Introduction
Sea buckthorn (genus, Hippophae; family,
Elaeagnaceae) is a cold-adapted thorny shrub native to Asia and
Europe (1). Of the three Indian
species of sea buckthorn [Hippophae rhamnoides (H.
rhamnoides), Hippophae salicifolia and Hippophae
tibetana], H. rhamnoides L. ssp. turkestanica is
the main one in the high-altitude Himalayan-Karakoram ranges. Sea
buckthorn has been traditionally used in Asian, Nordic and Baltic
countries for food, medicine, fodder, fuel and fencing (2,3). In
traditional Chinese medicine and the Tibetan Amchi system, its
leaves and berries are used to treat asthma, skin diseases and
gastric ulcers. Further experimental studies have revealed its
antioxidant, immune-modulatory, anti-atherogenic, anti-stress and
tissue-regenerative properties, as well as its efficacy against
jaundice, liver and gastrointestinal disorders (2-8).
Phytochemical studied of sea buckthorn have led to identification
of a range of bioactive compounds such as β-carotene, lycopene,
lutein, zeaxanthin, quercetin, kaempferol, isorhamnetin,
β-sitosterol, ursolic acid, oleanolic acid, and palmitic acid etc.
in its berries (9-14).
In addition, several bioactive flavonols, leucoanthocyanidins,
epicatechin, gallocatechin, epigallocatechin, gallic acid,
including quercetin and kaempferol derivatives have been reported
in its leaf extracts (15,16).
Hepatitis B virus (HBV) the DNA virus, remains a
globally crucial hepatovirus, which causes chronic liver diseases,
such as cirrhosis and hepatocellular carcinoma in approximately
three million individuals worldwide (17). Although there are effective vaccines
and potent anti-HBV drugs (polymerase inhibitors) available, the
emergence of drug-resistant viral mutants remains an obstacle to
its complete eradication (18,19).
In recent decades, several herbal formulations and phytochemicals,
such as flavonoids, terpenes, saponins, lignans, alkaloids and
anthraquinones have been reported as potential antiviral agents
against numerous pathogenic RNA and DNA viruses, including HBV
(20-24).
Quercetin, isorhamnetin and kaempferol are natural flavonoids with
various health protective salutations due to their potent
anti-inflammatory, antioxidant and anti-carcinogenic properties
(25). Notably, quercetin and
kaempferol, as well as their glucoside and rhamnoside derivatives
have been shown to possess enhanced antiviral activities against
human immunodeficiency virus (HIV) and herpes simplex virus (HSV),
genetically close to HBV (21). In
line with this finding, the authors have recently reported
quercetin and kaempferol, including their derivatives, as well as
anthraquinones and catechins with promising anti-HBV activities in
cell-culture model (26-32).
Sea buckthorn leaf extract has been reported to
exhibit anti-dengue virus (DNV) activity (33), and its phenol-rich fraction has been
demonstrated to include myricetin, quercetin, kaempferol and
isorhamnetin (34). In addition,
hiporamin isolated from its leaves has been identified for its
potent activities against influenza virus, HIV and HSV (35). However, to the best of our
knowledge, the anti-HBV activity of sea buckthorn has not yet been
investigated. Thus, the present study assessed the anti-HBV
potential of sea buckthorn aerial parts and its bioactive flavonols
using a HBV-reporter cell culture model.
Materials and methods
Plant material collection
The fresh aerial parts of sea buckthorn (H.
rhamnoides L.) were collected from the Karakoram-Indus valley
of Pheh, Ladakh (3180 m above sea level), India in late July, 2018
(Fig. 1, upper panel). The plant
was identified by a local herbalist-Amchi practitioner (Leh,
Ladakh), and further validated by an expert taxonomist, Dr Tariq
Husain (National Botanical Research Institute, Lucknow, India).
Extraction and fractionation
Analytical grade solvents (Sigma-Aldrich, Merck
KGaA) were used for the extraction and fractionation of sea
buckthorn, using standard methods. Briefly, the air-dried powder of
the aerial parts (410 g) of sea buckthorn were extracted with 90%
ethanol (3x250 ml) at room temperature with continuous shaking till
exhaustion. The combined alcoholic-extracts were filtered (Whatman
paper no. 1) and concentrated under reduced pressure at 40˚C, using
rotary evaporator (Buchi Rotavapor; Model R-215, Thermo Fisher
Scientific, Inc.). The obtained dried ethanol-extract (198.7 g) was
dissolved in water and successively fractionated with chloroform
(SB-Chl; 4.12 g), ethyl acetate (SB-Eac; 2.1 g) and finally with
n-butanol (SB-But; 62.9 g), along with the aqueous part
(SB-Aqu; 122.4 g) to yield the corresponding fractions. For each
fraction, solvents were completely evaporated to dryness, and
maintained at 4˚C until analysis.
High performance liquid chromatography
(HPLC)
Based on the published literature, the sea buckthorn
extract was subjected to HPLC analysis for the presence of the
antiviral flavonoids: Quercetin, kaempferol and isorhamnetin
(34,36). The Alliance chromatographic system
(Waters Instruments, Inc.) was used for HPLC analysis equipped with
built-in quaternary pump, dual wavelength absorbance detectors and
autosampler at 25˚C. For separation, an Agilent HC-C18 column (5
µm, 250x4.6 mm) was maintained with the gradient flow rate (1
ml/min) of the mobile phase (A, acidified water:1% acetic acid,
v/v; and B, methanol:acetonitrile, 80:20, v/v) and peaks were
detected at 370 nm. The gradient was programmed as follows: 0-9
min, 0-35% B; 9-13 min, 35-60% B; 13-18 min, 60-80% B; 18-30 min,
80-90% B. For obtaining the calibration curve, standard stock
solutions of quercetin, kaempferol, and isorhamnetin were prepared
in methanol (0.5 mg/ml). Furthermore, gradient concentrations
(0.01-100.0 µg/ml) of the three compounds were prepared by serial
dilution for identifying and quantifying them in the sea buckthorn
extract.
