Introduction
Pituitary adenoma (PA) is a space-occupying tumor
that typically arises from the anterior pituitary gland and
comprises ~15% of all primary intracranial tumors (1). Although the majority of PA cases are
benign, they can occasionally be either invasive, inoperable or
non-responsive (2). PA has the
potential of causing serious complications long-term due to its
compressive effect on the local cranial structure. In addition,
functional PA tumors cause hormone hypersecretion, which can have
serious clinical implications if left untreated (3). Complications associated with
compression and hormonal hypersecretion include blindness, diabetes
insipidus, pituitary hormonal deficiency, Cushing syndrome,
acromegaly, secondary hyperthyroidism and infertility (4). Recent epidemiological data showed a
markedly increasing trend in the prevalence of PA (5). This may be due to recent advancements
in diagnostic modalities and incidental finding during imaging
(6). PA can be categorized using a
number of methods, namely based on size [micro (<1 cm) or macro
(>1 cm)] and functionality (secretory or non-secretory)
(7).
Several therapeutic options [medical, surgical and
radiation therapy (RT)] are available for treating PA (8). However, the type of therapeutic
intervention used depends on the size and functional status of the
tumor (9). Except prolactinoma
which is treated medically with dopamine agonist (cabergoline,
bromocriptine), surgical intervention is normally the treatment of
choice for all PA (10). In
addition, tumor recurrence frequently occurs even after surgery as
complete resection is difficult to achieve due to its invasion in
local structures such as cavernous sinus, nasopharynx and orbital
extension (11,12). Therefore, RT is now being proposed
as a viable therapeutic option for postoperative remnant growth or
tumor recurrence (13).
Conventionally, RT is delivered in multiple fractions with high
radiation exposure (45-50, 1.8-2 Gy per fraction) to the PA tumors
(14). RT has demonstrated potency
in inhibiting PA tumor growth (15). However, RT is also associated with a
number of serious side effects, such as hypopituitarism, optic
nerve neuropathy, occasionally cerebrovascular events and even
second primary brain tumors (15,16).
Therefore, the aim of the present retrospective
study was to evaluate the rate of tumor control and the incidence
of RT side effects in patients with pituitary macroadenomas.
Materials and methods
Patient data
A total of 75 patients with pituitary macroadenoma
who received RT were included into the present retrospective study.
The institutional review board (IRB; approval no. EX-03-07-20-01)
of Shaukat Khanum Memorial Cancer Hospital and Research Centre
(Lahore, Pakistan) has approved the present study. The IRB of
Shaukat Khanum Memorial Cancer Hospital and Research Centre also
allowed the waiver for informed consent for the present study.
The medical records of all patients with PA who
received RT between January 2005 and November 2019 were reviewed.
Patient demographics, in addition to their diagnosis, type of
adenoma, size of tumor, pituitary hormonal profile, presence of
hypopituitarism, medical treatment for prolactinoma, initial
treatment provided, type or surgery performed, re-surgery, total
dose of RT, fraction dose of RT, fractions given, indication for
radiation therapy, outcome of RT, biochemical outcome, visual
acuity status, complications and final outcome of RT, were all
collected from the medical records of patients present in the
hospital electronic medical record system. Adult patients with PA
(functional or non-functional) who received RT for relapse,
recurrence or irresectable disease, and post RT follow up of at
least 1 year or more with required set of investigations (pituitary
magnetic resonance imaging and pituitary hormonal profile) were
included. Patient who lost follow up or had incomplete follow up
investigations were excluded.
Statistical analysis
Statistical analysis was performed using the SPSS
software (version 20.0; IBM Corp.). Continuous variables were
presented as the mean ± standard deviation whereas categorical
variables were presented as frequencies and percentages. To compare
the frequency distribution in the RT characteristics between
functional and non-functional tumors, the chi-squared
(χ2) test or Fisher's exact test (where appropriate) was
applied. The response to RT was considered complete if PA totally
resolved, partial if decreased in size or stable if not increased
in size on follow up. Normalization of excess hormonal production
in functional PA after RT was considered biochemical remission.
