acetonitrile gradient mode with UV-detection at 240 nm.
The commercially available ClinCal® Plasma Calibrator,
lyophil., for Amino Acids (lot. no. 10213; ClinCheck) calibrators
were used for HPLC-system calibration.
Laboratory quality control was routinely performed
with reference materials of human plasma amino acid levels using
ClinChek® Plasma Control, lyophil., for Amino Acids,
Levels I (cat. no. 10280) and II (cat. no. 10281). The obtained
values for all amino acids fitted the certified control range
specified by the manufacturer (ClinCheck). The recovery rates for
the studied amino acids varied from 94-109%.
Serum amino acid concentrations are expressed as
µmol/l. In addition, total glutamatergic metabolite concentration
(Glx), defined as a sum of Glu and Gln levels, was calculated as
described previously (17). The
obtained values of serum Gln, Glu, Hypro and Pro were used for
calculating the Gln/Glu and Pro/Hypro ratios.
Statistical analysis was performed using Statistica
version 10.1 (Statsoft, Inc.). Evaluation of data distribution
performed using a Shapiro-Wilk test revealed skewed distribution of
data on amino acid levels in the study groups. Therefore, the
median and the respective interquartile range (IQR) boundaries were
used as descriptive statistics. Raw data were log-transformed for
subsequent processing. Group comparisons were performed using
analysis of covariance (ANCOVA) with adjustment for age and sex as
covariates and subsequent Bonferroni adjustments. Multiple linear
regression was performed in order to evaluate the relative
association between serum amino acid levels (independent
predictors) and ADHD (dependent variable) after adjustment for age
and sex. Correlation analysis was performed using a Spearman's rank
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference, whereas 0.05<P<0.1 was
considered nearly significant.
Results
The obtained data demonstrate that ADHD is
associated with altered amino acid profiles in children. Evaluation
of serum essential amino acids revealed 29% lower His levels in
ADHD patients compared with the neurotypical controls (Table I). No significant group differences
in other essential amino acids levels were observed.
 | Table IEssential amino acid levels in the
serum of ADHD cases and neurotypical controls. |
Table I
Essential amino acid levels in the
serum of ADHD cases and neurotypical controls.
| Amino acid | Control,
µMa | ADHD, µMb | F-value | P-value |
|---|
| Histidine | 85.0 (50-120.6) | 60.7 (45.2-94.6) | 3.140 | 0.081 |
| Isoleucine | 56.1 (47.6-63.9) | 54.1 (43.5-70.8) | 0.029 | 0.923 |
| Leucine | 118.6
(103.6-133.7) | 117.5
(96.2-138.5) | 0.105 | 0.862 |
| Lysine | 170.6
(147.5-198.4) | 161.5
(120.8-212.1) | 1.242 | 0.360 |
| Methionine | 54.2 (46.2-69.1) | 51.6 (44.1-60.2) | 0.416 | 0.396 |
| Phenylalanine | 55.8 (47.5-68.2) | 57.8 (48.4-66.4) | 0.009 | 0.923 |
| Threonine | 108.9 (90.5-129) | 111.4
(86.8-143.1) | 0.768 | 0.605 |
| Tryptophan | 56.5 (43.3-70.6) | 58.1 (44.6-71.5) | 0.011 | 0.794 |
| Valine | 167.2
(136.9-212.3) | 167.2
(128-223.4) | 0.060 | 0.907 |
Greater differences were observed in the levels of
conditionally essential and non-essential amino acids (Table II). Particularly, serum Asp and Glu
levels were 7% higher compared with the healthy controls, although
the difference was significant only in the case of Glu levels. In
contrast, serum Gln and Pro concentrations were 10% and 20% lower
in ADHD cases compared with the neurotypical controls,
respectively. In corroboration with the overall decrease in serum
Gln levels, total Glx concentration (Glu+Gln) was also 12% lower in
children with ADHD [425.5 (351.5-533.6) µM] compared with the
control values [483.1 (406.0-577.4) µM] (F=4.007; P=0.048).
