Introduction
The skin is the largest organ, protecting the body
from external stimuli and preventing foreign matter from entering
the body. Sunlight is necessary for human life, but certain
components of sunlight can damage the skin. According to Halliday
et al (1) exposure to even
low doses of sunlight during everyday activities can suppress
immunity. UV irradiation is known to cause inflammation and thus
increases the risk of skin cancer with an enhanced frequency of
mutations (2-4).
For example, according to Martincorena et al (5), a substantial number of somatic
mutations (from 2-6 mutations per megabase per cell) were observed
in the sun-exposed epidermis (5).
This mutational frequency is similar to that seen in several types
of cancer and reflects the characteristic damage caused by UV
exposure (5). Studies of perceived
age, a valid biomarker of systematic aging (6,7),
suggest that exposure to UV can expedite the aging process
(8,9). Sunscreens can protect the skin from
UV-facilitated aging (10). Fatty
acids are also promising for photoprotection according to a
previous report. showing that the topical application of
eicosapentaenoic acid inhibited UV-induced epidermal damage
(11). Although sunscreens are
effective in protecting the skin against UV-induced aging (11), they fail to protect against
sub-erythemal UV exposure, resulting in severe DNA damage when
applied unevenly or insufficiently around the eyes, where topical
application is awkward (12).
UV exposure can cause DNA damage, either directly or
via active oxygen, to induce an inflammatory reaction (sunburn) and
prompt the release of inflammatory mediators (13). DNA damage also induces apoptosis - a
process of regulated cell death (14). The UV-induced damage also leads to
oxidative stress, but it complementarily activates nuclear factor
erythroid 2-related factor 2 (Nrf2), thus inducing Phase-II
drug-metabolizing enzymes (15).
The expression of γ-glutamylcysteine synthetase, the rate-limiting
enzyme in the production of reduced glutathione, is mediated by
Nrf2 expression (15). It has been
highlighted that certain food components influence the inflammasome
possibly through Nrf2-related enzymes (16).
Previous studies suggested that antioxidant
supplementation (such as vitamins C and E) could help combat
reactive oxygen species (17,18),
and augment the protective effects of sunscreens (19). Other effective treatments against UV
damage include green tea, coffee, fruit, and other products rich in
antioxidants (20-22);
some carotenoids have been reported to show similar effects
(17,23,24). A
systematic review of Lycii Fructus, namely goji berries (Lycium
barbarum and Lycium chinense), found that these plants
have been used as a folk medicine and traditional foods for
centuries (25). The body of
research demonstrated that Lycii Fructus is effective for enhancing
kidney and liver function, protecting ocular health, and boosting
immunity (25). Lycii Fructus is
rich in polysaccharides, water-soluble vitamins, carotenoids, and
polyphenols such as flavonoid glycosides and phenolic acids
(26). As noted in a review by
Ulbricht et al (25), some
of these components exhibit antioxidative activity and are able to
inhibit UVB-induced cell death (25). This view is supported by Amagase and
Farnsworth (26), who examined the
effects of goji berries on a man who had a pruriginous eruption on
a sun-exposed area of skin (27).
This study demonstrated that the man's minimal erythema dose for
UVB was decreased following intake of the goji berries (27). A study using rodents also showed
that orally consumed goji berry juice inhibited UV damage in mice
(28). In a study on human
participants, Kuwazuru et al (29) examined the effects of
supplementation containing goji berries or their ethanol extracts
(29). The results indicated that
the supplementation was effective in inhibiting UV-induced erythema
formation. However, the study was limited by its small size, and
the trial was not randomized.
In the present randomized, double-blind,
placebo-controlled clinical trial study, we examined the effects of
LFE on UV-induced epidermal damage to confirm whether LFE is
effective for inhibiting erythema formation. Considering previous
reports, 44 participants were recruited and 22 participants were
randomized to each treatment (17,21-24).
We also investigated the possibility that LFE exerts antioxidative
effects in vivo as LFE has been reported to facilitate
cytoprotective gene expression and enhance the production of
glutathione in hepatocytes (30).
To elucidate which polyphenols participate in protection against
UVB-induced skin damage, we examined the effects of the LFE
components on antioxidative capacity after human keratinocytes were
directly exposed to UV.
Patients and methods
Study design
The present study was a randomized,
placebo-controlled, double-blinded study, with a parallel group
test conducted by Derma Labo, Inc. This trial conformed to the
Declaration of Helsinki (31) and
was approved by the Ethics Committee of Tactics (Hokkaido
Activation Center) on December 14, 2016 (approval no. 2016-100).
Written informed consent was obtained from all potential
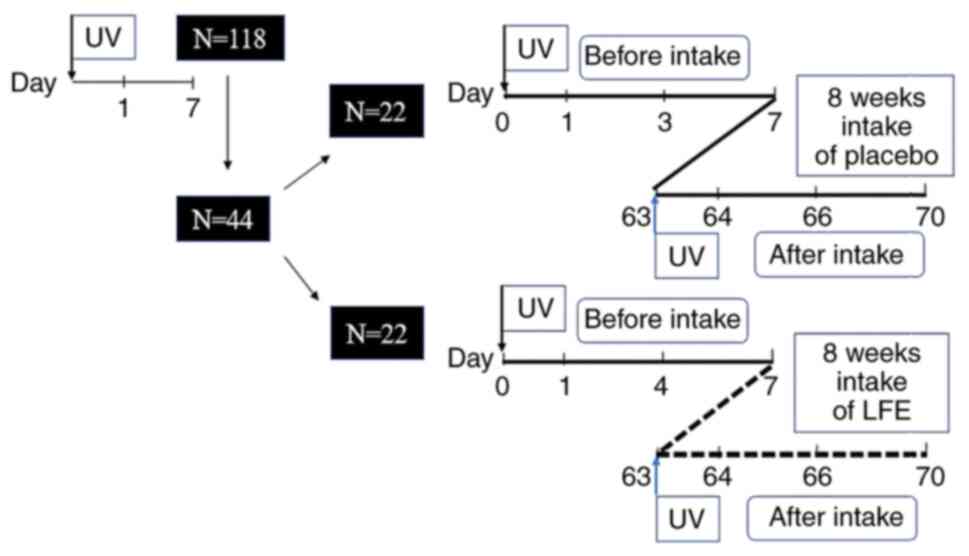
participants prior to participant selection. Fig. 1 shows the test schedule. The test
was conducted between January 16 and May 1, 2017.
The individual in charge of test materials
allocation performed random allocation, such that the primary
background factors were not biased amongst each group. The
allocation of the test materials was performed based on an
allocation table (key code) prepared from a random number table in
advance by the individual in charge of the test materials
allocation. The allocation table was sealed by the person in charge
of test materials allocation and kept secret until opening. After
confirming that all data was fixed, the individual in charge of
test materials' allocation opened the key after confirming the
sealing status of the allocation table and emergency key code. The
individual in charge of test materials allocation submitted the
allocation table after opening the key and created a record.
Participants
The purpose of the study was to clarify the effects
of LFE ingestion on UVB-induced skin erythema. After screening,
participants were recruited from among 118 individuals in the
Exam's volunteer bank, which was managed by Derma Labo. Inc, who
agreed to participate. Participants were Japanese nationals aged
between 20 and 60 years of age.
