Introduction
The BCR::ABL1 negative chronic
myeloproliferative neoplasms (MPN) represent a heterogeneous group
of clonal diseases of the hematopoietic progenitor cell, of which
the most classic are polycythemia vera (PV), essential
thrombocythemia (ET) and primary myelofibrosis (PMF) (1,2). In
the 5th Classification of Hematolymphoid Tumors, published in 2022,
the World Health Organization (WHO) revised certain aspects for the
category of MPN (1), establishing
as diagnostic criteria for the diagnosis of PV elevated hemoglobin
concentration and/or hematocrit, accompanied by panmyelosis and
detection of JAK2V617F or exon 12 variants in
JAK2.
The primary diagnostic criterion of ET is marked
thrombocytosis (platelet count
>450x103/mm3). PMF is characterized by a
proliferation of abnormal megakaryocytes and granulocytes in the
bone marrow, which is associated in fibrotic stages with a
polyclonal increase in fibroblasts that drive secondary reticulin
and/or collagen marrow fibrosis, osteosclerosis, and extramedullary
hematopoiesis. Thereby, these diseases are characterized by
increased cell proliferation, development of chronic inflammation,
and association with clonal hematopoiesis (1,3,4).
Missense mutations in the JAK/STAT pathway are the
primary causes of the development of chronic MPN (5). Variants in the driver genes JAK2,
CALR, and MPL are the most commonly associated with the
development of MPN (6). According
to National Center of Biotechnology Information (NCBI:https://www.ncbi.nlm.nih.gov/gene/3717),
the JAK2 gene is located on chromosome 9p24.1 and
encompasses 145,559 nucleotides, distributed across 28 exons, and
the JAK2 coding sequence has a length of 3,399 nucleotides,
distributed across 23 exons, from exon 3 to exon 25, which encodes
a protein of 1,132 α. amino acids, a non-receptor tyrosine kinase
named JAK2.
Most of the variants identified in JAK2
result in a gain of function, and are characterized as somatic
missense types that lead to unregulated production of hematopoietic
cells in bone marrow and accumulation of mature cells in peripheral
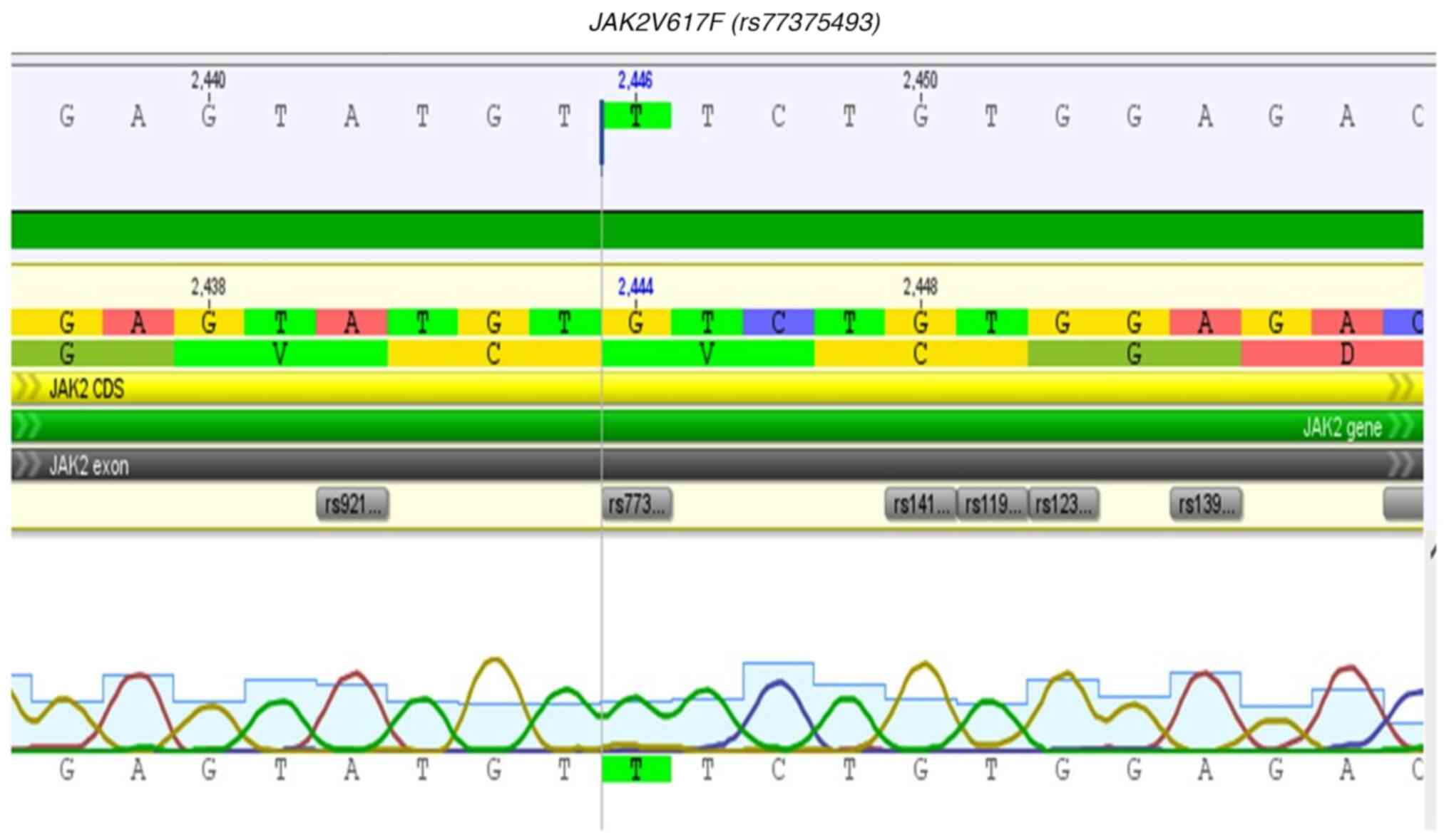
blood (7). JAK2V617F (dbSNP:
rs77375493) is the most commonly identified variant in MPN
and is found in up to 95% of cases of PV and between 50-60% of
cases of ET and PMF (8). This
variant is located in exon 14 of the JAK2 gene and is
characterized as a missense variant. It is a product of the
substitution of a guanine by a thymine at position 1,849, that
leads to a substitution of valine with phenylalanine at the amino
acid position 617 (V617F) of the protein structure (9,10), a
position that belongs to the pseudokinase domain, which is a region
of the primary positive and negative regulation of the protein
(10,11).
Variants in exon 12 of the JAK2 gene are
identified in ~3% of JAK2V617F-negative patients diagnosed
with PV (12). Genetic alterations
in this exon include missense and indel variations (13), which confer a marked erythrocytic
picture in individuals with PV, and appear at younger ages when
compared to the JAK2V617F variant (14).
The presence of coexisting non-driver variants can
modulate the JAK2V617F variant allele frequency (VAF). In
MPN, the determination of JAK2V617F VAF is pivotal when
evaluating laboratory and clinical implications. It is worth
mentioning that, in PV, a high VAF (≥50%) is associated with
fibrotic progression and positively associated with total white
blood cell count (WBC), neutrophil count, and thrombosis events,
especially in the presence of coexisting non-driver variants
(15), while in ET, a high VAF is
correlated with increased thrombo-hemorrhagic events,
hypercoagulable status, and low quantitation of hemostasis factors
(16,17).
Sanger sequencing and next-generation sequencing
have allowed the identification of variants in other JAK2
exons (18,19). Several variants have been identified
in the complete coding region of the JAK2 gene, which affect
other domains of the JAK2 protein (19,20)
and lead to constitutive activation of the JAK/STAT pathway, with
most of the described variants being somatic, with only a small
fraction of them being germinal. This finding suggests that certain
patients may develop a non-clonal myeloproliferative phenotype,
with variable penetrance at the familial level (21).
Certain variants that are acquired in the coding
region of JAK2 are described as benign or of uncertain
clinical significance, and the primarily affected exons are
6(22), 9-10(23), 11-15(19), and 19(24). According to certain studies, some
variants in these regions have been found in coexistence,
presenting cytokine-independent signaling (25), and are even associated with leukemic
transformation and development of non-hematological solid tumors
(23,24,26).
Thus, the present study aimed to molecularly characterize variants
in the complete coding region of the JAK2 gene in
individuals with BCR::ABL1 negative chronic
myeloproliferative neoplasms.
Materials and methods
Patients
In the present study, 97 patients from the state of
Amazonas, Brazil, diagnosed with PV (n=38), ET (n=55) and MF
(n=04), who were treated between July 2021 and March 2023 at
Hospital Foundation for Hematology and Hemotherapy of Amazonas
(which is the only reference institution in the state of Amazonas
for the diagnosis and treatment of hematological diseases) were
included. Participants showed an absence of BCR::ABL1
transcripts. Additionally, all the patients with a MF diagnosis who
agreed to participate in the investigation were included.
The prese study was performed in accordance with the
Declaration of Helsinki and Resolution 466/12 of the Brazilian
Ministry of Health. This study was approved by the National Ethics
Committee, which is responsible for approving relevant human
studies in Brazil (approval no. 4.450.813). Written informed
consent was obtained from all subjects involved in the study.
Clinical and laboratory data
Clinical data were obtained from medical records,
which included data regarding sex, age, splenomegaly, history of
thrombotic or hemorrhagic events, and treatments administered.
Laboratory data were obtained from blood samples and included red
blood cell count (RBC), hematocrit (Ht), hemoglobin (Hb), mean
corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), WBC,
percentage of segmented neutrophils, monocytes and lymphocytes;
Platelet count, prothrombin time-International Normalized Ratio
(PT-INR), activated partial thromboplastin time (aPTT), fibrinogen
(FIB), lactate dehydrogenase (LDH) and uric acid (UA). UA and LDH
analyses were performed after diagnosis and during treatment,
mentioning that several patients included in the study had received
several years of hydroxyurea administration. The median optimal
treatment regime in PV patients was 4 years (100-500 mg/per day of
hydroxyurea or 2 mg/per day of Anagrelide), in ET patients it was
10.5 years (100-300 mg/per day of hydroxyurea or 2 mg/per day of
Anagrelide), and in MF patients it was 2 years (2 mg/per day of
Anagrelide). Of note, administration of hydroxyurea can
significantly alter laboratory analysis.
Blood-sample processing and RNA
extraction
Total RNA was extracted from peripheral blood
samples with EDTA anticoagulant using TRIzol® (Ambion;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. cDNA was synthesized using SuperScript™ III
Reverse Transcriptase (Promega Corporation). Reverse transcription
was used to obtain cDNA, using the following thermocycling
parameters: 5 min at 25˚C and 60 min at 42˚C. After the reaction,
the cDNA was stored at -80˚C until used for PCR.
PCR and Sanger sequencing
analysis
Amplifications were performed using a total volume
of 25 µl. Reaction products were visualized using electrophoresis
on a 1.5% agarose gel stained with ethidium bromide. PCR products
were purified with the DNA precipitate and purification protocol
using polyethylene glycol 8000 (Promega Corporation) as described
previously (27-29).
