Introduction
Non-small cell lung cancers (NSCLCs) are the leading
cause of cancer-related mortality in the United States, and are
broadly comprised of two subtypes, including squamous cell
carcinoma (SCC) and adenocarcinoma (ADC) (1,2).
Patients with NSCLC have a poor prognosis, and this disease
accounts for 85-90% of all lung cancers. Notably, NSCLC may be
attributed to a number of genetic abnormalities, including genetic
mutations, deletions and amplifications in receptor tyrosine
kinases, such as epidermal growth factor receptor (EGFR),
mesenchymal-epithelial transition (MET) factor and anaplastic
lymphoma kinase, and activation of their downstream signaling
mediators including Kirsten rat sarcoma viral oncogene homolog
(KRAS), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), and
phosphatidylinositol-3-kinase (PI3K). These genetic aberrations
have brought about the clinical use of various kinase inhibitors as
secondary targeted treatment strategies beyond surgery and
radiation for patients diagnosed with NSCLC. Thus, numerous kinase
inhibitors, such as erlotinib, gefitinib and crizotinib, have been
used in clinical practice. Specifically, these inhibitors target
EGFR, MET and ALK for the treatment of NSCLC (3,4).
Despite the pharmacodynamic rationale for the treatment of NSCLCs
using these tyrosine kinase inhibitors, the clinical management and
overall survival of patients treated with these agents has not
significantly improved. This may be due to high levels of acquired
resistance to clinically used kinase inhibitors. These levels of
resistance may be a result of increased activation of compensatory
tyrosine kinases and downstream signaling mediators of the intended
targets, which may inhibit the efficacy of first and second
generation NSCLC kinase inhibitors (5-7).
Notably, in preclinical studies, the use of combined treatment
strategies that impair divergent signaling pathways have proven
efficacious in the treatment of NSCLC resistant to EGFR inhibitors.
More specifically, when apatinib, a vascular endothelial growth
factor (VEGF) receptor-2 inhibitor, was combined with the EGFR
inhibitor, gefitinib, it exerted an antitumorigenic effect in NSCLC
as a consequence of independently downregulating VEGFR-2 and EGFR
activity. This supported the notion that targeting multiple
receptors and pathways may help overcome EGFR inhibitor resistance
and treat NSCLC (8,9).
An additional contributor to the therapeutic
resistance and high mortality rates associated with NSCLCs are
brain metastases, estimated to occur in 20-40% of diagnosed cases,
with a ~100% mortality rate (9-11).
Thus, the biology of lung cancer brain metastasis and the
underlying molecular mechanisms remain poorly understood. However,
the receptor tyrosine kinases, EGFR and MET, are implicated in the
propagation of lung cancer brain metastases. MET exerts signaling
capacity in cancer cells via activation and stimulation of the
PI3K/AKT/mTOR pathway, and is expressed in 44% of NSCLC brain
metastatic tissues. In addition, activating mutations in EGFR may
induce DNA synthesis and tumor cell proliferation via signal
transduction activation of MAPK, AKT and JNK (12). Collectively, these results
demonstrate that a network of signaling kinases not only contribute
to the survival of primary NSCLC, but also impact the progression
of NSCLC brain metastases. Identification of novel therapeutic
agents is urgently required to overcome these clinical resistance
mechanisms of NSCLCs, to improve the management and overall
survival outcomes of patients diagnosed with these tumors.
PP121 is a novel dual kinase inhibitor that targets
tyrosine kinases and PI3K. It is often used in a single agent
approach to simultaneously target multiple pro-tumorigenic
signaling mediators in primary NSCLC and NSCLC that metastasizes to
the brain. Notably, results of preclinical studies have
demonstrated the antitumorigenic properties of PP121 in the
inhibition of esophageal and brain cancer cell proliferation, and
the impaired migration of anaplastic thyroid carcinoma cells
(13-15).
In addition, results of a previous study demonstrated that PP121
inhibited ovarian cancer metastasis (16). Mechanistically, downregulation of
PI3K-mTOR signaling mediators and inhibition of MEK have been
associated with the suppression of tumor cell proliferation and
metastatic progression of these solid tumors (17-19).
These results further demonstrate the potential specificity of
PP121 in targeting divergent receptor tyrosine kinase molecular
signaling pathways. Collectively, these results support the
hypothesis that PP121 may exhibit potential as a single
agent-targeted strategy to overcome multilateral compensatory
resistant mechanisms in primary NSCLC and NSCLC that metastasizes
to the brain.
Materials and methods
Cell culture conditions and
reagents
ADC (NCI-H1975; CRL-5908) and SCC (NCI-H2170;
CRL-5928) NSCLC cells were purchased from the American Type Culture
Collection. All cell lines were cultured in RPMI medium containing
10% FBS and penicillin-streptomycin (all from Invitrogen; Thermo
Fisher Scientific, Inc.) at 37˚C with 5% CO2. PP121 was
purchased from Tocris Bioscience. Healthy human astrocytes (product
no. 1800) were purchased from ScienCell Research Laboratories,
Inc., and cultured in astrocyte media (ScienCell Research
Laboratories, Inc.).
Crystal violet cell proliferation
assay
For dose-response experiments, cells were plated in
12-well plates with 1, 5 and 10 µM and 500 nM PP121, and incubated
for 48 h at 37˚C with 5% CO2. Vehicle controls were
treated with dimethyl sulfoxide (DMSO). Subsequently, tissue
culture medium was removed, the cell monolayer was fixed with 100%
methanol for 5 min at room temperature (22˚C) and stained with 0.5%
crystal violet in 25% methanol for 10 min at room temperature.
