1. Introduction
There is no clear definition of artificial
intelligence (AI), but it is generally considered to be a computer
system with the functions of human intelligence, such as learning,
inference, and judgment (1,2). Machine learning refers to the
technology by which computers learn large amounts of data and
automatically build algorithms and models to perform tasks, such as
classification and prediction (1,2). The
field of AI has evolved with the development of deep learning.
Neural networks are mathematical models that have properties
similar to those of brain functions, and deep learning is a
machine-learning technique that enhances expression and learning
ability by combining neural networks in multiple layers (3,4). Image
recognition is an ability developed through deep learning, which is
used for face recognition, automated driving, and other tasks
(5). The combination of robotics
and AI also has numerous benefits in daily life. When a robot is
equipped with AI, it functions as a machine that senses its
surroundings and determines how to act. AI is also being introduced
into the medical field; since it excels in image recognition,
previous studies have reported the use of X-ray, CT, ultrasonic,
and other images for the diagnosis of diseases through deep
learning (6,7). In addition, these studies describe the
use of deep learning to diagnose diseases and predict patient
prognosis based on information in medical records (6,7).
The use of minimally invasive surgery is now
widespread in most surgical fields. Among minimally invasive
surgery approaches, indications for robot-assisted surgery systems
such as da Vinci have recently been expanded (8).
In recent years, the number of studies on the use of
deep learning to analyze surgical videos and apply them to medical
care has increased (9,10), and a growing number of studies on
the development of autonomous surgical robots have been published
(11,12).
The aim of the present review was to examine the
possibility of fully autonomous surgical robots in the future.
First, studies on the analysis of surgical videos for laparoscopic
surgery and robot-assisted surgery using deep learning were
described. Subsequently, studies on the development of autonomous
surgical robots using AI were presented.
2. Analysis of laparoscopic surgery video
using deep learning
One important process in the development of
autonomous surgical robots is the recognition of surgical details.
A number of studies have used deep learning for laparoscopic
surgery and robot-assisted surgery (9,10).
Different aspects are described in the following sections: i) Organ
and instrument identification, ii) procedure and surgical phase
recognition, iii) safe surgical navigation, and iv) surgical
education (Table I).
 | Table IPrevious studies on surgical-related
artificial intelligence analysis. |
Table I
Previous studies on surgical-related
artificial intelligence analysis.
| Authors | Year | Procedure | Dataset | No. | Application | Performance
score | (Refs.) |
|---|
| Zadeh et
al | 2020 | Gynecologic
surgery | Mask R-CNN | 461 images | Organ
identification | Accuracy: 29.6%
(ovary) and 84.5% (uterus) | (10) |
| Mascagni et
al | 2022 | Laparoscopic
cholecystectomy | CNN | 2,854 images | Organ
identification | Average accuracy:
71.9% | (13) |
| Padovan et
al | 2022 | Urologic
surgery | Segmen- tation
CNN | 971 images | Organ
identification | IoU: 0.8067
(prostate) and 0.9069 (kidney) | (14) |
| Koo et
al | 2022 | Liver surgery | CNN | 133 videos | Organ
identification | Precision:
0.70-0.82 | (15) |
| Namazi et
al | 2022 | Laparoscopic
cholecystectomy | Recurrent CNN | 15 videos | Instrument
identification | Mean precision:
0.59 | (16) |
| Yamazaki et
al | 2022 | Laparoscopic
gastrectomy | CNN | 19,000 images | Instrument
identification | N.A. | (17) |
| Aspart et
al | 2022 | Laparoscopic
cholecystectomy | CNN | 122,470 images | Instrument
identification | AUROC: 0.9107;
specificity 66.15%; and sensitivity: 95% | (18) |
| Cheng et
al | 2022 | Laparoscopic
cholecystectomy | CNN | 156,584 images | Surgical phase
recognition | Accuracy: 91% | (19) |
| Kitaguchi et
al | 2022 | Transanal total
mesorectal excision | CNN | 42 images | Surgical phase
recognition | Accuracy:
93.2% | (20) |
| Kitaguchi et
al | 2020 | Laparoscopic
sigmoid colon resection | CNN | 71 cases | Surgical phase
recognition | Accuracy:
91.9% | (21) |
| Twinanda et
al | 2019 | Cholecystectomy and
gastric bypass | CNN and LSTM
network | 290 cases | Surgical time
prediction | N.A. | (23) |
| Bodenstedt et
al | 2019 | Laparoscopic
interventions of various types | Recurrent CNN | 3,800 frames | Surgical time
prediction | Overall average
error: 37% | (24) |
| Igaki et
al | 2022 | Total mesorectal
excision | CNN | 600 images | Safe surgical
navigation | Dice coefficient:
0.84 | (25) |
| Kumazu et
al | 2021 | Robot-assisted
gastrectomy | CNN | 630 images | Safe surgical
navigation | N.A. | (26) |
| Moglia et
al | 2022 | Virtual simulator
for robot-assisted surgery | CNN | 176 medical
students | Surgical
education | Accuracy:
>80% | (27) |
| Zheng et
al | 2022 | Box trainer for
laparoscopic surgery | Long-/ short-term
memory recurrent neural network | 30 medical
students | Surgical
education | Accuracy:
74.96% | (28) |
Organ and instrument
identification
Previous research has described the identification
of organs and anatomical structures in the analysis of laparoscopic
images using deep learning. Zadeh et al manually annotated
the uterus and ovaries on 461 gynecological laparoscopic videos.