A Sanger sequencing reaction (in both directions) was performed
using BigDye® Terminator v3.1 (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
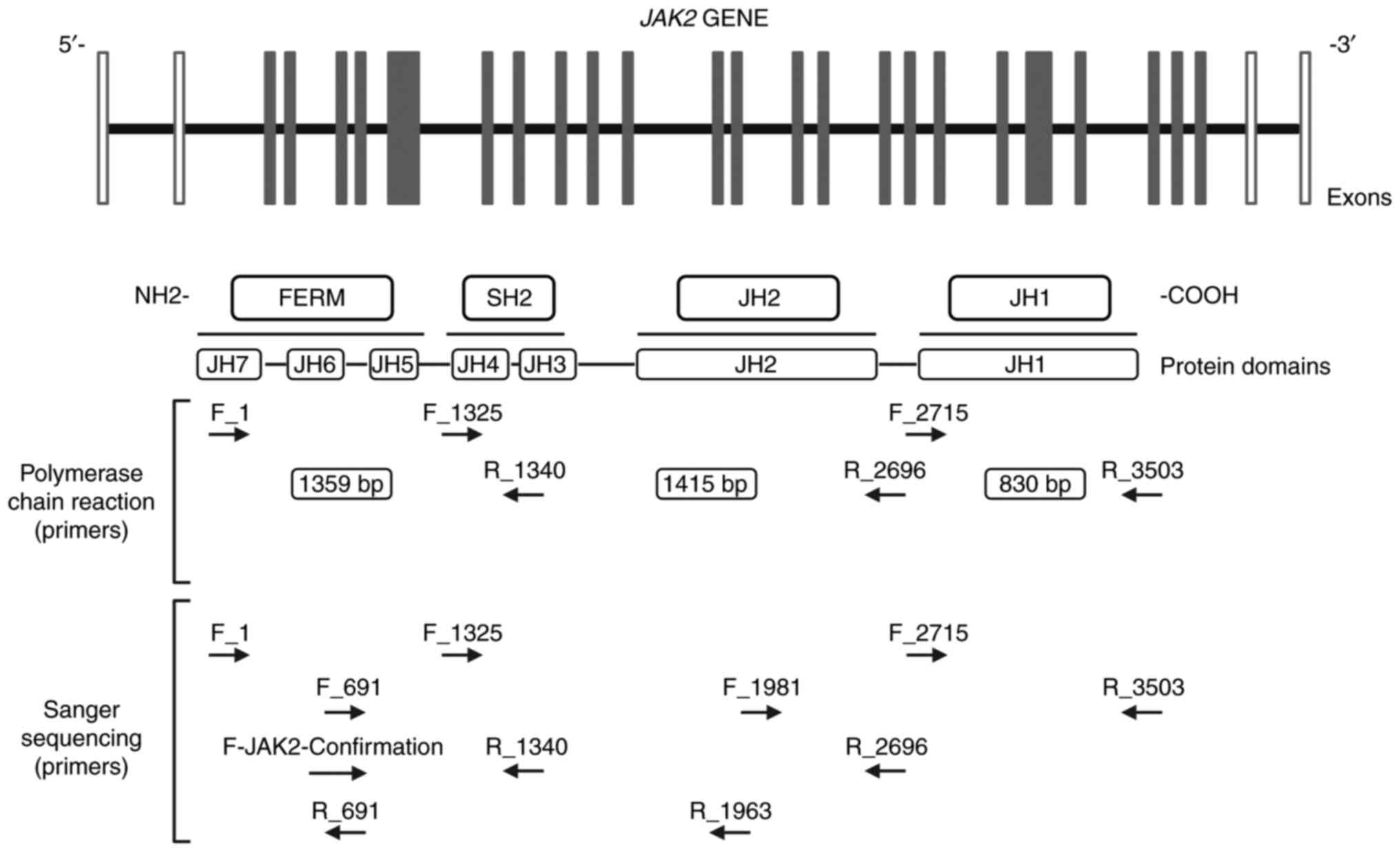
protocol. The sequences of the primers used are listed in Table I, and were designed using
Primer-BLAST-NCBI and OligoAnalyzer Tool-IDTDNA to evaluate the
percentage of GC, Tm, Hairpin capacity, and ΔG index, to flank the
complete coding region of JAK2, spanning from exon 3 to exon
25 (Fig. 1). The products of the
sequencing reaction were purified using the EDTA/ethanol protocol
and were subsequently evaluated in an automatic sequencer (3500 XL
Genetic Analyzer®, Applied Biosystems handbook; Thermo
Fisher Scientific, Inc., pag. 12) using the POP-7 polymer.
 | Table ISequences of the primers used for PCR
and Sanger sequencing. |
Table I
Sequences of the primers used for PCR
and Sanger sequencing.
| Primer name | Sequence
(5'à3') | Annealing
temperature |
|---|
| JAK2_Fow_1 |
GGCAACAGGAACAAGATGTGAA | 69˚C |
| JAK2_Rev_691 |
AGCTGATAGAGTTATAGATGGC | 64˚C |
| JAK2_Fow_691 |
AAACGATCAAACCCCACTGG | 68˚C |
| JAK2_Fow_1325 |
CCCAATTTCGATGGATTTTGCCA | 69˚C |
| JAK2_Rev_1340 |
TCCAGTCTGATTACCTGCTT | 65˚C |
| JAK2_Fow_1981 |
ATTCTGGTTCAGGAGTTTG | 62˚C |
| JAK2_Rev_1963 |
CAAACTCCTGAACCAGAAT | 62˚C |
| JAK2_Fow_2715 |
GGTATGACCCTCTACAGGAC | 66˚C |
| JAK2_Rev_2696 |
GTCCTGTAGAGGGTCATACC | 65˚C |
| JAK_Rev1_3503 |
TTGGTCTCAGAATGAAGGTC | 64˚C |
|
Fow-JAK2-Confirmation |
AGTGGTCCTTCAGGTGAGGAG | 56˚C |
Data analysis
The sequences obtained were initially analyzed using
the Sequencing Analysis software (Applied Biosystems; Thermo Fisher
Scientific, Inc.); only high-quality sequences were used for
variant analysis (Q score ≥30). Geneious software 6.0.6
(Biomatters, Inc.) was used to obtain contigs and compare them to
the Homosapien JAK2 reference sequence, transcript 2, mRNA
(NCBI: NM_001322194.2). Samples with the presence of rare variants
were sequenced and confirmed at least twice. VAF was measured in
JAK2V617F-positive individuals using Minor Variant Finder
(Applied Biosystems, Thermo Fisher Scientific, Inc.) and Edit R
software (moriaritylab.shinyapps.io/editr_v10). The clinical
significance of the variants identified in the research was
analyzed using the Polyphen2 tool and the ClinVar-NCBI site
(https://www.ncbi.nlm.nih.gov/clinvar/).
Statistical analysis
Categorical variables are presented as the frequency
(n, %). Continuous numeric variables are presented as the median
and interquartile range (IQR). The distribution of continuous
numerical variables was verified using a Shapiro-Wilk test.
Statistical analysis of categorical variables was performed using
a00202 test. Kruskal-Wallis and Mann-Whitney U tests
were used to analyze numerical variables, when appropriate. Data
from individuals with MF were excluded from the statistical
analysis between groups due to the number of patients with MF.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis of the data was performed using
GraphPad Prism version 8.2.1 (GraphPad Software, Inc.).
Results
Clinical and laboratory
characteristics of patients
Samples from 97 patients diagnosed with MPN were
evaluated, and these were distributed among PV (n=38), ET (n=55),
and MF (n=04). During the length of the study, none of the patients
showed transformation to acute leukemia, post-PV, or post-ET-MF.
Clinically, ET showed a predominance in females (P=0.0276),
compared with PV and MF. All individuals were between the fifth and
sixth decade of life (P=0.565; comparing the age between the PV and
TE groups). Splenomegaly was detected more frequently in MF, than
in PV and ET (75, 23.6, and 16.3%, P=0.0212, respectively)
patients.
Thrombotic and hemorrhagic events were more often
observed in ET cases (16.3 and 21.8%, P=0.6406 and P=0.0205,
respectively) when compared to PV cases. The thrombotic events
included deep venous thrombosis, thrombosis of the splenic vein,
esophageal varices, and miscarriage, and the following hemorrhagic
events were evaluated in the study: Hypermenorrhagia, ocular and
gingival hemorrhage, and hemorrhage of the gastrointestinal tract.
All medical records of the patients included in this study were
reviewed and none of these reported acquired von Willebrand
syndrome.
In the blood count, an increase in the erythrocyte
lineage was observed in individuals with PV compared to those with
ET and MF, with an increased RBC (5.03 x mm3,
P<0.0001), a finding that is complemented by Ht values (48%,
P<0.0001) and Hb concentration (15.2 g/dl, P<0.0001).
Hemometric values were found to be increased in ET cases [Mean
Corpuscular Volume, MCV: 103.9 fl; P=0.0013; Mean Corpuscular
Hemoglobin, MCH: 33.5 pg, P=0.006, and Mean Corpuscular Hemoglobin
Concentration (MCHC): 32.5 g/dl, P=0.1160] when compared to PV and
MF cases. The white blood cell count was within normal ranges in PV
and TE cases, compared with those with MF (P=0.0134). However, the
percentage of neutrophils was higher in MF patients (76.4%) when
compared to ET and PV patients (P=0.0232), and the lymphocyte count
was slightly higher in ET than in PV and MF patients (29.2%,
P=0.0005). In ET patients, a high platelet count was observed when
compared to PV and MF patients (470,500 x mm3,
P<0.0001). Erythropoietin measurements were not available in the
present study.
Values in the hemostasis tests of individuals with
PV, ET, and MF were closely related; however, a slight increase in
fibrinogen concentrations was observed in individuals with MF (321
mg/dl, P=0.400). Biochemical analyses demonstrated higher
concentrations of LDH and UA in subjects with MF (904.5 U/l,
P=0.0295 and 6.8 mg/dl, P=0.006; respectively) compared with PV and
ET patients. Clinical and laboratory values are described in
Table II.
 | Table IIDemographic, clinical, and laboratory
characteristics of patients. |
Table II
Demographic, clinical, and laboratory
characteristics of patients.