Human cell culture and drugs
Two human hepatoma cell lines [HepG2 and its
derivative, HepG2.2.15 (HBV-reporter cells)] were generously
provided by Dr Shahid Jameel (International Center for Genetic
Engineering and Biotechnology, New Delhi, India). The cells were
cultured in T25 flasks (BD Biosciences, San Jose, CA, USA) using
DMEM reconstituted with 10% bovine calf serum and 1X
penicillin-streptomycin mix (all from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37˚C with a 5%
CO2 supply. The anti-HBV drug lamivudine, as well as
natural quercetin, kaempferol and isorhamnetin (all from
Sigma-Aldrich, Merck KGaA) were procured.
Cell viability or cytotoxicity
assay
The four sea buckthorn fractions (SB-But, SB-Chl,
SB-Eac and SB-Aqu; 3 mg/ml each) were dissolved in 100 µl dimethyl
sulfoxide (DMSO; Sigma-Aldrich, Merck KGaA) and reconstituted in
DMEM to yield various working concentrations (50, 100 and 150
µg/ml). Likewise, isorhamnetin, quercetin and kaempferol (0.5 mg,
each) were first dissolved in 50 µl DMSO and reconstituted in DMEM
(1 mg/ml, final). HepG2 cells (0.5x105/100 µl/well) in a
96-well cell culture plate (BD Biosciences) were grown overnight
and treated with the sea buckthorn fractions (in triplicate) the
following day after an incubation period of 72 at 37˚C h. DMSO
(0.1%) served as a vehicle or untreated control. The cytotoxicity
induced by the sea buckthorn fractions, if any, was evaluated using
MTT assay (TACS MTT Cell Proliferation and Viability Assay kit,
Tervigen; Thermo Fisher Scientific, Inc.) according to instructions
provided with the kit. The optical density (OD) of each sample was
recorded using a microplate reader (ELx800; BioTek Instruments,
Inc.). Non-linear regression analysis was performed using Excel
software (2010; Microsoft Corporation) to estimate the 50% maximal
cytotoxic concentration (CC50) in relation to the
untreated control. The experiment was repeated twice under the same
conditions for reproducibility.
HBV surface protein (HBsAg) inhibition
assay
To assess the inhibitory effects of the sea
buckthorn fractions on HBsAg expression levels, the HepG2.2.15
cells were seeded (0.5x105 cells/100 µl/well) in a
96-well plate and grown overnight. The cells were treated with the
non-cytotoxic SB-But, SB-Eac and SB-Aqu fractions (25, 50 and 100
µg/ml, each) as well as with isorhamnetin (10, 20 and 50 µg/ml),
quercetin (10 µg/ml) and kaempferol (10 µg/ml). Lamivudine (2 µM)
served as the standard, whereas DMSO (0.1%) functioned as a
negative or untreated control. Following incubation at
37˚C, culture supernatants were collected on days 1, 3
and 5, and stored at -20˚C for further analysis. HBsAg production
was quantified using the Monolisa HBsAg ULTRA Elisa kit (Bio-Rad
Laboratories, Inc.) according to the instructions provided with the
kit. The OD (λ=570 nm) of the samples was recorded and analyzed in
relation to the untreated control, and non-linear regression was
performed using Excel software (2010; Microsoft Corporation) to
estimate percent inhibitions. All samples were tested in triplicate
and the experiment was repeated twice.
HBV pre-core protein (HBeAg)
inhibition assay
Based on the HBsAg inhibition data, the SB-But,
SB-Eac and SB-Aqu fractions (50 µg/ml, each) as well as IRM, QRC
and KMP (10 µg/ml, each) were further tested for their time-course
inhibitory effects on HBeAg synthesis in HepG2.2.15 cells. The
secretion of HBeAg was analyzed using the HBeAg/Anti-HBe Elisa kit
(DIAsource ImmunoAssays, Belgium) according to the instructions
provided with the kit. The OD (λ=570 nm) of the samples was
recorded and analyzed in relation to the untreated control, and
non-linear regression was performed to determine percent
inhibitions. All samples were tested in triplicate and the
experiment was repeated twice.
Statistical analysis
All data from all the analyzed samples (in
triplicate) are expressed as the mean ± SEM. In a set of data, the
determination of total variation was performed using one-way
analysis of variance (ANOVA), following the Dunnett's test.
Statistical analyses were performed using Excel software (2010;
Microsoft Corporation). A value of P<0.01 was considered to
indicate a statistically significant difference.