Kaplan-Meier survival curve was drawn to assess the progression
free survival proportion. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Demographic and clinical
characteristics
A total of 75 PA patients who fulfilled criteria
were included in this retrospective analysis. The mean age was
38.55±1.36 years. In addition, there were 43 males (57.3%) and 32
were females (42.7%). Of the 75 patients in the present study, 50
(66.7%) were diagnosed with non-functioning and 25 (33.3%) were
suffering from functioning tumors. Of the 25 functioning tumors
observed, 18 (72%) were growth hormone (GH)-secreting, 4 (16%) were
prolactin-secreting, 1 (4%) was adrenocorticotrophic
hormone-secreting and 2 (8%) were found to be secreting both GH and
prolactin. Furthermore, 42 (56%) patients were found to exhibit
hypopituitarism before RT. The baseline patient characteristics are
shown in Table I.
 | Table IPre-radiation therapy patient
characteristics. |
Table I
Pre-radiation therapy patient
characteristics.
| Characteristic | Frequency (%)/Mean
& SD |
|---|
| Age, years | 38.55±1.36 |
| Sex | |
|
Male | 43 (57.3%) |
|
Female | 32 (42.7%) |
| Hypopituitarism | |
|
Partial | 19 (25.3%) |
|
Complete | 23 (30.7%) |
|
None | 33 (44%) |
| Excess hormone
secretion | |
|
Functioning | 25 (33.3%) |
|
Prolactin | 4 (16%) |
|
GH | 18 (72%) |
|
ACTH | 1 (4%) |
|
GH
+ prolactin | 2 (8%) |
|
Non-functioning | 50 (66.7%) |
| Size of tumor,
cm | 3.84±1.43 cm |
Of the 75 patients, 59 (78.7%) received surgery 33
transcranial and 26 transsphenoidal approach) before RT. In total,
10 (13.3%) patients received RT as the initial mode of treatment,
whilst 6 (8%) patients (4 were prolactin-secreting and 2 were
co-secreting prolactin and GH) were initially managed with dopamine
agonist medical therapy before being treated with RT due to
resistance to medical therapy and not being eligible for surgery
due to age and comorbidities. All 75 patients received external
beam RT (EBRT). Specifically, the three-dimensional conformal RT
technique was used in all patients (two laterals and one
low-weighted vertex field). The majority of patients (45; 60%)
received RT radiation doses in the range of 5041-5400 cGy.
Furthermore, 61 patients (81.3%) received a dose of 180 cGy for
each fraction. The total RT radiation dose and dose per fraction
delivered for patients with functional and non-functional tumors
are shown in Table II.
 | Table IIRadiation therapy-related patient
characteristics in functional and non-functional tumors. |
Table II
Radiation therapy-related patient
characteristics in functional and non-functional tumors.
| Characteristic | Functional (%) | Non-functional
(%) | Total (%) | P-value |
|---|
| Total dose (cGy) | | | | |
|
4,500-5,040 | 9(36) | 20(40) | 29 (38.7) | |
|
5,041-5,400 | 15(60) | 30(60) | 45(60) | |
|
>5,400 | 1(4) | 0 (0) | 1 (1.3) | 0.474 |
| Dose per fraction
(cGy) | | | | |
|
180 | 18(72) | 43(86) | 61 (81.3) | |
|
181-200 | 7(28) | 6(12) | 13 (17.3) | 0.185 |
|
>200 | 0 (0) | 1(2) | 1 (1.3) | |
| Indication | | | | |
|
Pre-operative | 6(24) | 10(20) | 16 (21.3) | 0.208 |
|
Post-operative | 19(76) | 40(80) | 59 (78.7) | |
RT complications
Several complications were observed after RT in the
present study (Table III). Out of
the 75 patients included, pan-hypopituitarism was the most commonly
observed complication, with 29 patients being recorded (38.7%).