Individuals were excluded from the study if any of
the following circumstances applied: i) Pigmentation, inflammation,
or other significant reactions were present at the skin site; ii)
the participant had a history of photo sensitivity or presented
symptoms of such; iii) the participant was suspected to be allergic
to the test materials (for example, the participant had experienced
allergic reactions to the applied food in the past); iv) sunburn
had occurred in the skin site during the 3 months preceding the
study; v) they had chronic skin conditions (such as atopic
dermatitis) present at the skin site; vi) the participant was
pregnant (or planned to get pregnant during the study) or
lactating; vii) the participant had asthma or a similar chronic
condition and regularly took medication for the condition; viii)
the participant was taking medication that may affect the test
results, including medication for freckles, UV-induced
pigmentation, or liver spots (e.g., drugs containing L-cysteine,
vitamin C, or tranexamic acid); ix) during the 3 months preceding
the study, the participant was regularly taking (>5 days a week)
skin whitening supplements or other nutritional supplement with
claims of antioxidant properties (e.g., supplements rich in
catechins, flavonoids, or polyphenols); x) the participant was
currently undergoing, or had undergone during the past three
months, a trial that involved the ingestion of food or drugs, the
use of cosmetics, or something similar; xi) the participant was a
regular smoker; xii) the participant had applied topical medication
to the skin site during the past week; xiii) the participant
intended to travel abroad or to swim in the sea during the study
period (between screening and end of the study); xiv) the
participant was undergoing hormone replacement therapy following
the start of menopause or a menopausal disorder; and/or xv) the
participant was otherwise deemed ineligible for the study by the
physician-in-charge or principal investigator.
During the study, participants were required to
adhere to the following rules and prohibitions: i) Participants
were not allowed to apply topical medication to the skin site, take
medication for freckles, UV-induced pigmentation, or liver spots
(e.g., drugs containing L-cysteine, vitamin C, or tranexamic acid),
or use non-medical drugs (officially known in Japan as
ʻquasi-drugsʼ) or cosmetics. Participants were permitted to use
personal hygiene products such as soap and shower gel, but they
were not allowed to switch these products during the study period.
If participants unavoidably needed to use the above medication,
quasi-drugs, or cosmetics, they were instructed to report the
product name, volume used, period used, and reason for use in a
journal; ii) participants were not allowed to take medication (or
apply topical medication) or take or apply newly designated
ʻquasi-drugsʼ or traditional Chinese medicines; iii) participants
were not allowed to ingest newly designated health foods or foods
carrying health or nutritional claims (officially known in Japan as
ʻfood for specified health usesʼ or ʻfood with function claimsʼ).
If participants unavoidably needed to eat such foods, they were
instructed to either report the product name, ingredients, and
volume consumed in their journal or to affix the label (or a copy
thereof) to their records. Participants were also instructed to
report the name and amount consumed of any health food, ʻfood for
specified health uses,ʼ or ʻfood with function claimsʼ that they
were regularly consuming; iv) For a 2-week period preceding the
study, participants had to refrain from any activity that could
cause sunburn, such as participating in outdoor sports or using a
tanning bed. Participants were asked to take steps to avoid UV
exposure altogether (from both indoor and outdoor sources). They
were instructed, for example, to wear sun-protective hats and
clothing and to use sunscreen. If participants nonetheless suffered
sunburn, they were instructed to report the time, duration of
exposure, and severity (e.g., ʻskin red, feels soreʼ) in their
journal; v) on the evening prior to a test day, participants were
required to bathe or shower before going to bed. They were not
allowed to bathe or shower before testing on the test day; vi) on a
test day, participants were not allowed to engage in intense
exercise until the test was over; vii) on a test day, participants
were not allowed to consume any spicy food or drink such as curry,
chilies, or hot sauce (e.g., Tabasco); vii) participants were not
allowed to scrub the skin site with abrasive personal hygiene
products; ix) for a 1-month period preceding the study,
participants were not allowed to undergo beauty treatments such as
a chemical peel or spa treatment; and x) participants were not
allowed to start any regular supplement regimens.
Test materials
The supplement used in this study was a tablet
containing LFE, which was provided by Matsuura Yakugyo Co., Ltd.,
and consisted of microcrystalline cellulose, carmellose calcium,
and calcium stearate. This supplement was comparable to a placebo
containing the same components, except that the LFE was substituted
with potato starch (Table I). A
total of six LFE tablets (the daily dose) contained 900 mg LFE; the
amount of rutin and chlorogenic acid contained in the extract of
LFE in this human study was 926 and 876 µg/day, respectively, with
966 mg microcrystalline cellulose, 36 mg carmellose calcium, and 18
mg calcium stearate. A total of 6 placebo tablets contained 933 mg
microcrystalline cellulose, 36 mg carmellose calcium, 18 mg calcium
stearate, and 933 mg potato starch. The LFE and placebo tablets
were supplied by Shiseido Pharmaceutical. The tablets were
administered orally at a rate of six per day for 8 weeks.
 | Table IComposition of LFE and placebo
tablets. |
Table I
Composition of LFE and placebo
tablets.
| Component | LFE tablets, mg/6
tabletsa | Placebo tablets,
mg/6 tabletsa |
|---|
| LFE | 900 | - |
| Microcrystalline
cellulose | 966 | 933 |
| Carmellose
calcium | 36 | 36 |
| Calcium
stearate | 18 | 18 |
| White potato | - | 933 |
| Total | 1920 | 1920 |
Screening
The candidates reported their skin type with
reference to the Fitzpatrick scale (32). Those with Type I (always burns,
never tans) and Type II (usually burns, tans minimally) were
selected for screening. Solar Light's Model 601 Multiport solar
simulator was used to produce six stages of erythema on the
selected candidates' backs at increments of ~20% (11.4, 15.0, 19.2,
21.9, 27.6, and 30.6 mJ/cm2). Each irradiated surface
area was 0.5 cm2 (Φ=8 mm). UV irradiance was measured
using a Solar Light's PMA2100 radiometer and PMA2108 biologically
weighted UV-B detector. The following day, the irradiated areas
were examined to determine each participant's MED. Minimal tanning
dose (MTD) was determined on the 7th day following irradiation.
Based on the MED and MTD results, 44 candidates were selected for
participation in the study. These candidates were randomly assigned
to be divided into two groups to avoid bias in major background
factors among the groups. Allocation of test materials was carried
out based on the allocation table prepared in advance from the
random number table. The assignment table was sealed by the person
in charge of the trial food assignment and was strictly stored
until opening.
Other screening processes included a lifestyle
survey [with items on medical history, present symptoms, lifestyle,
medication, use of cosmetics and supplements, and physical
characteristics such as height (cm), weight (kg), BMI, systolic
blood pressure (mmHg), diastolic blood pressure (mmHg), and pulse
(beats/min)], a general biochemical blood test, a blood test for
in vitro antioxidant potential test, and a questionnaire
about diet (brief-type self-administered diet history
questionnaire) (33).
Induction of erythema and measurement
of skin color
Of the candidates judged eligible to participate by
the physician-in-charge, 44 individuals were finally selected. All
the candidates were highly UV-sensitive (8 men, 36 women; median
age was 50 years old; age range 22 to 59 years).
During the study, MED was determined as follows.
Before and after an 8-week course of the active agent or placebo
(intervention), the skin sites were exposed to six stages of
irradiation as in the screening. A total of 24 h after irradiation,
the pigmentation in the testing sites was measured, and the
pigmentation after intervention was compared with that before
intervention.
Three skin sites on the back were irradiated. The
irradiated UV intensity was 1.5x higher than that of the
participant's MED. The participants were divided into two groups:
An experimental (LFE) group and control (placebo) group. To control
for MED differences, the participants were assigned to groups such
that the groups had a similar average MED. Before intervention,
pigmentation was measured four times: Before irradiation, and on
days 1, 3, and 7 after irradiation. The participants then took
their tablets for 8 weeks; those in the LFE group took the
supplement containing LFE and those in the placebo group took the
placebo. After the 8-week intervention, the participants underwent
irradiation with the UV intensity at 1.5x their MED. As before,
pigmentation was measured before irradiation, and on days 1, 3, and
7 after irradiation (Fig. 1).
The devices used to measure skin pigmentation were a
Minolta CR-200 Chromameter (Konica Minolta), a Mexameter MX 16
(Courage + Khazaka Electric GmbH), and a C-Cube (Pixience SA)
(34). The measurements were
conducted in a room with a constant temperature (23±2˚C) and
humidity (45±5%), and the participants were given 20 mins to
acclimatize themselves to the room before measurement. Two skin
hydration values were measured: Stratum corneum hydration (SCH) and
transepidermal water loss (TEWL). The former was measured using a
Corneometer CM825 (Courage + Khazaka Electric GmbH). Each
irradiated site was measured five times and the average SCH was
determined as arbitrary units (AU). Non-irradiated sites were
measured as well to provide a control value.