| Characteristic | PV, n=38 | ET, n=55 | MF, n=4 | P-value | Reference
values |
|---|
| Male/Female, n | 18/20 | 12/43 | 2/2 | 0.0276a | |
| Age, median
(IQR) | 60.5
(48.75-70.25) | 57 (42-72) | 62 (54.2-75.7) | 0.565 | |
| RBC, x
mm3, median (IQR) | 5.03 (4.3-6.2) | 3.75 (3.2-4.5) | 4.3 (3.4-6.1) |
<0.0001d |
3.9-5.3x103/mm3 |
| Ht, %, median
(IQR) | 48 (43.4-52.2) | 37.9
(34.6-42.2) | 37.05
(33.5-48.5) |
<0.0001d | 36-48% |
| Hb, g/dl, median
(IQR) | 15.2
(13.7-16.2) | 12.7
(11.6-13.9) | 11.7
(10.6-15.9) |
<0.0001d | 12-16 g/dl |
| MCV, fL, median
(IQR) | 92.3
(82.6-103.6) | 103.9
(92.3-112.7) | 85.9
(78.6-92.2) | 0.0013b | 80-100 fl |
| MCH, pg, median
(IQR) | 30.3
(27.1-33.2) | 33.5
(30.1-36.7) | 27.6
(24.6-30.3) | 0.0006c | 27-33 pg |
| MCHC, g/dl, median
(IQR) | 31.8
(30.3-33.3) | 32.5 (32-33.6) | 32.1
(30.6-33.4) | 0.1160 | 32-36 g/dl |
| WBC, x
mm3, median (IQR) | 6,540
(5,170-8,060) | 5,370
(4,170-7,200) | 12,930
(5,783-15,678) | 0.0134a |
3,600-11,000x103/mm3 |
| Neutrophils, %,
median (IQR) | 68 (56.7-77.1) | 61.9
(56.1-69.2) | 76.4
(65.7-79.0) | 0.0232a | |
| Lymphocytes, %,
median (IQR) | 21.5
(15.9-29.8) | 29.2
(22.5-35.4) | 11.0
(11.0-17.6) | 0.0005c | |
| Monocytes, %,
median (IQR) | 5 (3.5-7.0) | 4.8 (3.9-6) | 2.4 (1.2-5.2) | 0.169 | |
| Platelets, x
mm3, median (IQR) x103/mm3 | 301,000
(180,000-403,000) | 470,500
(369,000-577,000) | 439,000
(253,250-839,250) |
<0.0001d |
150,000-400,000 |
| LDH, U/l, median
(IQR) | 439.8
(324.7-552.9) | 423.1
(348.5-494.2) | 904.5
(568.1-1210) | 0.0295a | 214-450 U/l (male)
195-453 U/l (female) |
| Uric acid, mg/dl,
median (IQR) | 4.4 (3.4-5.6) | 4.1 (2.9-4.8) | 6.8 (5.8-7.6) | 0.006b | 3.5-7.2 mg/dl
(male) 2.6-6.0 mg/dl (female) |
| PT, sec, median
(IQR) | 11.5
(10.9-12.6) | 11.4
(11.0-12.3) | 13.8
(13.0-14.1) | 0.0360a | 12-14 sec |
| INR, median
(IQR) | 0.99
(0.93-1.08) | 0.98
(0.95-1.06) | 1.18
(1.11-1.21) | 0.0342a | |
| aPTT, sec, median
(IQR) | 31.7
(27.9-36.3) | 30.7
(28.1-33.6) | 37.9
(35.1-42.7) | 0.0336a | 35-40 sec |
| Fibrinogen, mg/dl,
median (IQR) | 278 (228-320) | 291 (220-362) | 321
(218.8-493.8) | 0.400 | 180-350 mg/dl |
| Splenomegaly, n
(%) | 9 (23.6) | 9 (16.3) | 3(75) | 0.0212a | |
| Thrombotic events,