Mask Regional Convolutional Neuronal Network (Mask R-CNN), a
deep-learning method, was used to identify the datasets and
automatically segment the uterus, ovaries, and surgical
instruments. Segmentation accuracy was examined as the percentage
overlap between the segmented area of the manual annotation and
that of the Mask R-CNN. The accuracy values were 29.6, 84.5 and
54.5% for the ovary, uterus, and surgical instruments, respectively
(10). The segmentation results for
the ovaries were not as satisfactory as those for the uterus and
instruments. This can be due to the following two main reasons.
First, the training dataset contained a lower number of ovary
instances because this organ is often hidden by other structures
(the fallopian tube or uterus). Second, the ovaries often present
with a highly varying appearance across patients (10). There are no other studies on the
recognition of ovaries in surgical videos using deep learning. The
shape of the uterus also varies across patients; therefore, the
shapes of ovaries are not considered to be the only reason for the
low rate of correct diagnosis. In addition, it is considered that
the rate of correct diagnosis may be improved if the annotation is
performed well and the number of ovaries in the training dataset is
increased.
Mascagni et al (13) developed a deep-learning model to
automatically segment the liver and gall bladder in laparoscopic
images and evaluate safety criteria for laparoscopic
cholecystectomy (LC). Developments in the surgical field method
prevent surgical complications. The specific surgical field
deployment for safe LC is known as the critical view of safety
(CVS). There are three points to consider when developing CVS: i)
Fat and fibrous tissue must be removed from Calot's triangle, ii)
the neck of the gallbladder must be removed from the gallbladder
plate, and iii) ensure that only the cholecystic duct and
cholecystic artery are connected to the gallbladder. In this study,
2,854 images from 201 LC videos were annotated and 402 images were
segmented. A deep neural network was developed consisting of a
segmentation model to highlight the anatomy of the liver sac, and
the average accuracy of the classification model to predict the
achievement of CVS criteria was 71.9% (13). By increasing the rate of correct
diagnosis of this system in the future, the CVS may be determined
by this model before proceeding with surgery.
Padovan et al used deep learning from
laparoscopic surgery videos to construct 3D models that accounted
for position and rotation. They confirmed the accuracy by
superimposing the constructed data on actual organ images, and the
accuracy was >80% in all tests (14). The initial registration of a 3D
preoperative CT model to 2D laparoscopic images in an augmented
reality system for liver surgery may be useful for surgeons to
better identify the internal anatomy. This study aimed to develop a
system that automates this process using deep learning instead of
the conventional manual method. Specifically, the system automates
the construction of a 3D model, the identification of the hepatic
limbus in CT images, and the identification of the hepatic limbus
in 2D laparoscopic video images (15). Thus, the integration of laparoscopic
video and 3D CT may be useful for preoperative simulation in the
future.
In the analysis of laparoscopic surgery videos using
deep learning, the identification of surgical instruments is
equally important to the identification of organs. Therefore,
several studies on surgical tool identification have been reported.
For example, Namazi et al developed an AI model called
LapToolNet to detect surgical instruments, such as bipolar,
clipper, grasper, hook, irrigator, scissors, and specimen bag, in
each frame of a laparoscopic video, all with agreement rates of 80%
or higher (16). Several studies
have dealt with surgical instrument recognition in this way, but
the results are often not very different from those of organ
recognition (16,17). In another study, the patterns of
instrumentation use were compared among surgeons with different
skill levels during laparoscopic gastrectomy for gastric cancer. A
total of 33 cases of D2 suprapancreatic lymphadenectomy for gastric
cancer were evaluated in this retrospective study. Patterns of
surgical device use were compared between surgeons certified under
the Endoscopic Surgical Techniques Certification System of the
Japanese Society for Endoscopic Surgery and unqualified surgeons.
The percentage of time spent using incision forceps and clip
appliers was higher among non-technically-certified surgeons than
among technically-certified surgeons. For suprapancreatic
lymphadenectomy, the percentage of time spent using energy
instruments, clip appliers, and grasping tweezers was significantly
different between the two groups (17).
One of the key techniques in LC is clipping the
cholecystic artery before cutting. For safe clipping, it is
important to have full visibility of the clipper while surrounding
the artery and biliary duct with the clip applier jaws. Using
videos of 300 cholecystectomies, Aspart et al developed a
deep-learning model that provides real-time feedback on the proper
visibility of the clip applier (18). Notably, the study demonstrated the
difference in skills between skilled and unskilled surgeons by
means of deep learning. Such analysis can be used as an educational
tool to improve surgical skills by identifying the so-called ‘good
surgeries’ in the future.