The latter value, TEWL, was measured using a
Tewameter TM300 (Courage + Khazaka Electric GmbH). The TM300 was
placed on the target skin site for 15 secs and then activated for
20 secs. Five readings were taken after 10 secs following
activation, and the average (where the deviation was least) was
defined as the TEWL (g/hm2).
Analyses of antioxidants and oxidative
markers in serum
Blood samples were taken just before trial and at 8
weeks. In each case, the samples were taken before irradiation by a
nurse under a physician's supervision. The blood sample was used to
determine the in vivo antioxidative capacity. Each sample
was collected using a vacuum blood collection tube with a
separating agent. The blood was centrifugally separated for 15 min
at 2,000 x g at room temperature. The separated blood was then
stored at -30˚C before measurement. Antioxidant volume was used as
a measure of antioxidative capacity. The blood samples were sent to
Hoken Kagaku's Sapporo laboratory for biochemical and hematological
analysis. Four markers of in-serum antioxidative capacity were
used. The first was total glutathione disulfide (GSH+GSSG),
measured using an OxiSelect total glutathione assay kit (cat. no.
STA-312). The second was carbonylated protein, measured using an
OxiSelect protein carbonyl ELSIA kit (cat. no. STA-310-T). The
third was lipid peroxides, measured using an OxiSelect
8-iso-prostaglandin F2 α Elisa kit (cat. no. STA-337). These three
OxiSelect devices were provided by Cell Biolabs. The fourth marker
was 8-hydroxy-2-deoxyguanosine (8-OHdG), measured using a highly
sensitive 8-OHdG check ELISA kit (cat. no. KOG-HS10E) available on
the market by the Japan Institute for the Control of Aging (Nikken
Seil Co. Ltd.).
Daily diet
The participants' daily diet was ascertained using
the brief-type self-administered diet history questionnaire (BDHQ).
The results were analyzed by the survey provider's support center
(the ʻDHQ Support Centerʼ).
Analysis of antioxidative effect on
keratinocytes
An immortalized human keratinocyte cell line (HaCaT)
was used to analyze the antioxidative effect. HaCaT cells were
obtained from Deutsches Krebsforschungszentrum with a material
transfer agreement. The HaCaT cells were cultured using DMEM
(Thermo Fisher Scientific Inc.) supplemented with 10% FBS (Thermo
Fisher Scientific Inc.) and antibiotics (50 units/ml penicillin and
50 µg/ml streptomycin, both provided on the market by Fujifilm Wako
Pure Chemical Industries). Alongside LFE, two reagents were used on
the HaCaT cells: Chlorogenic acid and quercetin, both of which were
provided by Sigma-Aldrich; Merck KGaA. To obtain the LFE, hydrous
ethanol was dehydrated, and the remnants were dissolved in DMSO
(final concentration 0.1% (v/v). The protective effect of LFE was
measured in terms of cell viability after exposure to UVB. The
HaCaT cells were treated with the reagent for 24 h 1 day after
pre-culture. Then, the culture medium was replaced with Hank's
Balanced Salt Solution (Thermo Fisher Scientific Inc.) and exposed
to 50 mJ/cm2 UVB. The cells were incubated for an
additional 24 h after which the cell viability was determined using
an MTT (FUJIFILM Wako) assay.
Glutathione (GSH), a marker of intracellular
antioxidative capacity, was measured using the DTNB method (Total
Glutathione Quantification Kit, Dojindo Molecular Technologies,
Inc.). A total of 24 h after inoculation, the cells were treated
with LFE for 24 h. Cells were then collected, and the intracellular
GSH was measured.
Statistical analysis
Statistical analysis was performed using Microsoft
Excel 2013 (Microsoft Corporation). A paired Student's t-test was
used for intragroup comparisons. Multivariate regression analysis
was used to compare changes in MED between the two groups.
Covariance analysis was used for erythema time series results,
including for time point comparisons. An unpaired Student's t-test
was used for the corresponding time points. All values are
presented as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
UMIN registration
This study was registered at the University Hospital
Medical Information Network (registration no. UMIN000025593).
Results
Participants' age, MED, and BMI
All 44 healthy volunteers selected in the screening
process participated in this study. However, six participants were
subsequently discontinued; four were voluntarily withdrawn, one was
excluded after taking medication, and another was excluded after
the MED results indicated a concerning reaction to UV exposure. The
final trial consisted of 38 remaining participants, 20 of whom were
in the placebo group and 18 of whom were in the experimental group.
There were no significant inter-group differences in these items
(the placebo group's average MED was 17.5±4.1 mJ/cm2,
and the LFE group's was 18.2±3.8 mJ/cm2; the placebo
group's average BMI was 22.5±3.1 kg/m2, and the LFE
group's was 22.6±3.5 kg/m2). There were no harmful
effects of test material based on general biochemical blood
tests.
Change in MED following LFE
administration
Table II shows the
placebo and LFE groups' MED values at week 0 (pre-intervention) and
week 8 (post-intervention), as well as the change between the
two-time points. The LFE group's average MED was significantly
higher post-intervention (P=0.002). The week 8 figure was 20.6±3.9
mJ/cm2, which marks a 13% increase from the week 0
figure of 18.2±3.8 mJ/cm2. There was no significant
change in the placebo group's MED (P=0.38). The week 0 figure was
17.5±4.1 mJ/cm2 and the week 8 figure was 18.0±5.1
mJ/cm2. The degree of change significantly differed
between the groups (P=0.02). In the LFE group, MED changed by
2.4±2.6 mJ/cm2, while in the placebo group, it only
changed by 0.5±3.5 mJ/cm2.
 | Table IIChange in MED by the intake of
LFE. |
Table II
Change in MED by the intake of
LFE.
| | MED,
mJ/cm2 (%)b |
|---|
| Group | 0 week | 8 week | Δ8 weeks |
|---|
| Placebo | 17.5±4.1(100) | 18.0±5.1
(102.9) | 0.5±3.5 |
| LFE | 18.2±3.8(100) | 18.2±3.8
(113.2) |
2.4±2.6a |
Changes in erythema measurements
This section outlines the results for the erythemas
that formed at the three sites on the participants after exposure
to UV at 1.5x their MED. The data included 20 placebo participants
and the 18 remaining participants in the LFE group. Table III shows the changes in
pigmentation over the four time points before intervention (Day 0,
Day 1, Day 3, and Day 7) and after intervention (Day 63, Day 64,
Day 66, and Day 70). The a* values indicate CR-200 readings, while
ʻmelanin indexʼ indicates MX 16 readings. Table IV shows the changes in erythema
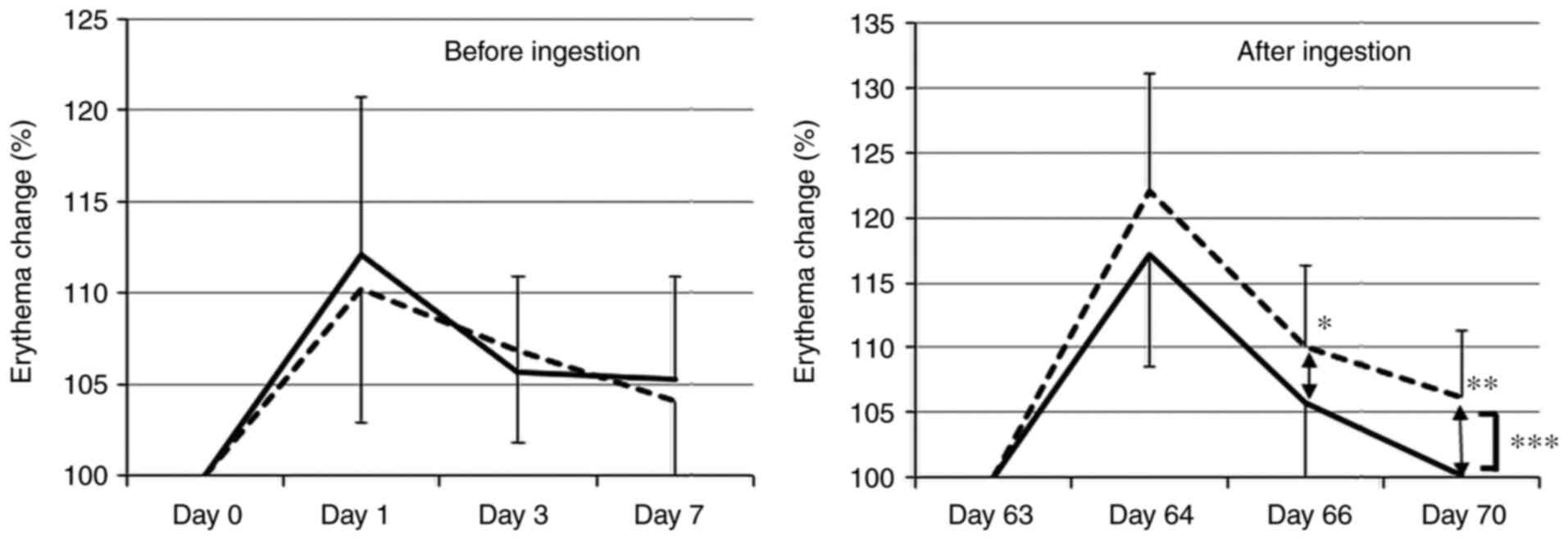
index as measured by the C-Cube. Fig.