Results and discussion
Quantification of antiviral flavonoids
in sea buckthorn extract
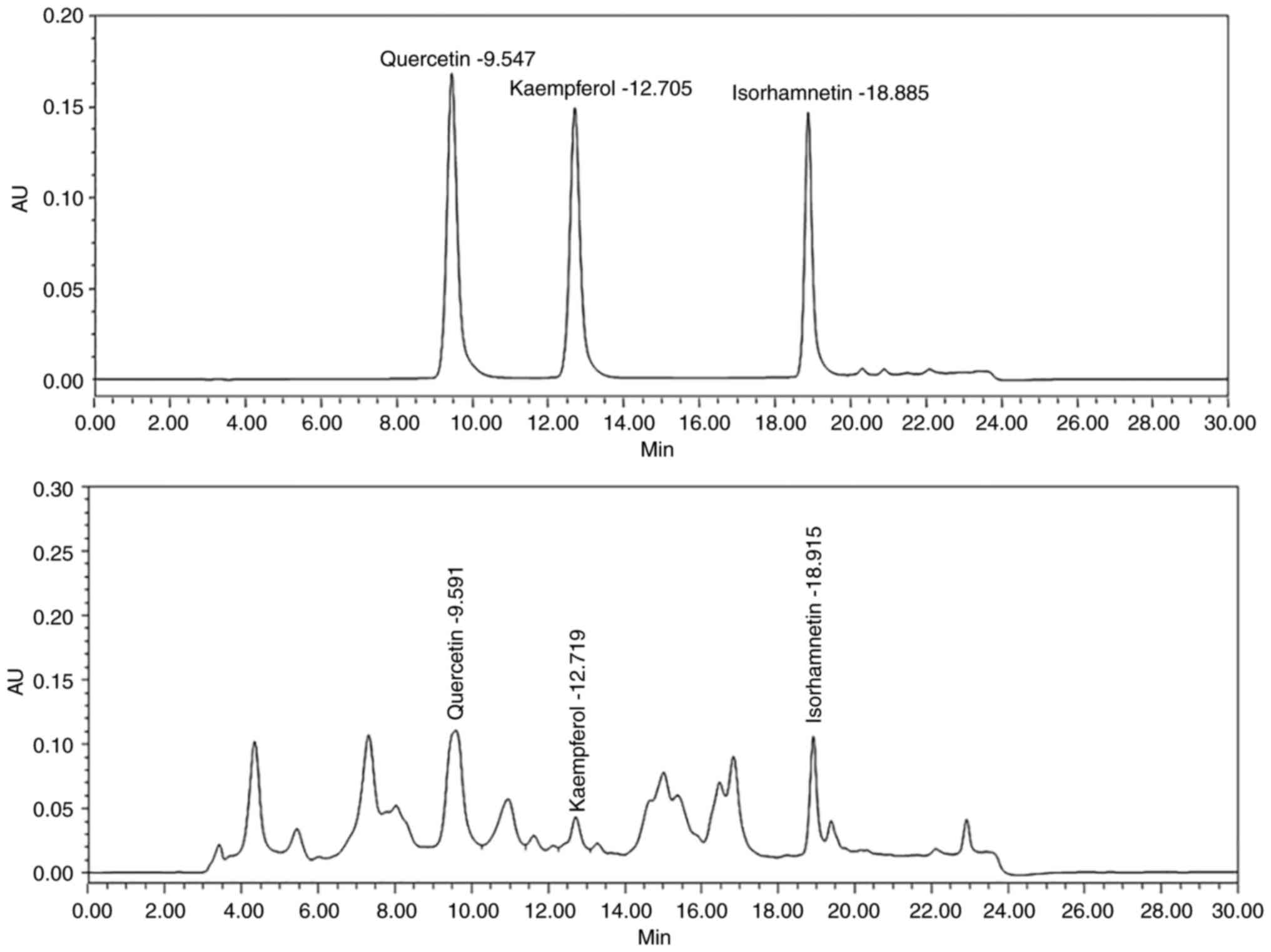
HPLC analysis of the sea buckthorn extract
identified three antiviral flavonols isorhamnetin, quercetin and
kaempferol (Fig. 2), and were
quantified to be 138.75, 93.09 and 44.19 µg/g, respectively. This
was in line with the previous demonstration of these flavonoids in
the phenol-rich fraction of sea buckthorn leaves (34). Flavonols are natural flavonoids with
variations in their heterocyclic
(C6-C3-C6) carbon ring (36). The detected flavonols are
structurally similar in having hydroxyl groups at same positions on
their A and B rings; however, isorhamnetin has an additional
3'-methyl group on the B ring as compared to quercetin and
kaempferol (Fig. 1, lower
panel).