Procedure and surgical phase recognition.
Recognition of the surgical technique and stage is important, as is
the dissection and identification of surgical instruments. Cheng
et al used deep learning to recognize and analyze the
surgical steps in multiple LC surgical videos. In this study, 163
LC videos sourced from four medical centers were evaluated. The
accuracy of the developed model in recognizing the surgical steps
was 91% (19). Breaking down the
surgical procedure into steps facilitates the acquisition, storage,
and organization of intraoperative video data. However, manual data
organization is time-consuming; therefore, Kitaguchi et al
developed a model using deep learning to automatically segment the
surgical steps of the transanal total mesorectal excision
procedure, achieving an overall accuracy of 93.2% (20). In another study, Kitaguchi et
al developed a model that uses CNN-based deep learning to
recognize the steps and procedures of a laparoscopic sigmoid colon
resection based on manually annotated data. The surgical steps were
classified into 11 procedures, and the accuracy of their
recognition was 91.9% (21).
Indocyanine green is sometimes used to investigate blood return
after bowel resection and bowel anastomosis in deep endometriosis.
In another study, a prediction model of blood return after bowel
anastomosis was developed by analyzing images of the bowel after
indocyanine green injection using deep learning (22). The course of a surgical procedure is
often not predictable, and it is difficult to evaluate the time of
the procedure in advance, which renders the scheduling of surgical
procedures difficult. Therefore, surgical time prediction is also
an important factor in terms of surgical recognition. Fewer studies
have been reported on surgical time prediction using AI than
others. Twinanda et al developed a deep-learning pipeline
called RSDNet that automatically predicts the remaining
intraoperative surgical time (RSD) using only visual information
from laparoscopic images. A key feature of RSDNet is that it can
easily accommodate various types of surgeries without relying on
manual annotation during training (23).
Bodenstedt et al developed a convolutional
neural network-based method for continuous prediction of
laparoscopic surgery time based on endoscopic images. Various types
of laparoscopic images were used, and these methods were evaluated.
The results showed an overall average error of 37% and an average
half-time error of approximately 28% (24). Recognition of surgical steps
provides evidence of the capture of changes in higher-order
features, such as surgical procedures, which will lead to the
development of surgical navigation systems and autonomous surgical
robots in the future.
Surface navigation for safe incisions. One of
the most important factors in surgery is making the incision in a
safe area, which can be difficult depending on the skill level of
the individual surgeon. Thus, several studies on the development of
surface navigation systems for safe incisions using AI have been
reported.
Igaki et al developed an AI-based navigation
of the entire mesorectal resection plane in laparoscopic colorectal
surgery. A total of 32 videos of laparoscopic left colorectal
resections were analyzed using deep learning. The developed model
helped identify and highlight the target area, however more images
are required to improve the accuracy (25). Kumazu et al defined the
surgical surface for safe incision as a loose connective tissue
fiber (LCTF) and developed a model that automatically segments the
LCTF. A surgical video of a robot-assisted gastrectomy was created
using U-NET-based deep learning, and the segmentation results were
output. The answers to two questions were then obtained from 20
surgeons with regard to the segmentation results including i) is
this AI highly sensitive in recognizing LCTF (on a 5-point scale)?
and ii) how many frames does the AI misrecognize? The mean value
for question 1 was 3.52, and the mean value for question 2 was
0.14. This suggests that AI can be used to recognize difficult
anatomical structures and assist surgeons in surgery (26). Regarding this study, it is
considered that the present model can be used for gastrectomy as
well as other surgeries if developed in the future. However, while
LCTF may lead to the recognition of a safe incision line, it may
not necessarily lead to the effective navigation of an appropriate
incision line, and it is surmised that resolving this issue is a
future challenge. In the future, it is important to develop such a
navigation system, and it is maintained that the development of
such a system will lead to the development of automated surgical
robots.
Surgical education. Deep learning has also
been applied to the field of surgical education. Moglia et
al used deep learning to develop a model to predict the
proficiency of medical students in a surgical simulator based on
their training data. Subsequently, they used the model to predict
proficiency from simulator data of untrained medical students with
an accuracy rate of >80% (27).
Excessive stress experienced by surgeons can negatively affect
their surgical procedures, and Zheng et al developed a
deep-learning model to detect, in real time, the movements of
surgical procedures in which surgeons appear to be under stress. In
this study, stress-sensitive procedures were identified, which may
be integrated into robotic-assisted surgical platforms and used for
stress management in the future (28). Future development of such a system
may render it possible to use deep learning in surgical
education.