2 shows the changes before and after intervention. In Tables IV and V, values in parentheses indicate change
relative to the pre-irradiation figure (Day 0/Day 63), which was
scaled at 100.
 | Table IIIChanges in the *a value and melanin
indexa. |
Table III
Changes in the *a value and melanin
indexa.
| A, *a value |
|---|
| | Before
ingestion | After
ingestion |
|---|
| Group | Day 0 | Day 1 | Day 4 | Day 7 | Day 63 | Day 64 | Day 66 | Day 70 |
|---|
| Placebo |
|
Value | 5.24±1.22 | 7.01±1.27 | 5.85±1.11 | 5.32±1.13 | 5.37±1.29 | 7.59±1.39 | 6.75±1.39 | 6.44±1.38 |
|
Relative
difference, %b | 100 | 137.05±22.19 | 113.45±11.95 | 102.37±8.17 | 100 | 144.48±22.49 | 127.11±12.62 | 121.06±12.49 |
| LFE |
|
Value | 4.81±1.56 | 6.50±1.37 | 5.41±1.48 | 4.94±1.53 | 5.01±1.33 | 7.13±1.52 | 6.21±1.30 | 5.88±1.24 |
|
Relative
difference, %b | 100 | 143.39±37.88 | 115.39±17.91 | 104.58±14.46 | 100 | 144.93±19.90 | 126.23±17.35 | 119.78±14.35 |
| B, Melanin
index |
| | Before
ingestion | After
ingestion |
| Group | Day 0 | Day 1 | Day 4 | Day 7 | Day 63 | Day 64 | Day 66 | Day 70 |
| Placebo |
|
Value | 92.67±33.94 | 94.28±31.29 | 100.66±35.93 | 98.80±33.80 | 82.03±39.83 | 84.79±38.08 | 90.35±43.23 | 87.07±33.80 |
|
Relative
difference, %b | 100 | 103.76±12.29 | 110.14±10.23 | 108.00±14.43 | 100 | 102.26±16.18 | 111.33±10.65 | 112.67±18.73 |
| LFE |
|
Value | 82.96±41.79 | 83.82±41.06 | 86.76±41.21 | 88.33±37.64 | 65.83±36.68 | 69.29±36.09 | 74.14±35.26 | 72.12±34.59 |
|
Relative
difference, %b | 100 | 101.44±6.90 | 108.09±19.06 | 114.67±35.95 | 100 | 107.47±19.90 | 118.39±25.54 | 112.85±16.30 |
 | Table IVChanges in Erythema
index.a |
Table IV
Changes in Erythema
index.a
| | Before
ingestion | After
ingestion |
|---|
| Erythema index | Day 0 | Day 1 | Day 4 | Day 7 | Day 63 | Day 64 | Day 66 | Day 70 |
|---|
| Placebo |
|
Value | 45.69±4.10 | 50.22±4.47 | 48.75±4.48 | 47.51±4.24 | 45.16±5.25 | 54.83±4.90 | 49.56±5.21 | 47.83±5.23 |
|
Relative
difference, %b | 100 | 110.05±7.30 | 106.82±4.99 | 104.12±4.99 | 100 | 122.07±9.06 | 110.05±6.27 | 106.12±5.20 |
| LFE |
|
Value | 44.63±5.57 | 49.90±6.41 | 47.06±5.78 | 47.04±5.81 | 45.99±5.81 | 53.81±6.91 | 48.49±5.49 | 45.90±5.14 |
|
Relative
difference, %b | 100 | 112.12±8.90 | 105.62±5.32 | 105.58±5.65 | 100 | 117.27±8.90 | 105.78±6.52 | 100.16±6.56 |
 | Table VComposition of LFE tablets and
placebo tablets. |
Table V
Composition of LFE tablets and
placebo tablets.
| | LFE, n=19 | Placebo, n=20 |
|---|
| Marker | Before | After | Before | Before |
|---|
| Total GSH, nM | 3.04±1.68 | 4.17±3.38 | 3.09±1.67 | 3.09±1.67 |
| 8-OHdG, ng/ml | 2.75±1.83 |
2.25±1.67a | 3.50±3.18 | 3.50±3.18 |
| Carbonylated
protein, ng/ml | 0.316±0.07 | 0.297±0.04 | 0.302±0.07 | 0.302±0.07 |
| 8-iso-PRO F2α,
nmol/ml | 81.72±40.92 |
36.58±31.91a | 104.51±62.75 | 104.51±62.75 |
As shown in Table
III, the a* value peaked at the first day (Day 1/Day 64) and
declined over the next two time points. Conversely, the melanin
index rose across the four time points. The LFE group did not
differ significantly from the placebo group in terms of the score
differences for either of the two indices before and after
intervention.
As shown in Table
IV, the C-Cube-measured erythema index rose between Day 0/Day
63 and Day 1/Day 64 before declining. Before intervention, the LFE
group's results for the C-Cube-measured erythema index did not
differ significantly from that of the placebo group (P=0.72). After
intervention however, the LFE group's results for the
C-Cube-measured erythema index differed significantly from that of
the placebo group (P=0.001). On Days 66 and 70 (the 4th
and 7th days after irradiation), the placebo group's
values were significantly higher than that of the LFE group. In
addition, UV exposure also affected SCH and TEWL, but the LFE
group's post-intervention results did not significantly differ from
that of the placebo group after 8 weeks of LFE ingestion (data not
shown).
Evaluation of antioxidative capacity
in the serum
Table V shows the
results for the four markers of in-serum antioxidative capacity.
Total GSSG increased in both groups after intervention, but not
significantly. In the placebo group, total GSH was 3.09±1.67 nM at
pre-intervention and 3.27±1.44 nM at post intervention; in the LFE
group, it was 3.04±1.68 nM at pre-intervention and 4.17±3.38 nM at
post intervention. While 8-OHdH in serum were significantly
suppressed after intervention in both the Placebo and LFE groups,
there were no differences between the two groups. Carbonylated
protein measurements showed no differences before or after
intervention, or between groups. Carbonylated protein levels
declined following intervention in both groups, but the decline was
not significant. The remaining two markers, lipid peroxides and
8-iso-pro F2 alpha, were significantly lower after intervention,
but there was no significant intergroup difference.