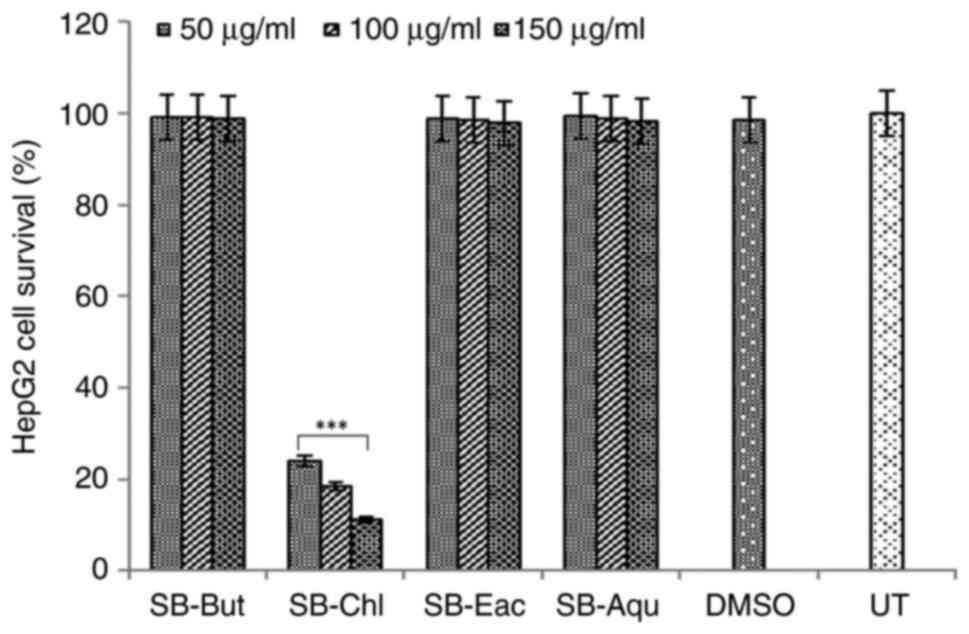
Cytotoxic assessment of sea buckthorn
fractions
The present study first examined the cytotoxic
effects of the SB-But, SB-Chl, SB-Eac and SB-Aqu fractions on HepG2
cells. Of these, while SB-Chl exhibited significantly high
cytotoxicity (CC50, 32.58 µg/ml; P<0.01), SB-Eac,
SB-But and SB-Aqu exhibited non-toxicity at concentrations of up to
150 µg/ml dose as compared to the untreated control (Fig. 3). Notably, HepG2 and HepG2.2.15
cells are biologically and physiologically the same, apart from the
fact that HepG2.2.15 cells allow for HBV DNA replication and gene
expression. In addition, while the HepG2 cells are widely
distributed and easily available, the accessibility of HepG2.2.15
cells, even commercially, is very limited worldwide (37). In view of this, in the present
study, MTT assay was performed on HepG2 cells, whereas the
HepG2.2.15 cells were only used for anti-HBV assays.
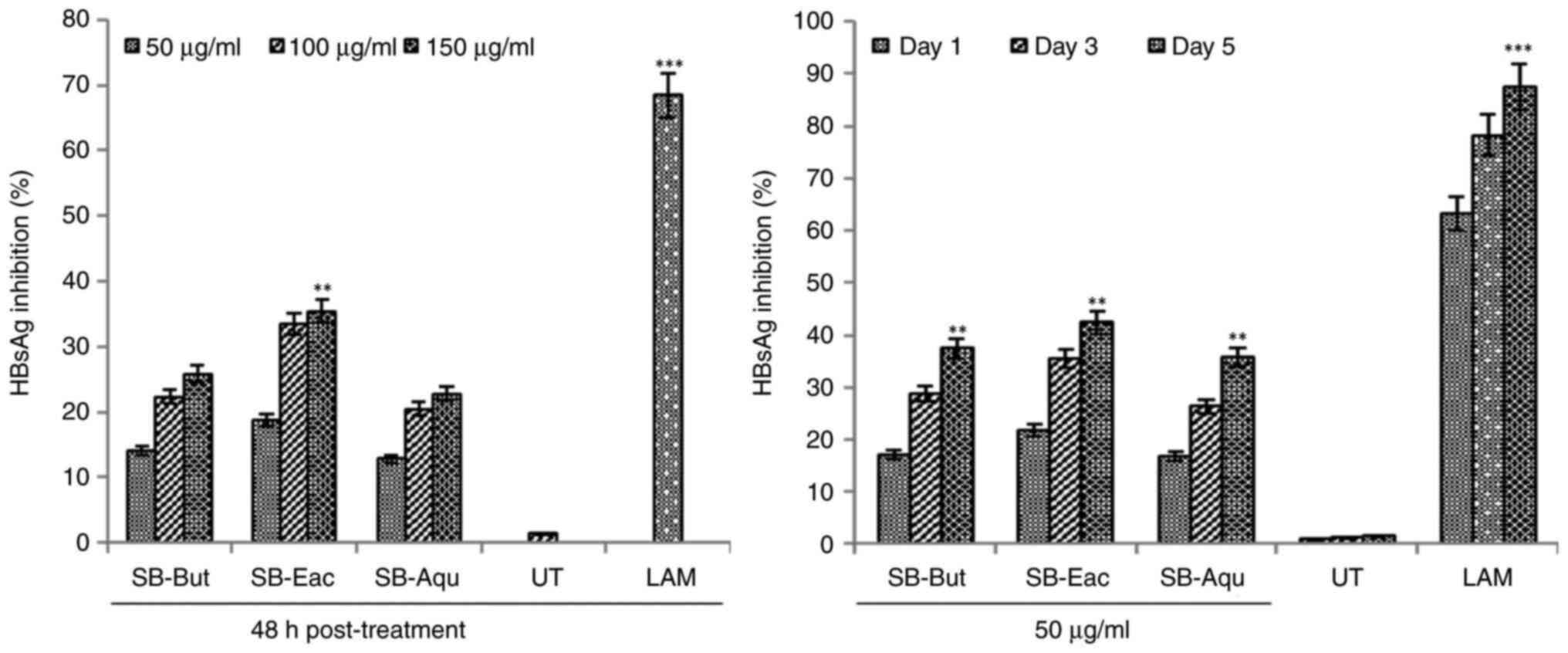
Anti-HBV activities of SB fractions
and flavonols
The concentration-dependent analysis of the SB-Eac,
SB-But and SB-Aqu fractions revealed marked inhibitory effects on
HBsAg production at 50 and 100 µg/ml (P<0.01) in relation to the
untreated control on 48 h (Fig. 4,
left panel). As no further considerable enhanced effect was
observed at 100 µg/ml when compared to 50 µg/ml, the 50 µg/ml
concentration was selected for time-course analysis. In the
time-course assay of the fractions (50 µg/ml), the optimal
inhibition of HBsAg was observed by SB-Eac (~42.6%; P<0.01)
followed by SB-But (~37.5%) and AB-Aqu (~36.2%) in relation to the
untreated control on day 5 (Fig. 4,
right panel). The concentration- and time-dependent analysis of
isorhamnetin revealed its optimal, but comparative HBsAg inhibitory
activity at the 10 and 20 µg/ml concentrations on day 5 (Fig. 5). At the selected 10 µg/ml
concentration, isorhamnetin suppressed HBsAg levels by ~30.5% as
compared to the high activities of quercetin (~67.5%; P<0.01)
and kaempferol (~62.3%; P<0.01) in relation to the untreated
control on day 5 (Fig. 5). The
reference drug lamivudine (2 mM) suppressed HBsAg synthesis by
~87.4% (P<0.001) in relation to the untreated control on day
5.