n (%) | 5 (13.1) | 9 (16.3) | 0 | 0.6406 | |
| Bleeding events, n
(%) | 1 (2.6) | 12 (21.8) | 0 | 0.0205a | |
| Treatment with HU,
n (%) | 27(71) | 49 (89.09) | 0 |
<0.0001d | |
| Treatment with
Anagrelide, n (%) | 0 | 5 (9.09) | 1(25) | 0.0565 | |
| Therapy with
phlebotomy, n (%) | 7 (18.4) | 0 | 0 | 0.0029b | |
Variants detected in chronic MPN
patients
In this study, missense variants were identified in
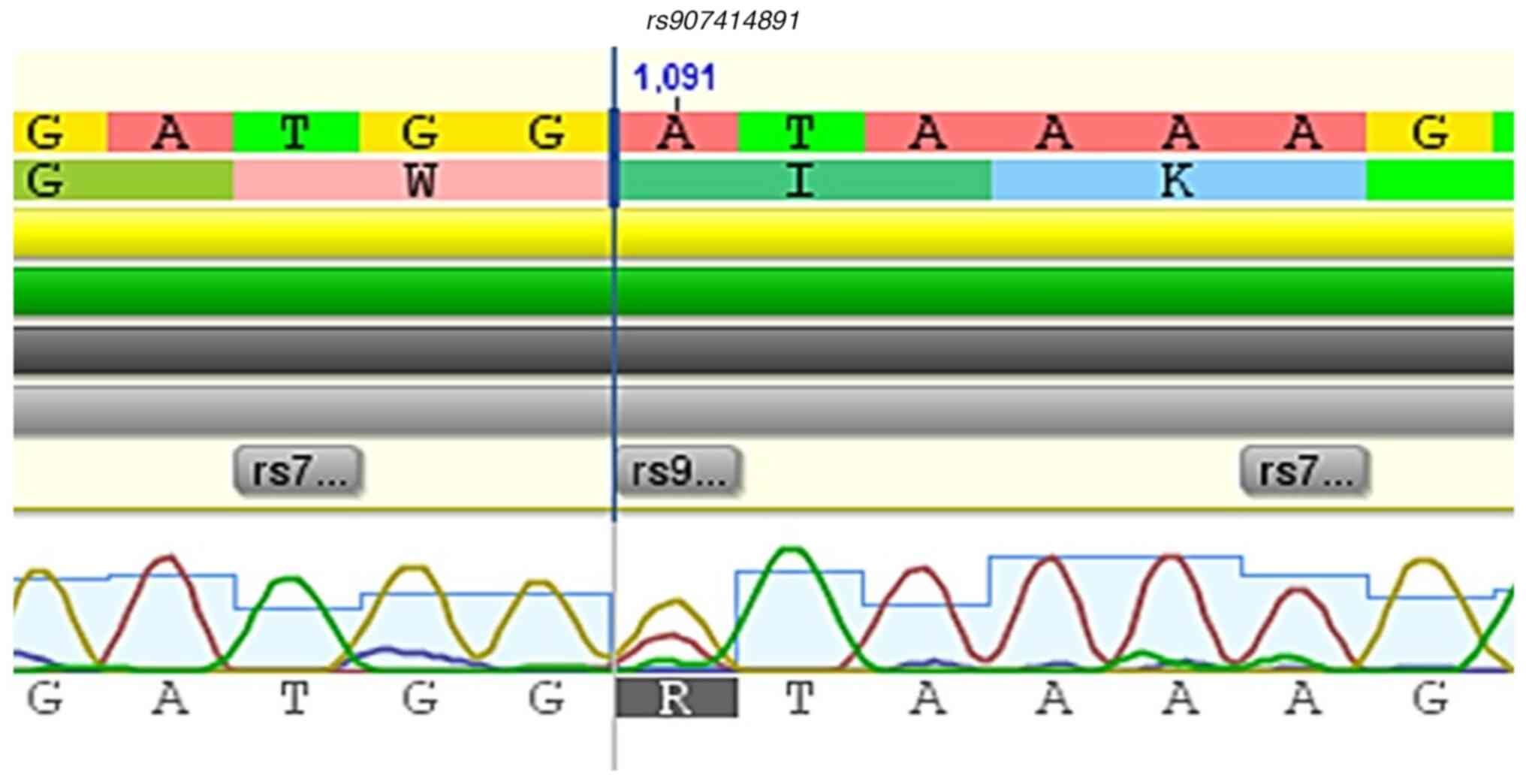
the FERM domain (rs907414891); 1 variant in the FERM-SH2
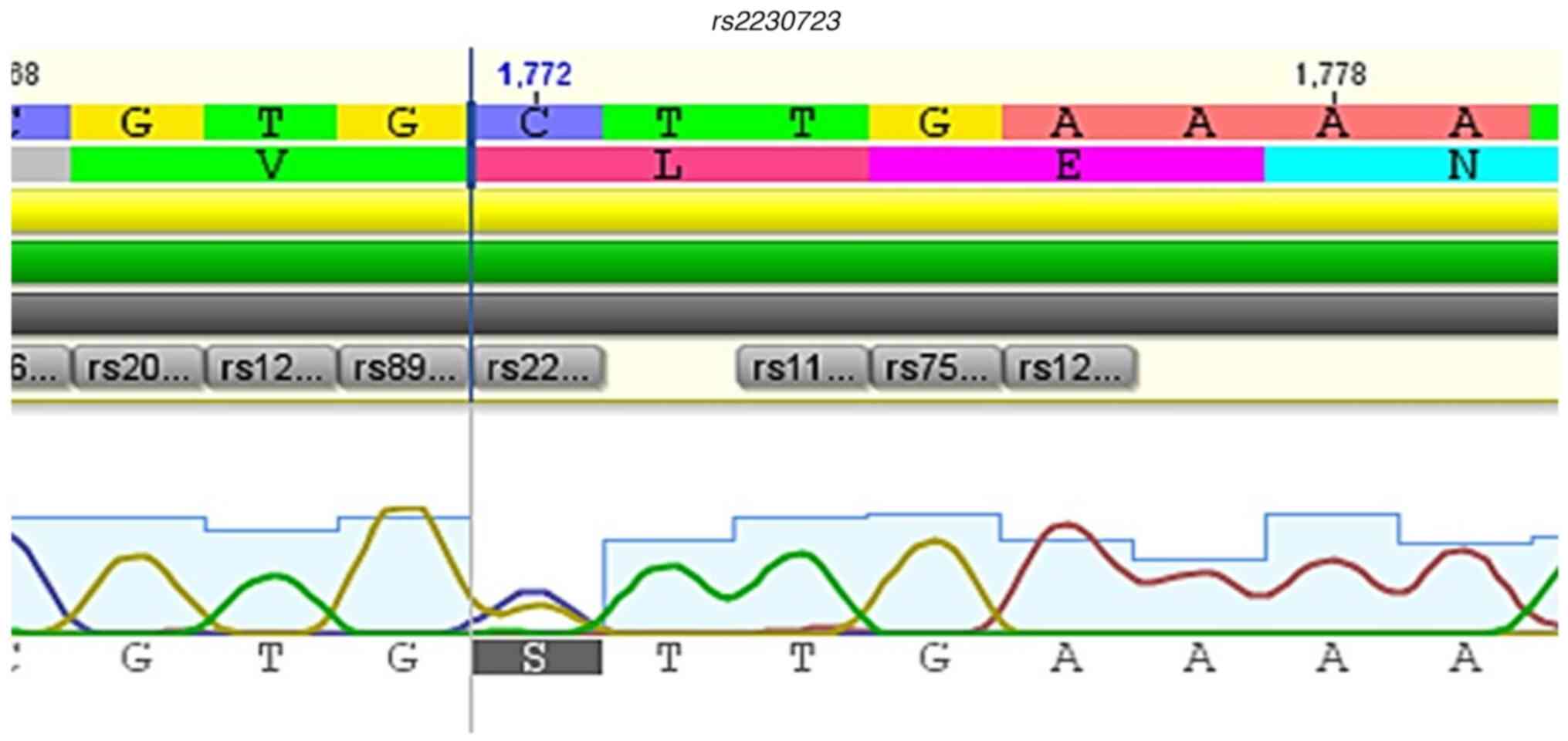
linker region (rs2230723), 1 variant in the pseudokinase
domain (rs77375493), and 1 variant in the kinase domain
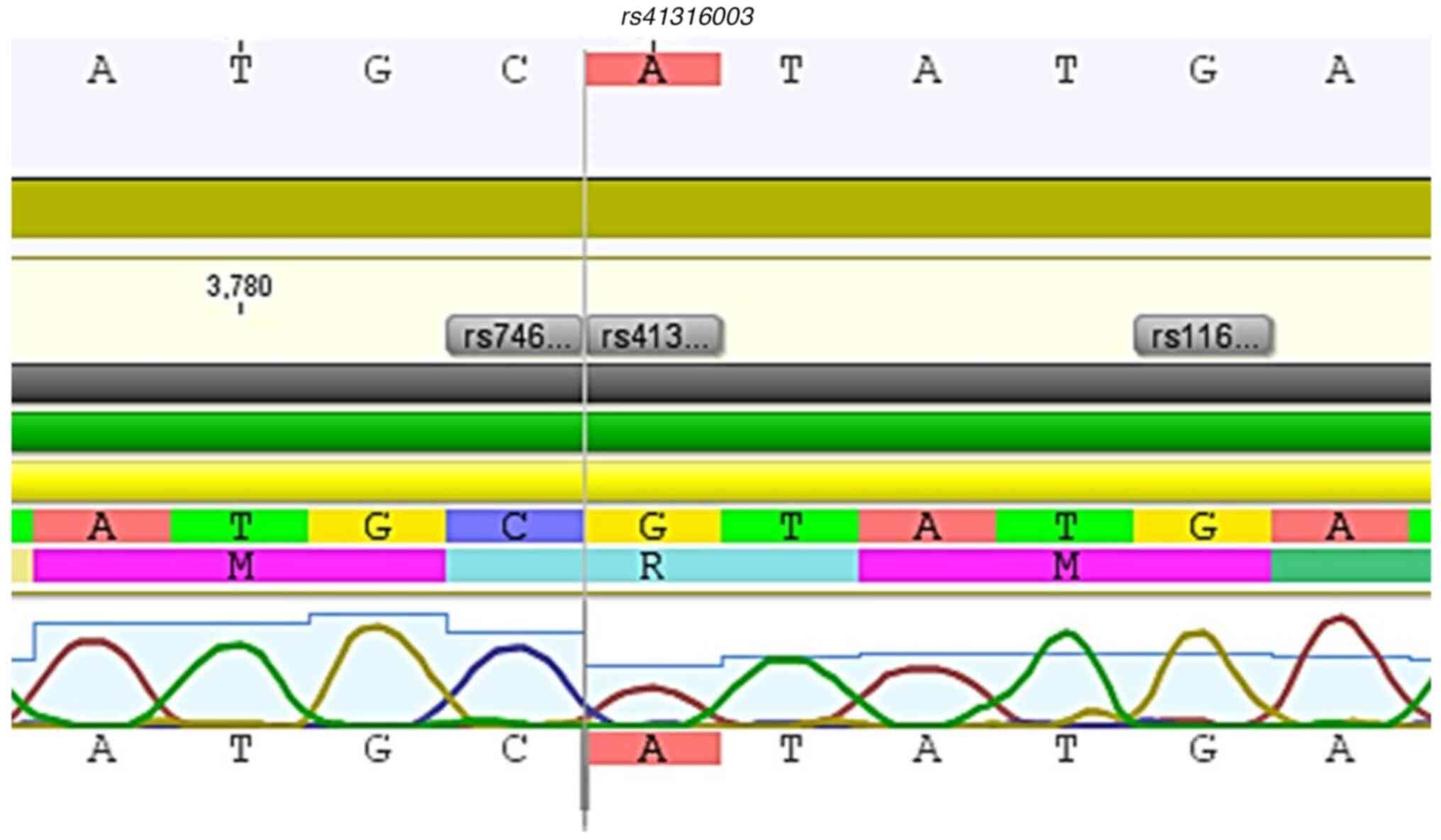
(rs41316003). This totals 4 missense variants identified in
the complete coding region of the JAK2 gene, as described in
Table III. In addition, other
synonyms and benign variants were detected in the complete coding
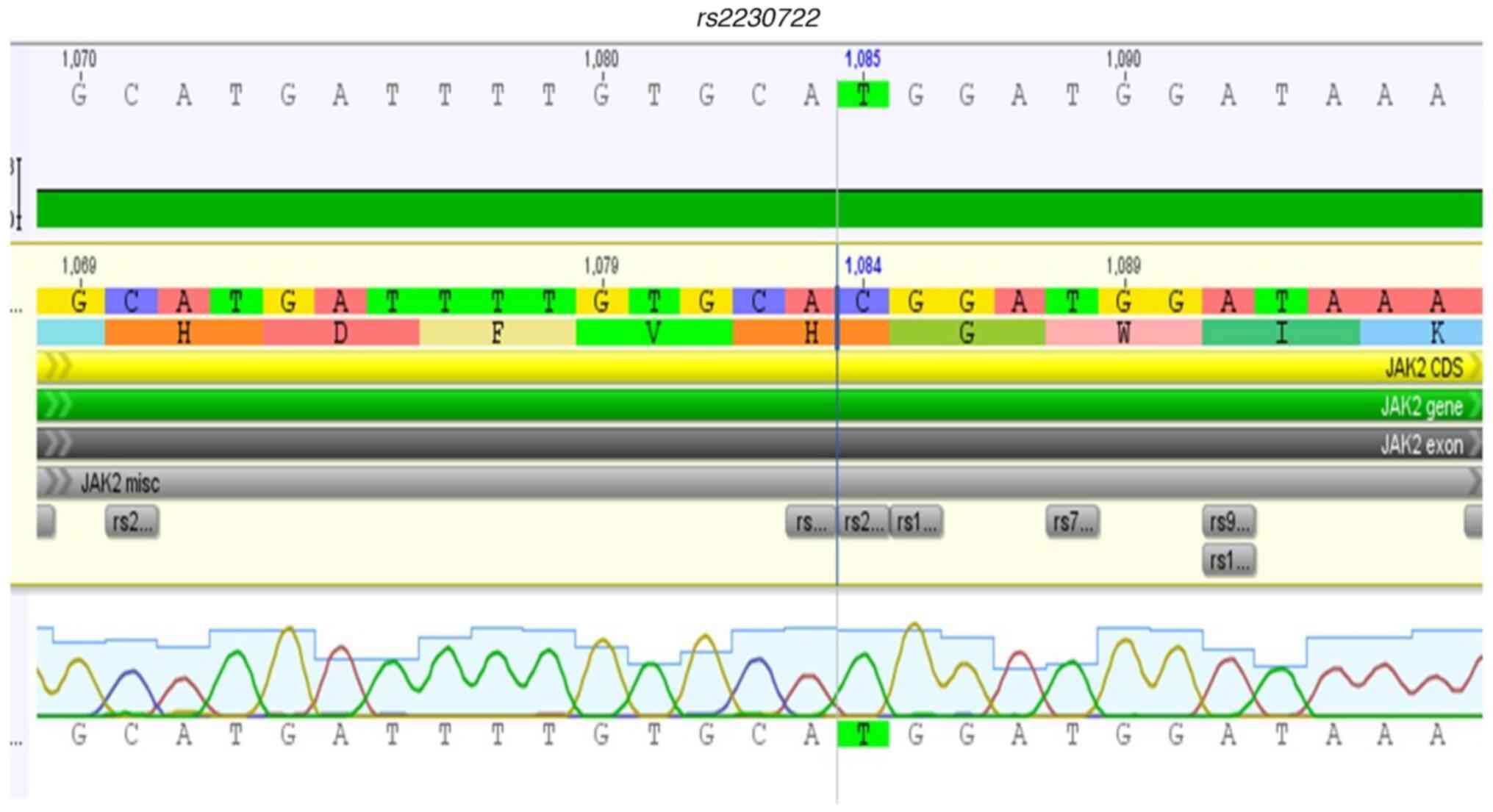
region of the JAK2 gene (rs2230722,
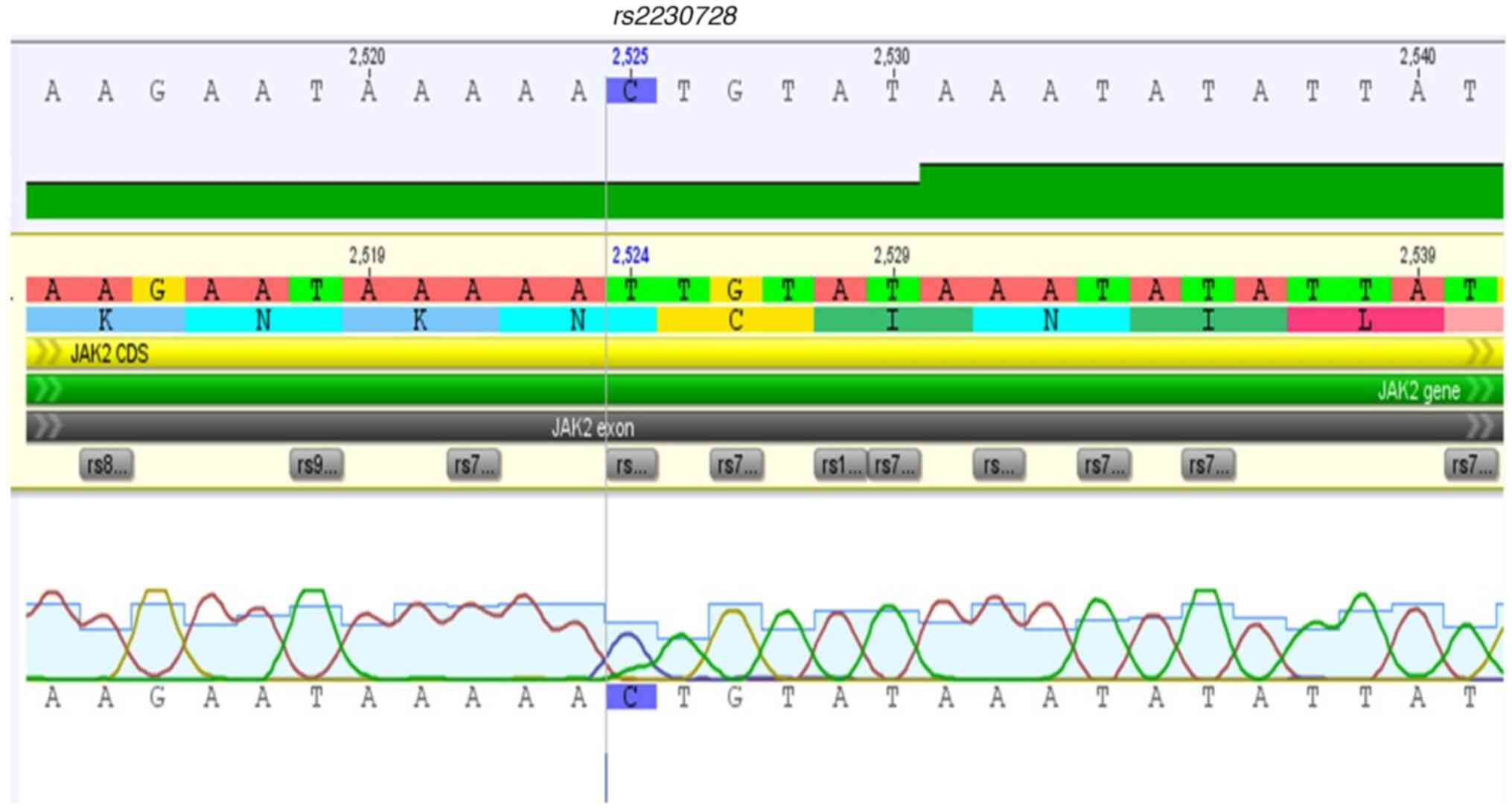
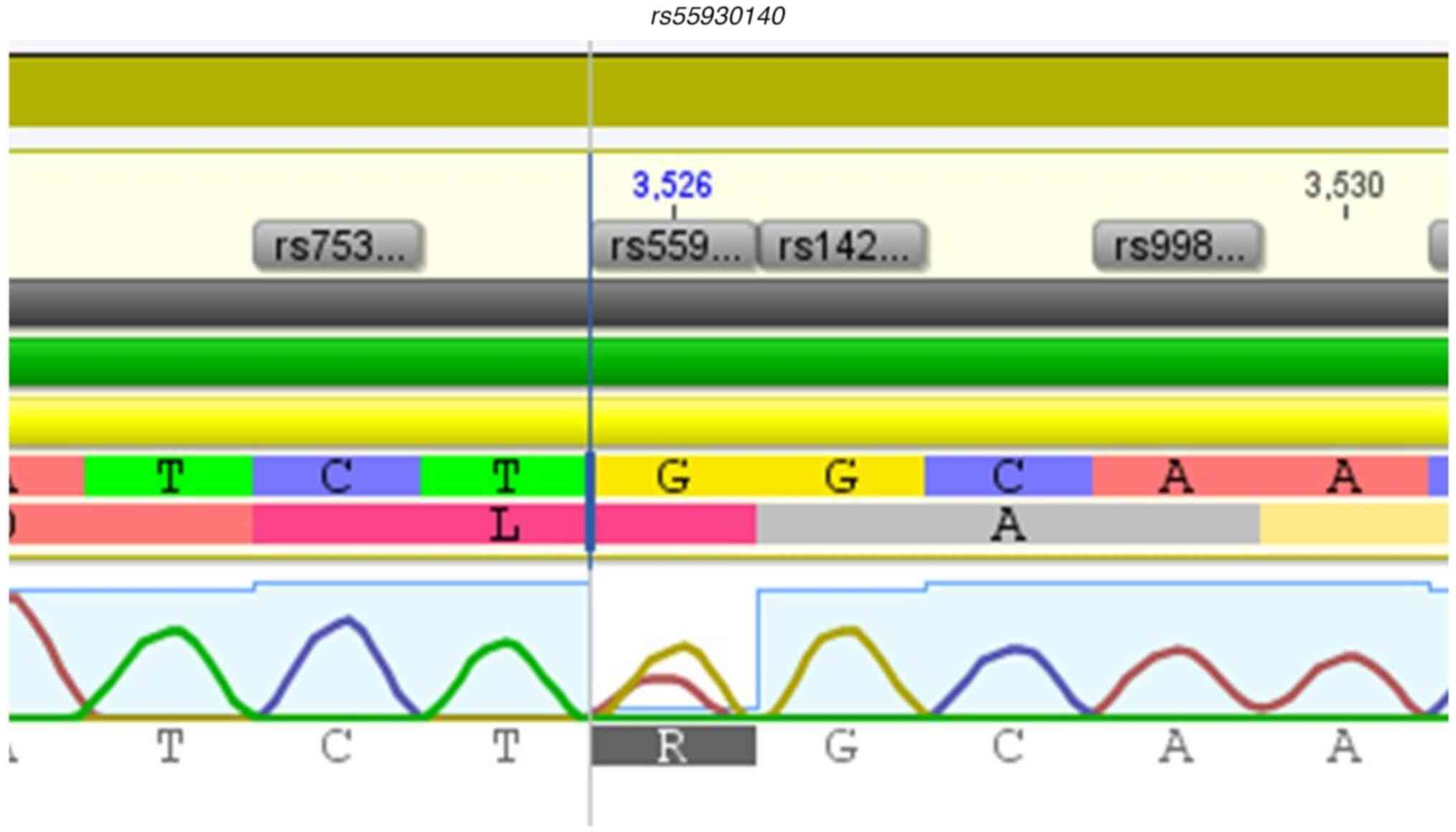
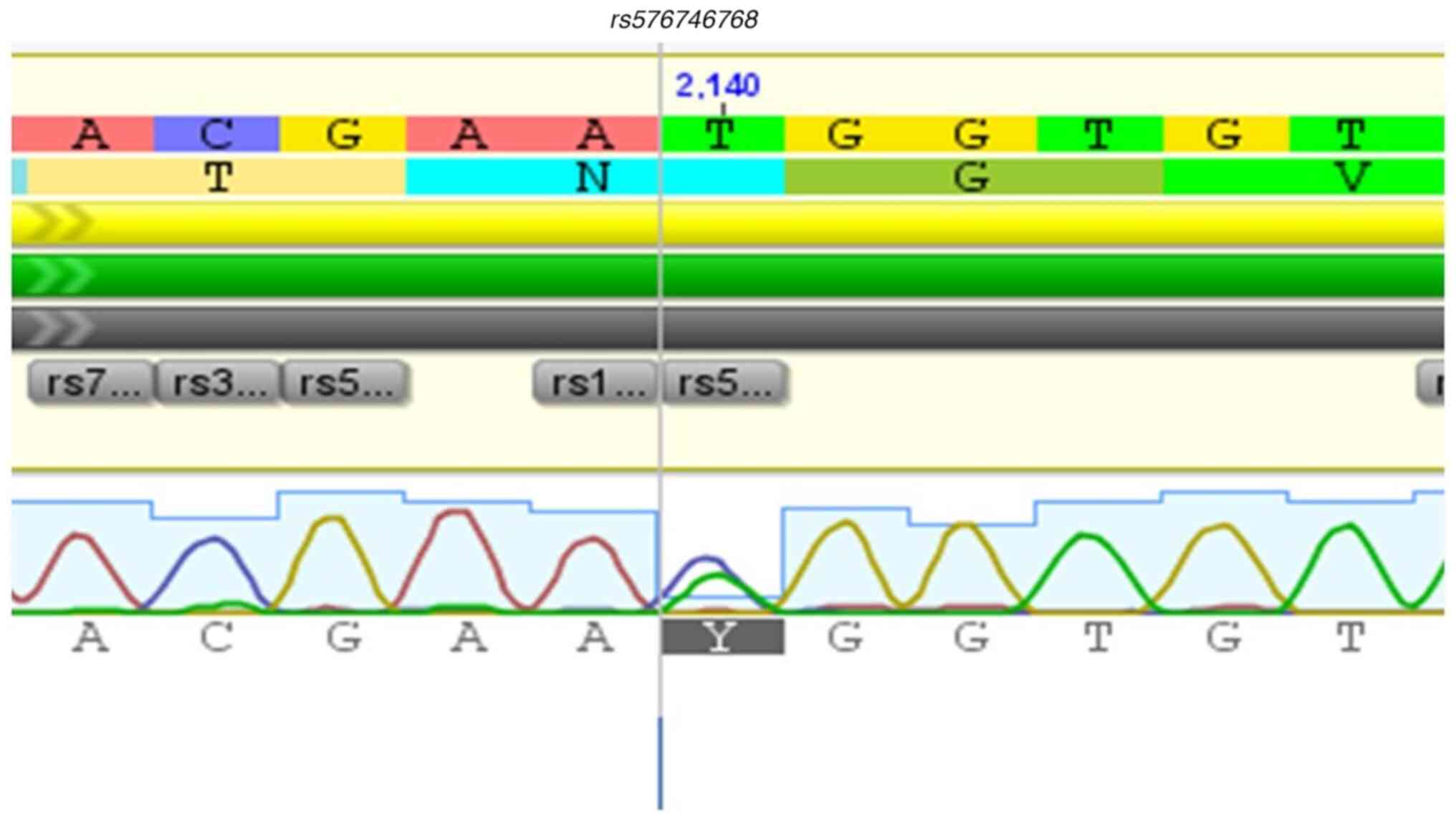
rs576746768, rs2230728, rs2230724, and
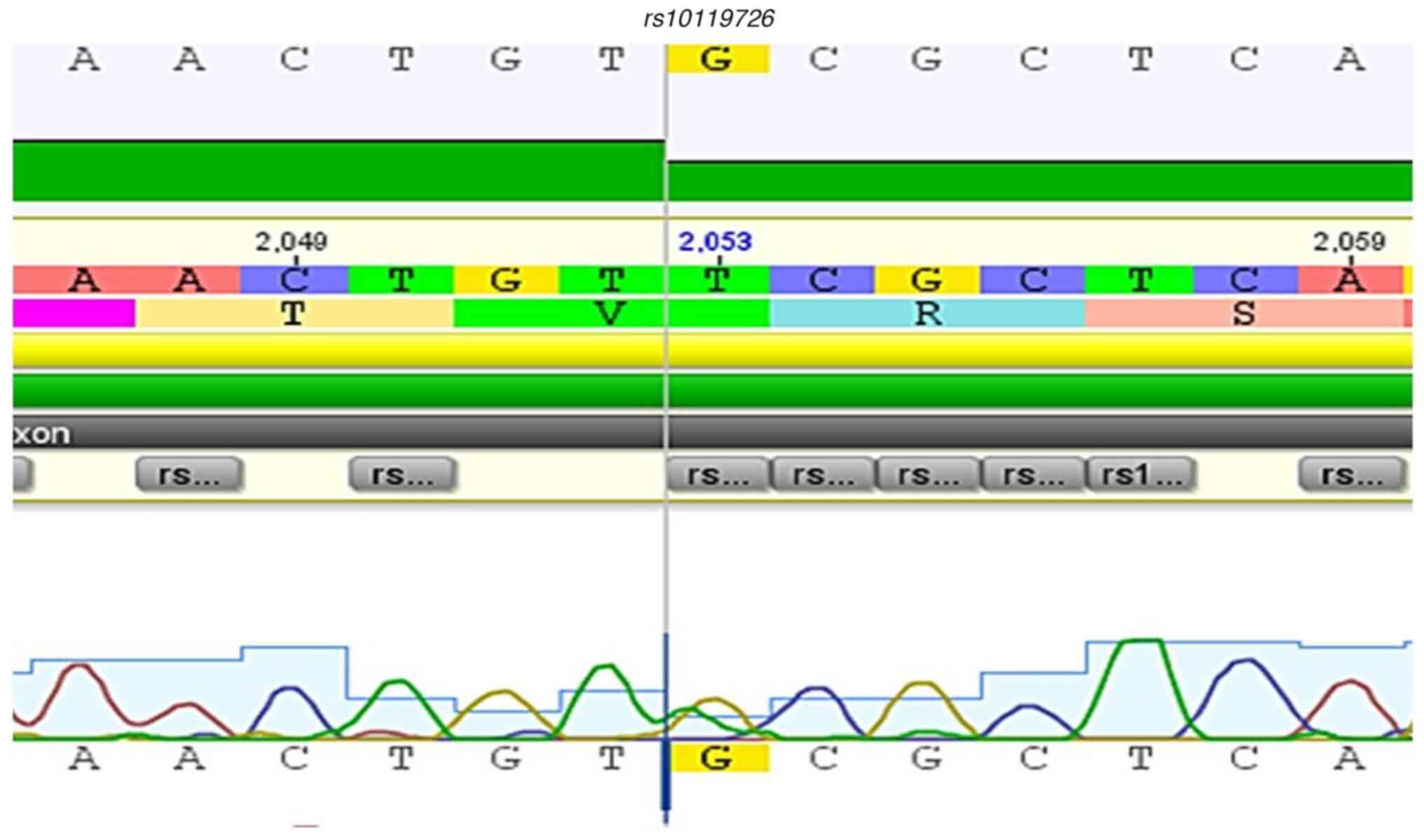
rs55930140). Conversely, the rs10119726 variant is a
synonymous variant and does not have a description of its clinical
significance on ClinVar. These variants are shown in
Fig. 2, Fig. 3, Fig.
4, Fig. 5, Fig. 6, Fig.
7, Fig. 8, Fig. 9, Fig.
10 and Fig. 11.
 | Table IIIMissenses variants detected by Sanger
sequencing in the entire coding region of the JAK2 gene in
patients with myeloproliferative neoplasms. |
Table III
Missenses variants detected by Sanger
sequencing in the entire coding region of the JAK2 gene in
patients with myeloproliferative neoplasms.
| Variant | Allele | Variation in
cDNA | Exon
localization | Variation in the
protein | Affected
domain | Functional
consequence | Clinical
significancea | Type of
variantb |
|---|
|
rs907414891 | A>G | c.496 | 6 | (p.Ile166Val) | FERM | Missense, no
functional evidence registered | No description | Somatic |
|
rs2230723 | C>G | c.1177 | 9 | (p.Leu393Val) | FERM-SH2 | Missense, no
functional evidence registered | Uncertain clinical