Cells were washed three times using distilled water for 5 min each
time to remove excess dye. Subsequently, cells were left to dry
overnight at room temperature. The incorporated dye was solubilized
in 0.1 M sodium citrate (Sigma-Aldrich; Merck KGaA) in 50% ethanol.
In total, 100 µl of treated and control samples were transferred to
96-well plates and optical densities were read at 540 nm using an
X-mark microplate absorbance spectrophotometer (BioRad
Laboratories, Inc.).
Patient-derived xenograft organoids
(PDXOs)
NSCLC-PDXO experiments were carried out in
collaboration with Crown BioScience. NSCLC-PDXOs (LU6471B-SCC and
LU5162B-ADC) were generated from histopathologically identified
patient-derived xenografts. Written informed consent was obtained
from patients and ethics approval was obtained from the
Integreview-Advarra ethical review board (Columbia, USA; approval
no. MRL01). Organoids were processed using Matrigel for subsequent
screening and size determination. Organoids were collected, plated
in triplicate at a density of 250 NSCLC-PDXOs/well and treated with
PP121 (0.0078-2 µM) or vehicle controls for 5 days. Cell viability
was assessed as an endpoint using the CellTiter-Glo®
Luminescent assay (cat. no. G9683; Promega Corporation).
Western blotting
Cells were plated and treated with PP121 or DMSO for
3 h, rinsed with PBS and lysed using CelLytic M Cell lysis reagent
(Sigma-Aldrich; Merck KGaA). Protein concentrations were
subsequently determined using Bradford reagent. Proteins (30 µg)
were separated via SDS-PAGE in 8% polyacrylamide gels and
transferred to PVDF membranes. Membranes were incubated with
primary antibodies at 1:500 against phosphorylated (p)-Akt (product
no. 4060L), Akt (product no. 4691), p-S6 ribosomal protein (p-RPS6;
product no. 4858S), S6 ribosomal protein (product no. 2317), and
cyclophilin B (product no. 43603; all from Cell Signaling
Technology, Inc.) overnight at 4˚C. Following primary incubation,
membranes were incubated with an HRP-conjugated secondary antibody
(product no. 7074S; Cell Signaling Technology, Inc.) at 1:1,000 for
1 h at room temperature, and visualized using an enhanced
chemiluminescence (ECL) detection system (Thermo Fisher Scientific,
Inc.), and a UVP BioSpectrum imaging system (Analytik Jena AG).
Radius cell migration assays
Radius motility assays were established by placing
inserts into 12-well plates and seeding 5x104 NSCLC
cells via openings at the top of the inserts (Cell Biolabs, Inc.)
and incubated for 24 h at 37˚C with 5% CO2.
Subsequently, inserts were removed and NSCLC cells were treated
with 500 nM PP121 for 96 h. At the end of the incubation period,
cells were stained with crystal violet as previously described, and
the cell-free zone was quantified using ImageJ [1.52a; Java
1.8.0_112 (64-bit) National Institutes of Health]. For radius cell
migration assays using cell co-culturing, NSCLC cells were plated
as previously described, followed by the plating of
1x105 healthy human astrocytes.
Immunofluorescence
Immunofluorescence labeling was performed 5 days
after cells were treated with 500 nM PP121. Cells were rinsed in
PBS and fixed in 4% paraformaldehyde for 5 min at room temperature.
Subsequently, cells were rinsed with PBS, permeabilized in 0.075%
Triton X-100/PBS for 5 min, rinsed again with PBS, and blocked with
3.0% bovine serum albumin and 1.5% horse serum (Vector
Laboratories, Inc.) in PBS for 1 h at room temperature. Cells were
incubated overnight at 4˚C with primary antibodies (1:500) against
thyroid transcription factor 1 (product no. 12373) and glial
fibrillary acidic protein (product no. 3656; Cell Signaling
Technology, Inc.). Samples were subsequently rinsed three times
with PBS, incubated with an Alexa 488 goat anti-mouse-conjugated
secondary antibody at 1:1,000 (product no. 4408; Cell Signaling
Technology, Inc.) for 1 h in the dark, rinsed again and examined
using an Olympus IX53 fluorescence microscope (Olympus
Corporation).
P-glycoprotein (P-gp) assay
A P-gp Glo assay kit was purchased from Promega
Corporation and the P-gp assay was performed following the
manufacturer's instructions. Untreated controls (Pgp-Glo assay
buffer), positive controls (0.05 mM Na3VO4
and 0.1 mM verapamil) and 50 µM PP121 were prepared following the
manufacturer's instructions. P-gp membranes were added to wells
containing untreated controls, 0.05 mM
Na3VO4, 0.1 mM verapamil or 50 µM PP121 and
incubated at 37˚C for 5 min. Reactions were subsequently initiated
following the addition of 5 mM magnesium adenosine triphosphate
(MgATP), and samples were incubated for 40 min at 37˚C.
Luminescence was initiated following the addition of ATP detection
reagent, followed by incubation at room temperature for 20 min.
Subsequently, luminescence was read using a luminometer.