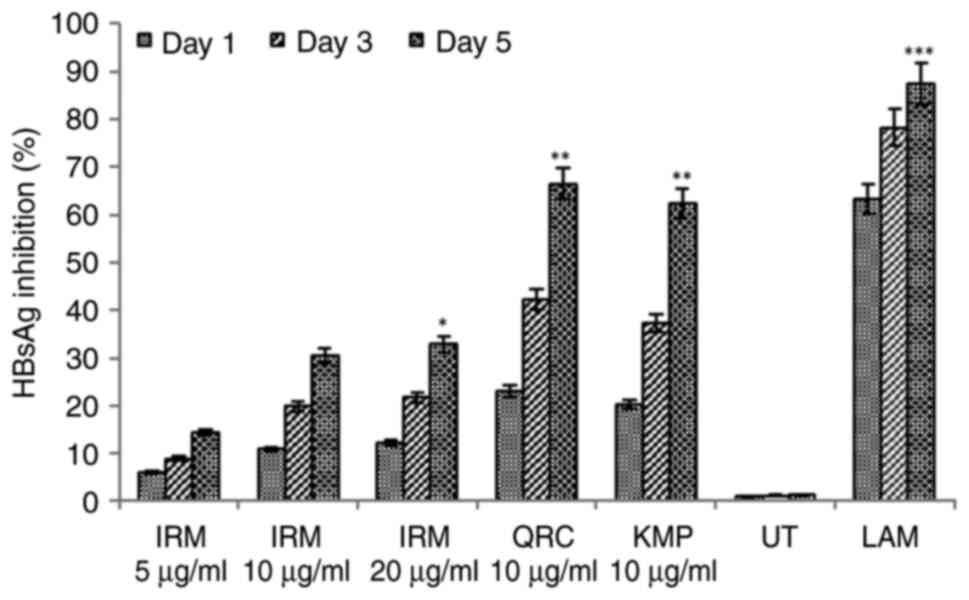
As HBeAg production is a serological hallmark of
active viral DNA replication (18),
the present study further tested the three fractions (50 µg/ml) and
the flavonoids (10 µg/ml) for their effects on HBeAg synthesis.
SB-Eac suppressed HBeAg production by ~43.2% in relation to the
untreated control on day 5. In addition, SB-But suppressed this by
~35.5% and AB-Aqu by ~32.5% in relation to the untreated control on
day 5 (Fig. 6). Isorhamnetin
decreased HBeAg production by ~28.4%, whereas quercetin and
kaempferol inhibited this by ~64.4% (P<0.01) and ~60.2%
(P<0.01), respectively in relation to the untreated control
(Fig. 6). Lamivudine (2 mM)
suppressed HBsAg production by ~83.5% (P<0.001) in relation to
the untreated control on day 5. In a previous study by the authors,
the assessment of the synergistic effects of quercetin with other
flavonoids, such as rutin and hesperidin revealed further
suppressions of HBsAg and HBeAg by ~10% in HepG2.2.15 cells
(28). Thus, the present study did
not examine the synergistic effects of quercetin with kaempferol or
isorhamnetin.