Protection against UV-induced
oxidative stress in HaCaT cells
The HaCaT, a spontaneously transformed human
keratinocyte cell line derived from the epidermis was used to
determine the protective effects of LFE as well as chlorogenic acid
(a major component of LFE) and quercetin (an intestinal metabolite
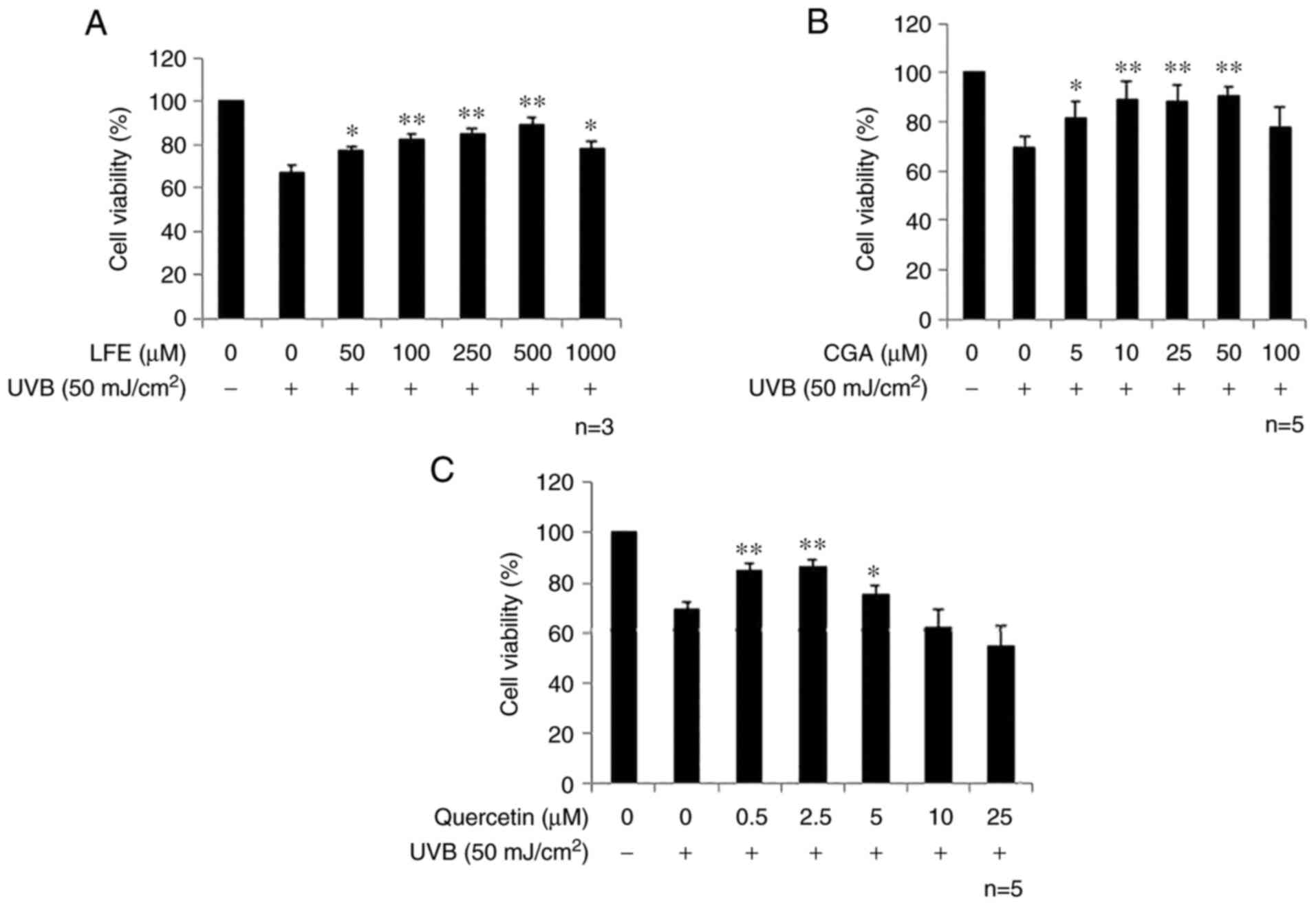
of rutin, another major component of LFE). Pretreatment with LFE
for 24 h exhibited a significant protective effect on the
UVB-induced cytotoxicity at a concentration of 50 µg/ml, but this
effect decreased at 1,000 µg/ml (Fig.
3A). Similarly, chlorogenic acid showed a significant effect at
5 µM, but this effect was absent at 100 µM (Fig. 3B). Quercetin also exhibited an
inhibitory effect from 0.5-5 µM (Fig.
3C). The pretreatment of LFE, chlorogenic acid or quercetin
significantly inhibited the hydrogen peroxide-induced cytotoxicity
(data not shown). Thus, chlorogenic acid and quercetin as well as
LFE are effective for inhibiting cell damage resulting from UV
exposure, possibly through their antioxidative effects.
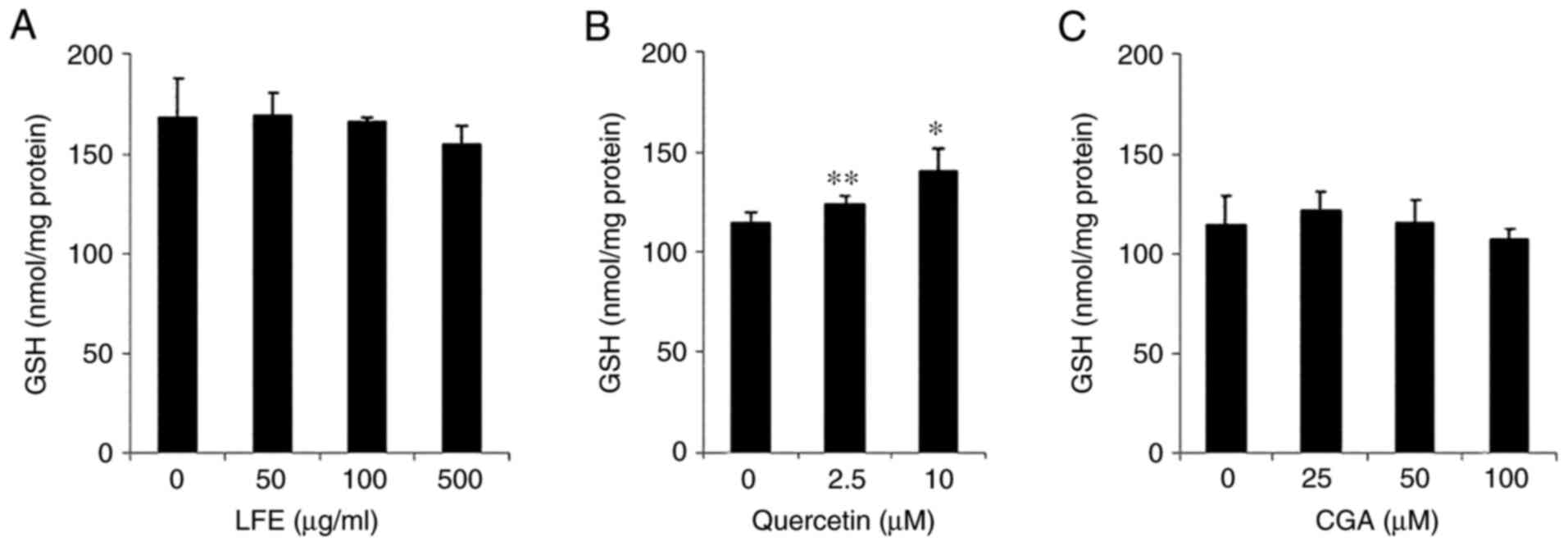
Fig. 4 shows the
effect of LFE upon intracellular total GSH, which was determined
using the DTNB method. Quercetin increased total GSH at
concentrations of 2.5 and 10 µM, LFE and chlorogenic acid showed no
significant effect for increasing total GSH in HaCaT cells, even
though LFE has been reported to enhance the cellular GSH level in
hepatocytes (30).
In the BDHQ survey, we asked the participants how
much tea and coffee they consumed. The responses indicated that
participants, who consumed plenty of tea or coffee prior to the
start of the test, tended to frequently have these beverages in the
second half of the study (at 8 weeks). Based on this, we assumed
that the increasing response in serum total GSH levels comes from
the participants who consumed low amounts of tea or coffee per day.