significance | Germline |
|
rs77375493 | G>T | c.1849 | 14 | (p.Val617Fen) | Pseudokinase | Missense, no
functional evidence registered | Pathogenic | Somatic |
|
rs41316003 | G>A | c.3188 | 24 | (p.Arg1063His) | Kinase | Missense, no
functional evidence registered | Benign | Germline |
Frequency and distribution of missense
variants in patients with variant alleles of the JAK2 gene
The frequency of variants was estimated in the
population (PV=38, ET=55, and MF=04), and it was noted that most of
them were in the first protein domains, especially in the FERM
domain, followed by the pseudokinase domain. The variant
rs77375493 (JAK2V617F) showed a high frequency in
individuals with PV when compared to those with ET (65.7 and 38.1%,
respectively, P=0.0116). Variant rs2230723 was found in
sporadic cases of PV and ET. Interestingly,
rs907414891 and rs41316003 were found only in cases
of ET, but not in cases of PV or MF. The frequency of missense
variants is presented in Table
IV.
 | Table IVFrequency and distribution of
missenses variants in patients. |
Table IV
Frequency and distribution of
missenses variants in patients.
| Variant | PV, n=38 | ET, n=55 | MF, n=4 | P-value |
|---|
| rs2230723, n
(%) | 1 (2.6) | 2 (3.6) | 0 | >0.9999 |
| rs77375493,
n (%) | 25 (65.7) | 21 (38.1) | 2(50) | 0.0116a |
| rs907414891,
n (%) | 0 | 1 (1.8) | 0 | NA |
| rs41316003,
n (%) | 0 | 1 (1.8) | 0 | NA |
Mutational landscape of the JAK2 gene
in individuals with chronic MPN
After estimating the frequency of the variants in
the complete coding region of the JAK2 gene, the mutational
profile of the individuals was mapped. It was observed that
patients with variant alleles in JAK2 simultaneously presented with
1-3 variants. Among the primary variants found simultaneously in
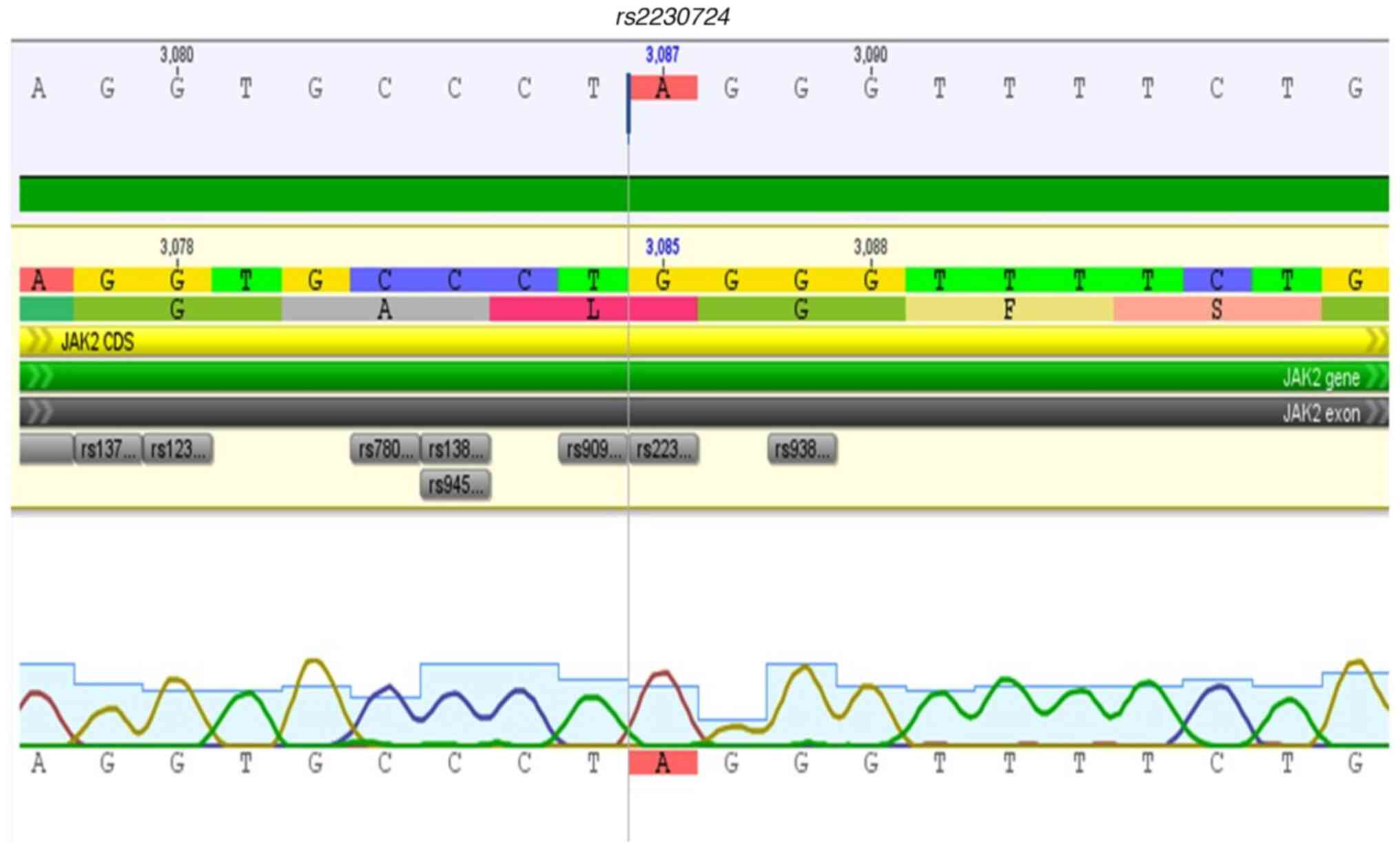
the three types of MPN were rs2230724, rs2230722, and
rs77375493, thus highlighting that most individuals with PV
presented with the three variants when compared to those with ET
(P=0.0023). In contrast, individuals with ET showed a predominance
of two variants (rs2230722 and rs2230724) compared to
those with PV (P=0.0253). The mutational landscape of the patients
is presented in Table V.