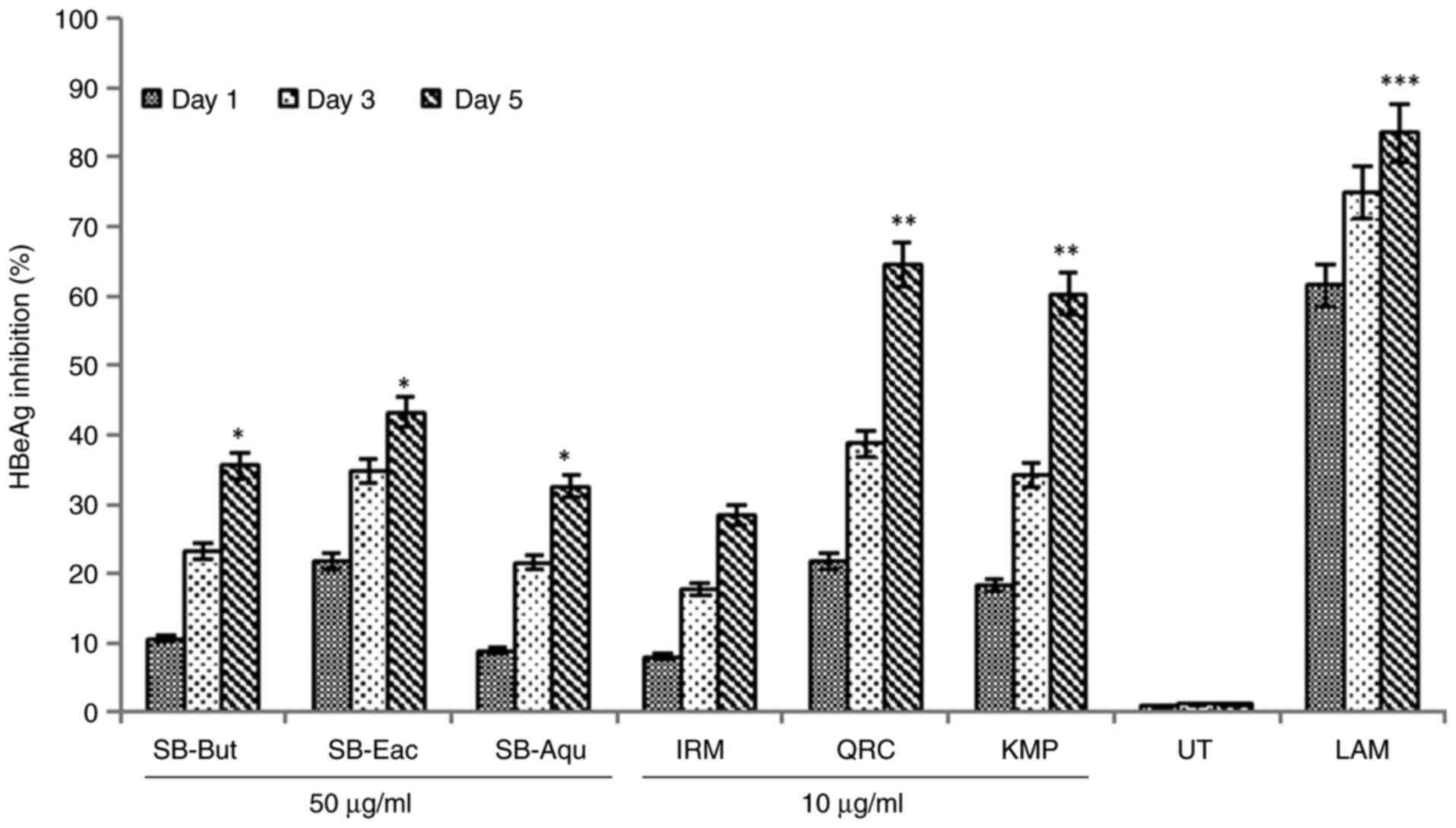 | Figure 6The anti-HBV assay showing
time-course inhibitions of HBV pre-core or ‘e’ antigen (HBeAg) by
the sea buckthorn SB-But, SB-Eac and SB-Aqu fractions (50 µg/ml,
each), as well as isorhamnetin (10 µg/ml), quercetin (10 µg/ml) and
kaempferol (10 µg/ml) in HepG2.2.15 cells. Lamivudine (2 µM) served
as a reference or positive control, while DMSO (0.1%) functioned as
the untreated control. Data are presented as the mean ± SEM (n=3);
*P<0.05, **P<0.01 and
***P<0.001 vs. UT. UT, untreated control; SB-But,
n-butanol fraction; SB-Eac, ethyl acetate fraction; SB-Aqu, aqueous
fraction; IRM, isorhamnetin; QRC, quercetin; LAM, lamivudine. |
Of note, although isorhamnetin was quantified as the
most abundant flavonol in the sea buckthorn extract, it exhibited
low anti-HBV activity, as compared to that observed for quercetin
and kaempferol (Figs. 5 and
6). As isorhamnetin has an
additional 3'-methyl group on its B ring as compared to quercetin
and kaempferol (Fig. 1, lower
panel), the structure-activity association may be attributed to its
weak antiviral activity. In contrast to the present data, in a
previous comparative antiviral study of structurally-related
flavonoids, isorhamnetin was reported to have the most potent
activity against influenza virus, which is an RNA virus (38). Notably, HBV is a DNA virus that has
different replication mechanisms than RNA viruses. Nonetheless, HBV
has a unique replication mechanism similar to HSV and HIV, the
retroviruses. Therefore, the majority of the anti-HSV and anti-HIV
drugs are also potent anti-HBV drugs. In previous studies, while
sea buckthorn leaf extract was reported for anti-DNV activity
(32), the isolated compound,
hiporamin, was found to exert potent antiviral effects against
influenza virus, HSV and HIV (34).
Of the three antiviral flavonoids identified in its leaves
(34), quercetin and kaempferol, as
well as their derivatives have been demonstrated to have marked
HBsAg and HBeAg inhibitory potentials in HepG2.2.15 cells (27-30).
Notably, although the broad antiviral potential of isorhamnetin
remains unexplored, a previous study reported its in vitro
activity against the influenza virus (37). Moreover, to further obtain insight
into the association between the chemical structures and anti-HBV
activities, molecular docking was previously performed for
quercetin, kaempferol and lamivudine with HBV-polymerase in
previous studies by the authors (28,29).
As in the present study, isorhamnetin exhibited a comparatively low
anti-HBV activity, molecular docking was not performed.
