Introduction
Primary penile squamous cell carcinoma (SCC) is an
uncommon neoplasm in men. In Europe and the US, the reported
incidence is ~<1% per 100,000 person-year (1). By contrast, in some countries such as
India or Brazil, the prevalence is high ranging from 2.3-8.3 per
100,000 person-year (2-4).
In Thailand, according to data from 2008-2012, the age-standardized
rate of penile cancer incidence is high (1.4 per 100,000) and
ranked top three in the world (5).
Penile cancer is an aggressive tumor with limited
systemic treatment options in a locally advanced and advanced stage
(1,3). Therefore, identifying prognostic
biomarkers is important and could be applied to predict treatment
outcomes and planning.
Programmed cell death ligand-1 (PD-L1) is a T-cell
regulatory protein expressed on the surface of tumor and
tumor-infiltrating lymphocytes. The PD-L1/PD-1 pathway is important
in cancer progression (6). The
binding between PD-L1 of cancer cells with PD-1 of immune cells
helps cancers evade the host immune response and prevents cancers
from being killed by cytotoxic T lymphocyte (6,7). In
the past few decades, major advances in immunotherapy, especially
the use of immune checkpoint inhibitors of anti-PD1 or anti-PDL1
have changed the treatment paradigm in a number of types of cancer.
Expression of PD-L1 by tumor cells and tumor-infiltrating
lymphocytes has been described in various types of cancer, such as
renal cell carcinoma, bladder, and lung cancer (8-11),
and has been identified as both a prognostic and predictive
marker.
Earlier studies reported high PD-L1 expression
positivity in penile cancer, in endemic and non-endemic areas
(12-15).
However, the results for its prognostic role remain
contradictory.
The present study examined the clinicopathological
characteristics of PD-L1 expression in penile cancer and the
association between PD-L1 expression in tumor cells and immune
cells in an endemic area.
Materials and methods
Patients and clinicopathological
data
The present study was a retrospective study. All
penile SCC patients who were diagnosed and underwent surgical
resection in Srinagarind Hospital between 2008 and 2018 were
included. The unavailable formalin-fixed paraffin-embedded (FFPE)
tissue and those surviving <30 days were excluded from the
present study. Finally, the FFPE tissues from 43 patients which
were all primary penile SCC, were available for the present
study.
Demographic data were collected including age,
performance status, and survival time of patients according to
Eastern Cooperative Oncology Group (ECOG) (16). The histologic subtype, histologic
grading, lymphovascular invasion (LVI) status, and perineural
invasion (PNI) status were evaluated using 2016 WHO classification
standard templates (17). The
pathological staging was performed according to the 8th
edition American Joint Committee on Cancer (AJCC) staging system
(18). The pre-treatment immune
profiles including hemoglobin, total white blood cells, neutrophil
count, lymphocyte count, and platelet count were recorded.
Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio were
calculated.
The present study was approved by the Institutional
Review Board of the Khon Kaen University Ethics Committee for Human
Research based on the Declaration of Helsinki and the ICH Good
Clinical Practice Guidelines (HE611509). For this type of study,
formal consent was not required in accordance with institutional
guideline.
PD-L1 immunohistochemistry
For all tissue, PD-L1 immunohistochemistry was
performed on a representative block of the whole slide section.
Tumor sections (4 µm) were deparaffinized and stained with an
anti-PD-L1 antibody (VENTANA PD-L1 clone SP263; Roche Diagnostics,
cat. no. 790-4905 (prediluted). PD-L1 was stained using Ventana
Benchmark XT IHC staining module (incubated at 37˚C for 1 h) and
detected by OptiView DAB Detection kit. The sections were
counterstained by hematoxylin followed by bluing reagent. The
slides were subsequently removed and rinsed, dehydrated, cleared
and mounted.
The percentage of tumor cells with membranous
staining was assessed separately by two evaluators (one senior
resident-in-training and one board-certified pathologist) blinded
to the patient's clinicopathological parameters. The tumor and
immune cells were considered PD-L1 expression status according to
the interpretation guide for the VENTANA PD-L1 (SP263) Assay
Scoring (Table I).
 | Table IThe scoring criteria of PD-L1
status. |
Table I
The scoring criteria of PD-L1
status.