Individuals with four variants were not found.
 | Table VFrequency and distribution of
variants in patients. |
Table V
Frequency and distribution of
variants in patients.
| Number of
variants | PV, n=38 | ET, n=55 | MF, n=4 | P-value |
|---|
| 1c, n (%) | 6 (15.7) | 11 (19.6) | 0 | 0.786 |
| 2d, n (%) | 7 (18.4) | 22 (40.7) | 0 | 0.0253a |
| 3e, n (%) | 21 (55.2) | 13 (23.6) | 3 | 0.0023b |
JAK2V617F VAF in patients with PV and
ET
Of the 97 patients included in this study, the
allele burden of JAK2V617F was measured in 46 individuals
who were JAK2V617F-positive (PV, n=25 and ET, n=21).
The allele burden of JAK2V617F was compared in individuals
with PV and ET. In each disease, two groups were considered to
describe the VAF of JAK2V617F: High VAF (≥50%) and low VAF
(<50%). Individuals with ET showed a low VAF JAK2V617F
(<0.0001) when compared to those with PV who showed VAF ≥50%
(0.0477). Individuals with MF were excluded from this comparison.
The comparison of the VAF of JAK2V617F among the groups is
presented in Table VI.
 | Table VIJAK2V617F variant allele
frequency in patients with PV and ET. |
Table VI
JAK2V617F variant allele
frequency in patients with PV and ET.
| Myeloproliferative
neoplasm | VAF <50%, n
(%) | VAF ≥50%, n
(%) | P-value |
|---|
| PV, n=25 | 9 (36%) | 16 (64%) | 0.0477a |
| ET, n=21 | 17 (80.9) | 4(19) |
<0.0001b |
Comparison of the clinical and
laboratory profile according to the VAF of JAK2V617F in patients
with PV
The clinical and laboratory profile of individuals
with PV and ET with JAK2 variants were compared considering
the VAF of JAK2V617F in both groups [high VAF (≥50%) and low
VAF (<50%)]. Regarding the clinical profile in individuals with
PV, thrombotic and hemorrhagic events were evenly distributed among
both groups. However, in the PV patients, splenomegaly was more
frequent in individuals with a high VAF. The clinical data of the
individuals with PV according to VAF of JAK2V617F are
presented in Table VII.
 | Table VIIClinical data in individuals with PV
according to the VAF of JAK2V617F. |
Table VII
Clinical data in individuals with PV
according to the VAF of JAK2V617F.
| | PV, n=25 | ET, n=21 |
|---|
| Parameter | VAF <50% | VAF ≥50% | P-value | VAF <50% | VAF ≥50% | P-value |
|---|
| Thrombotic events,
n (%) | 1 (4.0) | 2 (8.0) | 0.5515 | 2 (9.5) | 5 (23.8) | 0.214 |
| Hemorrhagic events,
n (%) | 1 (4.0) | 0 | 0.3124 | 6 (28.5) | 7 (33.3) | 0.738 |
| Splenomegaly, n
(%) | 0 | 7 (28.0) | 0.0043c | 1 (4.7) | 3 (14.2) | 0.293 |
| RBC, x
mm3, median (IQR) | 4.5 (4.05-5.6) | 4.7 (3.9-5.7) | 0.834 | 3.7 (3.2-4.4) | 5.1 (4.7-6.5) | 0.006b |
| Ht, %, median
(IQR) | 42.6
(40.1-49.1) | 46.9
(44.4-51.0) | 0.2325 | 40 (36.6-43.0) | 47.0
(44.6-54.2) | 0.0022b |
| Hb, g'dl, median
(IQR) | 14.5
(13.1-15.6) | 14.9
(13.6-16.1) | 0.7989 | 13.4
(12.0-13.8) | 15.5
(14.6-16.7) | 0.0023b |
| WBC, x
mm3, median (IQR) | 6,615
(4,748-8,065) | 6,860
(5,673-10,430) | 0.343 | 5,760
(4,645-7,335) | 5,320
(3,968-7,115) | 0.6977 |
| PLT, x
mm3, median (IQR) | 310,500
(190,500-448,000) | 373,500
(255,250-562,750) | 0.3576 | 429,000
(365,500-491,500) | 333,500
(167,250-458,500) | 0.1718 |
| LDH, U/l, median
(IQR) | 394.5
(331.1-623.6) | 486.5
(421.4-564.4) | 0.4523 | 387.1
(316.9-462.6) | 412.1
(386.5-493.7) | 0.517 |
| Uric acid, mg/dl,
median (IQR) | 4.1 (3.4-5.4) | 3.7 (2.7-5.1) | 0.4077 | 3.8 (2.7-4.3) | 4.0 (3.5-4.2) | 0.682 |
| PT (sec), median
(IQR) | 11.5
(10.8-11.7) | 12.0
(11.2-12.8) | 0.2576 | 11.1
(10.6-11.6) | 13.0
(12.1-14.5) | 0.0132a |
| INR, median
(IQR) | 0.98
(0.93-1.0) | 1.03
(0.96-1.10) | 0.2184 | 0.95
(0.91-1.00) | 1.11
(1.04-1.25) | 0.013a |
| aPTT (sec), median
(IQR) | 31.5
(28.2-35.6) | 34.4
(31.5-37.5) | 0.2076 | 28.4
(27.0-33.05) | 38.8
(33.8-40.3) | 0.0057b |
| Fibrinogen, mg/dl,
median (IQR) | 293.0
(209.8-365.0) | 257.5
(225.5-283.0) | 0.4438 | 315.0
(268.5-414.5) | 224.5
(124.5-296.0) | 0.0847 |
The comparison of laboratory profiles in individuals
with PV, according to their VAF of JAK2V617F, showed an
increase in hematimetric values (RBC, 4.7 x mm3; Ht,
46.9%; Hb, 14.9) in individuals who presented a VAF of
JAK2V617F ≥50%, compared with those with a VAF of <50%.
WBC and platelet count were slightly augmented in individuals with
a VAF of ≥50%. Likewise, LDH was elevated in individuals with a VAF
of JAK2V617F of ≥50% (486.5 U/l). Hemostasis tests were
relatively equivalent between both groups in PV patients. The
laboratory profiles of the individuals with PV, according to the
VAF of JAK2V617F, are presented in Table VII.
Comparison of the clinical and
laboratory profiles according to the VAF of JAK2V617F in patients
with ET
In the individuals with ET, the clinical and
laboratory profiles were also described based on the VAF of
JAK2V617F. Regarding the clinical characteristics in
individuals with ET, thrombo-hemorrhagic episodes were the most
commonly recorded clinical events in the patients, especially in
those with VAF of JAK2V617F of ≥50%; however, despite this
fact, it was not statically significant. Just as in the PV
individuals, splenomegaly was more frequent in individuals with a
high VAF. The clinical data of the individuals with ET according to
the VAF of JAK2V617F are presented in Table VII.
The laboratory profiles of individuals with ET,
according to the VAF of JAK2V617F, showed an increase in
hematimetric values (RBC, 5.1 x mm3; Ht, 47.0%; and Hb,
15.5 g/dl) in individuals who presented a VAF of JAK2V617F
of ≥50% when compared to those with a VAF of <50%. The WBC
showed equivalence in both groups. Interestingly, the platelet
count was increased in individuals with a VAF of <50%. Likewise,
for individuals with ET, LDH was elevated in individuals with a VAF
of JAK2V617F of ≥50% (412.1 U/l). Hemostasis was slightly
prolonged in individuals with a VAF of JAK2V617F of ≥50%.
The laboratory profiles of individuals with ET, according to VAF
JAK2V617F, are presented in Table VII.
Discussion
MPNs are generally characterized by an increase in
cell counts in the blood, which can lead to clonal evolution and
disease progression. Despite investigations in other Brazilian
states (30-32),
this study is the first to address JAK2V617F mutation
detection and the hematologic profile according to JAK2V617F
VAF in patients from the state of Amazonas diagnosed with MPN.
Regarding the proportion of MF patients, which is a
multifactorial issue, previous studies in Brazil have shown a lower
proportion of MF patients compared with PV and ET (30-32),
and it is noteworthy that MF is the most aggressive MPN, and shows
a high ratio of leukemic transformation. Silva et al
(32) determined the prevalence of
JAK2V617F in MPN in Pernambuco, Brazil, and found that few
patients had MF diagnosis compared with those with PV and ET.
Similarly, Macedo et al (30) investigated the association between
the JAK2 46/1 haplotype and acquisition of JAK2V617F.
They observed the lowest number of cases of MF. Furthermore, they
concluded that the JAK2 46/1 haplotype was present in
JAK2V617F positive individuals and associated with MPN
phenotype in Brazilian patients. Likewise, in another study, Macedo
et al (31) assessed the
association of TNF polymorphisms with JAK2V617F MPN in
Brazilian patients finding a low number cases of MF.
The present study showed that the increase in the
erythrocyte lineage was in fact a characteristic of individuals
with PV and that the increase in the platelet count was an
indicator that is suggestive of ET, according to the indicators
established by the WHO (1). RBC
counts are directly related to Hb and Ht concentrations; it is
hypothesized that these two hematological parameters are reliable
indices for the diagnosis of PV (33).
Currently, erythropoietin measurement is considered
a major diagnostic criterion for PV diagnosis (1,34). In
the present study, these measurements were not available; however,
MCV is considered a marker that can be used to differentiate
between PV and ET (33). In the
present study, MCV was found to be lower in patients with PV than
in those with ET. This finding may explain the iron deficiency and
the accelerated time for renewal of red blood cells in these
patients (33,35).
The role of the lymphocyte count in MPN is not well
described. Stefaniuk et al (36) found that there was little evidence
for the prognostic significance of the neutrophil-lymphocyte ratio
and lymphocyte-monocyte ratio in MPN, but they both may be higher
in patients with PMF compared to healthy individuals, and may be
associated with chronic inflammation and tumorigenesis. Likewise,
Mulas et al (37) described
that high a neutrophil-lymphocyte ratio had been reported in
JAK2-positive patients and this parameter could be used as an
indicator of chronic inflammation in MPN.
In addition, Vannucchi et al (38) reported that individuals with MPN
have an increased risk of developing lymphoproliferative neoplasms,
particularly in those that were JAK2V617F-positive.
