Introduction
Myxoid liposarcoma (MLS) is the second most common
type of liposarcoma, accounting for ~10% of all adult soft tissue
sarcomas. MLS typically develops in the deep soft tissue of the
extremities and tends to metastasize to nonpulmonary soft tissue
sites (1,2). Surgical resection with or without
radiotherapy is the standard treatment modality for localized MLS.
Although most MLS patients have favorable prognoses, a subgroup of
patients develops local recurrence and metastasis (2-4).
Most cases of MLS are characterized by specific chromosomal
translocations that generate the chimeric oncogenes encoding
translocated in liposarcoma (TLS)-CCAAT/enhancer-binding protein
homologous protein (CHOP), while cases with translocations in
Ewing's sarcoma (EWS)-CHOP are rare (5-7).
TLS is also called fused in sarcoma (FUS), and CHOP is also known
as DNA-damage-inducible transcript 3 (DDIT3) or growth arrest and
DNA damage-inducible gene 153 (GADD153). These chimeric
oncoproteins function as MLS-specific transcription factors that
inhibit adipocytic differentiation and promote malignant
transformation and tumor progression (8-12).
The expression of the antitumor cytokine
interleukin-24 (IL-24) is progressively decreased during the
development of melanoma from melanocytes, suggesting its
tumor-suppressive function in melanoma progression (13-16).
Indeed, ectopic expression of IL-24 has been revealed to induce
growth arrest and apoptotic cell death in human malignant tumor
cells from diverse origins (17-19);
however, it has minimal lethal effects on normal cells (20). Suppression of IL-24 has also been
demonstrated to be critical for MLS cell survival. It was
previously reported by the authors that proteoglycan 4 (PRG4), a
downstream molecule of TLS-CHOP and EWS-CHOP, supressed IL24
mRNA expression to sustain MLS cell proliferation (9,19,21).
In addition, the abundance of IL-24 protein was revealed to be
decreased by the ubiquitin-proteasome system in human ovarian and
lung cancer cells (22). However,
it remains unknown whether proteasomal degradation of IL-24 is
required for MLS cell survival.
Plasminogen activator inhibitor-1 (PAI-1) has been
revealed to be highly expressed in various types of cancer and to
promote tumor growth and metastasis as well as angiogenesis
(23). It has been previously
demonstrated that TLS-CHOP induced the expression of PAI-1, whereas
PAI-1 knockdown suppressed MLS cell proliferation (24). However, the molecular mechanism
underlying the growth-suppressive effects of PAI-1 knockdown
remains unclear.
The present study investigated whether IL-24 was
regulated by the ubiquitin-proteasome system in MLS cells and
whether PAI-1 was associated with the underlying mechanism. The
effects of a pharmacological inhibitor of PAI-1 on MLS cells was
also evaluated.
Materials and methods
Cell culture
The MLS-derived cell lines, 2645/94 and 1955/91,
were kindly provided by Professor David Ron (University of
Cambridge, Cambridge, UK) and cultured in Dulbecco's modified
Eagle's medium (product no. D5796; Sigma-Aldrich; Merck KGaA)
supplemented with 10% fetal bovine serum (Biowest) at 37˚C in a
humidified incubator with 5% CO2. The cell lines were
tested for mycoplasma contamination using Cycleave PCR Mycoplasma
Detection Kit (Takara Bio, Inc.). Phase-contrast images were
captured using an inverted microscope (ECLIPSE TS100; Nikon
Corporation) and a Microscope Camera Control Unit (DS-L3; Nikon
Corporation). For cell quantitation, cells were harvested from the
wells of a 12-well plate and resuspended in phosphate-buffered
saline (PBS). An equal volume of Trypan blue solution (0.4%;
Sigma-Aldrich Corporation; Merck KGaA) was then added to each
sample at room temperature, and immediately viable cell numbers
were calculated from four independent counts using a
hemocytometer.
Small interfering RNA (siRNA)
transfection
2645/94 and 1955/91 cells grown to ~10% confluence
were transfected with 10 nM of siRNAs using the
Lipofectamine® RNAiMAX Transfection Reagent and Opti-MEM
I Reduced Serum Medium (both from Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The cells were then cultured at 37˚C in a humidified incubator with
5% CO2 until the cells were harvested for cell
quantitation or preparation of protein or RNA samples. The target
nucleotide sequences of PRG4, SERPINE1 (encoding
PAI-1), IL24, and negative control siRNAs were as follows:
PRG4, 5'-CCACAAAAGCCCUGAUGAA-3'; SERPINE1,
5'-GGACAAAACUGGAGAUGCA-3'; IL24, 5'-GUGGAUGGGUGCUUAGUAA-3';
negative control, 5'-AUCCGCGCGAUAGUACGUA-3' (19,21,24).
Two FlexiTube siRNAs were used against IL24, namely,
Hs_IL24_5 and Hs_IL24_6 (GeneGlobe IDs SI02638139 and SI02638146,
respectively; Qiagen KK).