There were 10 participants in the LFE group who consumed <220 g
of tea or coffee a day and 12 such participants in the placebo
group (total GSH: from 2.7±1.6 to 4.7±3.2 nM). The increase in
serum GSH in the 10 LFE participants (total GSH: from 2.7±1.7 to
3.2±1.4 nM) was not significantly greater than in the placebo group
(P=0.11). It would be necessary to control tea/coffee consumption
to confirm the cause-effect relationship.
Discussion
When an erythema forms on the human skin following
exposure to UV, melanin production and deposition causes the skin
to darken (35). The damaging
effects of UV irradiation are significant not only in the
epidermis, but also in the hypodermis, resulting in inhibition of
an immune response and acceleration of skin aging (36). In addition to sunscreens,
consumption of certain nutrients can protect the skin from UV
irradiation. Green tea catechins, carotenoids in vegetables and
fruits, lycopene in tomatoes and collagen peptides have been
reported to inhibit erythema formation (20,37-39).
Similarly, Kuwazuru et al (29) and Gomez-Bernal et al
(27) suggested that LFE has the
potential to inhibit UV-induced damage. However, neither study
conclusively confirmed this effect.
The present study examined the effects of an 8-week
supplementation of LFE in Japanese men and women aged 20-60. The
protective effects were determined by measuring the participants'
MED and the change-over-time in erythema formation after exposure
to UV irradiation at 1.5x the MED. The study adopted a
placebo-controlled, double-blind design, and participants took
either the LFE supplement or a placebo at 900 mg a day for 8 weeks.
As shown in Table II, the LFE
group's average MED was increased significantly by 13%. MED
increased in the placebo group too, although this increase was not
significant. Moreover, the increased MED in the LFE group was
significant in comparison to that of the placebo group. As shown in
Table III, the LFE intervention
did not significantly affect the formation or change-over-time of
erythema as measured by a* values (CR-200) or melanin index (MX
16). However, the supplementation did significantly affect such
according to the C-Cube-measured erythema index (Table IV). Erythema formation in the LFE
group was significantly lower than that in the placebo group on the
day after irradiation (day 64); moreover, on subsequent days, the
erythemas disappeared significantly faster in the LFE group
compared with the placebo group (Table
IV and Fig. 2). The reason that
the C-Cube detected a significant difference where the other
measures did not, may be due to the fact that the C-Cube
measurement relies on a relatively large surface area (44.6
mm2), resulting in greater detection sensitivity.
A previous rodent study demonstrated that LFE
consumption inhibited UV-induced damage and suggested that LFE
could have the same effect in humans (28). The same study also showed that LFE
inhibited UV-induced immune responses with an upregulation in heme
oxygenase-1 (HO-1) protein expression. Polysaccharides are a major
active component of LFE (40).
Another report showed that the polysaccharides contained in LFE
protected epidermal cells from UV damage by inducing Nrf2 activity
and eliminating reactive oxygen species (41). The effects of polysaccharides were
also investigated in a study by Ding et al (42). However, that study failed to clarify
whether polysaccharides are absorbed intact; if they are
metabolized by the gut flora, they may be converted into an active
agent (42).
Other than polysaccharides, polyphenols such as
chlorogenic acid and rutin, which possess potent antioxidative
effects, are abundantly present in LFE. These components may be
absorbed during digestion intact directly or as metabolites, after
being metabolized by gut microbiota. Previous studies suggest that
chlorogenic acid is absorbed in the stomach or intestine intact as
it is and as metabolites (43-45).
Once absorbed, chlorogenic acid may spread to the skin via the
bloodstream. A study using topically applied chlorogenic acid
showed that the intradermal accumulation of chlorogenic acid
inhibited UV-induced erythema (46). Quercetin is produced when rutin is
metabolized by gut microbiota (47). Once produced in this manner, the
aglycone is absorbed into the bloodstream (48), whereupon it may be transferred to
and exert its antioxidative effects in the skin. In a study using
epidermal cells, quercetin inhibited UV irradiation-induced release
of certain inflammatory cytokines (49). Quercetin may protect against
UV-induced cell damage as it blocks UV-induced production of
reactive oxygen species and protects the mitochondria (50). Gut flora is suggested to mediate the
effects of ingested photoprotective agents. For example, Gueniche
et al (51) found that
probiotic bacteria facilitated an earlier recovery following
UV-induced immune response inhibition, while probiotic bacteria are
associated with an increased MED (52).
Exposure to UV damages the epidermal cells. The
causal factors include singlet oxygen production and DNA damage
(53). Singlet oxygen produces
8-OHdG in DNA, which can be inhibited by antioxidants (54). As shown in Fig. 3, the present study demonstrated that
UV-induced cytotoxicity was reduced by the pretreatment of the
epidermal cells with LFE or its components (chlorogenic acid and
quercetin). Another polyphenol (Chafuroside B) can reduce UV
damage, via the modulation of the inflammasome (55). Given this insight, the polyphenols
in LFE may inhibit the inflammasome, which remains to be examined.
The present study showed that, although LFE had no effect on
intracellular GSH production, quercetin significantly increased
total GSH levels in HaCaT cells (Fig.
4). Since LFE was reported to enhance the expression of HO-1,
an enzyme involved in antioxidant responses, and GCLC (the
catalytic subunit for the rate-limiting enzyme in glutathione
biosynthesis) as well as NQO1, a Phase-II drug-metabolizing enzyme
(30) in hepatocytes, similar
mechanisms might be involved in the antioxidative cytoprotective
effects of LFE in HaCaT cells. Although it is still unclear why an
increase in the expression of these genes is not correlated with
intracellular GSH level, quercetin is one of the possible
components of LFE that, once absorbed into the body, travels to the
skin via the bloodstream and enhances the antioxidative capacity in
the epidermal cells. In addition, the redox state also plays an
important role in intracellular antioxidant capacity, future
efforts will be concerned with modulating the effects of
antioxidative components of LFE on the GSSG/GSH ratio.
Other studies suggested that LFE ingestion was
associated with increased superoxide dismutase (SOD) (56), and that goji extract administration
reduced 8-OHdG levels in old mouse cavernosal tissue (57). More recently, our group demonstrated
that LFE significantly enhanced the intracellular GSH levels in
hepatocytes (30). However, as
shown in Table V, LFE ingestion did
not lead to increased antioxidative capacity in the blood as
measured by total in-serum GSH levels. This discrepancy ay be
attributable to the wide inter-individual variety in Japanese
dietary habits. Fukushima et al (58) reported that Japanese women who
consumed a substantial amount of coffee had fewer pigmented spots,
and suggested that the volume of coffee consumed might affect
in-serum antioxidative capacity. In an earlier study, Fukushima
et al (59) claimed that tea
and coffee are the typical beverages from which Japanese people
obtain chlorogenic acid, catechines, and other antioxidants. LFE
with antioxidants that inhibit UV-induced cytotoxicity, such as
chlorogenic acid, can be expected to function to help protect the
skin from UV irritation.
The related literature suggests that orally consumed
components can affect the epidermis as well as the hypodermis. A
systematic review by Wang et al (60), for example, revealed that eating
fish oil rich in polyunsaturated fatty acids (docosahexaenoic acid
and eicosapentaenoic acid) facilitates the expression of genes
associated with basement membrane formation and skin cell division.
Similarly, Li et al (16)
reported that oral consumption of sulforaphane, which is abundant
in cruciferous vegetables such as broccoli, inhibits inflammatory
cytokines and enhances antioxidative activity in the retina. They
concluded that sulforaphane both inhibits NLRP3 inflammasome and
activates the antioxidative Nrf2 pathway. The above findings can
support our findings of a reduction and accelerated decline in UV
damage as represented by erythema formation by LFE supplementation.