Statistical analysis
Cell viability, radius cell migration and Pg-p
activity experiments were performed at least three times using
duplicate or triplicate samples. Unpaired Student's t-tests and
one-way ANOVA followed by Tukey's post hoc analysis were performed
using GraphPad Prism 8.0 (GraphPad Software, Inc.) to determine the
statistical significance between groups. The results are presented
as the average means ± standard error of means. Coexpression
analyses of mRNA expression were performed using Spearman's
correlation test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PP121 reduces NSCLC cell viability and
migratory invasion
To the best of my knowledge, PP121 has not been
evaluated as a targeted agent for the treatment of lung cancers,
specifically NSCLC. To determine the use of PP121 as a novel single
drug agent for the treatment of NSCLC, an expression analysis of
the receptor tyrosine kinase, MET was carried out. The potential
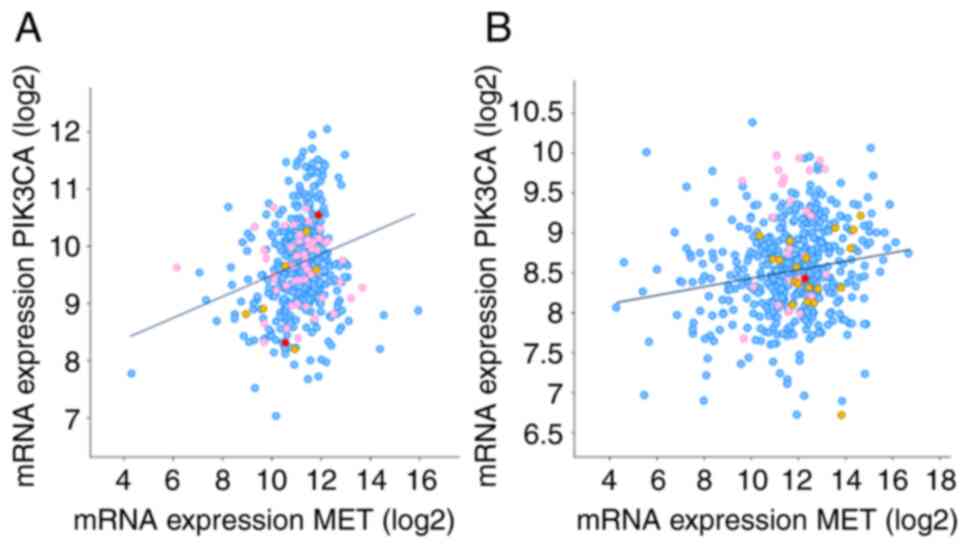
association between MET and PIK3CA was determined using The Cancer
Genome Atlas database. Results of the present study revealed a
positive association between PIK3CA and MET (Table I and Fig. 1). As drug doses differ between
experiments and clinical practice, a range of PP121 concentrations
(500 nM-10 µM) were evaluated to establish the lowest doses that
exerted antiproliferative effects on SCC and ADC cells.
 | Table ICo-expression of MET and PIK3CA in