Western blot analysis
Protein sample preparation was performed as
previously described (25). Protein
samples were quantified using the Pierce™ BCA Protein
Assay Kit (Thermo Fisher Scientific). The protein samples (10
µg/lane) were then separated using SDS-PAGE (14% gel) and
transferred to Hybond ECL nitrocellulose membranes (Amersham;
Cytiva). To confirm equal sample loading, the membranes were
stained with Ponceau S solution (Sigma-Aldrich; Merck KGaA) for 1-2
min at room temperature. After blocking with 2% bovine serum
albumin (BSA; FUJIFILM Wako Pure Chemical Corporation) in Tris
Buffered saline with 0.05% Tween-20 (TBS-T; Takara Bio, Inc.; for
the antibodies against PAI-1, IL-24 and α-tubulin) or 5% skim milk
powder (FUJIFILM Wako Pure Chemical Corporation) in PBS (for all
other primary antibodies) at room temperature for 30 min, the
membranes were probed with specific antibodies at room temperature
overnight. The primary antibodies used were as follows: Purified
mouse anti-PAI-1 (1:2,500; cat. no. 612024; BD Biosciences), human
IL-24 antibody (mouse monoclonal; 1:2,000; cat. no. K101, GenHunter
Corporation), anti-PRG4 antibody (1:2,000; cat. no. AB2200; Merck
KGaA), cleaved caspase-3 (Asp175) antibody (1:1,000; cat. no. 9661;
Cell Signaling Technology, Inc.), poly (ADP-ribose) polymerase
(PARP) antibody (1:1,000; cat. no. 9542; Cell Signaling Technology,
Inc.), and monoclonal anti-α-tubulin antibody clone B-5-1-2
(1:2,500; cat. no. T-5168; Sigma-Aldrich; Merck KGaA). The
membranes were then washed with TBS-T or PBS three times, incubated
with 2% BSA in TBS-T with goat anti-mouse IgG (HRP) H&L
(1:5,000; cat. no. ab205719; Abcam) or 5% skim milk powder in PBS
with goat anti-rabbit IgG (HRP) H&L (1:5,000; cat. no.
ab205718; Abcam) at room temperature for 1 h, and washed with TBS-T
or PBS three times. The signals were visualized using ECL Prime
Western Blotting Detection Reagent (Amersham; Cytiva) and detected
with Chemiluminescence CCD Imaging System (AE9300 Ez-Capture MG;
ATTO Corporation).
Reverse transcription-quantitative PCR
analysis (RT-qPCR)
Cellular RNA was extracted from 2645/94 and 1955/91
cells using ISOGEN (Nippon Gene Co., Ltd.). First-strand cDNA
synthesis and real-time polymerase chain reaction (PCR) analysis
were performed as previously described (21). The relative expression was
calculated using the standard curve. The nucleotide sequences of
the specific primers for ACTB (encoding β-actin) are
5'-GGGAAATCGTGCGTGACATTAAG-3' and 5'-TGTGTTGGCGTACAGGTCTTTG-3' and
those for IL24 are 5'-GTTTTCCATCAGAGACAGTG-3' and
5'-GTAGAATTTCTGCATCCAGG-3', as previously described (19,26).
The mRNA levels of IL24 were normalized to ACTB
levels. The PCR amplifications were performed in triplicate.
Chemicals
The proteasome inhibitor, MG-132 (product no. M7449;
Sigma-Aldrich; Merck KGaA), was added to the culture medium at a
final concentration of 1 µM and incubated at 37˚C for 1, 2, 6 and
24 h. The PAI-1 inhibitor, TM5275 sodium salt (product no. SML1398;
Sigma-Aldrich; Merck KGaA), was dissolved with dimethyl sulfoxide
at a stock concentration of 50 mM, added to the culture medium at a
final concentration of 60 µM and incubated at 37˚C for 48 h.
Statistical analysis
Data are expressed as the mean ± standard deviation
of at least three repeats. The individual groups were compared
using one-way analysis of variance (ANOVA) followed by
Tukey-Kramer's post hoc test. All statistical analyses were
performed using Excel 2013 software (Microsoft Corporation), with
the add-in software Statcel-the Useful Addin Forms on Excel-4th ed.
(OMS Publishing, Inc.; https://oms-publ.main.jp/main/). P<0.05 was
considered to indicate a statistically significant difference.
Results
Proteasomal degradation of IL-24
protein promotes MLS cell survival
The effects of the proteasome inhibitor, MG-132, on
MLS-derived 2645/94 cells was first analyzed. At 48 h after
transfection with IL24 siRNA or negative control siRNA, the
2645/94 cells were treated with MG-132 (Fig. 1A). In the negative control
siRNA-transfected cells (control), MG-132 induced cell death within
6 h (Fig. 1B), whereas the
IL24 siRNA-transfected cells (IL-24 knockdown) resisted
MG-132-induced cell death for at least 6 h (Fig. 1B). Both cells were destroyed 24 h
after MG-132 treatment (Fig. 1B).
Furthermore, the abundance of IL-24 protein was lower in the IL-24
knockdown cells than in the control cells at 1, 2 and 6 h after
MG-132 treatment (Fig. 1C). Similar
results were observed at 6 h after MG-132 treatment following
transfection with two additional siRNAs targeting different sites
in the IL24 mRNA (Hs_IL24_5 and Hs_IL24_6; Fig. 1D and E). On the other hand, it has been
previously demonstrated that IL-24 overexpression supresses the
growth of MLS cells (19).
Collectively, these results indicated that IL-24 degradation by the
ubiquitin-proteasome system contributed to the survival of MLS
cells.