| PD-L1
interpretation | Staining
description |
|---|
| High/positive | PD-L1 status is
considered high/positive if any of the following are met: |
| | • ≥25% of TC express
membrane (any intensity above background) in invasive area;
or, |
| | • ICP >1% and IC+
≥25%; or, |
| | • ICP=1% and
IC+=100%. |
| Low/negative | PD-L1 status is
considered low/negative if: |
| | • None of the
criteria for PD-L1 high status are met. |
Statistical analysis
SPSS software version 27 (IBM Corp.) was used to
analyze the association between PD-L1 expression and
clinicopathological parameters (including tumor size, histological
grading, histologic subtype, staging and survival time) with
χ2 or Fisher's exact test as appropriate. The
differences in continuous data between the two dependent groups
were analyzed by either an independent t-test (parametric test) or
Mann-Whitney test (non-parametric test). Values were presented as
the mean ± SD. The survival analysis was conducted and analyzed
using Kaplan-Meier estimation with Log-rank and Cox regression
tests. The analysis time was restricted to a 10-year period due to
the late crossover event. The present study selected a 10-year
period as it is the reasonable duration to declare the cure of the
disease (19). P<0.05 was
considered to indicate a statistically significant difference.
Results
PD-L1 expression and
clinicopathological features
The present study included 43 patients with penile
SCC. No patient received neoadjuvant chemotherapy or radiotherapy
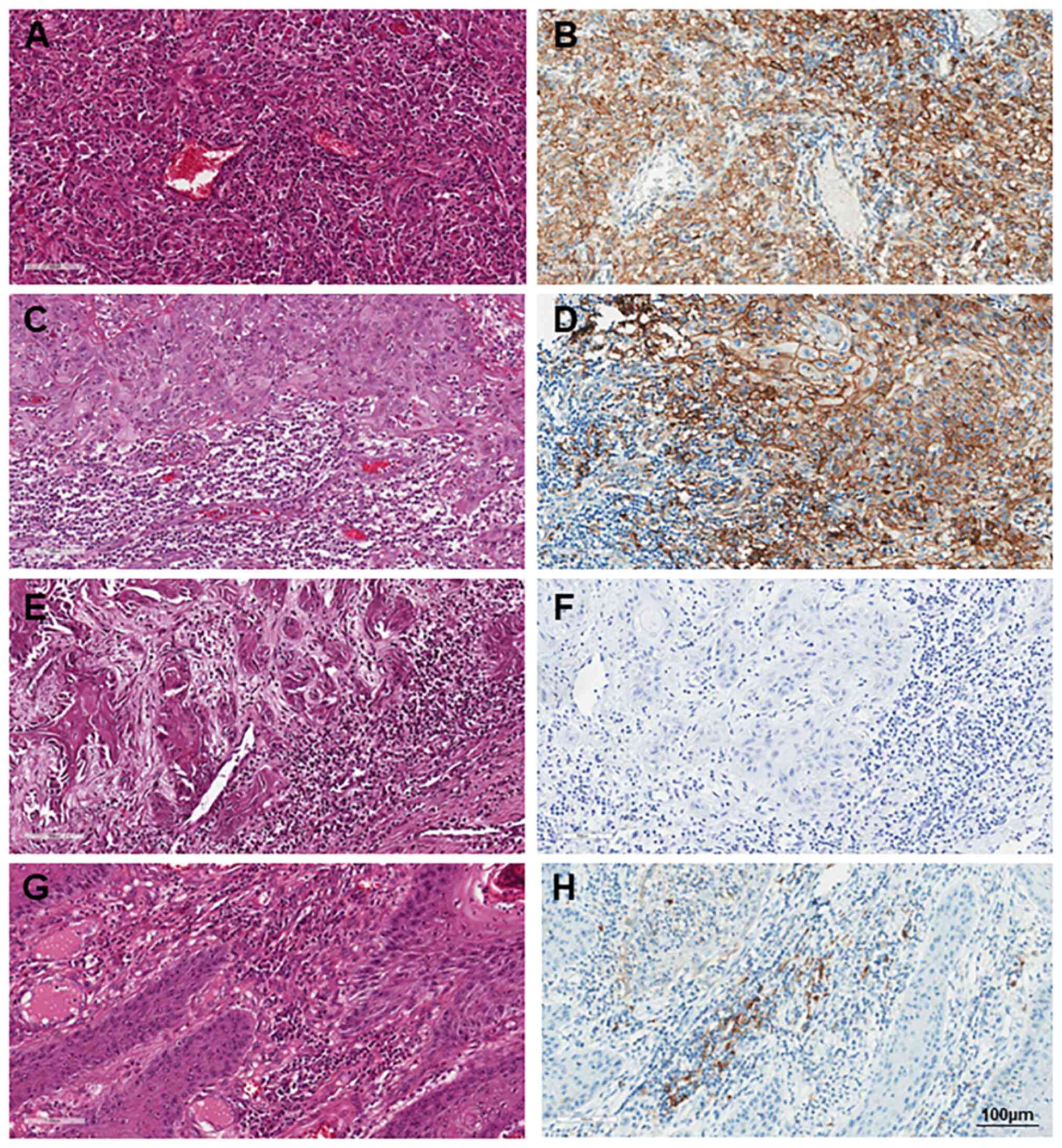
before complete resection. A total of eight out of 43 cases (18.6%)
were identified with positive PD-L1 expression, while 35 cases
(81.4%) exhibited negative PD-L1 expression (Fig. 1). PD-L1 immunoreactivity in tumor
cells (TC) >5% was found in 23 patients (53.5%).