Other complications include worsening of visual acuity [7 patients,
(9.3%)], optic neuropathy [2 patients, (2.7%)], brain atrophy [4
patients, (5.3%)], fits [3 patients, (4%)] and diabetes insipidus
[1 patient, (1.3%)].
 | Table IIIPost-radiotherapy complications. |
Table III
Post-radiotherapy complications.
| Complications | Frequency (%) |
|---|
| Visual acuity | |
|
Stable | 61 (81.3) |
|
Improved | 7 (9.3) |
|
Worsened | 7 (9.3) |
|
Panhypopituitarism | 29 (38.7) |
| Stroke | 2 (2.7) |
| Isolated growth
hormone deficiency | 2 (2.7) |
| Brain atrophy | 4 (5.3) |
| Fits | 3(4) |
| No
complication | 34 (45.3) |
Survival and outcome
Out of the 75 patients, 36 (48%) remained stable
(mean follow-up, 74±38 months), whereas tumor progression was
observed in 6 (8%) patients (mean follow-up, 11±6 months). In
total, 32 (42.7%) presented with partial response (mean follow-up,
85±33 months) and 1 (1.3%) patient showed a complete response
(follow-up, 116 months). Overall, the local tumor control was
observed in 92% of patients at 6.68 years median follow up. A total
of 2 (2.7%) patients succumbed to complications associated with
this disease. The specific causes of death were pulmonary embolism
and severe pneumonia. There was no difference in the frequency
distribution of the treatment outcomes between functional and
non-functional tumors (P=0.688; Table
IV). Additionally, of the 25 patients with functional tumors,
18 (72%) failed to control the biochemical excess, whilst remission
was observed in 7 (28%) patients after RT. The median hormonal
excess correction time was 69 months after RT (range, 24-112). The
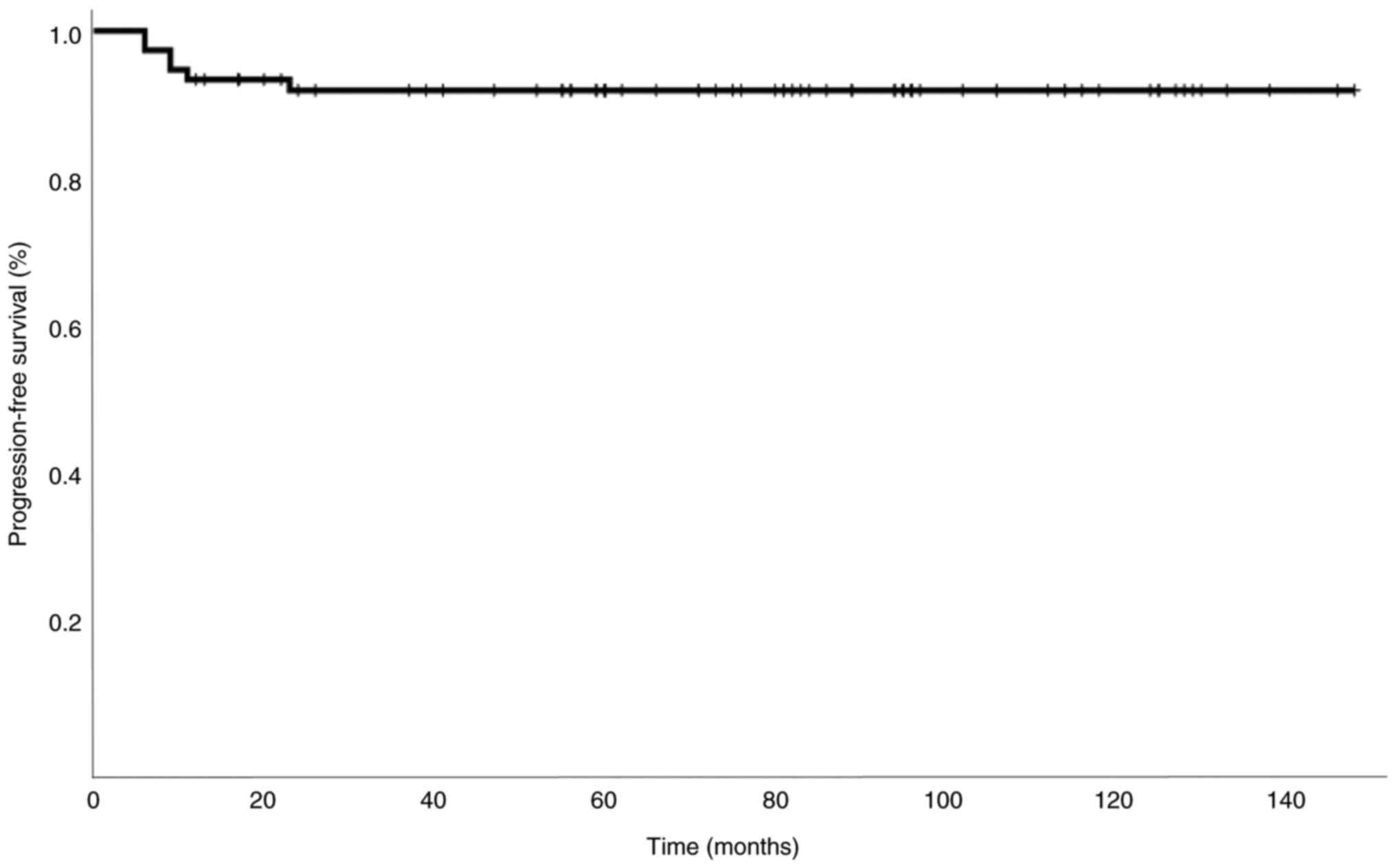
overall progression-free survival at 2 years was 92% (Fig. 1).
 | Table IVPost-radiation therapy outcomes. |
Table IV
Post-radiation therapy outcomes.
| Outcomes | Functional | Non-functional | Total | P-value |
|---|
| Local tumor
control | | | | |
|
Stable | 10 (40%) | 26 (52%) | 36 (48%) | |
|
Progress | 2 (8%) | 4 (8%) | 6 (8%) | 0.688 |
|
Partial
response | 13 (52%) | 19 (38%) | 32 (42.7) | |
|
Complete
response | 0 (0%) | 1 (2%) | 1 (1.3%) | |
| Post-radiation
therapy hormonal excess status | | | | |
|