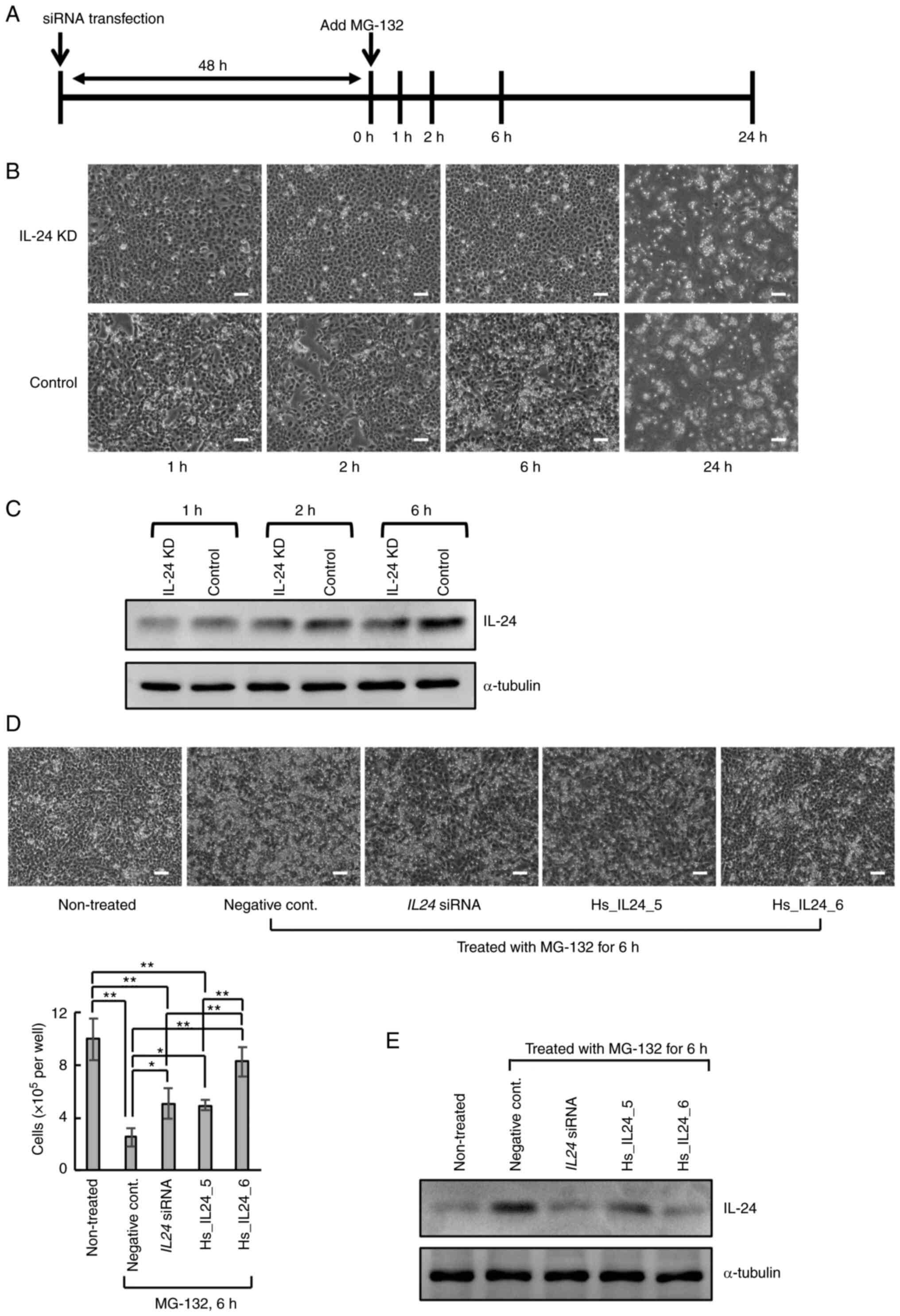 | Figure 1Effects of the proteasome inhibitor,
MG-132, on 2645/94 cells. (A) Graphical representation of the
experimental timeline. (B) Representative phase-contrast images of
2645/94 cells at 1, 2, 6 and 24 h after the addition of MG-132.
Magnification, x40. Scale bar, 100 µm. (C) Western blot analysis of
IL-24 expression in 2645/94 cells at 1, 2 and 6 h after the
addition of MG-132. α-Tubulin was used as a loading control. (D)
Representative phase-contrast images and viable cell counts of
2645/94 cells transfected with siRNA at 6 h after MG-132 treatment.
The image and the viable cell count of the 2645/94 cells without
siRNA transfection and MG-132 treatment (non-treated) are also
displayed. Magnification, x40; scale bar, 100 µm. Data is presented
as the mean ± SD. *P<0.05 and **P<0.01.
(E) Western blot analysis of IL-24 abundance in the cells shown in
D. α-Tubulin was used as a loading control. IL-24, interleukin-24;
siRNA, small interfering RNA; KD, knockdown. |
PAI-1 knockdown does not affect the
mRNA expression of IL24, but enhances the protein expression of
IL-24
Next, to determine whether IL-24 participates in the
growth suppression mechanism associated with PAI-1 knockdown,
knockdown experiments were performed in 2645/94 cells using
SERPINE1 (encoding PAI-1) and IL24 siRNAs (Fig. 2A, upper panels). PRG4 siRNA
was also used as a positive control for IL-24 induction. The
negative control siRNA-transfected cells (control) were used as a
negative control. Consistent with previous results (25), PRG4 knockdown suppressed MLS cell
growth, whereas PRG4 and IL-24 double knockdown canceled the
growth-suppressive effects of PRG4 single knockdown (Fig. 2A, upper panels). Interestingly,
double knockdown of PAI-1 and IL-24 also prevented the growth
suppression by PAI-1 single knockdown (Fig. 2A, upper panels). These experiments
were also performed using another MLS-derived cell line, 1955/91,
and similar results were obtained (Fig.
2A, lower panels). Thus, these results indicated that PAI-1 was
also involved in the mechanism of IL-24 supression in MLS
cells.