NSCLC. |
Table I
Co-expression of MET and PIK3CA in
NSCLC.
| Type of cancer | Spearman's
correlation | P-value |
|---|
| SCC | 0.19 |
2.65e-5 |
| ADC | 0.16 |
2.08e-4 |
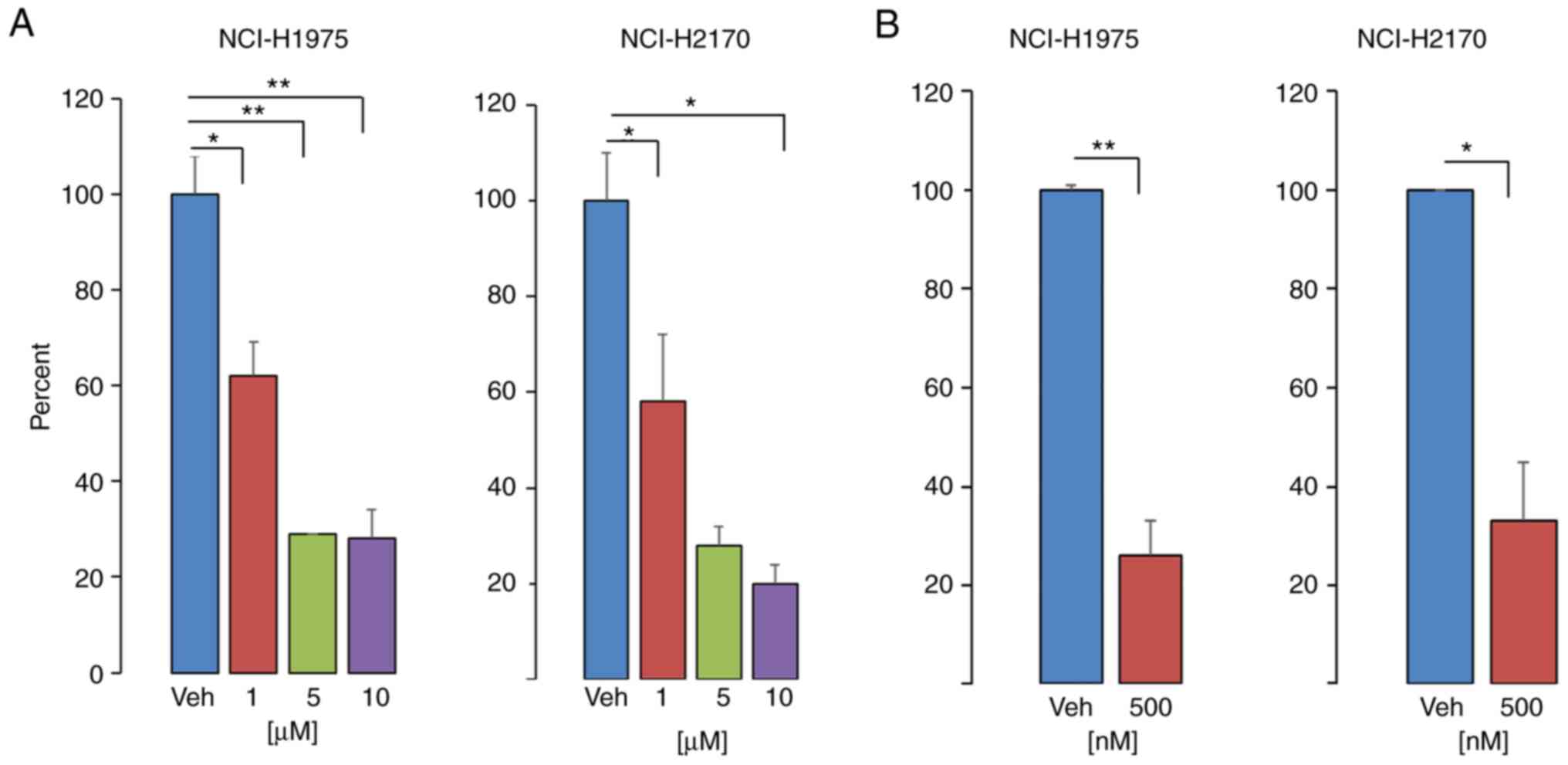
Results of the present study demonstrated that PP121
decreased NSCLC cell viability in a dose-dependent manner, and that
exposure to concentrations as low as 500 nM significantly decreased
cell viability by 75 and 70% in SCC and ADC cells, respectively
(Fig. 2). The observed decrease in
NSCLC cell viability following treatment with PP121 was consistent
with previous efficacy experiments, demonstrating that PP121
treatment reduced glioblastoma and breast cancer cell proliferation
(14). Notably, the
antiproliferative effect of PP121 was demonstrated in NCI-1975
cells possessing mutations in EGFR and PIK3CA.
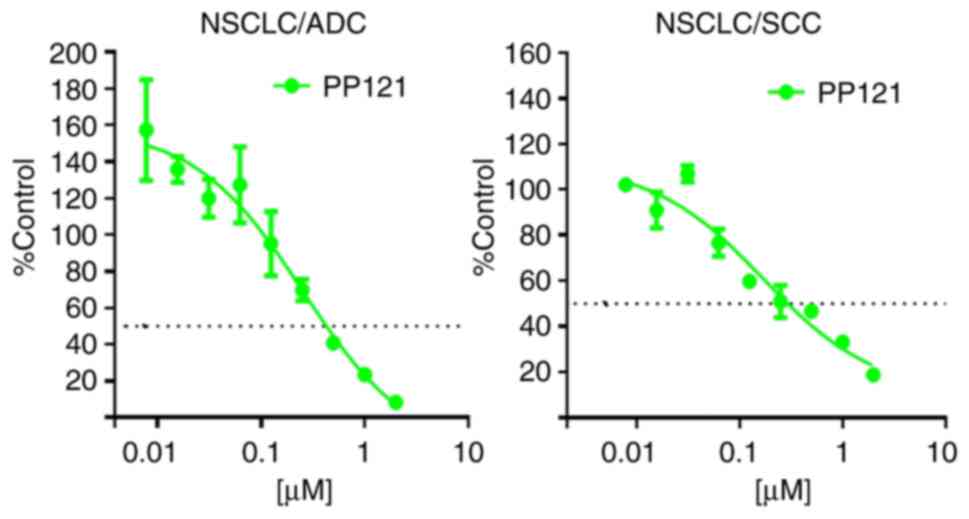
To assert a more clinically relevant approach for
evaluating the response of NSCLC to PP121, translational applicable
model systems were used. Specifically, PDXOs were used to further
determine the preclinical antitumorigenic effects of PP121
(Fig. 3). Notably, PDXOs are 3D
in vitro models developed from in vivo
patient-derived xenografts. These are useful preclinical models
that maintain the genetic heterogeneity of clinical tumors. Results
of the present study demonstrated that PP121 significantly
decreased the proliferative capacity of NSCLC-PDXOs, compared with
vehicle controls (Fig. 3).