 | Table IIConditionally essential and
non-essential amino acid levels the serum of children with ADHD and
healthy controls. |
Table II
Conditionally essential and
non-essential amino acid levels the serum of children with ADHD and
healthy controls.
| Amino acid | Control,
µMb | ADHD,
µMb | F-value | P-value |
|---|
| Alanine | 323.5
(248.3-395.5) | 342.7
(298.1-401.9) | 0.779 | 0.342 |
| Arginine | 68.9
(55.7-85.8) | 66.5
(55.9-81.6) | 0.008 | 0.988 |
| Asparagine | 77.8
(67.7-85.0) | 78.5
(64.8-91.6) | 0.032 | 0.872 |
| Aspartate | 13.9
(10.5-17.9) | 14.9
(11.1-20.3) | 2.335 | 0.103 |
| Glutamine | 433.4
(379.5-534.4) | 389
(312.8-474.7) | 5.935 | 0.024a |
| Glutamate | 39.3
(27.2-46.5) | 42.0
(34.2-53.5) | 4.130 | 0.039a |
| Glycine | 418.4
(374.7-455.2) | 396.7
(340.5-469.7) | 0.181 | 0.724 |
| Proline | 316.3
(219.0-418.1) | 254.6
(207.2-319.8) | 3.690 | 0.045a |
| Serine | 83.2
(68.7-92.8) | 82.5
(66.1-97.0) | 0.034 | 0.934 |
| Taurine | 64.5
(50.6-82.1) | 66.4
(55.6-82.7) | 0.709 | 0.416 |
| Tyrosine | 82.9
(72.6-98.3) | 85.5
(75.7-101.0) | 0.090 | 0.718 |
| Citrulline | 54.1
(42.1-70.1) | 50.9
(42.4-64.9) | 0.548 | 0.541 |
| Ornithine | 58.2
(46.5-71.1) | 60.4
(45.8-83.0) | 0.068 | 0.882 |
| Phosphoserine | 54.7
(26.9-89.8) | 50.3
(31.5-66.1) | 0.502 | 0.435 |
| Hydroxyproline | 11.4
(1.6-18.7) | 16.2
(11.3-20.8) | 4.752 | 0.018a |
Amongst the amino acid derivatives analyzed, only
serum Hypro concentrations were significantly elevated, being 42%
higher in ADHD cases compared with the healthy controls.
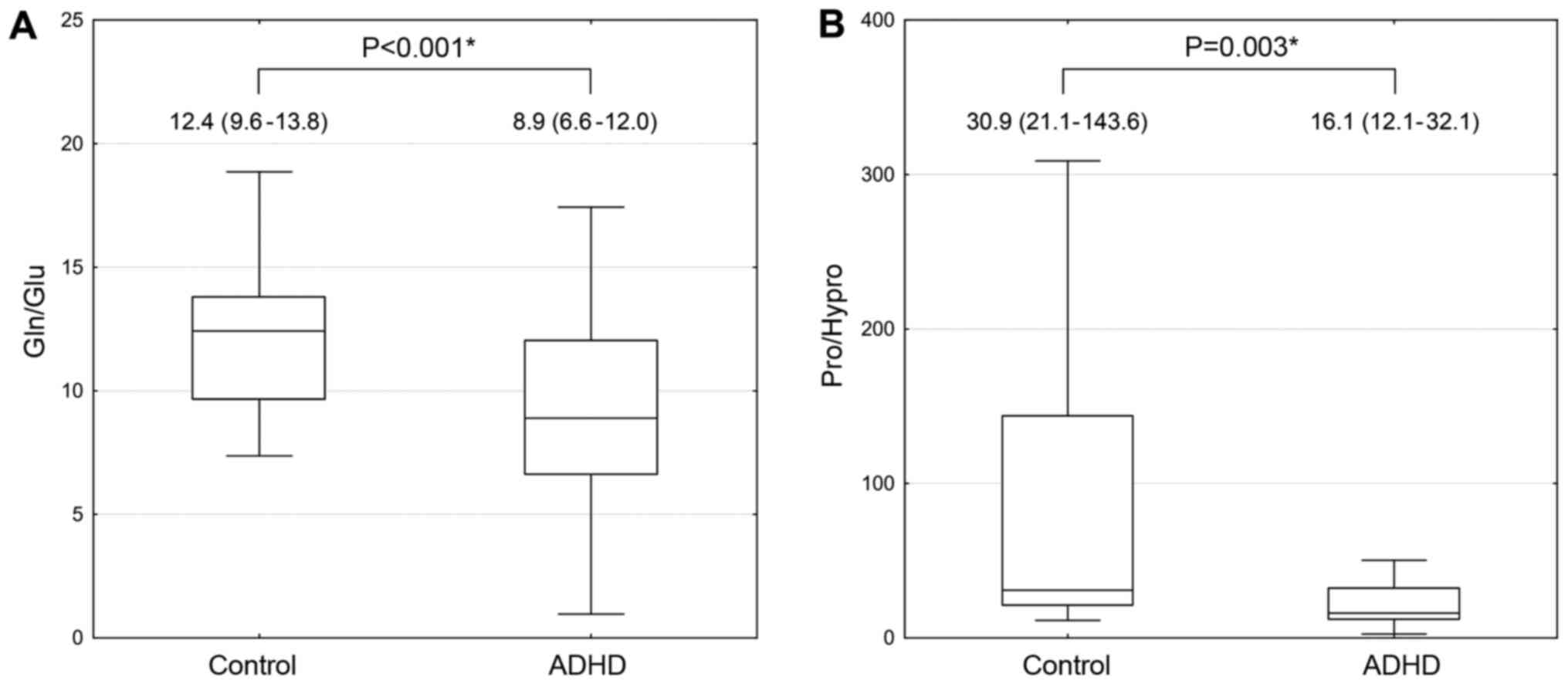