3. Autonomous surgical robots
Description of autonomy
Autonomy is defined as being independent and capable
of making decisions; however, its definition is ambiguous and
without standards, and it is often used inappropriately. For
example, the da Vinci system, which is currently used in numerous
hospitals, is misnamed a surgical robot, even though it is,
narrowly defined, as a high-tech motion repeater manipulator. This
designation of the term ‘robot’ is rather incorrect, but
unfortunately, the name has stuck. Therefore, when developing an
autonomous surgical system, it is better to define the term
‘autonomy’ to avoid misnomers (11). Han et al and Yang et
al proposed six frameworks describing the levels of autonomy
for medical robots, similar to that for autonomous vehicles. Level
0, which is the no autonomy group, includes surgical robots with
motion scaling capabilities that respond to the commands of the
surgeon. Level 1, or the robotic assistance group, includes robots
that provide some assistance while humans predominantly manage the
system. Level 2, which is the task autonomy group, includes robots
that autonomously perform a specific task that is started by a
human. A feature that differs from that of Level 1 is that the
operator controls the system discretely rather than continuously.
An example is an automated suturing system in a surgical procedure.
The surgeon instructs the robot where to suture, and the robot
performs the task autonomously, with the surgeon monitoring and
intervening as needed. Level 3, which is the conditional autonomy
group, includes robots that can perform system-generated tasks but
rely on humans to select among different plans. This surgical robot
can perform tasks without close supervision. Level 4, or the high
autonomy group, includes robots that can make decisions in surgery,
but it is only allowed to do so under the supervision of a
qualified physician. Level 5, or the full autonomy group, includes
robots that do not require a human at all. This is a ‘robotic
surgeon’ who can perform the entire surgery (12,29).
Two additional factors are important for autonomous surgery:
Recognition and task. The classification level of recognition is as
follows: Level 1, awareness of the environment; Level 2,
understanding the current status; and Level 3, prediction of the
future status. The classification level of tasks, or Level of Task
Complexity (LoTC), is as follows: LoTC 1, simple training tasks
that are limited to surgical tasks such as distance considerations;
LoTC 2, high training tasks; LoTC 3, simple surgical tasks; LoTC 4,
advanced surgical tasks, such as suturing; and LoTC 5, complex
surgical tasks, such as stopping sudden bleeding (30). The development of autonomous
surgical robots should be aimed at accounting for the
abovementioned classification levels.
Autonomous (or semi-automatic) surgical robots
developed to date. In this section, the autonomous (or
semi-autonomous) surgical robots that have been developed thus far
are introduced. Despite the increasing adoption of robot-assisted
surgery, surgical procedures on soft tissues are still performed
completely manually by human surgeons. Shademan et al have
developed a supervised surgical robot called Smart Tissue
Autonomous Robot (STAR), which is a monitored surgical robot that
can perform complex surgical procedures that could previously only
be performed by humans (31).
The first surgical techniques STAR aimed to perform
were anastomosis and suture. These techniques are important because
suturing soft organs (such as the intestinal tract, urinary system,
and gynecological vaginal segments) is a common surgical procedure
that requires repeatability, accuracy, and efficiency and thus
supports the development of autonomous surgical robots. Autonomous
robotic surgery offers benefits in terms of efficacy, safety, and
reproducibility, regardless of the skill and experience of the
individual surgeon. In this context, autonomous anastomosis is
challenging because it requires complex imaging, navigation, and
highly adaptable and precise execution. As reported in 2016, STAR
performed intestinal anastomosis in open surgery in pigs. This STAR
phase was characterized by the following two points: i) A 3D visual
tracking system using near-infrared fluorescence imaging and ii) an
automated suture algorithm. Suture consistency, anastomotic leak
pressure, number of mistakes, and completion time were compared
with those of robot-assisted surgery and manual laparoscopic
surgery, and STAR was superior (31). Since then, STAR has undergone a
number of improvements. For example, an autonomous 3D path planning
system was developed for STAR that utilizes biocompatible
near-infrared markings and aims at precise incisions in complex 3D
soft tissues. This plan was able to reduce the incision progress
error compared to previous autonomous path planning (32). Other improvements include 3D imaging
endoscopy and the development of a laparoscopic suture tool to
generate a suture planning strategy for automated anastomosis. This
tool was 2.9 times more accurate than manual suturing (33). Anastomosis performed by an
autonomous robot may improve surgical outcomes by ensuring more
accurate suture spacing and suture size than manual anastomosis.
However, it is difficult for those robots without features such as
continuous tissue detection and 3D path planning, because soft
tissues have irregular shapes and unpredictable deformations.
Therefore, Kam et al developed a new 3D path planning
strategy for STAR that allows semi-autonomous robotic anastomosis
in deformable tissues. A comparison was performed between STAR
using the completed algorithm and a surgeon-completed anastomosis
of synthetic vaginal cuff tissue. The results revealed that STAR
with the newly developed method achieved 2.6 times better
consistency in suture spacing and 2.4 times better consistency in
suture bite size than manual performance (34). STAR was also used to create and
analyze shared control strategies for human-robot collaboration in
surgical scenarios. Specifically, a shared control strategy was
developed based on trust, and the accuracy of the developed
strategy was analyzed by evaluating the pattern tracking
performance, both autonomous and manual. In an experiment with pig
fat samples, by combining the advantages of autonomous robot
control with complementary human skills, the control strategy
improved cutting accuracy by 6.4% while reducing operator work time
by 44% compared to manual control (35).