Similarly, Garcia-Gisbert et al (39) found that certain patients with a
diagnosis of MPN showed CD3+ JAK2V617F-positive lymphocytes,
These findings may support the hypothesis that
JAK2V617F-positive lymphocytes may be related to leukemic
transformation.
Furthermore, it has been highlighted that MPN is
associated with a high risk of thrombotic and thromboembolic events
when compared with the general population, and is also associated
with increased hematopoietic counts (40), which was also observed in the
present study. This fact may be explained by the presence of a high
VAF of JAK2V617F (≥50%), which likely stimulates
deregulation signaling in hematopoietic progenitor cells and may be
potentialized by the presence of other variants in genes such as
CALR and MPL; these are directly implicated in
platelet activation and increased platelet account (40).
Administration of hydroxyurea is frequently used in
cases of PV and ET for the normalization of hematological counts
(41,42). The results of the present study
showed that the high platelet count observed in individuals with ET
was directly related to the increase in the frequency of
thrombo-hemorrhagic events, which indicates that platelets could in
fact be the primary mediators of thrombotic activation in these
patients. As such, the study by Buxhofer-Ausch et al
(43) demonstrated that platelet
count normalization is an important factor in reducing thrombotic
risk, regardless of the leukocyte count. However, further studies
are needed to confirm what the cut-off point in the platelet count
is to trigger these risks.
Esophageal and gastric complications are often
described in patients with myeloproliferative neoplasms diagnosis
(44), and this is typically due to
portal system hypertension or von Willebrand syndrome, which is the
result of excessive thrombocytosis. However, in the present study,
bleeding complications were relatively high, especially in patients
with ET. This fact may be due to an increased platelet count with
functional platelet disorders, such as impaired platelet
aggregation response to collagen and reduced number of dense
granules in platelets (45). In
addition, current literature notes that ET is more common in
females, and bleeding and thrombotic risks are the major
complications in MPN patients (40,46).
Nevertheless, female biology may play a role in the development of
bleeding and thrombotic events, likely due to pregnancy and the use
of contraceptives interfering with the interactions of platelets
and other molecules in the endothelium.
Other variants in the JAK2 gene have been
reported, and most of these variants are of the somatic type
(21,22). The existence of germline variants in
MPN has also been described, and this includes showing patterns of
erythropoietin (EPO) hypersensitivity and weak constitutive
signaling of the JAK2/STAT5 pathway compared to JAK2V617F
(47).
Therefore, by applying Sanger sequencing in the
complete coding region of the JAK2 gene, the results of the
present study demonstrated the existence of somatic and germline
variants in individuals with MPN other than JAK2V617F, with
somatic variants being the most frequent. This is also corroborated
by previous studies (19,48,49).
Moreover, germline variants in individuals with MPN at an early age
in individuals with a familial predisposition, compared with those
with somatic variants, have been described (50). Age differences between patients with
somatic and germline mutations were not investigated in the present
study, and this will form a future research direction.
JAK2V617F is the most common variant in
BCR::ABL1 negative MPN (51), with constitutive activity of the
JAK2/STAT5/STAT3 pathway, and it is highly associated with the
development of cardiovascular and thrombotic complications
(15). In the present study,
JAK2V617F was identified in 65.7% of the patients with a
diagnosis of PV. This may be related to the median optimal
treatment regimes, as these individuals have been treated with
cytoreductive therapy for several years.
The effects of JAK2 VAF are well
established; however, the specific populations affected are poorly
understood. Through the comparison of JAK2V617F VAF, it was
shown that patients from the state of Amazonas with PV had a
JAK2V617F VAF that was higher than those diagnosed with ET,
and individuals with a VAF of ≥50% had more thrombo-hemorrhagic
events and a slight prolongation in coagulation tests, especially
in PT-INR and aPTT when compared with those with a VAF of <50%,
which is that not dissimilar to previous studies (40,46).
This fact directly suggests that individuals with a high
JAK2V617F VAF exhibit increased intracellular signaling,
cellular activation, and possible alterations in coagulation
factors, thus contributing to the deregulation of hemostasis.
Furthermore, the results of the present study are
in agreement with the results of Hu et al (16) who demonstrated that individuals with
PV had a high JAK2V617F VAF (≥50%) compared with those with
ET. In addition, the results of the present study demonstrated that
patients from the state of Amazonas with a diagnosis of PV had a
mutational landscape that was more complex than that of individuals
with ET from the same state. This landscape showed at least three
mutations in concomitance in the JAK2 gene, suggesting
genomic instability and, subsequently, the instability of
regulatory mechanisms at the protein level and possibly in the
myeloproliferative phenotype of individuals with MPN.
According to data available on the ClinVar-NCBI
website, a number of the acquired variants located in the extension
of the JAK2 coding region are either benign or of uncertain
clinical significance. This indicates that most of the variants
reported to date are in the FERM domains, kinase, and binding
regions (19,22-24),
and this finding relates to the present study, since the detected
variants are located in the aforementioned regions.
Thus, it is highlighted that the presence of
variants in the FERM domain may result in increased basal activity
of JAK2 (52,53), which is a phenomenon that may
explain the myeloproliferative phenotype in
JAK2V617F-negative individuals who present with other
variants in the JAK2 gene, and could possibly be related to
the clinical phenotype in the different subtypes of neoplasms, a
phenomenon that is still not well understood. The present study
identified the rs907414891 variant, located in the FERM
domain, which results in the exchange of isoleucine for valine at
position 166 of the JAK2 protein (p. Ile166Val). Currently, this
variant has no description in the literature regarding its clinical
impact. However, the exclusive presence of rs907414891,
rs576746768, rs413160003, and rs55930140 in ET
individuals may represent novel clonal biomarkers in ET.
Nevertheless, it is necessary to perform additional molecular and
functional tests to verify their possible association with MPN.
The SNV rs2230722, located in exon 6 of
JAK2, was frequently observed in the present study and had a
higher predominance in females, in agreement with Sokol et
al (22). This variant was more
frequent in women with platelet aggregation syndrome compared to
men; and was significantly associated with deep vein thrombosis. As
such, the variant could be correlated with the clinical picture of
MPN, especially in individuals with thrombotic complications. The
SNV rs2230724, a variant that is present in exon 19 of
JAK2, was detected in the present study in the JH2-JH1
linker region. Although variants in this region are not frequently
described in MPN, alterations in the JH1-JH2 interaction may
generate dysregulation in the inhibition of catalytic activity and,
therefore, alter its function. This SNV, together with
rs2230728, are reported in hematologic cancers and
associated with the progression to acute leukemia, especially in
individuals older than 45 years old (23); and may thus serve as genetic markers
of leukemic progression in MPN.
The coexistence of JAK2 variants is not
often described in MPN; however, this could have greater
repercussions in the individual's clinical picture (50). In the present study, concomitance
was observed in up to three variants, in the presence of
JAK2V617F, and presented laboratory profiles with slight
increases in cell counts, including red blood cells and platelet
counts, which indicates that these variants may confer genomic
instability and increase intracellular signaling of the JAK/STAT,
PI3K, MAPK, NF-κB, and HIF1-α pathways, to induce tumorigenesis and
facilitate the acquisition of other variants within the same gene
(50,54).
Using Sanger sequencing, Lanikova et al
(55) demonstrated the presence of
SNV rs2230723 in coexistence with JAK2V617F and, in
this case, described normalized hematological counts after
administration of hydroxyurea. In other experiments, both variants
showed increased STAT1, STAT3, and STAT5 signaling, which suggested
the potential of both variants in the predisposition to
malignancies. Likewise, other variants in JAK2 may confer
weak constitutive signaling of the JAK/STAT pathway, resulting in a
‘more attenuated’ myeloproliferative phenotype, with slightly
altered cell counts. However, further studies are needed to assess
the functional behaviors of these variants, both individually and
when combined.
Although the present study highlights the
importance of detecting other variants in the entire coding region
and the coexistence of variants in the same gene with possible
repercussions on the clinical and laboratory status of individuals
with MPN, it has several limitations. Among the primary limitations
of this study is the small sample size due to the lack of patients
from various centers. Future studies will aim to recruit a larger
cohort from several centers to confirm the results. Here, only
patients from the Hospital Foundation of Hematology and Hemotherapy
of Amazon were included (a unique reference institution in the
state of Amazonas for the diagnosis and treatment of hematological
diseases). Another limitation is the lack of functional studies
that confirm the myeloproliferative activity of these variants, the
lack of allelic association of variants with outcomes, which may
explain the possible predispositions for the development of MPN,
and JAK2 analysis was performed once along of the study.
Likewise, the individuals included in the present study were
treated with hydroxyurea and anagrelide, decreasing the probability
of the detection of JAK2V617F mutations. The results also
may be affected by the low sensitivity of Sanger sequencing.
In conclusion, individuals with negative
BCR::ABL1 MPN may present with more than one variant in the
JAK2 gene, in particular rs2230722, rs2230724,
and rs77375493 variants, both separately and together, and
those with a high JAK2V617F VAF show alterations in the
clinical-laboratory profiles compared with those with a low
JAK2V617F VAF.
Acknowledgements
The authors would like to thank Dr Nadja Garcia
Romero (Genomics Laboratory-HEMOAM), Dr Luciana Cassa (Genomics
Laboratory-HEMOAM), Rechfy Kasen Abou Ali (MSc.; Genomics
Laboratory-HEMOAM) and Dr Enedina Nogueira (Genomics
Laboratory-CAM/UFAM).
Funding
Funding: The present study was supported by the Fundação de
Amparo à Pesquisa do Estado do Amazonas (Pro-Estado Program; grant
nos. #002/2008, #007/2018 and #005/2019, and POSGRAD Program grant
nos. #008/2021), Conselho Nacional de Desenvolvimento Científico e
Tecnológico, and Coordenação de Aperfeiçoamento de Pessoal de Nivel
Superior.
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request. The GenBank accession nos. for the nucleotide
sequences are ON706985 and ON706994.
Authors' contributions
AMT designed the study. DGT, GAVS, LPDSM and AMT
prepared the manuscript and performed the literature search. LPDSM,
AM, EVBA, MADS, WHL, JP, EA, DC, NAF, RA and LN acquired all the
data. AGC, GAVS, AM, EVBA, MADS, WHL, JP, EA, DC, and AMT
interpreted the data. AMT, AGC, GAVS, and DGT analyzed the data.
AMT, AGC, NAF, RA, LN, and GAVS edited the manuscript. AMT, DGT,
GAVS, LPDSM confirm the authenticity of all the data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and Resolution 466/12 of the Brazilian
Ministry of Health. The present study was approved by the National
Ethics Committee, which is responsible for approving relevant human
studies in Brazil (approval no. 4.450.813). Written informed
consent was obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barbui T, Thiele J, Gisslinger H,
Kvasnicka HM, Vannucchi AM, Guglielmelli P, Orazi A and Tefferi A:
The 2016 WHO classification and diagnostic criteria for
myeloproliferative neoplasms: Document summary and in-depth
discussion. Blood Cancer J. 8(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lussana F and Rambaldi A: Inflammation and
myeloproliferative neoplasms. J Autoimmun. 85:58–63.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Challen GA and Goodell MA: Clonal
hematopoiesis: Mechanisms driving dominance of stem cell clones.
Blood. 136:1590–1598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bousoik E and Montazeri Aliabadi H: ‘Do we
know jack’ about JAK? A closer look at JAK/STAT signaling pathway.
Front Oncol. 8(287)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Palumbo GA, Stella S, Pennisi MS, Pirosa
C, Fermo E, Fabris S, Cattaneo D and Iurlo A: The role of new
technologies in myeloproliferative neoplasms. Front Oncol.
9(321)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Campbell PJ and Green AR: The
myeloproliferative disorders. N Engl J Med. 355:2452–2466.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tefferi A: Myeloproliferative neoplasms: A
decade of discoveries and treatment advances. Am J Hematol.
91:50–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Baxter EJ, Scott LM, Campbell PJ, East C,
Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N,
et al: Acquired mutation of the tyrosine kinase JAK2 in human
myeloproliferative disorders. Lancet. 365:1054–1061.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen E and Mullally A: How does JAK2V617F
contribute to the pathogenesis of myeloproliferative neoplasms?