The correlation of PD-L1 status with
clinic-pathological characteristics is shown in Table II. Briefly, the median age was 58
years. The PD-L1 expression showed no significant difference
between primary tumor locations including tip and shaft
(P=0.390).
 | Table IIClinicopathological characteristics of
PD-L1 positive and negative in tumors. |
Table II
Clinicopathological characteristics of
PD-L1 positive and negative in tumors.
| Features | All patients | PD-L1 positive
(n=8) | PD-L1 negative
(n=35) | P-value |
|---|
| Age, median
(range) | 58 (26-84) | 58; 48-84 | 58; 26-80 | 0.490 |
| ECOG, n (%) | | | | |
|
0 | 36 (83.7) | 6(75) | 30 (85.7) | 0.390 |
|
1 | 7 (16.3) | 2(25) | 5 (14.3) | |
| Location | | | | |
|
Tip | 33 (76.7) | 7 (87.5) | 26 (74.3) | 0.390 |
|
Shaft | 10 (23.3) | 1 (12.5) | 9 (25.7) | |
| Histological
grade | | | | |
|
1 | 36 (83.7) | 6(75) | 30 (85.7) | 0.390 |
|
2-3 | 7 (16.3) | 2(25) | 5 (14.3) | |
| LVI positive | 5 (11.6) | 0 | 5 (14.3) | 0.340 |
| PNI positive | 3(7) | 0 | 3 (8.6) | 0.530 |
| T stage | | | | |
|
T1 | 14 (32.6) | 6(75) | 8 (22.9) | 0.014 |
|
T2-4 | 29 (67.4) | 2(25) | 27 (77.1) | |
| Lymph node
metastasis (N stage) | | | | |
|
Negative | 27 (62.8) | 4(50) | 23 (65.7) | 0.330 |
|
Positive | 16 (37.2) | 4(50) | 12 (34.3) | |
| Stage | | | | |
|
I-II | 24 (55.8) | 4(50) | 20 (57.1) | 0.510 |
|
III-IV | 19 (44.2) | 4(50) | 15 (42.9) | |
PD-L1 expression was not significantly correlated
with histological grade (P=0.390), lymphovascular or perineural
invasion (P=0.340 and 0.530, respectively). Moreover, no
association was observed between PD-L1 expression and nodal
involvement (P=0.330). Notably, the present study found that
pathological T staging, which represented the depth of primary
tumor invasion, displayed a statically significant correlation with
PD-L1 positivity; 75% of pT1 stage SCC patients were PD-L1
positive, while only 25% of patients with pT2-pT4 were positive for
PD-L1 expression (P=0.014; Table
II).
Univariate and multivariate analysis
of PD-L1 expression and clinicopathological features in the
survival of penile SCC patients
At the time of data analysis, 24 patients (55.8%)
had succumbed and the median follow-up time was 89.7 months. The
median survival time was 7.4 years (95% Confidence Interval
3.7-9.7). The 1, 5, and 10-year OS rates were 78.6, 63.6, and 25%
respectively.