The latest study by STAR describes a novel in
vivo autonomous robotic laparoscopic surgical technique. The
autonomous system developed in this study is characterized by its
ability to track tissue position and deformation, interact with
humans, and execute complex surgical plans (36). Owing to this improved autonomous
strategy, the operator can select among surgical plans generated by
this system, and the robot can perform various tasks independently.
Furthermore, using the enhanced autonomous strategy, needle
placement compensation, suture spacing, completion time, and the
rate of intestinal suture failure were compared with those of
skilled surgeons and robot-assisted surgical techniques, and the
developed STAR outperformed both. Another unique feature reported
by this study was the ability to perform subperitoneal surgery
(36). However, the issue remains
as to whether STAR can also automate incision and hemostasis, as it
has only been developed for suturing in the past.
Another reported autonomous surgical robot is the
RAVEN-II system. The features of the RAVEN-II system are as
follows: i) Provides software and hardware to support research and
development of surgical robots; ii) provides advanced robotics such
as computer vision, motion planning, and machine learning; iii)
establishes a software environment compatible with functions; and
iv) provides a hardware platform that allows for solid evaluation
of experiments (37). Using the
RAVEN-II system, a prototype medical robotic system designed to
autonomously detect and remove residual brain tumors after the
majority of the tumor has been removed by conventional surgery has
been developed. The scenario assumes that the majority of the brain
tumor has been removed, and then the cavity, the size of a
ping-pong ball is exposed. The system is equipped with a multimodal
scanning fiber endoscope, a suction machine for blood removal, and
multiple robotic arms with devices for brain tissue resection. The
automated surgical procedure is performed in six subtasks: i)
Medical image acquisition, which involves scanning of the surgical
cavity after tumor removal; ii) medical image processing, which
involves 3D construction of the aforementioned surgical cavity and
recognition of the residual tumor; iii) ablation plan creation; iv)
selection of the plan by the surgeon; v) performance of the plan by
the robot; and vi) verification of the ablation results (38).
Another experimental system is the da Vinci Research
Kit (dVRK). The dVRK is a joint industry-academia effort to
repurpose the obsolete da Vinci system (Intuitive Surgical, Inc.)
as a research platform to promote surgical robotics research
(https://research.intusurg.com/index.php/Main_Page).
This is important to facilitate the entry of new research groups
into the field of surgical robotics. For example, in the master
tool manipulator of the dVRK, hysteresis forces from the electrical
cables of the robotic joints often prevent accurate parameter
estimation in gravity-compensation models due to the magnitude of
gravity and identification. Therefore, a strategy to classify these
two hybrid forces and evaluate them in individual learning-based
algorithms has been proposed. A specially designed Elastic
Hysteresis Neural Network model was employed to capture the
external hysteresis. The gravity compensation method developed in
this study for the master tool manipulator of the dVRK exhibits
improvement over the previous one (39).
Ethics. Although it will still require a long
time before the development of a fully autonomous surgical system,
the development of a hybrid system may be realized in the near
future. Therefore, it is necessary to discuss ethical issues
related to autonomous surgical systems (27).
One of the most controversial ethical issues to be
discussed is responsibility. In a three-part sequence of
operations, including input, internal state (deep learning
algorithm), and output, full transparency is not ensured. This is
especially true for the algorithm, owing to the ‘black box’ design
inherent in deep-learning systems and the non-disclosure of source
code for reasons of copyright law and protection of trade secrets
(12,27). This prevents physicians and patients
from trusting robots. Another concern is responsibility for the
system used. Is it the developer or the physician using the system
who is responsible? For example, who can be held responsible for
surgical complications caused by an autonomous surgical robot? Who
is guilty and who should be punished if an anomaly occurs, such as
loss of communication during an aggravated teleoperation? One
proposed solution regarding liability is that, as in the case of
autonomous vehicles, the surgeon stays in the same room as the
surgical robot, which can be controlled by the physician at any
time. Another option is to use a limited system that does not give
full autonomy but assists the surgeon in routine surgery (12,27).
Discussion of ethical and other regulations should be completed by
the time an autonomous surgical robot is developed that is safe and
has more consistent performance than a human surgeon.