Notably, based on the safe usages of sea buckthorn
in traditional medicine, several clinical studies have revealed the
therapeutic effects of sea buckthorn against ulcerative stomatitis
(39), chronic cervicitis and
(40) and atopic dermatitis
(41). In another study, sea
buckthorn juice was reported to be beneficial in reducing the risk
factors for coronary heart disease, possibly due to its high
anti-oxidant property (42),
Moreover, when used in cirrhotic patients, sea buckthorn was found
to prevent the progression of liver fibrosis (8). In view of this, as well as in the
absence of a primate model of chronic HBV infection, these data
suggest that it is worthy of further clinical assessment.
In conclusion, the present study demonstrates that
the in vitro anti-HBV potential of sea buckthorn is
attributed to its well-known antiviral flavonols, quercetin and
kaempferol. Notably, although with a comparatively minimal effect,
isorhamnetin was found to exhibit anti-HBV activity, for the first
time, at least to the best of our knowledge. Nonetheless, further
molecular and pharmacological studies are warranted in order to
validate and develop anti-HBV therapeutics.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Researchers
Supporting Project (grant no. RSP-2021/379), King Saud University,
Riyadh, Saudi Arabia.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MKP and MSAD conceptualized and designed the study,
carried out experiments, collected and analyzed data, and wrote the
manuscript. OAB processed and prepared the plant sample. RNH
performed the HPLC analysis. MKP and MSAD confirm the authenticity
of all raw data. All authors have read and approved the final
manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li TSC: Taxonomy, natural distribution and
botany. In: Sea Buckthorn (Hippophae rhamnoides L.):
Production and Utilization. Li TSC and Beveridge T (eds). NRC
Research Press, Ottawa, ON, pp 7-11, 2003.
|
|
2
|
Suryakumar G and Gupta A: Medicinal and
therapeutic potential of sea buckthorn (Hippophae rhamnoides
L.). J Ethnopharmacol. 138:268–278. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dhyani D, Maikhuri RK, Misra S and Rao KS:
Endorsing the declining indigenous ethnobotanical knowledge system
of sea buckthorn in central Himalaya, India. J Ethnopharmacol.
127:329–334. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Suleyman H, Demirezer LO, Buyukokuroglu
ME, Akcay MF, Gepdiremen A, Banoglu ZN and Gocer F: Antiulcerogenic
effect of Hippophae rhamnoides. Phytother Res. 15:625–627.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Geetha S, Ram MS, Singh V, Ilavazhagan G
and Sawhney RC: Antioxidant and immunomodulatory properties of sea
buckthorn (Hippophae rhamnoides)-an in vitro study. J
Ethnopharmacol. 79:373–378. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xing J, Yang B, Dong Y, Wang B, Wang J and
Kallio PH: Effects of sea buckthorn (Hippophae rhamnoides
L.) seed and pulp oils on experimental models of gastric ulcer
in rats. Fitoterapia. 73:644–650. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Geetha S, Jayamurthy P, Pal K, Pandey S
and Sawhney RC: Hepatoprotective activity of Sea buckthorn
(Hippophae rhamnoides L.) against carbon tetrachloride
induced hepatic damage in rats. J Sci Food Agri. 88:1592–1597.
2007.
|
|
8
|
Gao ZL, Gu XH, Cheng FT and Jiang FH:
Effect of sea buckthorn on liver fibrosis: A clinical study. World
J Gastroenterol. 9:1615–1617. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang B and Kallio HP: Fatty acid
composition of lipids in Sea buckthorn (Hippophae rhamnoides
L.) berries of different origins. J Agri Food Chem.
49:1939–1947. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pintea A, Varga A, Stepnowski P, Socaciu
C, Culea M and Diehl HA: Chromatographic analysis of carotenol
fatty acid esters in Physalis alkekengi and Hippophae
rhamnoides. Phytochem Anal. 16:188–195. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Zheng RX, Xu XD, Tian Z and Yang JS:
Chemical constituents from the fruits of Hippophae
rhamnoides. Nat Product Res. 23:1451–1456. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pengfei L, Tiansheng D, Xianglin H and
Jianguo W: Antioxidant properties of isolated isorhamnetin from the
sea buckthorn marc. Plant Foods Human Nutr. 64:141–145.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Andersson SC, Olsson ME, Johansson E and
Rumpunen K: Carotenoids in sea buckthorn (Hippophae rhamnoides
L.) berries during ripening and use of pheophytin a as a
maturity marker. J Agri Food Chem. 57:250–258. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sajfrtova M, Lickova I, Wimmerova M,
Sovova H and Wimmer Z: b-Sitosterol: Supercritical carbon dioxide
extraction from sea buckthorn (Hippophae rhamnoides L.)
seeds. Intl J Mol Sci. 11:1842–1850. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Upadhyay NK, Kumar MSY and Gupta A:
Antioxidant, cytoprotective and antibacterial effects of Sea
buckthorn (Hippophae rhamnoides L.) leaves. Food Chem
Toxicol. 48:3443–3448. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim JS, Kwon YS, Sa YJ and Kim MJ:
Isolation and identification of sea buckthorn (Hippophae
rhamnoides) phenolics with antioxidant activity and glucosidase
inhibitory effect. J Agri Food Chem. 59:138–144. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
World Health Organization (WHO): Hepatitis
B. WHO, Geneva, 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
Accessed August 29, 2022.
|
|
18
|
Devi U and Locarnini S: Hepatitis B
antivirals and resistance. Curr Opin Virol. 3:495–500.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Parvez MK and Mechkarska M: Currently
available anti-hepatitis viruses drugs. J Gastroenterol Hepatol
Res. 9:3155–3157. 2020.
|
|
20
|
Wang G, Zhang L and Bonkovsky HL: Chinese
medicine for treatment of chronic hepatitis B. Chin J Integr Med.