Given the observed group differences between serum
Gln and Glu levels, as well as the levels of Pro and its derivative
Hypro, Gln-to-Glu and Pro-to-Hypro ratios were evaluated (Fig. 1). The obtained data demonstrate that
Gln/Glu values in ADHD cases were 28% lower compared with the
healthy children (F=14.202). In turn, ADHD patients had almost
2-fold lower levels of Pro/Hypro levels compared with the
neurotypical controls (F=8.936).
Correlation analysis demonstrated that serum Hypro
and Glu concentrations were correlated significantly (r=0.270;
P=0.006) and nearly significantly (r=0.194; P=0.051) with total
ADHD-RS scores, respectively. Concomitantly, circulating Gln levels
(r=-0.207; P=0.037) as well as Gln/Glu ratio (r=-0.376; P<0.001)
were characterized by a significant direct correlation with the
latter.
Multiple regression analysis was also performed in
order to determine if there was an independent association between
the serum amino acid levels and total ADHD-RS scores (Table III). In a crude model incorporating
all amino acids analyzed, serum Gln and Lys levels were found to be
inversely associated with total ADHD-RS scores. The overall model
trended towards statistically significant (P=0.072), accounting for
only 12% of total ADHD-RS scores. In a model incorporating amino
acids considered to be significantly and almost significantly
associated with ADHD scores (Model 2), serum Gln and Lys remained
significantly associated with ADHD, whereas circulating Glu levels
appeared to be positively associated with total ADHD-RS scores.