The survival rate was analyzed using Kaplan-Meier
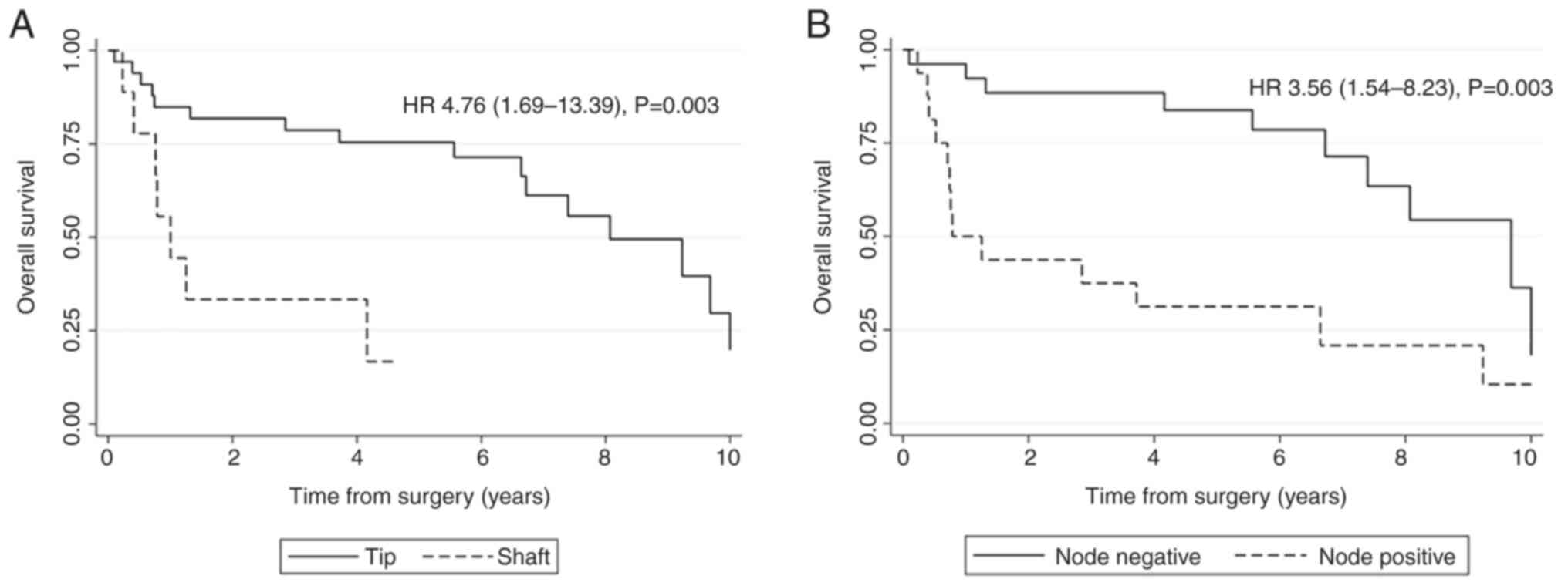
estimation with a log-rank test. There was a statistically
significant difference in clinicopathological features such as
tumor location [Hazard ratio (HR)=4.76, P=0.003], histological
grade (HR=4.25, P=0.005), LVI (HR=4.89, P=0.002), PNI (HR=4.75,
P=0.02), T category (HR=4.31, P=0.002) and lymph node metastasis
(HR=3.56, P=0.003) compared with their references. While there was
no statistical significance in the patient's age, ECOG score and
PD-L1 expression. The significant clinicopathological features of
survival analysis were further analyzed to identify independent
prognostic factors using the Cox regression test. The result showed
that tumor at shaft and positive lymph node metastasis were
independent factors for poor survival of SCC patients (HR=4.81 and
2.59, P=0.015 and 0.009, respectively; Table III and Fig. 2).
 | Table IIIUnivariate and multivariate analysis
for overall survival. |
Table III
Univariate and multivariate analysis
for overall survival.
| | Univariate | Multivariate |
|---|
| Characteristic | Median survival
(years) | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Age | | | | | |
|
<58 | 8.07 | Reference | | - | |
|
>58 | 6.64 | 1.33
(0.58-3.03) | 0.5 | - | - |
| ECOG | | | | | |
|
0 | 7.40 | Reference | | - | |
|
1 | 0.74 | 4.18
(0.92-19.0) | 0.06 | - | - |
| Histological
Grade | | | | | |
|
1 | 8.07 | Reference | | Reference | |
|
2-3 | 0.79 | 4.25
(1.54-11.75) | 0.005a | 1.37
(0.27-6.84) | 0.70 |
| Location | | | | | |
|
Tip | 8.07 | Reference | | Reference | |
|
Shaft | 1.00 | 4.76
(1.69-13.39) | 0.003a | 4.81
(1.35-17.16) | 0.015a |
| T stage | | | | | |
|
T1-2 | 9.23 | Reference | | Reference | |
|
T3-4 | 1.32 | 4.31
(1.71-10.85) | 0.002a | 2.39
(0.67-8.46) | 0.18 |
| Lymph node
metastasis (N) | | | | | |
|
Node
negative | 9.68 | Reference | | Reference | |
|
Node
positive | 0.79 | 3.56
(1.54-8.23) | 0.003a | 2.59
(0.87-7.73) | 0.09a |
| LVI | | | | | |
|
Negative | 8.07 | Reference | | Reference | |
|
Positive | 1.25 | 4.89
(1.62-14.81) | 0.005a | 1.40
(0.31-6.41) | 0.66 |
| PNI | | | | | |
|
Negative | 8.07 | Reference | | Reference | |
|
Positive | 1.00 | 4.73
(1.29-17.41) | 0.02a | 2.06
(0.43-9.86) | 0.37 |
| PD-L1 status | | | | | |
|
Negative | 6.72 | Reference | | - | |
|
Positive | NR | 0.41
(0.09-1.76)b | 0.23 | - | - |
Expression of PD-L1 in tumor cells and
immune cells
The immune profile and inflammatory markers were
compared among PD-L1 positive and negative tumors as shown in
Table IV. The number of peripheral
white blood cell count, total lymphocytes, neutrophil-lymphocyte
ratio, and platelet-lymphocyte ratio were comparable. No
statistically significant difference was observed in the immune
profile and inflammatory markers between PD-L1 positive and
negative.