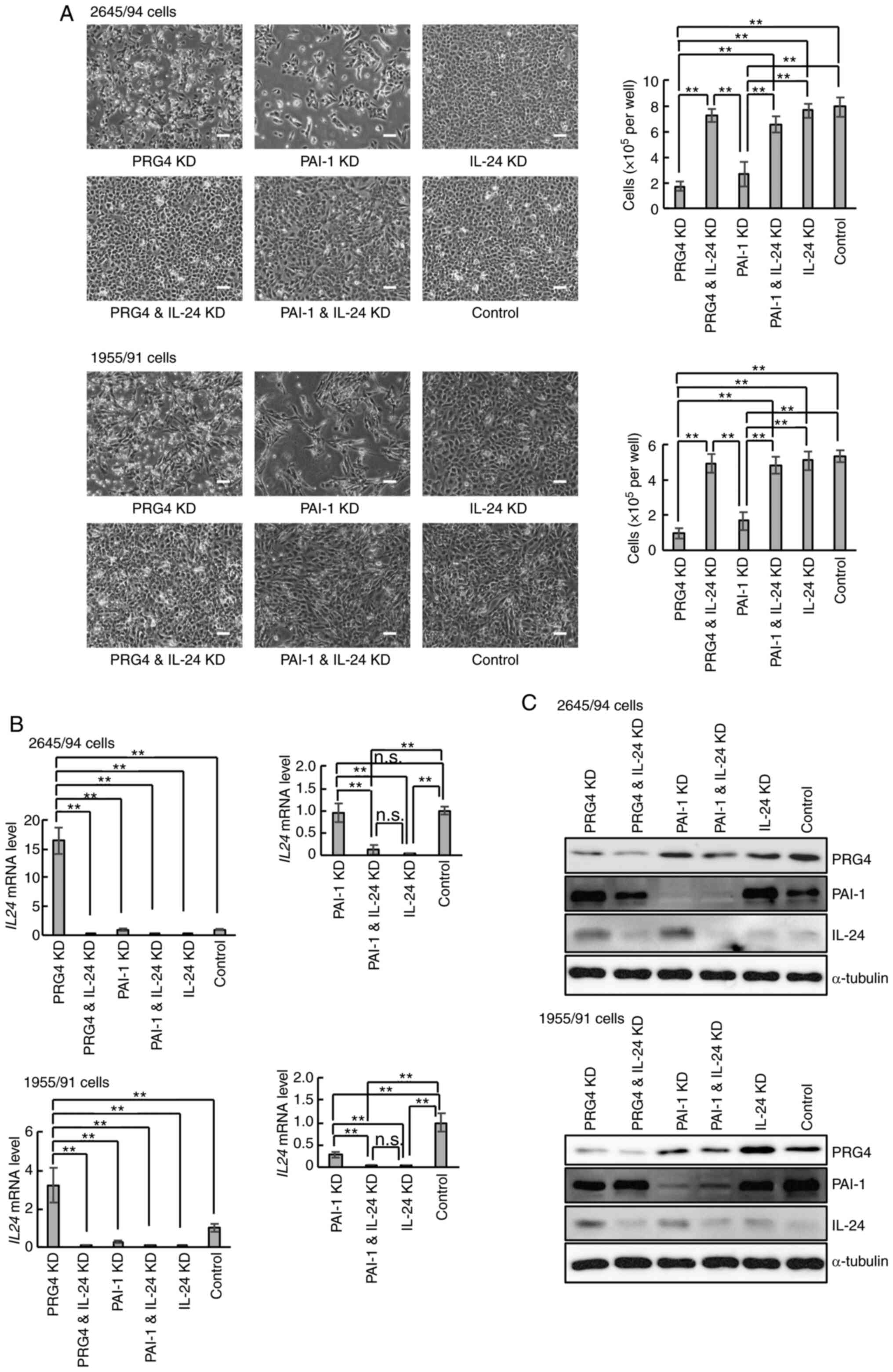 | Figure 2Effects of PAI-1 knockdown on myxoid
liposarcoma-derived cells. (A) Representative phase-contrast images
and viable cell numbers of 2645/94 and 1955/91 cells at 48 h after
siRNA transfection. Magnification, x40; scale bar, 100 µm. Data is
presented as the mean ± SD. **P<0.01. (B) Reverse
transcription-quantitative PCR analysis demonstrating the mRNA
levels of IL24 in 2645/94 and 1955/91 cells at 48 h after
siRNA transfection. Left panels displaying the relative mRNA levels
of IL24 in each cell line. Right panels displaying the
relative mRNA levels of IL24 in each cell line, excluding
cells with PRG4 knockdown. The right panel graphs present data
extracted from the left panel graphs, respectively, to clarify the
differences in the mRNA levels of IL24 between the indicated
cells. The mRNA level of IL24 in the control cells was
arbitrarily set to 1 in the graphical presentation, and all other
mRNA signals were normalized to this value. Data are presented as
the mean ± SD. **P<0.01; n.s., not significant. (C)
Western blot analysis of PRG4, PAI-1 and IL-24 abundance in
2645/94 and 1955/91 cells at 48 h after siRNA transfection.
α-Tubulin was used as a loading control. PAI-1, plasminogen
activator inhibitor-1; siRNA, small interfering RNA; IL24 or
IL-24, interleukin-24; PRG4, proteoglycan 4; KD, knockdown. |
To confirm whether PAI-1 knockdown induced IL-24
expression, IL24 mRNA expression in both cell lines was
analyzed using RT-qPCR. In contrast to the PRG4-knockdown results,
PAI-1 knockdown did not increase IL24 mRNA expression
(Fig. 2B, left panels). However,
the expression of IL24 mRNA was further downregulated in
PAI-1 and IL-24 double-knockdown cells (Fig. 2B, right panels). Thus, although
PAI-1 knockdown did not affect IL24 mRNA, downregulation of
IL-24 in PAI-1-knockdown cells may be required for MLS cell
survival. Subsequent analysis of IL-24 protein abundance in the
cells revealed an increase in IL-24 protein in PAI-1-knockdown
cells compared to control cells (Fig.
2C), further supporting the notion that PAI-1 affects IL-24
expression at the protein level.