Serum Hypro levels were almost significantly associated with ADHD
scores. Although the predictive value of Model 2 was significant,
it accounted for only 17% of score variability. Neither age nor sex
were associated significantly with ADHD in both regression
models.
 | Table IIIMultivariate linear regression
analysis of the association between serum amino acid levels
(independent predictor) and attention deficit/hyperactivity
disorder Rating Scale-IV scores (dependent variable). |
Table III
Multivariate linear regression
analysis of the association between serum amino acid levels
(independent predictor) and attention deficit/hyperactivity
disorder Rating Scale-IV scores (dependent variable).
| | Model 1 | Model 2 |
|---|
| Amino acid | β | P-value | β | P-value |
|---|
| Ala | 0.140 | 0.460 | 0.050 | 0.724 |
| Asp | -0.009 | 0.965 | -0.077 | 0.596 |
| Gln | -0.353 | 0.022a | -0.370 | 0.002b |
| Glu | 0.258 | 0.206 | 0.350 | 0.029a |
| His | -0.150 | 0.218 | -0.057 | 0.583 |
| Hypro | 0.227 | 0.111 | 0.211 | 0.065 |
| Lys | -0.527 | 0.027a | -0.339 | 0.021a |
| Orn | 0.281 | 0.087 | 0.161 | 0.248 |
| Pro | -0.051 | 0.643 | -0.082 | 0.391 |
| Thr | 0.327 | 0.081 | 0.169 | 0.214 |
| Multiple R | 0.576 | 0.518 | | |
| Multiple
R2 | 0.332 | 0.268 | | |
| Adjusted
R2 | 0.100 | 0.170 | | |
| P for the
model | 0.116 | 0.004b | | |
Discussion
The results of the present study demonstrated that
children with ADHD were characterized by distinct amino acid
profiles compared with the controls, indicative of predominant
alteration in Glu, Pro and Lys metabolism. Significant group
differences in Glu/Gln ratio may be indicative of altered
neurotransmission in children with ADHD, whereas high Hypro levels
and a high Hypro/Pro ratio may be considered as a marker of
collagen catabolism and connective tissue pathology.
Existing data demonstrate that alterations in
glutamatergic signaling may contribute significantly to ADHD
pathogenesis (6). Specifically, it
has been demonstrated that ADHD patients are characterized by
significantly lower Gln plus Glu levels in basal ganglia (18), including in the striatum (19). An increase in anterior cingulate
cortex Glu content was also associated with hyperactivity and
impulsivity in adult ADHD patients (20). Pertinent genome-wide analyses for
ADHD risk genes revealed altered expression profiles of genes
associated with glutamatergic neurotransmission (21). Correspondingly, altered
AMPAR-mediated transmission in pyramidal neurons of the prefrontal
cortex was also found to be associated with ADHD in an experimental
rat model (22).
In view of the role of Hypro as a marker of
connective tissue pathology (23),
the earlier proposed association between ADHD and joint
hypermobility (24) may underlie the
observed increase in plasma Hypro levels in children with ADHD.
Particularly, joint hypermobility was found to be >2-fold more
prevalent in ADHD subjects compared with the reference population
(25). Moreover, connective tissue
disorders are also closely associated with other neurodevelopmental
disorders (26). These data also
corroborate our earlier findings on increased Hypro levels in
autism spectrum disorder (27) and
cerebral palsy (28). In addition,
in ADHD subjects, Pro levels were found to be reduced with a
decreased Pro/Hypro ratio, and levels were found to be inversely
associated with S100B and positively related to IL-10 levels
(29), indicative of the potential
contribution of dysregulated Pro metabolism in this
neurodevelopmental disorder.
The results of serum amino acid profiling also
demonstrated group differences in serum His, Asp and Lys levels
between ADHD patients and neurotypical controls. Although an
essential role of His in brain physiology has been demonstrated
(30), data on the relationship
between ADHD and His metabolism are insufficient. His
supplementation was shown to reduce fatigue and improve mental
performance in subjects with high fatigue and sleep disruption
scores (31). In turn, experimental
data demonstrated that a His-deficient diet resulted in formation
of anxiety-like behaviors in mice through reduction of brain
histamine levels (32). In turn,
increased serum Asp levels in patients with ADHD generally
correspond with an earlier observed higher Asp intake in ADHD
patients (33).