4. Conclusion
Although the clinical applications of fully
autonomous surgical robots may yet take some time, partially
autonomous surgical robots may see practical applications in the
not-too-distant future. For this purpose, it is necessary to
advance surgical recognition and robotics through deep learning
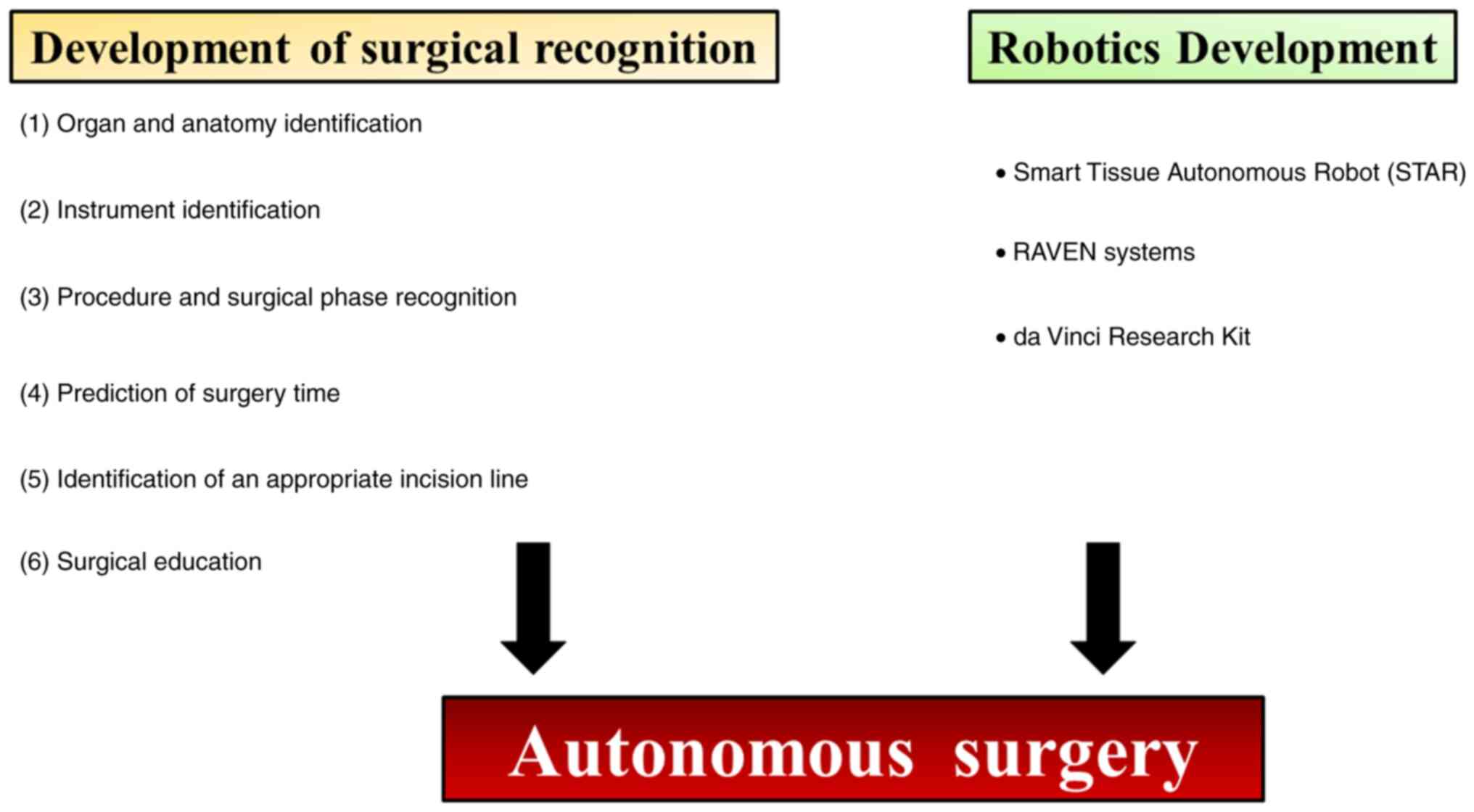
using surgical videos (Fig. 1). In
this review, various studies on the recognition of surgical videos,
including organ recognition, surgical instrument recognition, and
surgical education were investigated. Among these tasks, it is
considered that identifying the resection site is the most
important. However, it is inferred that the accuracy of the
currently reported AI models is still far from that required for
clinical application. Strictly speaking, the ultimate goal is for
physicians to be able to perform surgery safely according to
navigation assistance provided by the AI model. To improve
diagnostic accuracy, it is necessary to use public databases of
moving images and develop programs. Furthermore, in this review,
autonomous surgical robots were described. The STAR system is the
most widely reported, and the development of automatic suturing and
anastomosis systems is progressing with further innovations. In the
future, it will be necessary to develop applications for other
surgical techniques, such as incisions. For the time being, it is
necessary to develop semi-automatic systems that can perform simple
tasks and systems with a human surgeon on standby, who can
intervene in case of an emergency. To ultimately develop an
autonomous surgical robot, it is necessary to integrate the
navigation system with the surgical robot as aforementioned.
Particularly, it is necessary to develop a deep-learning model that
can feed back the results recognized by the navigation system to
the surgical technique.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KS, ST, YT, AT, YM, MM, TI, OWH, and YO contributed
to literature research, as well as manuscript writing and review.
KS, ST, YT, and AT designed the figure and table. KS, MM, TI, OWH,
and YO conceptualized and supervised the study. Data authentication
is not applicable. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moor J: The Dartmouth College artificial
intelligence conference: The next fifty years. AI Mag. 27:87–89.