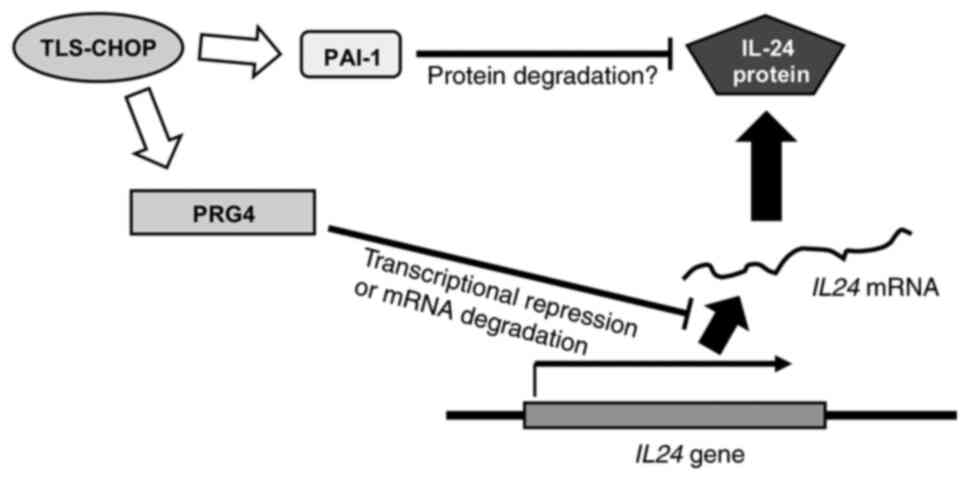
These findings indicated that the MLS-specific
chimeric oncoprotein TLS-CHOP may suppress IL-24 through two
separate mechanisms at the mRNA and protein levels, as described in
Fig. 3. It is posited that PAI-1 is
involved in the degradation of IL-24 protein in MLS cells; however,
it remains unclear whether PRG4 transcriptionally regulates or
degrades IL24 mRNA.
PAI-1 may serve as a candidate
therapeutic target for MLS treatment
Several pharmacological inhibitors of PAI-1,
including TM5275, have exhibited antitumor activity in various
tumor cells (27-32).
Therefore, it was examined whether TM5275 has tumor-suppressive
effects in MLS cells. To confirm the role of IL-24 in
TM5275-treated MLS cells, the effects of TM5275 on IL-24 knockdown
cells were also evaluated. At 24 h after transfection with
IL24 siRNA or negative control siRNA, the 2645/94 cells were
treated with TM5275 (Fig. 4A).
Although TM5275 induced cell death within 48 h in the control
cells, the IL-24-knockdown cells resisted the effect of TM5275
(Fig. 4B). IL-24 protein expression
was induced in the control cells treated with TM5275 (Fig. 4C). Furthermore, the TM5275-treated
control cells exhibited high expression of the apoptosis markers
cleaved caspase-3 and cleaved PARP, suggesting that PAI-1
inhibition by TM5275 induced apoptosis in MLS cells (Fig. 4C). Collectively, these results
provided further evidence that PAI-1 inhibition induced MLS cell
death through the induction of IL-24 expression and indicated that
PAI-1 may be a candidate therapeutic target for MLS treatment.
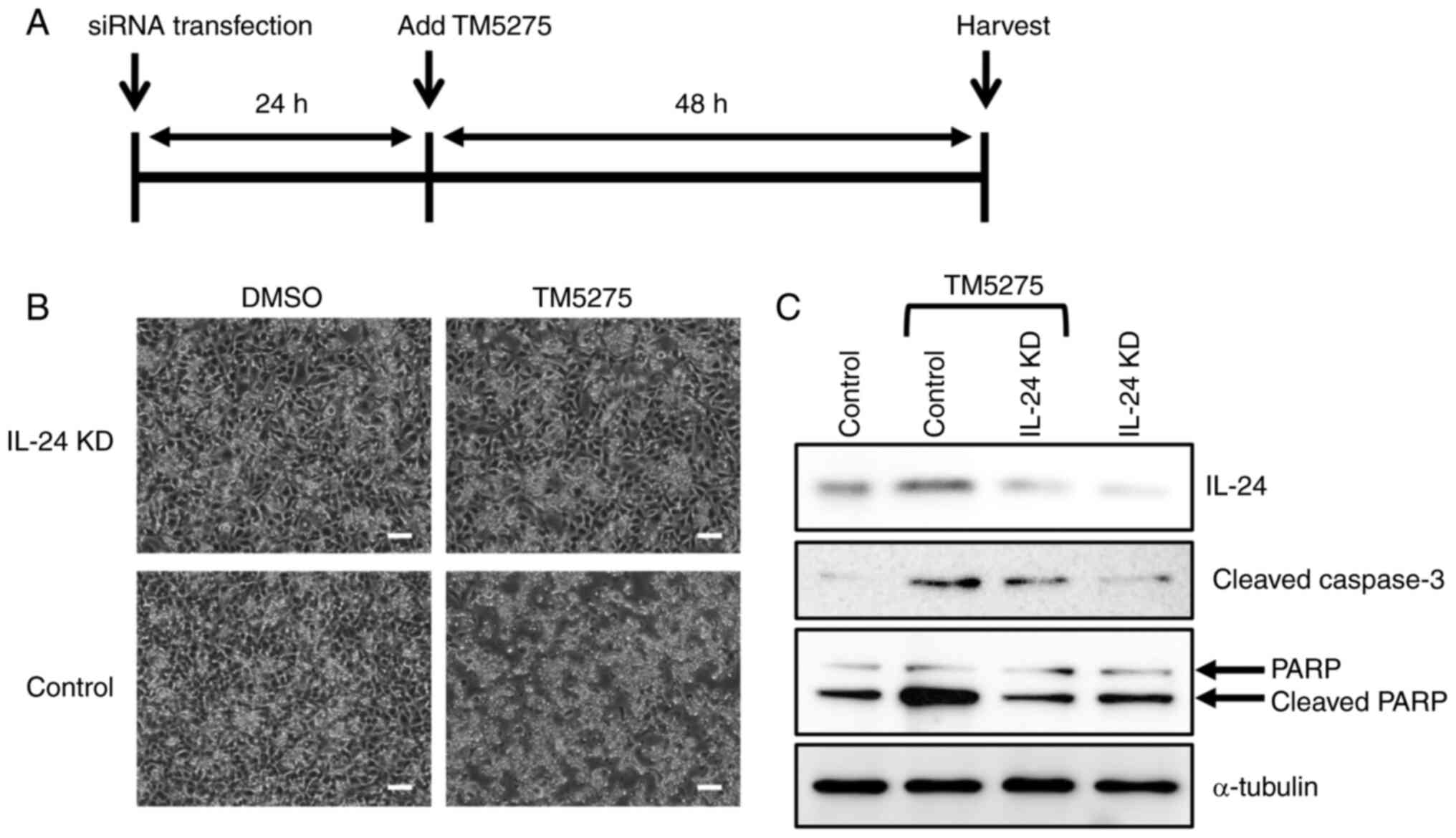 | Figure 4Effects of the inhibitor of
plasminogen activator inhibitor-1, TM5275, on 2645/94 cells. (A)
Graphical representation of the experimental timeline. (B)
Representative phase-contrast images of 2645/94 cells at 48 h after
the addition of TM5275. Magnification, x40; scale bar, 100 µm. (C)
Western blot analysis of IL-24, cleaved caspase-3, and total PARP
in 2645/94 cells at 48 h after the addition of TM5275. α-tubulin
was used as a loading control. IL-24, interleukin-24; PARP, poly
(ADP-ribose) polymerase; siRNA, small interfering RNA; DMSO,
dimethyl sulfoxide; KD, knockdown. |
Discussion
The present study demonstrated that PAI-1 is a key
molecule that supressed IL-24 expression in MLS cells. IL-24 is a
unique antitumor cytokine with various tumor-suppressive
activities, including the suppression of tumor growth, invasion,
and metastasis (33). IL-24
regulates key proteins involved in the regulation of endoplasmic
reticulum stress and mitochondrial function, and induces apoptosis
with toxic autophagy in diverse malignant tumor cells (33,34).
Thus, supression of IL-24 may be crucial for MLS cell survival.
Transcriptional regulation of IL-24 can occur via
multiple recognition sites on the IL24 gene, including those
of activator protein-1 (AP-1) and CCAAT/enhancer-binding protein
(C/EBP) (35). CHOP is a member of
the C/EBP family, and TLS-CHOP, which includes the full-length
CHOP, appears to directly affect IL24 gene expression.
However, it was previously demonstrated that the TLS-CHOP
downstream molecule PRG4 inhibited IL24 mRNA expression
(21). Furthermore, Madireddi et
al reported that C/EBP did not supress but rather promoted
IL-24 transcription (35). Thus, it
was considered that TLS-CHOP does not supress IL24 mRNA
expression directly but indirectly via PRG4 induction. Epigenetic