It is noteworthy that the present study revealed a
significant association between lower serum Lys levels and ADHD.
Despite the role of Lys metabolism in brain physiology (34), earlier data on the involvement of Lys
in ADHD pathophysiology are lacking. Nonetheless, Lys
supplementation has been used in ADHD management (35), based on observations that Lys as well
as Arg treatments reduce anxiety in stressed adults characterized
by high cortisol levels (36).
In contrast to earlier reports (11,12),
significant group differences in tyrosine, phenylalanine or Trp
levels were not observed in the present study, in agreement with a
study by Bergwerff et al (13) who did not reveal any significant
group differences in serum and urinary levels of the studied amino
acids (13).
The present study also has several limitations.
First, this was a cross-sectional study involving a relatively
small number of patients, and studying larger cohorts of both cases
and controls may improve the statistical power of the results.
Second, follow-up design of the study with evaluation of ADHD
incidence and severity would be beneficial to provide an insight
into the causal relationships between ADHD and the observed
alterations in amino acid metabolism. Third, although the results
demonstrate the potential alterations of amino acid-derived
neurotransmitters in patients with ADHD, serum levels do not
necessarily reflect brain levels of amino acids and their
derivatives. Therefore, evaluation of cerebrospinal fluid amino
acid levels or the use of techniques for direct brain metabolite
assessment, such as proton magnetic resonance spectroscopy, would
assist in highlighting ADHD-associated alterations in metabolism of
brain amino acids and neurotransmitters.
In conclusion, the results of the present study
demonstrated significant alterations in the serum amino acid
profile of children with ADHD. The observed alterations of
Pro/Hypro and Gln/Glu levels and their ratios may be associated
with the coexisting connective tissue pathology and alterations of
glutamatergic neurotransmission in ADHD, respectively. Altered
circulating levels of His, Lys and Asp may also be implicated in
the pathogenesis of ADHD. However, further in vitro and
in vivo studies are required to investigate the specific
underlying mechanisms linking amino acid metabolism with ADHD
pathogenesis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by funding from Russian
Foundation for Basic Research (RFBR) (grant no. 19-013-00528).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AVS, MGS and AAT conceived the study. AVS, AAT, AT,
DAS and MA designed the study. AVS, AAT, AAS, ALM, IPZ, YNL and MGS
performed the experiments. ALM, IPZ, AVS, AT and DAS analyzed the
data. ALM, AAT, AAS, IPZ, YNL, AVS, MGS, AT, DAS and MA wrote and
edited the manuscript. All authors have read and approved the final
manuscript. AVS, MGS and AAT confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present study was performed in accordance with
the ethical principles of the Declaration of Helsinki and its
amendments (2013). The protocol of the present study was considered
and approved by the Local Ethics Committee (Yaroslavl State
University, Yaroslavl, Russia). Parents or legal representatives
signed an informed consent form for participation of their children
in the present study prior to investigation. Examination and blood
sample collection was performed only in the presence of the
parents/guardians.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Posner J, Polanczyk GV and Sonuga-Barke E:
Attention-deficit hyperactivity disorder. Lancet. 395:450–462.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harpin VA: The effect of ADHD on the life
of an individual, their family, and community from preschool to
adult life. Arch Dis Child. 90 (Suppl 1):2–7. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Peasgood T, Bhardwaj A, Biggs K, Brazier
JE, Coghill D, Cooper CL, Daley D, De Silva C, Harpin V, Hodgkins
P, et al: The impact of ADHD on the health and well-being of ADHD
children and their siblings. Eur Child Adolesc Psychiatry.