Normal | 7 (28%) | 0 (0%) | 7 (9.3%) | |
|
Failed to
improve | 6 (24%) | 0 (0%) | 6 (8%) | |
|
Improved but
not normalized | 12 (48%) | 0 (0%) | 12 (16%) | |
Discussion
Pituitary macroadenoma is not acquiescent to
complete resection due to its invasive characteristics in the local
structure (12,17). Therefore, the majority of patients
with incompletely resected tumors are recommended for RT (18). The aim of the present study was to
examine the efficacy and toxicity of RT for pituitary macroadenoma.
This was achieved by studying the degree of local tumor control,
hormonal control rate and complications following RT.
All patients included in the present study had
pituitary macroadenoma. Specifically, 59 patients (78.7%) needed RT
post-surgery for recurrent/residual tumors, whilst 16 patients
(21.3%) received RT without surgery either due to being
unresponsive to medical therapy dopamine agonist in case of
prolactin secreting PA or not fit for surgery due to comorbidities
in case of non-functional PA. Furthermore, local tumor control was
achieved in the majority of patients (92% at 6.68 years median
follow up) with both functional and nonfunctional tumors. These
results are consistent with those previously reported by
Langsenlehner et al (19),
where the overall local tumor control rate for both functional and
non-functional tumors was 95.4% after 15 years.
The effectiveness of RT has been frequently reported
to alleviate pathological hormone hypersecretion (19). In the present study, normalization
of increased hormone levels after RT was attained in 7 (28%)
patients. By contrast, the biochemical remission rate achieved in
the present study was lower compared with that reported in previous
studies (20,21). This may be due to the shorter median
follow up time of 6.68 years. Additionally, the lack of
anti-hormonal medication after RT due to financial constraints may
be another underlying cause. It has been previously found that for
patients undergoing conventional external RT the time required for
the raised hormone levels to return to normal is relatively long
(median follow up of 5-8 years), thereby necessitating additional
antihormonal therapy (20,22).