Taken together, three factors may play a pivotal role as follows:
i) once absorbed, the LFE components may facilitate the GSH
production in the liver; ii) once transferred to the epidermis
through the bloodstream, they may enhance antioxidant gene
expression and inhibit inflammatory responses in Nrf2-dependent
manners; and iii) the antioxidative components may directly protect
the epidermis.
Further research is needed to determine the extent
to which the effect of LFE on the intestinal function affects the
skin's photoprotective capacity.
A limitation of the study is the sample size.
Sampling errors may occur when a survey is conducted using the
probability sampling method.
In conclusion, this randomized, double-blind,
placebo-controlled trial, combined with the in vitro
research using the human HaCaT cells on the effects of LFE against
UVB-induced damage, strongly suggests that LFE ingestion can
protect epidermal cells from UVB-induced oxidative stress. These
effects can, in turn, facilitate antioxidative capacity throughout
the body, such as in the skin. LFE may augment the effect of
sunscreen in protecting the skin from damage. However, the
molecular mechanisms underlying the metabolism, absorption, and
transportation to the skin of the LFE components are still unclear.
Whether these components influence the skin function directly or
indirectly through the actions of other organs is the subject of
further study. In addition, to find an optimal combination of food
ingredients having multiple action points, is another step in
obtaining more effective remedies for skin health.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported in part by MEXT KAKENHI (grant
nos. 17H03818 and 20H02933).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT, WX, TN, and TK performed the experiments. TM,
TN, and TK performed the data analysis. YN and OU conceived and
designed the study. YN and OU confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This trial conformed to the Declaration of Helsinki
and was approved by the Ethics Committee of Tactics (Hokkaido
Activation Center) on December 14, 2016 (approval no. 2016-100).
Written informed consent was obtained from all potential
participants prior to participant selection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Halliday GM, Damian DL, Rana S and Byrne
SN: The suppressive effects of ultraviolet radiation on immunity in
the skin and internal organs: Implications for autoimmunity. J
Dermatol Sci. 66:176–182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Heck DE, Gerecke DR, Vetrano AM and Laskin
JD: Solar ultraviolet radiation as a trigger of cell signal
transduction. Toxicol Appl Pharmacol. 195:288–297. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ley RD and Reeve VE: Chemoprevention of
ultraviolet radiation-induced skin cancer. Environ Health Perspect.
105 (Suppl 4):S981–S984. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang B, Xu QY, Guo CY, Huang JW, Wang SM,
Li YM, Tu Y, He L, Bi ZG, Ji C and Cheng B: MHY1485 ameliorates
UV-induced skin cell damages via activating mTOR-Nrf2 signaling.
Oncotarget. 8:12775–12783. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martincorena I, Roshan A, Gerstung M,
Ellis P, Van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB,
Tubio JM, et al: Tumor evolution. High burden and pervasive
positive selection of somatic mutations in normal human skin.
Science. 348:880–886. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gunn DA, de Craen AJ, Dick JL, Tomlin CC,
van Heemst D, Catt SD, Griffiths T, Ogden S, Maier AB, Murray PG,
et al: Facial appearance reflects human familial longevity and
cardiovascular disease risk in healthy individuals. J Gerontol A
Biol Sci Med Sci. 68:145–152. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gunn DA, Larsen LA, Lall JS, Rexbye H and
Christensen K: Mortality is written on the face. J Gerontol A Biol
Sci Med Sci. 71:72–77. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sveikata K, Balciuniene I and Tutkuviene
J: Factors influencing face aging. Literature review.
Stomatologija. 13:113–116. 2011.PubMed/NCBI
|
|
9
|
Gordon JR and Brieva JC: Images in
clinical medicine. Unilateral dermatoheliosis. N Engl J Med.
366(e25)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Young AR, Sheehan JM, Chadwick CA and
Potten CS: Protection by ultraviolet A and B sunscreens against in
situ dipyrimidine photolesions in human epidermis is comparable to
protection against sunburn. J Invest Dermatol. 115:37–41.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim HH, Cho S, Lee S, Kim KH, Cho KH, Eun
HC and Chung JH: Photoprotective and anti-skin-aging effects of
eicosapentaenoic acid in human skin in vivo. J Lipid Res.
47:921–930. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seite S, Fourtanier A, Moyal D and Young
AR: Photodamage to human skin by suberythemal exposure to solar
ultraviolet radiation can be attenuated by sunscreens: A review. Br
J Dermatol. 163:903–914. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hasegawa T, Nakashima M and Suzuki Y:
Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes
UVB-induced inflammatory responses in human keratinocytes. Biochem
Biophys Res Commun. 477:329–335. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
D'Errico M, Lemma T, Calcagnile A,
Proietti De Santis L and Dogliotti E: Cell type and DNA damage
specific response of human skin cells to environmental agents.
Mutat Res. 614:37–47. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kobayashi M and Yamamoto M: Nrf2-Keap1
regulation of cellular defense mechanisms against electrophiles and
reactive oxygen species. Adv Enzyme Regul. 46:113–140.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li S, Yang H and Chen X: Protective
effects of sulforaphane on diabetic retinopathy: Activation of the
Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Exp
Anim. 68:221–231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Swindells K and Rhodes LE: Influence of
oral antioxidants on ultraviolet radiation-induced skin damage in
humans. Photodermatol Photoimmunol Photomed. 20:297–304.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yimcharoen M, Kittikunnathum S, Suknikorn
C, Nak-On W, Yeethong P, Anthony TG and Bunpo P: Effects of
ascorbic acid supplementation on oxidative stress markers in
healthy women following a single bout of exercise. J Int Soc Sports
Nutr. 16(2)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hanson KM and Clegg RM: Bioconvertible
vitamin antioxidants improve sunscreen photoprotection against
UV-induced reactive oxygen species. J Cosmet Sci. 54:589–598.
2003.PubMed/NCBI
|
|
20
|
Jeon HY, Kim JK, Kim WG and Lee SJ:
Effects of oral epigallocatechin gallate supplementation on the
minimal erythema dose and UV-induced skin damage. Skin Pharmacol
Physiol. 22:137–141. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Puglia C, Offerta A, Saija A, Trombetta D
and Venera C: Protective effect of red orange extract
supplementation against UV-induced skin damages: Photoaging and
solar lentigines. J Cosmet Dermatol. 13:151–157. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Heinrich U, Moore CE, De Spirt S, Tronnier
H and Stahl W: Green tea polyphenols provide photoprotection,
increase microcirculation, and modulate skin properties of women. J
Nutr. 141:1202–1208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rizwan M, Rodriguez-Blanco I, Harbottle A,
Birch-Machin MA, Watson RE and Rhodes LE: Tomato paste rich in
lycopene protects against cutaneous photodamage in humans in vivo:
A randomized controlled trial. Br J Dermatol. 164:154–162.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Juturu V, Bowman JP and Deshpande J:
Overall skin tone and skin-lightening-improving effects with oral
supplementation of lutein and zeaxanthin isomers: A double-blind,
placebo-controlled clinical trial. Clin Cosmet Investig Dermatol.
9:325–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ulbricht C, Bryan JK, Costa D, Culwell S,
Giese N, Isaac R, Nummy K, Pham T, Rapp C, Rusie E, et al: An
evidence-based systematic review of Goji Lycium spp.) by the
Natural Standard Research Collaboration. J Diet Suppl. 12:184–240.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Amagase H and Farnsworth NR: A review of
botanical characteristics, phytochemistry, clinical relevance in
efficacy and safety of Lycium barbarum fruit (Goji). Food Res Int.
44:1702–1717. 2011.
|
|
27
|
Gomez-Bernal S, Rodriguez-Pazos L,
Martinez FJ, Ginarte M, Rodríguez-Granados MT and Toribio J:
Systemic photosensitivity due to Goji berries. Photodermatol
Photoimmunol Photomed. 27:245–247. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Reeve VE, Allanson M, Arun SJ, Domanski D
and Painter N: Mice drinking goji berry juice (Lycium barbarum) are
protected from UV radiation-induced skin damage via antioxidant
pathways. Photochem Photobiol Sci. 9:601–607. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kuwazuru S, Nakashima M, Honda E and
Fukaya Y: Effects of oral lycii fructus extracts on skin erythema
and skin pigmentation induced by solar-simulated ultraviolet
irradiation in healthy men. Pharmacometrics. 83:39–45. 2012.
|
|
30
|
Xu W, Saiki S, Myojin T, Liu Y, Zhu B,
Murata Y, Ashida H, Tsunenaga M and Nakamura Y: Lycii fructus
extract ameliorates hydrogen peroxide-induced cytotoxicity through
indirect antioxidant action. Biosci Biotechnol Biochem.