processes such as histone acetylation are also suggested to
modulate IL-24 transcription (36).
However, no evidence, to date, indicates that epigenetic
modifications affect TLS-CHOP-mediated supression of IL-24
transcription.
IL-24 protein abundance has been revealed to be
controlled by the ubiquitin-proteasome system (22). The present study revealed that PAI-1
knockdown did not impact IL24 mRNA expression but rather
increased its protein abundance in MLS cells. Given that PAI-1
reportedly interacts with proteasomes and regulates their activity
(37), it was postulated that PAI-1
may directly or indirectly modulate IL-24 degradation through the
ubiquitin-proteasome system. In fact, the results of the present
study revealed that the proteasome inhibitor, MG-132, induced MLS
cell death; while IL-24 knockdown did not fully abrogate these
effects, they were diminished. Thus, among numerous substrates for
the ubiquitin-proteasome system, IL-24 degradation appears to be
important in MLS cells. Collectively, previous studies by the
authors (19,21) and the results of the present study
indicated that IL-24 was suppressed by TLS-CHOP through two
separate mechanisms at the mRNA and protein level in MLS cells.
PAI-1, an inhibitor of urokinase-plasminogen
activator and tissue-type plasminogen activator, has multiple
functions in diverse pathological processes, including
cardiovascular disease and cancer, in addition to its involvement
in several biological processes such as fibrinolysis and wound
healing (38). However, the role of
PAI-1 in apoptosis remains controversial and has not been
established in MLS cells (39). The
known anti-apoptotic effects of PAI-1 include inhibition of
pro-apoptotic mediators (e.g., FasL and caspase-3) and production
of anti-apoptotic proteins (e.g., Bcl-2 and Bcl-xL) via induction
of c-Jun/ERK signaling. The inhibition of cell adhesion to
vitronectin by PAI-1 was revealed to exert both pro- and
anti-apoptotic activities depending on the condition (40). In the present study, PAI-1
inhibition increased the levels of the apoptotic markers, cleaved
caspase-3 and cleaved PARP, in association with the induction of
IL-24 expression and induced cell death in MLS cells. Thus, the
results suggest a novel molecular mechanism of PAI-1-mediated
anti-apoptotic effects in MLS cells.
PAI-1 is overexpressed in various cancer cells and
has been demonstrated to be a promising candidate target for their
treatment (23,41). Furthermore, PRG4 is expressed not
only in MLS, but also in various sarcomas (42,43).
Thus, the mechanisms to suppress IL-24 by PRG4 and PAI-1,
respectively, may be important for the growth of different types of
cancer cells. As such, disruption of these mechanisms may be a
promising therapeutic strategy for numerous cancers. Although the
present study has limitations, including the molecular mechanism of
the regulation of IL24 mRNA expression which was not
elucidated, and IL-24-knockout MLS cells which were not constructed
and analyzed, the results of the present study aid in advancing the
current understanding regarding the mechanisms underlying MLS
tumorigenesis and progression.
Acknowledgements
The authors would like to thank Professor David Ron
(Cambridge Institute for Medical Research, University of Cambridge,
Cambridge, UK) for providing the MLS-derived 2645/94 and 1955/91
cells. The authors are grateful to Professor Emeritus Yasuteru
Muragki (Wakayama Medical University) for his academic support.