18:253–255. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Parvez MK, Arab AH, Al-Dosari MS and
Al-Rehaily AJ: . Antiviral natural products against chronic
hepatitis B: recent developments. Curr Pharm Des. 3:286–293.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zakaryan H, Arabyan E, Oo A and Zandi K:
Flavonoids: Promising natural compounds against viral infections.
Arch Virol. 162:2539–2551. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Badshah SL, Faisal S, Muhammad A, Poulson
BG, Emwas AH and Jaremko M: Antiviral activities of flavonoids.
Biomed Pharmacother. 140(111596)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Di Petrillo A, Orrù G, Fais A and Fantini
MC: Quercetin and its derivates as antiviral potentials: A
comprehensive review. Phytother Res. 36:266–278. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5(e47)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arbab AH, Parvez MK, Al-Dosari MS,
Al-Rehaily AJ, Al-Sohaibani M, Zaroug EE, AlSaid MS and Rafatullah
S: Hepatoprotective and antiviral efficacy of Acacia
mellifera leaves fractions against hepatitis B virus. Biomed
Res Int. 2015(929131)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arbab AH, Parvez MK, Al-Dosari MS and
Al-Rehaily AJ: In vitro evaluation of novel antiviral
activities of 60 medicinal plants extracts against hepatitis B
virus. Exp Ther Med. 14:626–634. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Parvez MK, Rehman MT, Alam P, Al-Dosari
MS, Alqasoumi SI and Alajmi MF: Plant-derived antiviral drugs as
novel hepatitis B virus inhibitors: Cell culture and molecular
docking study. Saudi Pharm J. 27:389–400. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Parvez MK, Al-Dosari MS, Arbab AH,
Al-Rehaily AJ and Abdelwahid MAS: Bioassay-guided isolation of
anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along
with quercetin from Guiera senegalensis leaves. Saudi Pharm
J. 28:550–559. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parvez MK, Ahmed S, Al-Dosari MS,
Abdelwahid MAS, Arbab AH, Al-Rehaily AJ and Al-Oqail MM: The novel
anti-hepatitis B virus activity of Euphorbia schimperi and
its quercetin and kaempferol derivatives. ACS Omega. 6:2900–19110.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Parvez MK, Al-dosari MS, Abdelwahid MAS,
Alqahtani AS and Alanzi AR: Novel anti-hepatitis B virus-active
catechin and epicatechin from Rhus tripartita. Exp Ther Med.
3(398)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ahmed S, Parvez MK, Zia K, Nur-e-Alam M,
Ul-Haq Z, Al-Dosari MS and Al-Rehaily AJ: Natural anti-hepatitis B
virus flavones isolated from schimperi vatke growing in
Saudi Arabia: Cell culture and molecular docking study. Phramcog
Mag. 18:386–392. 2022.
|
|
33
|
Jain M, Ganju L, Katiyal A, Padwad Y,
Mishra KP, Chanda S, Karan D, Yogendra KM and Sawhney RC: Effect of
Hippophae rhamnoides leaf extract against Dengue virus
infection in human blood-derived macrophages. Phytomedicine.
15:793–799. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maheshwari DT, Kumar Y, Verma SK, Singh VK
and Singh SN: Antioxidant and hepatoprotective activities of
phenolic rich fraction of Sea buckthorn (Hippophae rhamnoides
L.) leaves. Food Chem Toxicol. 49:2422–2428. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shipulina LD, Tolkachev ON, Krepkova LV,
Bortnikova VV, Shkarenkov AA, et al: Anti-viral anti-microbial and
toxicological studies on Sea buckthorn (Hippophae
rhamnoides). In: Sea buckthorn (Hippophae L.): A
multipurpose wonder plant. Singh, V (ed). Vol 2. Daya Publishing
House, New Delhi, India, pp471-483, 2005.
|
|
36
|
Kumar S and Pandey AK: Chemistry and
biological activities of flavonoids: An overview.
ScientificWorldJournal. 2013(162750)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li F, Wang Z, Hu F and Su L: Cell culture
models and animal models for HBV study. Adv Exp Med Biol.
1179:109–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dayem AA, Choi HY, Kim YB and Cho SG:
Antiviral effect of methylated flavonol isorhamnetin against
influenza. PLoS One. 10(e0121610)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang LJ: Sea buckthorn oil and
chymotrypsin are effective in treating ulcerative stomatitis of
children. Hippophae. 5:32–34. 1992.
|
|
40
|
Ahani H and Attaran S: Therapeutic
potential of Seabuckthorn (Hippophae rhamnoides L.) in
medical sciences. Cell Mol Biomed Rep. 2:22–32. 2022.
|
|
41
|
Yang B, Kalimo KO, Tahvonen RL, Mattila
LM, Katajisto JK and Kallio HP: Effect of dietary supplementation
with sea buckthorn (Hippophae rhamnoides) seed and pulp oils
on the fatty acid composition of skin glycerophospholipids of
patients with atopic dermatitis. J Nutr Biochem. 11:338–340.
2000.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Eccleston C, Baoru Y, Tahvonen R, Kallio
H, Rimbach GH and Minihane AM: Effects of an antioxidant-rich juice
(sea buckthorn) on risk factors for coronary heart disease in
humans. J Nutr Biochem. 13:346–354. 2002.PubMed/NCBI View Article : Google Scholar
|