 | Table IVComparison of immune profile between
the PD-L1 expression status of SCC patients. |
Table IV
Comparison of immune profile between
the PD-L1 expression status of SCC patients.
| Immune profile | PD-L1 positive
(n=8) | PD-L1 negative
(n=35) | P-value |
|---|
| PD-L1 expression on
tumor cells (%) median, IQR | 57.5, 35-67.5 | 3, 0.5-15 |
<0.001a |
| PD-L1 expression on
immune cells (%) median, IQR | 12.5, 4-18.7 | 3, 0.5-7.5 | 0.012a |
| Percent of
tumor-associated immune cells in the tumor area (%) median,
IQR | 52.5, 28.7-65 | 25, 12.5-50 | 0.091 |
| Hb (g/dl) median,
IQR | 13.5, 11.7-14 | 12.5, 11-13.6 | 0.250 |
| White blood cells
(103/µl) median, IQR | 10.8, 7.8-17.9 | 9.2, 7.2-12.4 | 0.430 |
| Total PMN
(103/µl) median, IQR | 5.6, 4.4-13.8 | 5.8, 4.2-9.0 | 0.640 |
| Total lymphocyte
(103/µl) median, IQR | 2.4, 1.8-2.8 | 2.1, 1.4-2.9 | 0.640 |
| Platelet
(103/µl) median, IQR | 274, 217-356 | 297, 249-391 | 0.280 |
|
Neutrophil-lymphocyte ratio median,
IQR | 2.6, 1.9-8.2 | 2.7, 2.0-5.2 | 0.840 |
| Platelet-lymphocyte
ratio median, IQR | 111.1,
69.4-395.5 | 133,
86.8-241.5 | 0.660 |
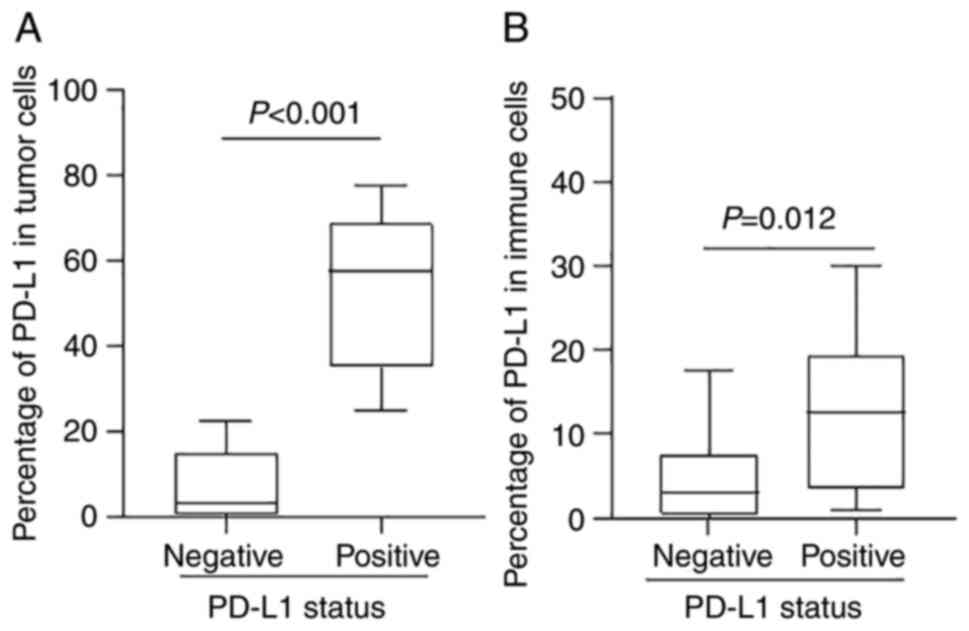
Comparing PD-L1 positive and negative cases, the
percentage of PD-L1 expression was higher in both tumor cells (57.5
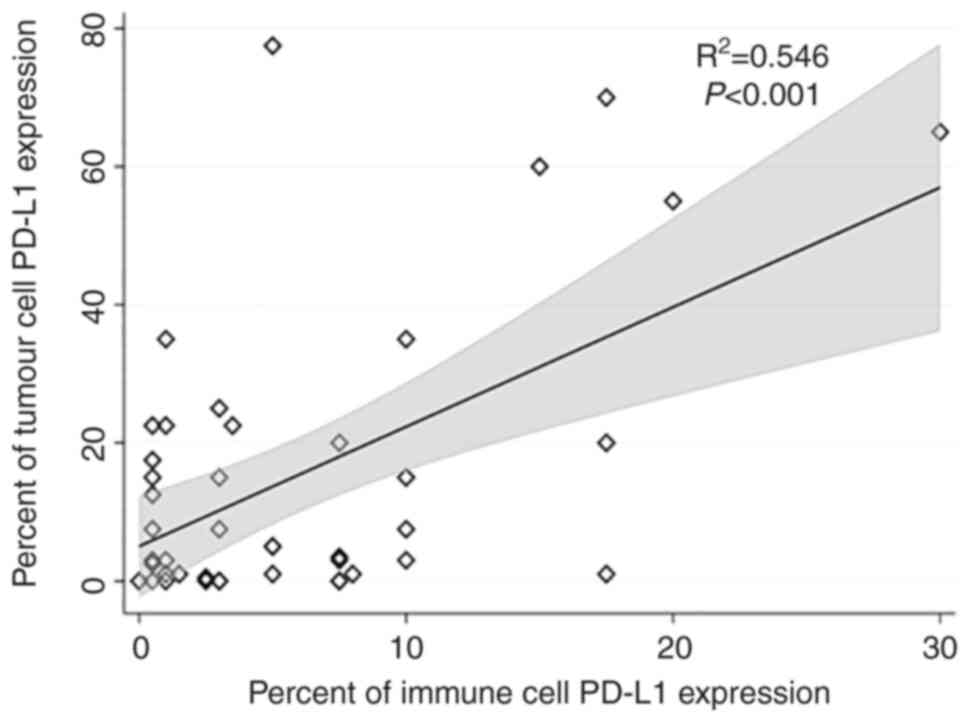
vs. 3%; P=0.0001) and immune cells (12.5 vs. 3%; P=0.012) (Table IV and Fig. 3). Moreover, the correlation analysis
between PD-L1 expression in tumor and immune cells has shown that
there was a high positive correlation between tumor cells and