Funding
Funding: The present study was partly supported by the
Grant-in-Aid for Scientific Research from the Japan Society for the
Promotion of Science (grant nos. 17K08768 and 22K09384 to KO).
Availability of data and materials
The datasets shown and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KO and MK conceived and designed the study. KO
performed experiments, analyzed the data and wrote the manuscript.
SE assisted with the experimental design, data analysis, and
writing of the manuscript. KO and SE confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spillane AJ, Fisher C and Thomas JM:
Myxoid liposarcoma-the frequency and the natural history of
nonpulmonary soft tissue metastases. Ann Surg Oncol. 6:389–394.
1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Manji GA and Schwartz GK: Managing
liposarcomas: Cutting through the fat. J Oncol Pract. 12:221–227.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zheng K, Yu XC, Xu M and Yang Y: Surgical
outcomes and prognostic factors of myxoid liposarcoma in
extremities: A retrospective study. Orthop Surg. 11:1020–1028.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Shinoda Y, Kobayashi E, Kobayashi H, Mori
T, Asano N, Nakayama R, Morioka H, Iwata S, Yonemoto T, Ishii T, et
al: Prognostic factors of metastatic myxoid liposarcoma. BMC
Cancer. 20(883)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crozat A, Aman P, Mandahl N and Ron D:
Fusion of CHOP to a novel RNA-binding protein in human myxoid
liposarcoma. Nature. 363:640–644. 1993.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Rabbitts TH, Forster A, Larson R and
Nathan P: Fusion of the dominant negative transcription regulator
CHOP with a novel gene FUS by translocation t(12;16) in malignant
liposarcoma. Nat Genet. 4:175–180. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Panagopoulos I, Höglund M, Mertens F,
Mandahl N, Mitelman F and Aman P: Fusion of the EWS and CHOP genes
in myxoid liposarcoma. Oncogene. 12:489–494. 1996.PubMed/NCBI
|
|
8
|
Sánchez-García I and Rabbitts TH:
Transcriptional activation by TAL1 and FUS-CHOP proteins expressed
in acute malignancies as a result of chromosomal abnormalities.
Proc Natl Acad Sci USA. 91:7869–7873. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kuroda M, Wang X, Sok J, Yin Y, Chung P,
Giannotti JW, Jacobs KA, Fitz LJ, Murtha-Riel P, Turner KJ and Ron
D: Induction of a secreted protein by the myxoid liposarcoma
oncogene. Proc Natl Acad Sci USA. 96:5025–5030. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Thelin-Järnum S, Lassen C, Panagopoulos I,
Mandahl N and Aman P: Identification of genes differentially
expressed in TLS-CHOP carrying myxoid liposarcomas. Int J Cancer.
83:30–33. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Riggi N, Cironi L, Provero P, Suvà ML,
Stehle JC, Baumer K, Guillou L and Stamenkovic I: Expression of the
FUS-CHOP fusion protein in primary mesenchymal progenitor cells
gives rise to a model of myxoid liposarcoma. Cancer Res.
66:7016–7023. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pérez-Mancera PA, Bermejo-Rodríguez C,
Sánchez-Martín M, Abollo-Jiménez F, Pintado B and Sánchez-García I:
FUS-DDIT3 prevents the development of adipocytic precursors in
liposarcoma by repressing PPARgamma and C/EBPalpha and activating
eIF4E. PLoS One. 3(e2569)2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fisher PB, Gopalkrishnan RV, Chada S,
Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT and Dent P:
mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine
gene: from the laboratory into the clinic. Cancer Biol Ther. 2 (4
Suppl 1):S23–S37. 2003.PubMed/NCBI
|
|
14
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
15
|
Huang EY, Madireddi MT, Gopalkrishnan RV,
Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ,
Alexandre D, et al: Genomic structure, chromosomal localization and
expression profile of a novel melanoma differentiation associated
(mda-7) gene with cancer specific growth suppressing and apoptosis
inducing properties. Oncogene. 20:7051–7063. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ellerhorst JA, Prieto VG, Ekmekcioglu S,
Broemeling L, Yekell S, Chada S and Grimm EA: Loss of MDA-7
expression with progression of melanoma. J Clin Oncol.
20:1069–1074. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dash R, Bhutia SK, Azab B, Su ZZ, Quinn
BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, et al:
mda-7/IL-24: A unique member of the IL-10 gene family promoting
cancer-targeted toxicity. Cytokine Growth Factor Rev. 21:381–391.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rahmani M, Mayo M, Dash R, Sokhi UK,
Dmitriev IP, Sarkar D, Dent P, Curiel DT, Fisher PB and Grant S:
Melanoma differentiation associated gene-7/interleukin-24 potently
induces apoptosis in human myeloid leukemia cells through a process
regulated by endoplasmic reticulum stress. Mol Pharmacol.
78:1096–1104. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Oikawa K, Tanaka M, Itoh S, Takanashi M,
Ozaki T, Muragaki Y and Kuroda M: A novel oncogenic pathway by
TLS-CHOP involving repression of MDA-7/IL-24 expression. Br J
Cancer. 106:1976–1979. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gupta P, Su ZZ, Lebedeva IV, Sarkar D,
Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P and
Fisher PB: mda-7/IL-24: Multifunctional cancer-specific
apoptosis-inducing cytokine. Pharmacol Ther. 111:596–628.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Oikawa K, Mizusaki A, Takanashi M, Ozaki
T, Sato F, Kuroda M and Muragaki Y: PRG4 expression in myxoid
liposarcoma maintains tumor cell growth through suppression of an
antitumor cytokine IL-24. Biochem Biophys Res Commun. 485:209–214.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gopalan B, Shanker M, Scott A, Branch CD,
Chada S and Ramesh R: MDA-7/IL-24, a novel tumor
suppressor/cytokine is ubiquitinated and regulated by the
ubiquitin-proteasome system, and inhibition of MDA-7/IL-24
degradation enhances the antitumor activity. Cancer Gene Ther.