2006.
|
|
2
|
Russell S and Norvig P: Artificial
intelligence: A modern approach. Prentice Hall, Upper Saddle River,
NJ, 1995.
|
|
3
|
Nilsson NJ: Artificial intelligence: A new
synthesis. Morgan Kaufmann, Burlington, MA, 1998.
|
|
4
|
Shinde PP and Shah S: A review of machine
learning and deep learning applications. In: Fourth International
Conference on Computing Communication Control and Automation
(ICCUBEA). IEEE, 2018.
|
|
5
|
Emmert-Streib F, Yang Z, Feng H, Tripathi
S and Dehmer M: An introductory review of deep learning for
prediction models with big data. Front Artif Intell.
3(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hamamoto R, Suvarna K, Yamada M, Kobayashi
K, Shinkai N, Miyake M, Takahashi M, Jinnai S, Shimoyama R, Sakai
A, et al: Application of artificial intelligence technology in
oncology: Towards the establishment of precision medicine. Cancers
(Basel). 12(3532)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sone K, Toyohara Y, Taguchi A, Miyamoto Y,
Tanikawa M, Uchino-Mori M, Iriyama T, Tsuruga T and Osuga Y:
Application of artificial intelligence in gynecologic malignancies:
A review. J Obstet Gynaecol Res. 47:2577–2585. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miyamoto Y, Tanikawa M, Sone K,
Mori-Uchino M, Tsuruga T and Osuga Y: Introduction of minimally
invasive surgery for the treatment of endometrial cancer in Japan:
A review. Eur J Gynaecol Oncol. 42:10–17. 2021.
|
|
9
|
Moglia A, Georgiou K, Georgiou E, Satava
RM and Cuschieri A: A systematic review on artificial intelligence
in robot-assisted surgery Int J. Surg. 95(106151)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Madad Zadeh S, Francois T, Calvet L,
Chauvet P, Canis M, Bartoli A and Bourdel N: SurgAI: Deep learning
for computerized laparoscopic image understanding in gynaecology.
Surg Endosc. 34:5377–5383. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gültekin IB, Karabük E and Köse MF: ‘Hey
Siri! Perform a type 3 hysterectomy. Please watch out for the
ureter!’ What is autonomous surgery and what are the latest
developments? J Turk Ger Gynecol Assoc. 22:58–70. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Han J, Davids J, Ashrafian H, Darzi A,
Elson DS and Sodergren M: A systematic review of robotic surgery:
From supervised paradigms to fully autonomous robotic approaches.
Int J Med Robot. 18(e2358)2022.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Mascagni P, Vardazaryan A, Alapatt D,
Urade T, Emre T, Fiorillo C, Pessaux P, Mutter D, Marescaux J,
Costamagna G, et al: Artificial intelligence for surgical safety:
Automatic assessment of the critical view of safety in laparoscopic
cholecystectomy using deep learning. Ann Surg. 275:955–961.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Padovan E, Marullo G, Tanzi L, Piazzolla
P, Moos S, Porpiglia F and Vezzetti E: A deep learning framework
for real-time 3D model registration in robot-assisted laparoscopic
surgery. Int J Med Robot. 18(e2387)2022.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Koo B, Robu MR, Allam M, Pfeiffer M,
Thompson S, Gurusamy K, Davidson B, Speidel S, Hawkes D, Stoyanov D
and Clarkson MJ: Automatic, global registration in laparoscopic
liver surgery. Int J Comput Assist Radiol Surg. 17:167–176.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Namazi B, Sankaranarayanan G and Devarajan
V: A contextual detector of surgical tools in laparoscopic videos
using deep learning. Surg Endosc. 36:679–688. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamazaki Y, Kanaji S, Kudo T, Takiguchi G,
Urakawa N, Hasegawa H, Yamamoto M, Matsuda Y, Yamashita K, Matsuda
T, et al: Quantitative comparison of surgical device usage in
laparoscopic gastrectomy between surgeons' skill levels: An
automated analysis using a neural network. J Gastrointest Surg.
26:1006–1014. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aspart F, Bolmgren JL, Lavanchy JL, Beldi
G, Woods MS, Padoy N and Hosgor E: ClipAssistNet: Bringing
real-time safety feedback to operating rooms. Int J Comput Assist
Radiol Surg. 17:5–13. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cheng K, You J, Wu S, Chen Z, Zhou Z, Guan
J, Peng B and Wang X: Artificial intelligence-based automated
laparoscopic cholecystectomy surgical phase recognition and
analysis. Surg Endosc. 36:3160–3168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kitaguchi D, Takeshita N, Matsuzaki H,
Hasegawa H, Igaki T, Oda T and Ito M: Deep learning-based automatic
surgical step recognition in intraoperative videos for transanal
total mesorectal excision. Surg Endosc. 36:1143–1151.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kitaguchi D, Takeshita N, Matsuzaki H,
Takano H, Owada Y, Enomoto T, Oda T, Miura H, Yamanashi T, Watanabe
M, et al: Real-time automatic surgical phase recognition in
laparoscopic sigmoidectomy using the convolutional neural
network-based deep learning approach. Surg Endosc. 34:4924–4931.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hernández A, Robles de Zulueta P, Spagnolo
E, Soguero C, Cristobal I, Pascual I, López A and Ramiro-Cortijo D:
Deep learning to measure the intensity of indocyanine green in
endometriosis surgeries with intestinal resection. J Pers Med.