25:1217–1231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Barkley RA: The high economic costs
associated with ADHD. ADHD Rep. 28:10–12. 2020.
|
|
5
|
Mehta TR, Monegro A, Nene Y, Fayyaz M and
Bollu PC: Neurobiology of ADHD: A review. Curr Dev Disord Rep.
6:235–240. 2019.
|
|
6
|
Levy F and de Leon J: Dopamine ADHD/OCD
theories: Is glutamine part of the story? Neurotransmitter (Houst).
2(e891)2015.
|
|
7
|
Wu G: Functional amino acids in growth,
reproduction, and health. Adv Nutr. 1:31–37. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valenzuela CF, Puglia MP and Zucca S:
Focus on: Neurotransmitter systems. Alcohol Res Health. 34:106–120.
2011.PubMed/NCBI
|
|
9
|
Saudubray JM and Garcia-Cazorla A: An
overview of inborn errors of metabolism affecting the brain: From
neurodevelopment to neurodegenerative disorders. Dialogues Clin
Neurosci. 20:301–325. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bornstein RA, Baker GB, Carroll A, King G,
Wong JT and Douglass AB: Plasma amino acids in attention deficit
disorder. Psychiatry Res. 33:301–306. 1990.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zavala M, Castejón HV, Ortega PA, Castejón
OJ, Marcano de Hidalgo A and Montiel N: Imbalance of plasma amino
acids in patients with autism and subjects with attention
deficit/hyperactivity disorder. Rev Neurol. 33:401–408.
2001.PubMed/NCBI
|
|
12
|
Stern M, Perez L, Johnstone J, Gracious B,
Leung B, Tost G, Arnold E, Hatsu I and Kopec R: Using Metabolomics
to classify the underlying effects of multi-nutrient
supplementation in ADHD youth. Curr Dev Nutr. 4 (Suppl
2)(1351)2020.
|
|
13
|
Bergwerff CE, Luman M, Blom HJ and
Oosterlaan J: No tryptophan, tyrosine and phenylalanine
abnormalities in children with attention-deficit/hyperactivity
disorder. PLoS One. 11(e0151100)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
General Assembly of the World Medical
Association. World Medical Association Declaration of Helsinki:
Ethical principles for medical research involving human subjects. J
Am Coll Dent. 81:14–18. 2014.PubMed/NCBI
|
|
15
|
International Statistical Classification
of Diseases and Related Health Problems 10th Revision (ICD-10):
Chapter V Mental and behavioural disorders (F00-F99). Behavioural
and emotional disorders with onset usually occurring in childhood
and adolescence (F90-F98). https://icd.who.int/browse10/2019/en#/F90-F98.
Accessed December 8, 2020.
|
|
16
|
DuPaul GJ, Power TJ, Anastopoulos AD and
Reid R: ADHD Rating Scale-IV: Checklists, norms, and clinical
interpretation. Guilford Press, 1998.
|
|
17
|
Goff DC, Hennen J, Lyoo IK, Tsai G, Wald
LL, Evins AE, Yurgelun-Todd DA and Renshaw PF: Modulation of brain
and serum glutamatergic concentrations following a switch from
conventional neuroleptics to olanzapine. Biol Psychiatry.
51:493–497. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Maltezos S, Horder J, Coghlan S, Skirrow
C, O'Gorman R, Lavender TJ, Mendez MA, Mehta M, Daly E, Xenitidis
K, et al: Glutamate/glutamine and neuronal integrity in adults with
ADHD: A proton MRS study. Transl Psychiatry. 4(e373)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Carrey NJ, MacMaster FP, Gaudet L and
Schmidt MH: Striatal creatine and glutamate/glutamine in
attention-deficit/hyperactivity disorder. J Child Adolesc
Psychopharmacol. 17:11–17. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bauer J, Werner A, Kohl W, Kugel H,
Shushakova A, Pedersen A and Ohrmann P: Hyperactivity and
impulsivity in adult attention-deficit/hyperactivity disorder is
related to glutamatergic dysfunction in the anterior cingulate
cortex. World J Biol Psychiatry. 19:538–546. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lesch KP, Merker S, Reif A and Novak M:
Dances with black widow spiders: Dysregulation of glutamate
signalling enters centre stage in ADHD. Eur Neuropsychopharmacol.