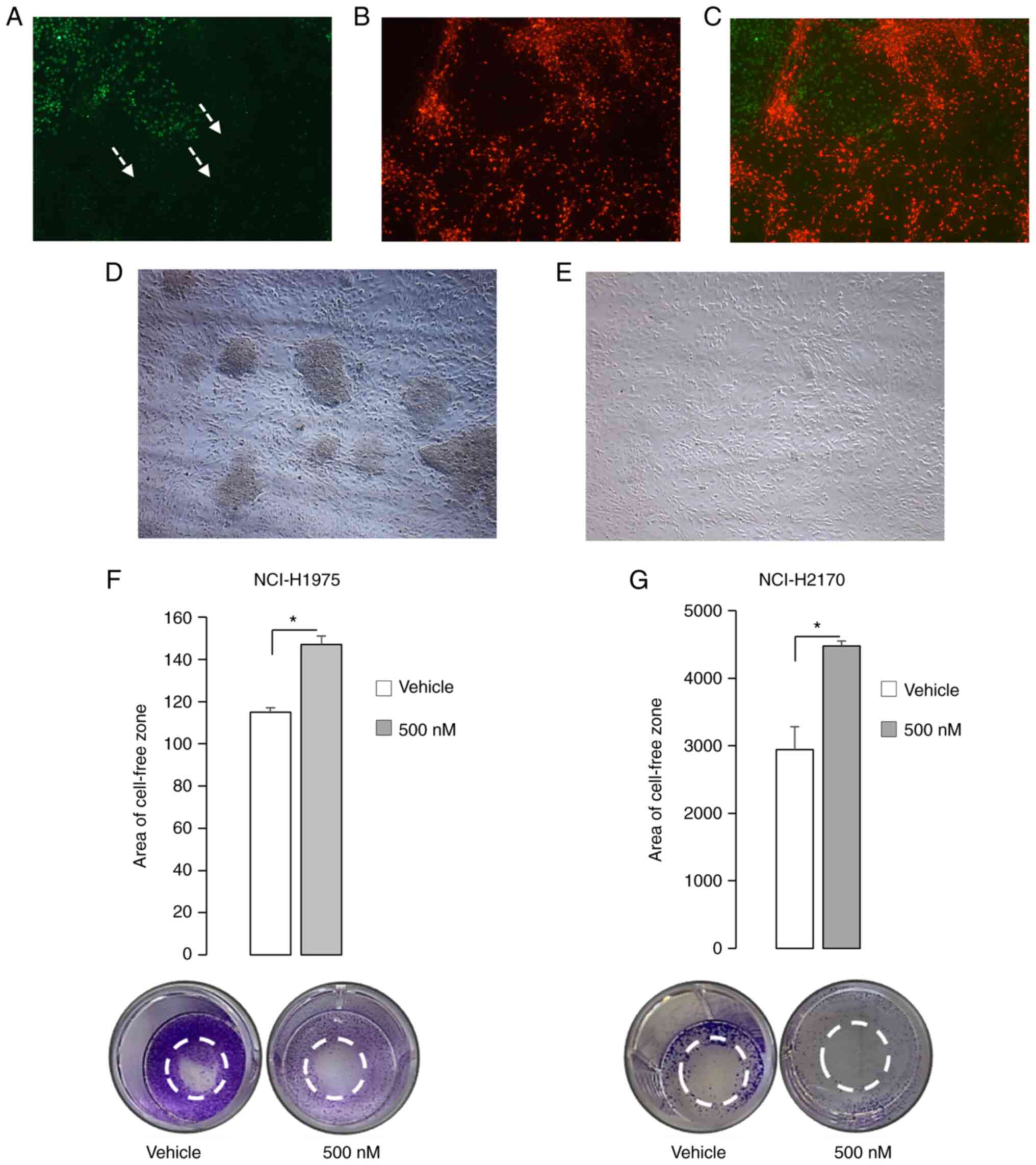
NSCLC brain metastasis is a major contributor to
NSCLC disease progression, as a consequence of therapeutic evasion
and resistance. To recapitulate NSCLC brain metastases, NCI-H1975
ADC cells were co-cultured with healthy human astrocytes. Results
of the present study demonstrated that NCI-H1975 ADC cells migrated
and penetrated healthy human astrocytes (Fig. 4A-C). However, treatment with PP121
inhibited SCC cell migration and the invasion of healthy human
astrocytes (Fig. 4D and E). In addition, the results of radius cell
migration studies demonstrated that PP121 decreased the migration
of ADC and SCC cells, consequently increasing the cell-free zone by
28 and 52%, indicative of reduced NSCLC cell migration (Fig. 4F and G). Moreover, the impact of PP121 on NSCLC
cell migration was evaluated in a co-cultured model system
comprised of NSCLC cells and healthy human astrocytes, a resident
glial cell located in the mammalian brain (Fig. 4). Results of the present study
demonstrated that clinically relevant concentrations of PP121
markedly reduced NSCLC cell migration in both SCC and ADC cells
(Fig. 4). Although few studies have
previously examined the efficacy of PP121 in the inhibition of
cancer cell migration, the capacity of PP121 to suppress NSCLC cell