12(982)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Twinanda AP, Yengera G, Mutter D,
Marescaux J and Padoy N: RSDNet: Learning to predict remaining
surgery duration from laparoscopic videos without manual
annotations. IEEE Trans Med Imaging. 38:1069–1078. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bodenstedt S, Wagner M, Mündermann L,
Kenngott H, Müller-Stich B, Breucha M, Torge Mees S, Weitz J and
Speidel S: Prediction of laparoscopic procedure duration using
unlabeled, multimodal sensor data. Int J Comput Assist Radiol Surg.
14:1089–1095. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Igaki T, Kitaguchi D, Kojima S, Hasegawa
H, Takeshita N, Mori K, Kinugasa Y and Ito M: Artificial
intelligence-based total mesorectal excision plane navigation in
laparoscopic colorectal surgery. Dis Colon Rectum. 65:e329–e333.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kumazu Y, Kobayashi N, Kitamura N, Rayan
E, Neculoiu P, Misumi T, Hojo Y, Nakamura T, Kumamoto T, Kurahashi
Y, et al: Automated segmentation by deep learning of loose
connective tissue fibers to define safe dissection planes in
robot-assisted gastrectomy. Sci Rep. 11(21198)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moglia A, Morelli L, D'Ischia R, Fatucchi
LM, Pucci V, Berchiolli R, Ferrari M and Cuschieri A: Ensemble deep
learning for the prediction of proficiency at a virtual simulator
for robot-assisted surgery. Surg Endosc. 36:6473–6479.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zheng Y, Leonard G, Zeh H and Fey AM:
Frame-wise detection of surgeon stress levels during laparoscopic
training using kinematic data. Int J Comput Assist Radiol Surg.
17:785–794. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang GZ, Cambias J, Cleary K, Daimler E,
Drake J, Dupont PE, Hata N, Kazanzides P, Martel S, Patel RV, et
al: Medical robotics-Regulatory, ethical, and legal considerations
for increasing levels of autonomy. Sci Robot.
2(eaam8638)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nagy TD and Haidegger T: Performance and
capability assessment in surgical subtask automation. Sensors
(Basel). 22(2501)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shademan A, Decker RS, Opfermann JD,
Leonard S, Krieger A and Kim PCW: Supervised autonomous robotic
soft tissue surgery. Sci. Transl. Med. 8(337ra64)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Saeidi H, Ge J, Kam M, Opfermann JD,
Leonard S, Joshi AS and Krieger A: Supervised autonomous
electrosurgery via biocompatible near-infrared tissue tracking
techniques. IEEE Trans Med Robot Bionics. 1:228–236.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saeidi H, Le HND, Opfermann JD, Leonard S,
Kim A, Hsieh MH, Kang JU and Krieger A: Autonomous laparoscopic
robotic suturing with a novel actuated suturing tool and 3D
endoscope. IEEE Int Conf Robot Autom. 2019:1541–1547.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kam M, Saeidi H, Wei W, Opfermann JD,
Leonard S, Hsieh MH, Kang JU and Krieger A: Semi-autonomous robotic
anastomoses of vaginal cuffs using marker enhanced 3D imaging and
path planning. Med Image Comput Comput Assist Interv. 11768:65–73.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Saeidi H, Opfermann JD, Kam M, Raghunathan
S, Leonard S and Krieger A: A confidence-based shared control
strategy for the Smart Tissue Autonomous Robot (STAR). Rep US.
1268-1275:2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Saeidi H, Opfermann JD, Kam M, Wei W,
Leonard S, Hsieh MH, Kang JU and Krieger A: Autonomous robotic
laparoscopic surgery for intestinal anastomosis. Sci Robot.
7(eabj2908)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hannaford B, Rosen J, Friedman DW, King H,
Roan P, Cheng L, Glozman D, Ma J, Kosari SN and White L: Raven-II:
An open platform for surgical robotics research. IEEE Trans Biomed
Eng. 60:954–959. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hu D, Gong Y, Seibel EJ, Sekhar LN and
Hannaford B: Semi-autonomous image-guided brain tumour resection
using an integrated robotic system: A bench-top study. Int J Med
Robot. 14(e1872)2018.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Gao Q, Tan N and Sun Z: A hybrid
learning-based hysteresis compensation strategy for surgical
robots. Int J Med Robot. 17(e2275)2021.PubMed/NCBI View
Article : Google Scholar
|