23:479–491. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng J, Liu A, Shi MY and Yan Z:
Disrupted glutamatergic transmission in prefrontal cortex
contributes to behavioral abnormality in an animal model of ADHD.
Neuropsychopharmacology. 42:2096–2104. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Srivastava AK, Khare P, Nagar HK,
Raghuwanshi N and Srivastava R: Hydroxyproline: A potential
biochemical marker and its role in the pathogenesis of different
diseases. Curr Protein Pept Sci. 17:596–602. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baeza-Velasco C, Sinibaldi L and Castori
M: Attention-deficit/hyperactivity disorder, joint
hypermobility-related disorders and pain: Expanding body-mind
connections to the developmental age. Atten Defic Hyperact Disord.
10:163–175. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Doğan ŞK, Taner Y and Evcik D: Benign
joint hypermobility syndrome in patients with attention
deficit/hyperactivity disorders. Arch Rheumatol. 26:187–192.
2011.
|
|
26
|
Baeza-Velasco C, Grahame R and Bravo JF: A
connective tissue disorder may underlie ESSENCE problems in
childhood. Res Dev Disabil. 60:232–242. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Skalny AV, Skalny AA, Lobanova YN,
Korobeinikova TV, Ajsuvakova OP, Notova SV, Burtseva TI, Skalnaya
MG and Tinkov AA: Serum amino acid spectrum in children with autism
spectrum disorder (ASD). Res Autism Spectr Disord.
77(101605)2020.
|
|
28
|
Tinkov AA, Skalnaya MG and Skalny AV:
Serum trace element and amino acid profile in children with
cerebral palsy. J Trace Elem Med Biol. 64(126685)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oades RD, Dauvermann MR, Schimmelmann BG,
Schwarz MJ and Myint AM: Attention-deficit hyperactivity disorder
(ADHD) and glial integrity: S100B, cytokines and kynurenine
metabolism - effects of medication. Behav Brain Funct.
6(29)2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Holeček M: Histidine in health and
disease: Metabolism, physiological importance, and use as a
supplement. Nutrients. 12(848)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sasahara I, Fujimura N, Nozawa Y, Furuhata
Y and Sato H: The effect of histidine on mental fatigue and
cognitive performance in subjects with high fatigue and sleep
disruption scores. Physiol Behav. 147:238–244. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoshikawa T, Nakamura T, Shibakusa T,
Sugita M, Naganuma F, Iida T, Miura Y, Mohsen A, Harada R and Yanai
K: Insufficient intake of L-histidine reduces brain histamine and
causes anxiety-like behaviors in male mice. J Nutr. 144:1637–1641.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Holton KF, Johnstone JM, Brandley ET and
Nigg JT: Evaluation of dietary intake in children and college
students with and without attention-deficit/hyperactivity disorder.
Nutr Neurosci. 22:664–677. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hallen A, Jamie JF and Cooper AJ: Lysine
metabolism in mammalian brain: An update on the importance of
recent discoveries. Amino Acids. 45:1249–1272. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mikirova NA, Rogers AM, Taylor PR,
Hunninghake RE, Miranda-Massari JR and Gonzalez MJ: Metabolic
correction for attention deficit/hyperactivity disorder: A
biochemical-physiological therapeutic approach. Funct Food Health
Dis. 3:1–20. 2013.
|
|
36
|
Smriga M, Ando T, Akutsu M, Furukawa Y,
Miwa K and Morinaga Y: Oral treatment with L-lysine and L-arginine
reduces anxiety and basal cortisol levels in healthy humans. Biomed
Res. 28:85–90. 2007.PubMed/NCBI View Article : Google Scholar
|