migration is supported by its inhibition of anaplastic thyroid
carcinoma cell migration and invasion (15).
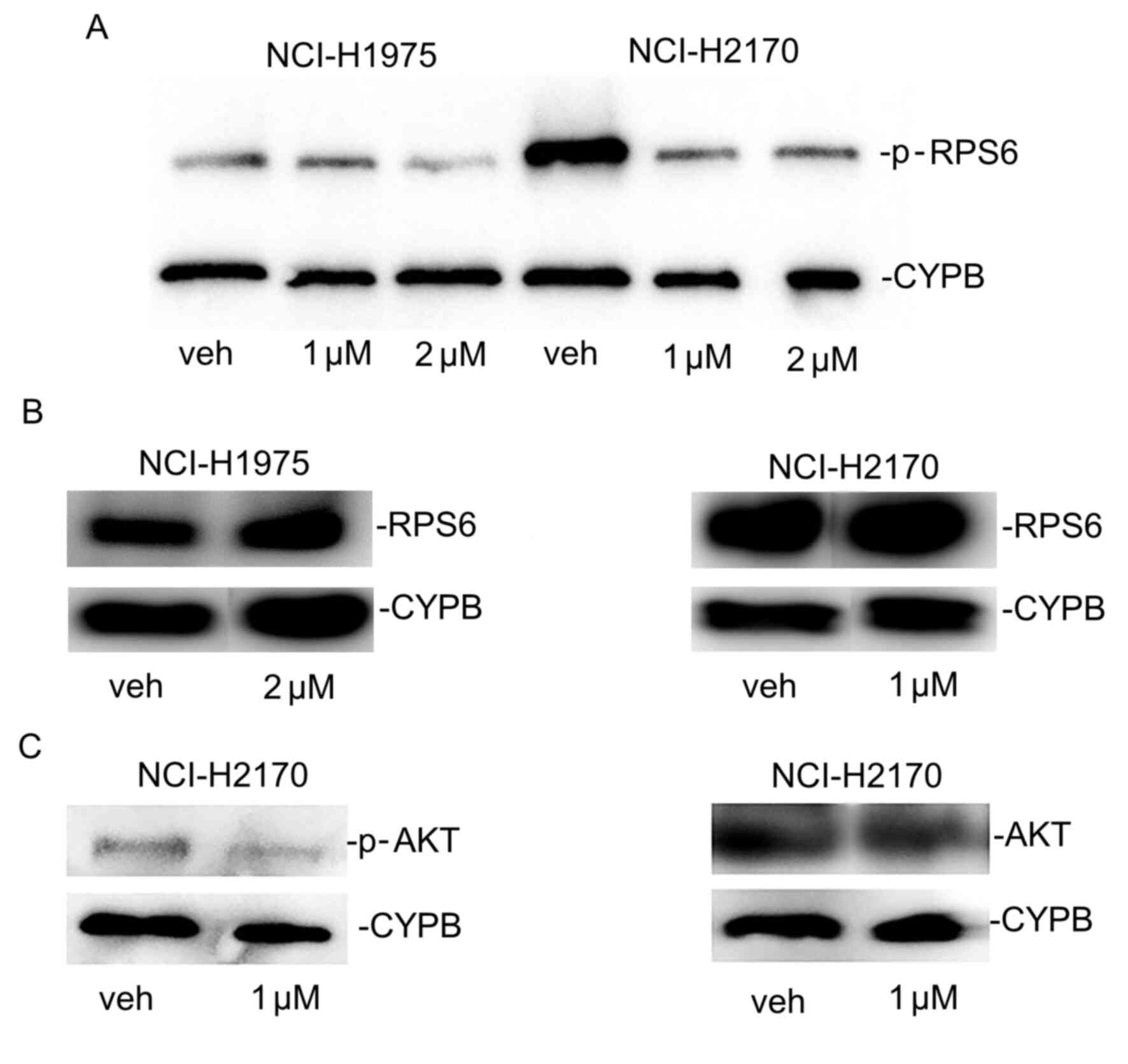
Downregulation of pharmacodynamic and
kinetic targets of PP121
PP121 antagonizes PI3K and tyrosine kinases
(12). Results of the present study
demonstrated that the protein expression levels of p-RPS6, a
downstream effector of PI3K and mTOR, were markedly reduced
following treatment with PP121 in SCC and ADC cells (Fig. 5A), but the levels of
unphosphorylated RPS6 were not reduced (Fig. 5B). Additionally, PP121 decreased
p-Akt protein expression in SCC cells but it did not downregulate
unphosphorylated Akt (Fig. 5C). The
reduced expression of these signaling mediators as a mechanistic
response to PP121 in NSCLC is comparable with previous findings in
esophageal cancer cells, glioblastoma, and thyroid carcinoma. These
results demonstrated reduced p-Akt and p-RPS6 protein expression
following treatment with this targeted agent without decreasing
total Akt and RPS6 protein levels in these solid cancers treated
with PP121 concentrations as high as 10 µM (13-15).
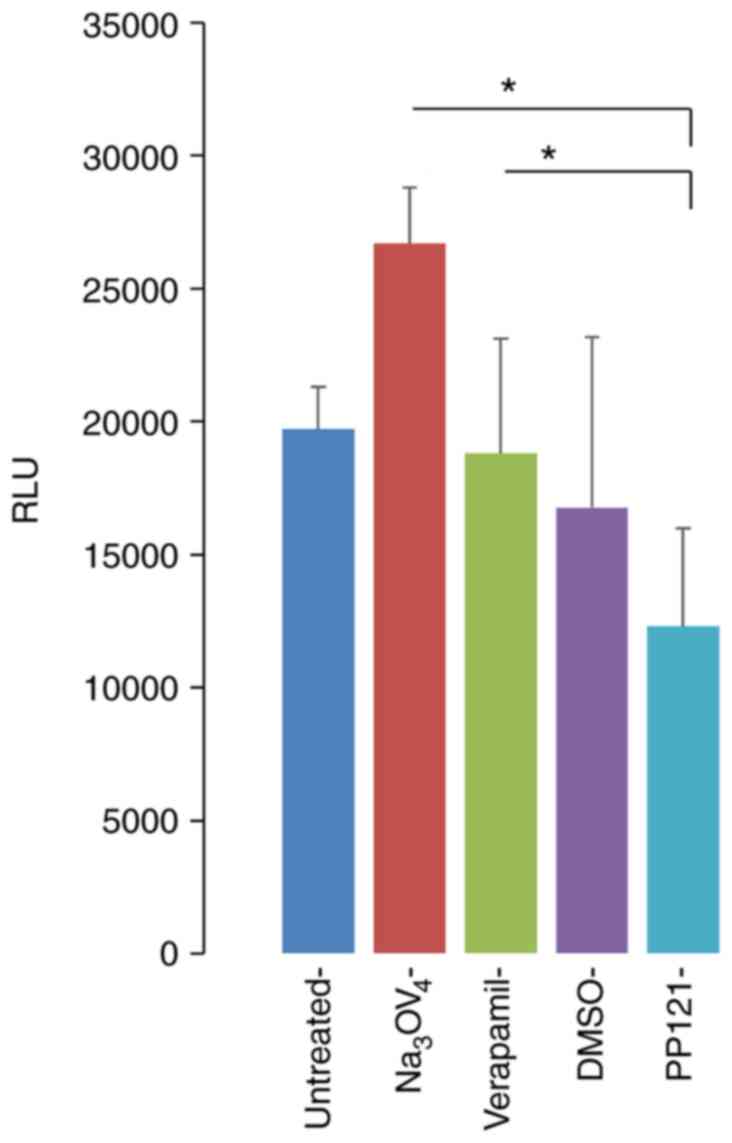
In addition, the effects of PP121 on P-gp, a drug efflux
transporter that plays a role in drug metabolism, clearing and drug
resistance were determined (20,21).