immune cells PD-L1 expression, R2=0.55, P<0.001 as in
Fig. 4.
Discussion
Immune checkpoint inhibitors have been shown to
possess significant benefits in various types of cancer (6). The percentage of PD-L1 expression in
tumor and immune cells is the prognostic and predictive biomarker
for PD-1/PD-L1 blockade agents in several tumors including
non-small cell lung cancer and gastric carcinoma (9,20).
However, in certain types of cancer, such as renal cell carcinoma,
bladder cancer and melanoma, PD-L1 expression does not predict the
benefit of an anti-PD1 agent (21-23).
Even patients identified as PD-L1 negative may derive benefits from
therapy.
In the present study in an endemic area, the PD-L1
status of tumor cells and immune cells in a cohort of primary SCC
of the penis was evaluated using the SP263 antibody. It was found
that 18.6% of the tumors were identified as PD-L1 positive. The
PD-L1 positive rate in penile cancer varies greatly according to
the type of antibody and the cut-off value. Studies have validated
PD-L1 in penile cancer and report a positive rate ranging from
7.3-87% (12,15). Udager et al (15) were among the first to report the
PD-L1 expression in 37 patients with penile SCC and the PD-L1
expression was positive in 62.2% of cases. The lower reported
positive rates in this study could be the higher cut-off point in
tumor cells; TC ≥25% in this study vs. TC ≥1-5% in other studies.
When the cut-off value to TC >5% was re-examined in the present
study, the PD-L1 positive rate was 53.5% which is comparable to the
results from China and Brazil (4,13).