Hematology Am Soc Hematol Educ Program. 2014:268–276.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hubbard SR: Mechanistic insights into
regulation of JAK2 tyrosine kinase. Front Endocrinol (Lausanne).
8(361)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Geay A, Aral B, Bourgeois V, Martin P,
Airaud F, Garrec C, Bézieau S, Gardie B and Girodon F: Diagnosis of
exon 12-positive polycythemia vera rescued by NGS. Clin Case Rep.
8:790–792. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bader MS and Meyer SC: JAK2 in
myeloproliferative neoplasms: Still a protagonist. Pharmaceuticals
(Basel) 15:. 160:1–13. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scott LM, Tong W, Levine RL, Scott MA,
Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison
CN, et al: JAK2 exon 12 mutations in polycythemia vera and
idiopathic erythrocytosis. N Engl J Med. 356:459–468.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kanduła Z, Janowski M, Więckowska B,
Paczkowska E and Lewandowski K: JAK2V617F variant allele frequency,
non-driver mutations, single-nucleotide variants and polycythemia
vera outcome. J Cancer Res Clin Oncol. 149:4789–4803.
2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hu L, Pu L, Ding Y, Li M, Cabanero M, Xie
J, Zhou D, Yang D, Zhang C, Wang H, et al: Relationship between
JAK2V617F mutation, allele burden and coagulation function in
Ph-negative myeloproliferative neoplasms. Hematology. 22:354–360.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Arellano-Rodrigo E, Alvarez-Larrán A,
Reverter JC, Colomer D, Villamor N, Bellosillo B and Cervantes F:
Platelet turnover, coagulation factors, and soluble markers of
platelet and endothelial activation in essential thrombocythemia:
Relationship with thrombosis occurrence and JAK 2 V617F allele
burden. Am J Hematol. 84:102–108. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Villanueva A, Poon KS, Gallardo CA, Chai
CN, Chiu L, Yan B, Ding CSL, Yong KJ, Zhou J, Lee J, et al: A novel
JAK2 R564* variant in a patient with thrombocytosis. Int
J Lab Hematol. 42:e38–e41. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alghasham N, Alnouri Y, Abalkhail H and
Khalil S: Detection of mutations in JAK2 exons 12-15 by Sanger
sequencing. Int J Lab Hematol. 38:34–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Skov V: Next generation sequencing in
MPNs. Lessons from the past and prospects for use as predictors of
prognosis and treatment responses. Cancers (Basel).
12(2194)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Loscocco GG, Guglielmelli P and Vannucchi
AM: Impact of mutational profile on the management of
myeloproliferative neoplasms: A short review of the emerging data.
Onco Targets Ther. 13:12367–12382. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sokol J, Skerenova M, Ivankova J, Simurda
T and Stasko J: Association of genetic variability in selected
genes in patients with deep vein thrombosis and platelet
hyperaggregability. Clin Appl Thromb Hemost. 24:1027–1032.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhong Y, Wu J, Ma R, Cao H, Wang Z, Ding
J, Cheng L, Feng J and Chen B: Association of Janus kinase 2 (JAK2)
polymorphisms with acute leukemia susceptibility. Int J Lab
Hematol. 34:248–253. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang L, Liu D, Liang S, Guo R, Zhang Z, Xu
H, Yang C and Zhu Y: Janus kinase 2 polymorphisms are associated
with risk in patients with gastric cancer in a Chinese population.
PLoS One. 8(e64628)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maslah N, Verger E, Schlageter MH, Miclea
JM, Kiladjian JJ, Giraudier S, Chomienne C and Cassinat B:
Next-generation sequencing for JAK2 mutation testing: Advantages
and pitfalls. Ann Hematol. 98:111–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
De Carvalho TG, De Carvalho AC, Maia DC,
Ogawa JK, Carvalho AL and Vettore AL: Search for mutations in
signaling pathways in head and neck squamous cell carcinoma. Oncol
Rep. 30:334–340. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Silva GA, Ramasawmy R, Boechat AL, Morais
AC, Carvalho BK, Sousa KB, Souza VC, Cunha MG, Barletta-Naveca RH,
Santos MP and Naveca FG: Association of TNF-1031 C/C as a potential
protection marker for leprosy development in Amazonas state
patients, Brazil. Hum. Immunol. 76:137–141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lis JT: Fractionation of DNA fragments by
polyethylene glycol induced precipitation. Methods Enzymol.
65:347–353. 1980.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Paithankar KR and Prasad KS: Precipitation
of DNA by polyethylene glycol and ethanol. Nucleic Acids Res.
19(1346)1991.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Macedo LC, Santos BC, Pagliarini-e-Silva
S, Pagnano KB, Rodrigues C, Quintero FC, Ferreira ME, Baraldi EC,
Ambrosio-Albuquerque EP, Sell AM and Visentainer JE: JAK2 46/1
haplotype is associated with JAK2 V617F-positive myeloproliferative
neoplasms in Brazilian patients. Int J Lab Hematol. 37:654–660.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Macedo LC, de Cesare Quintero F,
Pagliari-E-Silva S, Pagnano KB, Rodrigues C, de Alencar JB, Sell AM
and Visentainer JE: Association of TNF polymorphisms with JAK2
(V617F) myeloproliferative neoplasms in Brazilian patients. Blood
Cells Mol Dis. 57:54–57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
da Silva RR, Domingues Hatzlhofer BL,
Machado CG, Lima AS, de Albuquerque DM, dos Santos MN, Fertrin KY,
Costa FF, Araújo Ada S and Bezerra MA: JAK2 V617F mutation
prevalence in myeloproliferative neoplasms in Pernambuco, Brazil.
Genet Test Mol Biomarkers. 16:802–805. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hasselbalch HC: Time for revival of the
red blood cell count and red cell mass in the differential
diagnosis between essential thrombocythemia and polycythemia vera?
Haematologica. 104:2119–2125. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Langabeer SE: The role of a low
erythropoietin level in the diagnosis of JAK2 exon 12-mutated
polycythemia vera. Blood Cells Mol Dis. 80(102377)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Maslah N, Soret J, Dosquet C, Vercellino
L, Belkhodja C, Schlageter MH, Cassinat B, Kiladjian JJ, Chomienne
C and Giraudier S: Masked polycythemia vera: analysis of a single
center cohort of 2480 red cell masses. Haematologica. 105:e95–e97.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stefaniuk P, Szymczyk A and Podhorecka M:
The neutrophil to lymphocyte and lymphocyte to monocyte ratios as
new prognostic factors in hematological malignancies-a narrative
review. Cancer Manag Res. 12:2961–2977. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mulas O, Mola B, Madeddu C, Caocci G,
Macciò A and Nasa GL: Prognostic role of cell blood count in
chronic myeloid neoplasm and acute myeloid leukemia and its
possible implications in hematopoietic stem cell transplantation.
Diagnostics (Basel). 12(2493)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Vannucchi AM, Masala G, Antonioli E,
Chiara Susini M, Guglielmelli P, Pieri L, Maggi L, Caini S, Palli
D, Bogani C, et al: Increased risk of lymphoid neoplasms in
patients with Philadelphia chromosome-negative myeloproliferative
neoplasms. Cancer Epidemiol. Biomarkers Prev. 18:2068–2073.
2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Garcia-Gisbert N, Camacho L,
Fernández-Ibarrondo L, Fernández-Rodriguez C, Longarón R, Gibert J,
Angona A, Andrade-Campos M, Salar A, Besses C and Bellosillo B:
Analysis of saliva samples and cluster of differentiation 3 (CD3)+
lymphocytes as a source of germline DNA in myeloproliferative
neoplasms. Br J Haematol. 189:e204–e207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sunu C, Gunes AK, Akat GK, Kalpakci Y,
Ceran F, Dagdas S and Ozet G: The evaluation of patients with
essential thrombocythemia in terms of risk of thrombosis. Rev Assoc
Med Bras (1992). 67:385–389. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yahouédéhou SCMA, da Guarda CC, Figueiredo
CVB, Santiago RP, Carvalho SP, Fiuza LM, Ndidi US, Oliveira RM,
Carvalho MOS, Nascimento VML, et al: Hydroxyurea alters
hematological, biochemical and inflammatory biomarkers in Brazilian
children with SCA: Investigating associations with βS haplotype and
α-thalassemia. PLoS One. 14(e0218040)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Davidson TM: The good, the bad, and the
ugly. Arch Otolaryngol Head Neck Surg. 123(115)1997.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Buxhofer-Ausch V, Steurer M, Sormann S,
Schloegl E, Schimetta W, Gisslinger B, Ruckser R, Gastl G and
Gisslinger H: Influence of platelet and white blood cell counts on
major thrombosis-analysis from a patient registry in essential
thrombocythemia. Eur J Haematol. 97:511–516. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kaifie A, Kirschner M, Wolf D, Maintz C,
Hänel M, Gattermann N, Gökkurt E, Platzbecker U, Hollburg W,
Göthert JR, et al: Bleeding, thrombosis, and anticoagulation in
myeloproliferative neoplasms (MPN): Analysis from the German
SAL-MPN-registry. J Hematol Oncol. 9(18)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Matsuura S, Thompson CR, Belghasem ME,
Bekendam RH, Piasecki A, Leiva O, Ray A, Italiano J, Yang M,
Merill-Skoloff G, et al: Platelet dysfunction and thrombosis in
JAK2V617F-mutated primary myelofibrotic mice.
Arterioscler Thromb Vasc Biol. 40:e262–e272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Accurso V, Santoro M, Mancuso S,
Napolitano M, Carlisi M, Mattana M, Russo C, Di Stefano A, Sirocchi
D and Siragusa S: The essential thrombocythemia in 2020: What we
know and where we still have to dig deep. Clin Med Insights Blood
Disord. 13(2634853520978210)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chang YC, Lin HC, Chiang YH, Chen CG,
Huang L, Wang WT, Cheng CC, Lin J, Chang YF, Chang MC, et al:
Targeted next-generation sequencing identified novel mutations in
triple-negative myeloproliferative neoplasms. Med Oncol.
34(83)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Milosevic Feenstra JD, Nivarthi H,
Gisslinger H, Leroy E, Rumi E, Chachoua I, Bagienski K, Kubesova B,
Pietra D, Gisslinger B, et al: Whole-exome sequencing identifies
novel MPL and JAK2 mutations in triple-negative myeloproliferative
neoplasms. Blood. 127:325–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Schulze S, Stengel R, Jaekel N, Wang SY,
Franke GN, Roskos M, Schneider M, Niederwieser D and Al-Ali HK:
Concomitant and noncanonical JAK2 and MPL mutations in JAK2V617F-
and MPLW515 L-positive myelofibrosis. Genes Chromosom Cancer.
58:747–755. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Marty C, Saint-Martin C, Pecquet C,
Grosjean S, Saliba J, Mouton C, Leroy E, Harutyunyan AS, Abgrall
JF, Favier R, et al: Germ-line JAK2 mutations in the kinase domain
are responsible for hereditary thrombocytosis and are resistant to
JAK2 and HSP90 inhibitors. Blood. 123:1372–1383. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Etheridge SL, Cosgrove ME, Sangkhae V,
Corbo LM, Roh ME, Seeliger MA, Chan EL and Hitchcock IS: A novel
activating, germline JAK2 mutation, JAK2R564Q, causes familial
essential thrombocytosis. Blood. 123:1059–1068. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gou P, Zhang W and Giraudier S: Insights
into the potential mechanisms of JAK2V617F somatic mutation
contributing distinct phenotypes in myeloproliferative neoplasms.
Int J Mol Sci. 23(1013)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhao L, Ma Y, Seemann J and Huang LJ: A
regulating role of the JAK2 FERM domain in hyperactivation of
JAK2(V617F). Biochem J. 426:91–98. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kapralova K, Horvathova M, Pecquet C,
Fialova Kucerova J, Pospisilova D, Leroy E, Kralova B, Milosevic
Feenstra JD, Schischlik F, Kralovics R, et al: Cooperation of germ
line JAK2 mutations E846D and R1063H in hereditary erythrocytosis
with megakaryocytic atypia. Blood. 128:1418–1423. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lanikova L, Babosova O, Swierczek S, Wang
L, Wheeler DA, Divoky V, Korinek V and Prchal JT: Coexistence of
gain-of-function JAK2 germ line mutations with
JAK2V617F in polycythemia vera. Blood.
128:2266–2270. 2016.PubMed/NCBI View Article : Google Scholar
|