Notably, P-gp is expressed at high levels in the human brain for
protection against cytotoxic agents (22). Moreover, P-gp contributes to the
resistance of drugs used to treat diseases of the brain (23) such as NSCLC brain metastases.
Therefore, inhibition of P-gp function enhances drug
bioavailability and the subsequent therapeutic efficacy. Using an
activity assay approach, the results of the present study
demonstrated that PP121 decreased P-gp activity, compared with the
activity of the vehicle and positive controls, sodium orthovanadate
and verpamil (Fig. 6).
Discussion
Pro-tumorigenic signaling cascades that promote
cancer cell survival and recurrence enable the resistance of NSCLC
to clinical therapeutic approaches. Kinase inhibitors have been
used as a primary strategic approach to prevent disease
progression. Kinase inhibitors targeting EGFR have demonstrated
efficacy in wild-type or mutant EGFR NSCLC cells, but NSCLC was not
cured and metastatic disease was not prevented (24). Notably, small molecule inhibitors
activate compensatory pathways that contribute to disease evolution
and resistance. Thus, an improved targeted therapeutic approach,
such as the use of single agents capable of simultaneously
targeting multiple signaling pathways, is required. Results of the
present study demonstrated that PP121, a dual inhibitor of tyrosine
kinases and PI3K, decreased SCC and ADC cell viability, comparable
with the anti-tumorigenic effects of anlotinib and famitinib in
Phase III clinical trials and in vivo mouse studies,
respectively (25,26). Notably, NSCLC cells demonstrated an
increased sensitivity to PP121 exposure compared with famitinib,
which required higher concentrations to reduce cell viability. This
is likely attributed to the differential targets, including VEGF
receptor 2/3, stem cell factor receptor and platelet-derived growth
factor receptor.
A major therapeutic consideration for individuals
diagnosed with NSCLC is the treatment of brain metastases, which
occur in ~40% of cases. NSCLC brain metastases cause a median
survival rate of 3-6 months, highlighting an urgent and unmet
requirement for the identification of novel treatment strategies
for this progressive disease (27).
Astrocytes, a type of glial cell located in the brain, has been
described as an inducer and cultivator of NSCLC brain metastasis,
acting as the soil for NSCLC cells, as part of the seed and soil
hypothesis (28-31).
Results of the present study demonstrated that PP121 inhibited
NSCLC migratory invasion in an astrocytic environment, and
decreased P-gp activity. Notably, high expression levels of P-gp
are present at the blood brain barrier and P-gp also plays a role
in drug efflux functions (22).
These factors may contribute to therapeutic resistance and reduce
drug accessibility to tumors residing in the brain (23). Collectively, these findings provide
pre-clinical experimental evidence that PP121 may be an effective
strategy for the treatment of NSCLC brain metastasis, as a
consequence of enhanced bioavailability and distribution.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate the anti-tumorigenic
capacity of PP121 in NSCLC, and the subsequent ability to impede
NSCLC brain metastases which contribute to high mortality rates.
Moreover, the novelty of these findings provides insight into the
pharmacokinetic properties of this dual kinase inhibitor. Future
experimental studies should evaluate the pharmacokinetics of PP121
in vivo, to further determine the bioavailability of PP121.
More specifically, further pharmacokinetic parameters will be
examined in a murine model system, including the half-life, time of
maximum plasma concentration, area under the curve, peak
concentration and elimination rates. In addition, further in
vivo studies should assess the effects of PP121 on NSCLC brain
metastases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The author will make reagents and data available
upon requests.
Authors' contributions
QQ was responsible for all aspects of this study
that included conceptualization, experimentation, and data
analysis. QQ confirms the authenticity of all the raw data. QQ read
and approved the final manuscript and agrees to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that there are no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yuan M, Huang LL, Chen JH, Wu J and Xu Q:
The emerging treatment landscape of targeted therapy in
non-small-cell lung cancer. Sig Transduct Target Ther.
4(61)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
König D, Savic Prince S and Rothschild SI:
Targeted therapy in advanced and metastatic non-small cell lung
cancer. An update on treatment of the most important actionable
oncogenic driver alterations. Cancers (Basel).
13(804)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gazdar AF: Activating and resistance
mutations of EGFR in non-small-cell lung cancer: Role in clinical
response to EGFR tyrosine kinase inhibitors. Oncogene. 28 (Suppl
1):S24–S31. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Clark J, Cools J and Gilliland DG: EGFR
inhibition in non-small cell lung cancer: Resistance, once again,
rears its ugly head. PLoS Med. 2(e75)2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Suda K, Murakami I, Katayama T, Tomizawa
K, Osada H, Sekido Y, Maehara Y, Yatabe Y and Mitsudomi T:
Reciprocal and complementary role of MET amplification and EGFR
T790M mutation in acquired resistance to kinase inhibitors in lung
cancer. Clin Cancer Res. 16:5489–5498. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li F, Zhu T, Cao B, Wang J and Liang L:
Apatinib enhances antitumour activity of EGFR-TKIs in non-small
cell lung cancer with EGFR-TKI resistance. Eur J Cancer.