Montella et al (24) demonstrated the highest proportion of
positive PD-L1 expression in tumor cells and immune cells using
either SP142 or SP263 antibody in T1 stage and lower PD-L1
positivity in T2, T3, and T4 accordingly. Similarly the present
study also found a statistically significant correlation between
PD-L1 expression and pT staging in which 75% of SCC patients with
PD-L1 positive correlated with pT1 stage.
PD-L1 expression has been associated with regional
lymph node metastasis and decreased cancer-specific survival in
several studies (12,15,25,26).
By contrast, in this cohort, PD-L1 positivity did not show worse
survival outcomes when compared with negative patients. The present
study further examined the PD-L1 expression by tumor cells at the
cut-off value of 1 and 5%, but no survival difference was found
between those with positive or negative PD-L1.
In a recent meta-analysis, higher PD-L1 expression
was associated with shorter cancer-specific survival in Caucasians
but not in Asians (Chinese study). Furthermore, it was not
associated with overall survival (27). The different races of patients and
etiology of penile cancer along with different PD-L1 antibody,
detection technique and cut-off level could explain the variations
of the results. Further standardization of the technique designated
for penile cancer is warranted.
With limited data, immune checkpoint inhibitors,
either single agent anti PD-1 or a combination of anti PD-1/anti
CTLA4, did not provide an impressive outcome compared with other
types of tumor (28,29). PD-L1 expression as a predictive
biomarker for ICIs in advanced penile cancer remains controversial.
However, more data regarding ICI combined with chemotherapy or
radiotherapy in the future is expected (30).
The major strength of the present study included the
use of whole tissue sections, which has significant advantages over
tissue microarray (TMA), as whole tissue sections allow a more
comprehensive assessment of tumor protein expression. This is
especially important for PD-L1 immunochemistry, which can reduce
interpretative bias from tumor heterogeneity compared with the
tissue microarray technique. Moreover, the present study was the
first conducted in the high incidence area of southeast Asia. The
present study had a long follow up period and a high proportion of
node positive disease.
There are a few limitations in the present study.
First, the number of PD-L1 positive cases was small and only single
PD-L1 antibody was used. Second, in vitro experiments for
PD-L1 positivity were not performed. Third, the present study did
not evaluate the HPV status of p16 expression in the samples.
Larger number of tumor samples and in vitro validations are
needed in future studies.
In summary, PD-L1 expression was found in 18% of
primary penile SCC and PD-L1 positivity (high expression) was more
common in the early pT stage (pT1).
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Faculty of
Medicine, Khon Kaen University, Thailand (grant no. IN63132).
Authors' contributions
SS and JC conceived the present study. SS, NK, WS,
UR, PK, AS, KW, PT, PW and JC were responsible for data curation.
SS, NK, WS, UR, PK, AS, KW, PT, PW and JC were responsible for
experiments. SS, NK, WS, UR, PK, AS, KW, PT, PW and JC were
responsible for methodology. JC and SS confirm the authenticity of
all the raw data. SS, NK, WS and JC were responsible for writing,
reviewing and editing the manuscript. All authors read and approved
the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Khon Kaen University Ethics Committee for Human
Research based on the Declaration of Helsinki and the ICH Good
Clinical Practice Guidelines (HE611509). For this type of study,
formal consent was not required in accordance with institutional
guideline.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hakenberg OW, Comperat EM, Minhas S,
Necchi A, Protzel C and Watkin N: EAU guidelines on penile cancer:
2014 Update. Eur Urol. 67:142–150. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Christodoulidou M, Sahdev V, Houssein S
and Muneer A: Epidemiology of penile cancer. Curr Probl Cancer.
39:126–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Deng X, Liu Y, Zhan X, Chen T, Jiang M,
Jiang X, Chen L and Fu B: Trends in incidence, mortality, and
survival of penile cancer in the United States: A population-based
study. Front Oncol. 12(891623)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vieira CB, Feitoza L, Pinho J,
Teixeira-Júnior A, Lages J, Calixto J, Coelho R, Nogueira L, Cunha
I, Soares F and Silva GEB: Profile of patients with penile cancer
in the region with the highest worldwide incidence. Sci Rep.
10(2965)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fu L, Tian T, Yao K, Chen XF, Luo G, Gao
Y, Lin YF, Wang B, Sun Y, Zheng W, et al: Global pattern and trends
in penile cancer incidence: Population-based study. JMIR Public
Health Surveill. 8(e34874)2022.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ribas A: Tumor immunotherapy directed at
PD-1. N Engl J Med. 366:2517–2519. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang Y, Zhang SD, McCrudden C, Chan KW,
Lin Y and Kwok HF: The prognostic significance of PD-L1 in bladder
cancer. Oncol Rep. 33:3075–3084. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ueda K, Suekane S, Kurose H, Chikui K,
Nakiri M, Nishihara K, Matsuo M, Kawahara A, Yano H and Igawa T:
Prognostic value of PD-1 and PD-L1 expression in patients with
metastatic clear cell renal cell carcinoma. Urol Oncol.