15:1–8. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li S, Wei X, He J, Tian X, Yuan S and Sun
L: Plasminogen activator inhibitor-1 in cancer research. Biomed
Pharmacother. 105:83–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Borjigin N, Ohno S, Wu W, Tanaka M, Suzuki
R, Fujita K, Takanashi M, Oikawa K, Goto T, Motoi T, et al:
TLS-CHOP represses miR-486 expression, inducing upregulation of a
metastasis regulator PAI-1 in human myxoid liposarcoma. Biochem
Biophys Res Commun. 427:355–360. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Oikawa K, Ohbayashi T, Mimura J,
Fujii-Kuriyama Y, Teshima S, Rokutan K, Mukai K and Kuroda M:
Dioxin stimulates synthesis and secretion of IgE-dependent
histamine-releasing factor. Biochem Biophys Res Commun.
290:984–987. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Oikawa K, Ohbayashi T, Kiyono T, Nishi H,
Isaka K, Umezawa A, Kuroda M and Mukai K: Expression of a novel
human gene, human wings apart-like (hWAPL), is associated with
cervical carcinogenesis and tumor progression. Cancer Res.
64:3545–3549. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Placencio VR, Ichimura A, Miyata T and
DeClerck YA: Small molecule inhibitors of plasminogen activator
inhibitor-1 elicit anti-tumorigenic and anti-angiogenic activity.
PLoS One. 10(e0133786)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mashiko S, Kitatani K, Toyoshima M,
Ichimura A, Dan T, Usui T, Ishibashi M, Shigeta S, Nagase S, Miyata
T and Yaegashi N: Inhibition of plasminogen activator inhibitor-1
is a potential therapeutic strategy in ovarian cancer. Cancer Biol
Ther. 16:253–260. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakatsuka E, Sawada K, Nakamura K,
Yoshimura A, Kinose Y, Kodama M, Hashimoto K, Mabuchi S, Makino H,
Morii E, et al: Plasminogen activator inhibitor-1 is an independent
prognostic factor of ovarian cancer and IMD-4482, a novel
plasminogen activator inhibitor-1 inhibitor, inhibits ovarian
cancer peritoneal dissemination. Oncotarget. 8:89887–89902.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tsuge M, Osaki M, Sasaki R, Hirahata M and
Okada F: SK-216, a novel inhibitor of plasminogen activator
inhibitor-1, suppresses lung metastasis of human osteosarcoma. Int
J Mol Sci. 19(736)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xi X, Liu N, Wang Q, Chu Y, Yin Z, Ding Y
and Lu Y: ACT001, a novel PAI-1 inhibitor, exerts synergistic
effects in combination with cisplatin by inhibiting PI3K/AKT
pathway in glioma. Cell Death Dis. 10(757)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tseng YJ, Lee CH, Chen WY, Yang JL and
Tzeng HT: Inhibition of PAI-1 blocks PD-L1 endocytosis and improves
the response of melanoma cells to immune checkpoint blockade. J
Invest Dermatol. 141:2690–2698.e6. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Modi J, Roy A, Pradhan AK, Kumar A,
Talukdar S, Bhoopathi P, Maji S, Mannangatti P, Sanchez De La Rosa
D, Li J, et al: Insights into the mechanisms of action of
MDA-7/IL-24: A ubiquitous cancer-suppressing protein. Int J Mol
Sci. 23(72)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Emdad L, Bhoopathi P, Talukdar S, Pradhan
AK, Sarkar D, Wang XY, Das SK and Fisher PB: Recent insights into
apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a
multidimensional anti-cancer therapeutic. Semin Cancer Biol.
66:140–154. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Madireddi MT, Dent P and Fisher PB: AP-1
and C/EBP transcription factors contribute to mda-7 gene promoter
activity during human melanoma differentiation. J Cell Physiol.
185:36–46. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pan L, Pan H, Jiang H, Du J, Wang X, Huang
B and Lu J: HDAC4 inhibits the transcriptional activation of
mda-7/IL-24 induced by Sp1. Cell Mol Immunol. 7:221–226.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Boncela J, Przygodzka P, Papiewska-Pajak
I, Wyroba E, Osinska M and Cierniewski CS: Plasminogen activator
inhibitor type 1 interacts with alpha3 subunit of proteasome and
modulates its activity. J Biol Chem. 286:6820–6831. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sillen M, Miyata T, Vaughan DE, Strelkov
SV and Declerck PJ: Structural insight into the two-step mechanism
of PAI-1 inhibition by small molecule TM5484. Int J Mol Sci.
22(1482)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Balsara RD and Ploplis VA: Plasminogen
activator inhibitor-1: The double-edged sword in apoptosis. Thromb
Haemost. 100:1029–1036. 2008.PubMed/NCBI
|
|
40
|
Kubala MH and DeClerck YA: The plasminogen
activator inhibitor-1 paradox in cancer: A mechanistic
understanding. Cancer Metastasis Rev. 38:483–492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fang H, Placencio VR and DeClerck YA:
Protumorigenic activity of plasminogen activator inhibitor-1
through an antiapoptotic function. J Natl Cancer Inst.
104:1470–1484. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Domoto H, Hosaka T, Oikawa K, Ohbayashi T,
Ishida T, Izumi M, Iwaya K, Toguchida J, Kuroda M and Mukai K:
TLS-CHOP target gene DOL54 expression in liposarcomas and malignant
fibrous histiocytomas. Pathol Int. 52:497–500. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Panagopoulos I, Mertens F, Isaksson M and
Mandahl N: Expression of DOL54 is not restricted to myxoid
liposarcomas with the FUS-DDIT3 chimera but is found in various
sarcomas. Oncol Rep. 12:107–110. 2004.PubMed/NCBI
|