84:184–192. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Z, Zhang Y, Luo F, Ma Y, Fang W,
Zhan J, Li S, Yang Y, Zhao Y, Hong S, et al: Dual blockade of EGFR
and VEGFR pathways: Results from a pilot study evaluating apatinib
plus gefitinib as a first-line treatment for advanced EGFR-mutant
non-small cell lung cancer. Clin Transl Med. 10(e33)2020.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Benedettini E, Sholl LM, Peyton M, Reilly
J, Ware C, Davis L, Vena N, Bailey D, Yeap BY, Fiorentino M, et al:
Met activation in non-small cell lung cancer is associated with de
novo resistance to EGFR inhibitors and the development of brain
metastasis. Am J Pathol. 177:415–423. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Preusser M, Streubel B, Berghoff AS,
Hainfellner JA, von Deimling A, Widhalm G, Dieckmann K, Wöhrer A,
Hackl M, Zielinski C and Birner P: Amplification and overexpression
of CMET is a common event in brain metastases of non-small cell
lung cancer. Histopathology. 65:684–692. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Breindel JL, Haskins JW, Cowell EP, Zhao
M, Nguyen DX and Stern DF: EGF receptor activates MET through MAPK
to enhance non-small cell lung carcinoma invasion and brain
metastasis. Cancer Res. 73:5053–5065. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Peng Y, Zhou Y, Cheng L, Hu D, Zhou X,
Wang Z, Xie C and Zhou F: The anti-esophageal cancer cell activity
by a novel tyrosine/phosphoinositide kinase inhibitor PP121.
Biochem Biophys Res Commun. 465:137–144. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Apsel B, Blair JA, Gonzalez B, Nazif TM,
Feldman ME, Aizenstein B, Hoffman R, Williams RL, Shokat KM and
Knight ZA: Targeted polypharmacology: Discovery of dual inhibitors
of tyrosine and phosphoinositide kinases. Nat Chem Biol. 4:691–699.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Che HY, Guo HY, Si XW, You QY and Lou WY:
PP121, a dual inhibitor of tyrosine and phosphoinositide kinases,
inhibits anaplastic thyroid carcinoma cell proliferation and
migration. Tumour Biol. 35:8659–8664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kenny HA, Lal-Nag M, Shen M, Kara B,
Nahotko DA, Wroblewski K, Fazal S, Chen S, Chiang CY, Chen YJ, et
al: Quantitative high-throughput screening using an organotypic
model identifies compounds that inhibit ovarian cancer metastasis.
Mol Cancer Ther. 19:52–62. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moore G, Lightner C, Elbai S, Brady L,
Nicholson S, Ryan R, O'Sullivan KE, O'Byrne KJ, Blanco-Aparicio C,
Cuffe S, et al: Co-Targeting PIM Kinase and PI3K/mTOR in NSCLC.
Cancers (Basel). 13(2139)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Montaudon E, El Botty R, Vacher S, Déas O,
Naguez A, Chateau-Joubert S, Treguer D, de Plater L, Zemoura L,
Némati F, et al: High in vitro and in vivo synergistic activity
between mTORC1 and PLK1 inhibition in adenocarcinoma NSCLC.
Oncotarget. 12:859–872. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han J, Liu Y, Yang S, Wu X, Li H and Wang
Q: MEK inhibitors for the treatment of non-small cell lung cancer.
J Hematol Oncol. 14(1)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pérez-Tomás R: Multidrug resistance:
Retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen KG, Valencia JC, Gillet JP, Hearing
VJ and Gottesman MM: Involvement of ABC transporters in
melanogenesis and the development of multidrug resistance of
melanoma. Pigment Cell Melanoma Res. 22:740–749. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chai AB, Callaghan R and Gelissen IC:
Regulation of P-Glycoprotein in the Brain. Int J Mol Sci.
23(14667)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Haar CP, Hebbar P, Wallace GC IV, Das A,
Vandergrift WA III, Smith JA, Giglio P, Patel SJ, Ray SK and Banik
NL: Drug resistance in glioblastoma: A mini review. Neurochem Res.
37:1192–1200. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:20932–20937. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang M, Quan H, Fu L, Li Y, Fu H and Lou
L: Third-generation EGFR inhibitor HS-10296 in combination with
famitinib, a multi-targeted tyrosine kinase inhibitor, exerts
synergistic antitumor effects through enhanced inhibition of
downstream signaling in EGFR-mutant non-small cell lung cancer
cells. Thorac Cancer. 12:1210–1218. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun YW, Xu J, Zhou J and Liu WJ: Targeted
drugs for systemic therapy of lung cancer with brain metastases.
Oncotarget. 9:5459–5472. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dai W, Zhu H, Chen G, Gu H, Gu Y, Sun X
and Zeng X: Orchestration of the crosstalk between astrocytes and
cancer cells affects the treatment and prognosis of lung cancer
sufferers with brain metastasis. J Thorac Dis. 8:E1450–E1454.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin J, Jandial R, Nesbit A, Badie B and
Chen M: Current and emerging treatments for brain metastases.
Oncology (Williston Park). 29:250–257. 2015.PubMed/NCBI
|
|
30
|
Ni W, Chen W and Lu Y: Emerging findings
into molecular mechanism of brain metastasis. Cancer Med.
7:3820–3833. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hanibuchi M, Kim SJ, Fidler IJ and
Nishioka Y: The molecular biology of lung cancer brain metastasis:
An overview of current comprehensions and future perspectives. J
Med Invest. 61:241–253. 2014.PubMed/NCBI View Article : Google Scholar
|