With regards to toxicity, the most frequently
encountered late complication following RT in patients with
pituitary macroadenoma is hypopituitarism (23). Pan-hypopituitarism following RT is a
gradual process (24). In the
present study, panhypopituitarism following RT was observed in 29
(38.7%) patients. These results are in accordance with those
previously reported (20,23,25).
Only 7 (9.3%) patients developed visual acuity deterioration, which
is consistent with the data reported by Wilson et al
(26). Furthermore, other
complications, such as optic neuropathy, stroke, diabetes
insipidus, brain atrophy, cognitive decline and fits, were also
observed. Therefore, these data suggested that RT is a relatively
safe modality. However, the risk of these complications, except for
hypopituitarism, can be reduced further with development of novel
stereotactic RT techniques, including stereotactic radiosurgery and
fractionated stereotactic RT (15,27).
This is because they can deliver high doses of RT to the tumor more
precisely with lesser exposure to the adjacent structures (14,26).
The present study has the limitation of only assessing
complications associated with EBRT, since stereotactic RT was not
available in the Centre in the present study.
To conclude, data from the present study showed that
local tumor control in non-functional and functional pituitary
macroadenoma can be managed well with RT. However, the biochemical
control in functional pituitary macroadenoma was not as effective
as local tumor control. To optimize the outcome in biochemical
control, other treatment modalities may be considered alongside
RT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SAR conceived the idea and participated in the
design, data analysis, interpretation and writing of the present
study. WS contributed in the design of the study and carried out
critical review for important intellectual content. UA contributed
in the design of the study, and participated in the writing of the
manuscript. AIS contributed in the design of the study, and
participated in the writing of manuscript. KA contributed in the
design of the study, analysis and interpretation of the data,
participated in the writing of manuscript and critically review the
manuscript. HI, SA and AMA contributed in the acquisition, analysis
and interpretation of the data. MAB performed statistical analysis,
analyzed the data, and participated in the writing of the
manuscript. SAR and WS confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The institutional review board (approval no.
EX-03-07-20-01) of Shaukat Khanum Memorial Cancer Hospital and
Research Centre (Lahore, Pakistan) has approved the present study.
Shaukat Khanum Memorial Cancer Hospital and Research Centre also
allowed the waiving of informed consent for the present study due
to the retrospective nature that does not involve direct contact
with the patients. The clinical information already existed in the
hospital records. Private information of the human subjects was
recorded without any identifiers and the resulting research dataset
is completely anonymous (i.e., the dataset cannot be linked back to
the individuals).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Melmed S: Pathogenesis of pituitary
tumors. Nat Rev Endocrinol. 7:257–266. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lim CT and Korbonits MK: Update on the
clinicopathology of pituitary adenomas. Endocr Pract. 24:473–488.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brue T and Castinetti F: The risks of
overlooking the diagnosis of secreting pituitary adenomas. Orphanet
J Rare Dis. 11(135)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Russ S, Anastasopoulou C and Shafiq I:
Pituitary adenoma. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island, FL, 2021.
|
|
5
|
Chin SO: Epidemiology of functioning
pituitary adenomas. Endocrinol Metab (Seoul). 35:237–242.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hemminki K, Försti A and Ji J: Incidence
and familial risks in pituitary adenoma and associated tumors.
Endocr Relat Cancer. 14:103–109. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Russ S, Anastasopoulou C and Shafiq I:
Pituitary adenoma. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island, FL, 2022.
|
|
8
|
Molitch ME: Diagnosis and treatment of
pituitary adenomas: A review. JAMA. 317:516–524. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ding D, Starke RM and Sheehan JP:
Treatment paradigms for pituitary adenomas: Defining the roles of
radiosurgery and radiation therapy. J Neurooncol. 117:445–457.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Freda PU and Wardlaw SL: Clinical review
110: Diagnosis and treatment of pituitary tumors. J Clin Endocrinol
Metab. 84:3859–3866. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ciric I, Mikhael M, Stafford T, Lawson L
and Garces R: Transsphenoidal microsurgery of pituitary
macroadenomas with long-term follow-up results. J Neurosurg.