36:499.e9–499.e16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu L, Sun J, Wang L and Li Z, Wang L and
Li Z: Prognostic and clinicopathological significance of PD-L1 in
patients with bladder cancer: A meta-analysis. Front Pharmacol.
10(962)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Davidsson S, Carlsson J, Giunchi F, Harlow
A, Kirrander P, Rider J, Fiorentino M and Andrén O: PD-L1
expression in men with penile cancer and its association with
clinical outcomes. Eur Urol Oncol. 2:214–221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Bacco MW, Carvalhal GF, MacGregor B,
Marcal JMB, Wagner MB, Sonpavde GP and Fay AP: PD-L1 and p16
expression in penile squamous cell carcinoma from an endemic
region. Clin Genitourin Cancer. 18:e254–e259. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Müller T, Demes M, Lehn A, Köllermann J,
Vallo S, Wild PJ and Winkelmann R: The peri- and intratumoral
immune cell infiltrate and PD-L1 status in invasive squamous cell
carcinomas of the penis. Clin Transl Oncol. 24:331–341.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Udager AM, Liu TY, Skala SL, Magers MJ,
McDaniel AS, Spratt DE, Feng FY, Siddiqui J, Cao X, Fields KL, et
al: Frequent PD-L1 expression in primary and metastatic penile
squamous cell carcinoma: Potential opportunities for
immunotherapeutic approaches. Ann Oncol. 27:1706–1712.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
17
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC (eds), et al: AJCC cancer staging manual (8th edition).
Springer International Publishing: American Joint Commission on
Cancer, 2017.
|
|
19
|
Tai P, Yu E, Cserni G, Vlastos G, Royce M,
Kunkler I and Vinh-Hung V: Minimum follow-up time required for the
estimation of statistical cure of cancer patients: Verification
using data from 42 cancer sites in the SEER database. BMC Cancer.
5(48)2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shitara K, Ajani JA, Moehler M, Garrido M,
Gallardo C, Shen L, Yamaguchi K, Wyrwicz L, Skoczylas T, Bragagnoli
AC, et al: Nivolumab plus chemotherapy or ipilimumab in
gastro-oesophageal cancer. Nature. 603:942–948. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL,
Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi
A, et al: Randomized phase III KEYNOTE-045 trial of pembrolizumab
versus paclitaxel, docetaxel, or vinflunine in recurrent advanced
urothelial cancer: Results of >2 years of follow-up. Ann Oncol.
30:970–976. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Munhoz RR and Postow MA: Clinical
development of PD-1 in advanced melanoma. Cancer J. 24:7–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Montella M, Sabetta R, Ronchi A, De Sio M,
Arcaniolo D, De Vita F, Tirino G, Caputo A, D'Antonio A, Fiorentino
F, et al: Immunotherapy in penile squamous cell carcinoma: Present
or future? Multi-target analysis of programmed cell death ligand 1
expression and microsatellite instability. Front Med (Lausanne).
9(874213)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Joshi VB, Spiess PE, Necchi A, Pettaway CA
and Chahoud J: Immune-based therapies in penile cancer. Nat Rev
Urol. 19:457–474. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ottenhof SR, Djajadiningrat RS, de Jong J,
Thygesen HH, Horenblas S and Jordanova ES: Expression of programmed
death ligand 1 in penile cancer is of prognostic value and
associated with HPV status. J Urol. 197:690–697. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lu Y, Wang Y, Su H and Li H: PD-L1 is
associated with the prognosis of penile cancer: A systematic review
and meta-analysis. Front Oncol. 12(1013806)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Buonerba C, Scafuri L, Costabile F,
D'Ambrosio B, Gatani S, Verolino P, Trolio RD, Cosimato V, Verde A
and Lorenzo GD: Immune checkpoint inhibitors in penile cancer.
Future Sci OA. 7(FSO714)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang Y, Hu X, Wu K and Li X: Immune
landscape and immunotherapy for penile cancer. Front Immunol.
13(1055235)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Long XY, Zhang S, Tang LS, Li X and Liu
JY: Conversion therapy for advanced penile cancer with tislelizumab
combined with chemotherapy: A case report and review of literature.
World J Clin Cases. 10:12305–12312. 2022.PubMed/NCBI View Article : Google Scholar
|