82:1812–1820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
World Medical Association. World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fitzpatrick TB: The validity and
practicality of sun-reactive skin types I through VI. Arch
Dermatol. 124:869–871. 1988.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kobayashi S, Murakami K, Sasaki S, Okubo
H, Hirota N, Notsu A, Fukui M and Date C: Comparison of relative
validity of food group intakes estimated by comprehensive and
brief-type self-administered diet history questionnaires against 16
d dietary records in Japanese adults. Public Health Nutr.
14:1200–1211. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Delalleau A, Lagarde JM and George J: An a
priori shading correction technique for contact imaging devices.
IEEE Trans Image Process. 20:2876–2885. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lim SH, Kim SM, Lee YW, Ahn KJ and Choe
YB: Change of biophysical properties of the skin caused by
ultraviolet radiation-induced photodamage in Koreans. Skin Res
Technol. 14:93–102. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ambach W and Blumthaler M: Biological
effectiveness of solar UV radiation in humans. Experientia.
49:747–753. 1993.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stahl W, Heinrich U, Aust O, Tronnier H
and Sies H: Lycopene-rich products and dietary photoprotection.
Photochem Photobiol Sci. 5:238–242. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Carrascosa JM, Floriach N, Sala E and
Aguilera J: Increase in minimal erythemal dose following oral
administration of an antioxidant complex based on a mix of
carotenoids: Double-blind, placebo-controlled trial. Photodermatol
Photoimmunol Photomed. 33:284–286. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Koyama YI, Kuwaba K, Kondo S and Tsukada
Y: Supplemental ingestion of collagen peptide suppresses
uitraviolet-induced erythema. Jpn Pharmacol Ther. 42:781–790.
2014.
|
|
40
|
Pengjiao Z, Juan L, Yulong C and Lijuan Z:
The structures and biological functions of polysaccharides from
traditional Chinese herbs. Prog Mol Biol Transl Sci. 163:423–444.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li H, Li Z, Peng L, Jiang N, Liu Q, Zhang
E, Liang B, Li R and Zhu H: Lycium barbarum polysaccharide protects
human keratinocytes against UVB-induced photo-damage. Free Radic
Res. 51:200–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ding Y, Yan Y, Peng Y, Chen D, Mi J, Lu L,
Luo Q, Li X, Zeng X and Cao Y: In vitro digestion under simulated
saliva, gastric and small intestinal conditions and fermentation by
human gut microbiota of polysaccharides from the fruits of Lycium
barbarum. Int J Biol Macromol. 125:751–760. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nakamura S, Matsui Y, Watanabe T, Kondou N
and Masukawa Y: Pharmacokinetics of chlorogenic acids absorbed in
human plasma and their metabolites following oral ingestion of
coffee. drink. 34:1239–1246. 2006.
|
|
44
|
Lafay S, Gil-Izquierdo A, Manach C, Morand
C, Besson C and Scalbert A: Chlorogenic acid is absorbed in its
intact form in the stomach of rats. J Nutr. 136:1192–1197.
2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Renouf M, Marmet C, Giuffrida F, Lepage M,
Barron D, Beaumont M, Williamson G and Dionisi F: Dose-response
plasma appearance of coffee chlorogenic and phenolic acids in
adults. Mol Nutr Food Res. 58:301–309. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kitagawa S, Yoshii K, Morita SY and
Teraoka R: Efficient topical delivery of chlorogenic acid by an
oil-in-water microemulsion to protect skin against UV-induced
damage. Chem Pharm Bull (Tokyo). 59:793–796. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang J, Qian D, Jian S, Shang EX, Guo J
and Duan JA: Identification of rutin deglycosylated metabolites
produced by human intestinal bacteria using UPLC-Q-TOF/MS. J
Chromatogr B Analyt Technol Biomed Life Sci. 898:95–100.
2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Erlund I, Kosonen T, Alfthan G, Mäenpää J,
Perttunen K, Kenraali J, Parantainen J and Aro A: Pharmacokinetics
of quercetin from quercetin aglycone and rutin in healthy
volunteers. Eur J Clin Pharmacol. 56:545–553. 2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Vicentini FT, He T, Shao Y, Fonseca MJ,
Verri WA Jr, Fisher GJ and Xu Y: Quercetin inhibits UV
irradiation-induced inflammatory cytokine production in primary
human keratinocytes by suppressing NF-kappaB pathway. J Dermatol
Sci. 61:162–168. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhu X, Li N, Wang Y, Ding L, Chen H, Yu Y
and Shi X: Protective effects of quercetin on UVB
irradiationinduced cytotoxicity through ROS clearance in
keratinocyte cells. Oncol Rep. 37:209–218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gueniche A, Philippe D, Bastien P, Blum S,
Buyukpamukcu E and Castiel-Higounenc I: Probiotics for
photoprotection. Dermatoendocrinol. 1:275–279. 2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bouilly-Gauthier D, Jeannes C, Maubert Y,
Duteil L, Queille-Roussel C, Piccardi N, Montastier C, Manissier P,
Piérard G and Ortonne JP: Clinical evidence of benefits of a
dietary supplement containing probiotic and carotenoids on
ultraviolet-induced skin damage. Br J Dermatol. 163:536–543.
2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gueranger Q, Li F, Peacock M,
Larnicol-Fery A, Brem R, Macpherson P, Egly JM and Karran P:
Protein oxidation and DNA repair inhibition by 6-thioguanine and
UVA radiation. J Invest Dermatol. 134:1408–1417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hakozaki T, Date A, Yoshii T, Toyokuni S,
Yasui H and Sakurai H: Visualization and characterization of
UVB-induced reactive oxygen species in a human skin equivalent
model. Arch Dermatol Res. 300 (Suppl 1):S51–S56. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hasegawa T, Shimada S, Ishida H and
Nakashima M: Chafuroside B, an Oolong tea polyphenol, ameliorates
UVB-induced DNA damage and generation of photo-immunosuppression
related mediators in human keratinocytes. PLoS One.
8(e77308)2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Amagase H, Sun B and Borek C: Lycium
barbarum (goji) juice improves in vivo antioxidant biomarkers in
serum of healthy adults. Nutr Res. 29:19–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Moon HW, Park JW, Lee KW, Jeong HC, Choi
JB, Choi SW, Bae WJ, Cho HJ, Ha US, Hong SH, et al: Administration
of Goji (lycium chinense mill.) extracts improves erectile function
in old aged rat model. World J Mens Health. 35:43–50.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Fukushima Y, Takahashi Y, Hori Y,
Kishimoto Y, Shiga K, Tanaka Y, Masunaga E, Tani M, Yokoyama M and
Kondo K: Skin photoprotection and consumption of coffee and
polyphenols in healthy middle-aged Japanese females. Int J
Dermatol. 54:410–418. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fukushima Y, Tashiro T, Kumagai A,
Ohyanagi H, Horiuchi T, Takizawa K, Sugihara N, Kishimoto Y,
Taguchi C, et al: Coffee and beverages are the major contributors
to polyphenol consumption from food and beverages in Japanese
middle-aged women. J Nutr Sci. 3(e48)2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang P, Sun M, Ren J, Aslam MN, Xu Y,
Fisher GJ, Voorhees JJ, Wang X and Li Y: Dietary fish oil
supplementation enhances expression of genes involved in cornified
cell envelope formation in rat skin. J Invest Dermatol.
138:981–983. 2018.PubMed/NCBI View Article : Google Scholar
|