59:395–401. 1983.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Serioli S, Doglietto F, Fiorindi A, Biroli
A, Mattavelli D, Buffoli B, Ferrari M, Cornali C, Rodella L,
Maroldi R, et al: Pituitary adenomas and invasiveness from
anatomo-surgical, radiological, and histological perspectives: A
systematic literature review. Cancers (Basel).
11(1936)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chanson P, Dormoy A and Dekkers OM: Use of
radiotherapy after pituitary surgery for non-functioning pituitary
adenomas. Eur J Endocrinol. 181:D1–D3. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scheick S, Amdur RJ, Kirwan JM, Morris CG,
Mendenhall WM, Roper S and Friedman W: Long-term outcome after
fractionated radiotherapy for pituitary adenoma: The curse of the
secretory tumor. Am J Clin Oncol. 39:49–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sebastian P, Balakrishnan R, Yadav B and
John S: Outcome of radiotherapy for pituitary adenomas. Rep Pract
Oncol Radiother. 21:466–472. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA
and Kim CY: Radiotherapy for pituitary adenomas: Long-term outcome
and complications. Radiat Oncol J. 29:156–163. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rutkowski M and Zada G: Management of
pituitary adenomas invading the cavernous sinus. Neurosurg Clin N
Am. 30:445–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Loeffler J and Shih HA: Radiation therapy
in the management of pituitary adenomas. J Clin Endocrinol Metab.
96:1992–2003. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Langsenlehner T, Stiegler C, Quehenberger
F, Feigl GC, Jakse G, Mokry M, Langsenlehner U, Kapp KS and Mayer
R: Long-term follow-up of patients with pituitary macroadenomas
after postoperative radiation therapy: Analysis of tumor control
and functional outcome. Strahlenther Onkol. 183:241–247.
2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Colin P, Jovenin N, Delemer B, Caron J,
Grulet H, Hecart AC, Lukas C, Bazin A, Bernard MH, Scherpereel B,
et al: Treatment of pituitary adenomas by fractionated stereotactic
radiotherapy: A prospective study of 110 patients. Int J Radiat
Oncol Biol Phys. 62:333–341. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sasaki R, Murakami M, Okamoto Y, Kono K,
Yoden E, Nakajima T, Nabeshima S and Kuroda Y: The efficacy of
conventional radiation therapy in the management of pituitary
adenoma. Int J Radiat Oncol Biol Phys. 47:1337–1345.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Becker G, Kocher M, Kortmann RD, Paulsen
F, Jeremic B, Müller RP and Bamberg M: Radiation therapy in the
multimodal treatment approach of pituitary adenoma. Strahlenther
Onkol. 178:173–186. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kokubo M, Sasai K, Shibamoto Y, Aoki T,
Oya N, Mitsumori M, Takahashi JA, Hashimoto N and Hiraoka M:
Long-term results of radiation therapy for pituitary adenoma. J
Neurooncol. 47:79–84. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Minniti G, Jaffrain-Rea ML, Osti M,
Esposito V, Santoro A, Solda F, Gargiulo P, Tamburrano G and Enrici
RM: The long-term efficacy of conventional radiotherapy in patients
with GH-secreting pituitary adenomas. Clin Endocrinol (Oxf).
62:210–216. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jallad RS, Musolino NR, Salgado LR and
Bronstein MD: Treatment of acromegaly: Is there still a place for
radiotherapy? Pituitary. 10:53–59. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wilson PJ, De-Loyde KJ, Williams JR and
Smee RI: A single centre's experience of stereotactic radiosurgery
and radiotherapy for non-functioning pituitary adenomas with the
linear accelerator (Linac). J Clin Neurosci. 19:370–374.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gheorghiu ML and Fleseriu M: Stereotactic
radiation therapy in pituitary adenomas, is it better than
conventional radiation therapy? Acta Endocrinol (Buchar).
13:476–490. 2017.PubMed/NCBI View Article : Google Scholar
|