Introduction
The chronic cholestatic liver illness known as
primary biliary cholangitis (PBC), previously referred to as
primary biliary cirrhosis, is characterized by a cycle of
immune-mediated destruction of intrahepatic bile ducts. At least
two of the following criteria can be used to diagnose PBC in
patients: Tests indicating cholestasis in the serum [such as
unexplained increase of alkaline phosphatase (ALP) or γ-glutamyl
transferase (GGT) levels], positivity for antimitochondrial
antibodies (AMA) or PBC-specific antinuclear antibodies, and the
presence of cholangitis and nonsuppurative interlobular bile duct
damage on histopathology (1). The
gold standard for measuring histologic lesions and determining the
stage of liver fibrosis remains liver biopsy. However, it has
several significant drawbacks, including invasiveness, sampling
errors, unpredictability in histological evaluation, risk of severe
procedure-related consequences and high cost (2). Considering the benefits-to-risk ratio,
liver biopsy is seldom required for diagnosing PBC (1), unless the cases are unusual or it is
essential to rule out concurrent liver diseases such as autoimmune
hepatitis. PBC has the same potential for progression as other
chronic liver disorders, including fibrosis, cirrhosis and
consequences of portal hypertension. The major worldwide health
issue of liver cirrhosis, which has high morbidity and mortality
rates, may be prevented by detecting liver fibrosis early (3). Given the limitations of liver biopsy,
there is substantial clinical value in exploring noninvasive,
cost-effective, accurate, readily available and reproducible
indicators for assessing hepatic fibrosis in individuals with PBC.
Hematological parameters are considered reliable indicators of
prognosis in PBC (4). Blood indices
may be highly informative in evaluating concurrent conditions
linked to non-alcoholic fatty liver disease (NAFLD), including
atherosclerosis (5). Serum lipid
levels are often markedly elevated in PBC, although it has remained
uncertain whether this hyperlipidemia is linked to an accelerated
development of atherosclerosis. This question was initially
inconclusive due to the disease's progressive nature but has gained
clinical significance in light of improved survival rates (6).
Various studies have demonstrated the influence of
numerous noninvasive markers (7-9)
and the application potential of serum indices in the prediction of
(10-12)
the fibrosis degree, particularly for NAFLD and viral hepatitis.
Examples include the aspartate aminotransferase (AST) to platelet
(PLT) ratio index (APRI), magnetic resonance elastography,
ultrasound elastography and the fibrosis index based on four
variables (FIB-4). However, there are fewer options available for
PBC. The diagnostic performances of these noninvasive predictors
are diversiform and need to be further confirmed. It is widely
recognized that an increase in serum bilirubin and a decrease in
serum albumin (Alb) indicate exacerbation and poor prognosis of PBC
(1,13). Currently, the bilirubin to Alb ratio
(BAR) is mainly used to predict hemolytic disease in a newborn
(14) and assess the prognosis of
hepatic encephalopathy (15). The
association between PBC and BAR has rarely been reported. In order
to forecast the phases of liver fibrosis in individuals with PBC,
laboratory parameters were analyzed in the present study and the
diagnostic value of noninvasive serum indicators was
investigated.
Materials and methods
Patient population
Patients with PBC treated at the Affiliated Hospital
of Qinghai University (Xining, China) from January 2015 to
September 2022 were gathered for this retrospective analysis. The
diagnostic criteria are based on recommendations for PBC
identification and treatment (1).
Adults with PBC were included in the study if they had undergone a
liver biopsy with at least 10 portal tracts visible in the
pathological examination. Patients were excluded from the study if
they met any of the following criteria: i) Concurrent presence of
other factors causing chronic liver diseases, such as hepatitis
viruses, drug-induced liver damage or other autoimmune hepatitis,
and ii) the presence of hematological system disorders or other
systemic illnesses.
Data collection
The demographic and laboratory data acquired during
the preceding week before the biopsy included age, gender, AST, red
cell distribution width, PLT count, alanine aminotransferase (ALT),
ALP, GGT, total bilirubin (TBil) and Alb.
Liver biopsy
Informed consent was obtained from all of the
patients before liver biopsy. Under computerized tomography or
ultrasonographic supervision, a needle biopsy was carried out using
a 16G disposable needle. The liver specimens were pierced and at
least 1.5 cm of the length was required. The liver specimens were
then collected, fixed with 4% formaldehyde solution, embedded in
paraffin, sliced into 2-3 mm slices and stained for pathological
analysis using hematoxylin-eosin and Masson's trichrome stains. Two
qualified pathologists used Scheuer's classification to analyze the
results of liver histology as follows: F1, periportal fibrosis; F2,
a few fibrotic septa; F3, several septa; and F4, cirrhosis. Staging
as F1 was considered to indicate significant fibrosis; otherwise,
it suggested minimal fibrosis. Staging as F3 was used to identify
advanced fibrosis; otherwise, it denoted early fibrosis.
Index computation without
intervention
The following formulae were used to determine the
AST to ALT ratio (AAR), APRI (ULN:upper limit of normal value),
bilirubin to Alb ratio (BAR), FIB-4, GGT to PLT ratio (GPR), red
blood cell distribution width (RDW) to PLT ratio (RPR) and TBil to
PLT ratio (TPR):
i) AAR=AST (IU/l)/ALT (IU/l)
ii) APRI=[AST (IU/l)/ULN (IU/l)/PLT
(109/l)]x100
iii) BAR=TBil (mg/dl)/Alb (g/dl)
iv) FIB-4=age (years) x AST (IU/l)/[PLT
(109/l) x ALT (IU/l)1/2]
v) GPR=GGT (IU/l)/PLT (109/l)
vi) RPR=RDW (%)/PLT (109/l)
vii) TPR=TBil (µmol/l)/PLT (109/l)
Statistical analysis
All statistical analyses were conducted using the
MedCalc statistical program version 20.0.4 (MedCalc Software Ltd.)
and SPSS software version 22.0 (IBM Corp.). The mean and standard
deviation were used to describe quantitative data with a normal
distribution, while the median (interquartile range) was used to
express continuous data with a non-normal distribution. Numbers
(percentages) were used for presenting categorical data. Student's
t-tests were used for normally distributed variables, Mann-Whitney
U-tests for non-normally distributed continuous variables and the
Chi-squared test for categorical variables to compare groups. To
identify fibrosis predictors, single-variable logistic regression
analysis was performed. Subsequently, multiple logistic regression
models were constructed by incorporating fibrosis-related
variables. The receiver operating characteristic (ROC) curve was
utilized to calculate the diagnostic accuracies of noninvasive
indices. The highest sum of specificity and sensitivity was used to
determine the best cut-off values for the fibrosis diagnosis. Using
DeLong's test, the area under the ROC curve (AUROC) was used to
assess the diagnostic performance and compare the AUROCs of various
noninvasive markers. A 2-sided P<0.05 was considered to indicate
statistical significance.
Results
Characteristics of the study
population
The present study included 78 patients with PBC who
underwent liver biopsies. Their average age was 54.0±9.3 years and
the cohort comprised 68 (87.2%) women and 10 (12.8%) men. Among
them, 67 (85.9%) were positive for AMA. In terms of the Scheuer
fibrosis staging, there were 12 (15.4%) patients with F1, 42
(53.8%) patients with F2, 12 (15.4%) patients with F3 and 12
(15.4%) patients with F4. Significant variations in TBil, BAR, GPR
and TPR were observed between negligible fibrosis (<F2) and
considerable fibrosis (≥F2). Patients with advanced fibrosis (≥F3)
showed decreased PLT and Alb and elevated levels of TBil, AAR,
APRI, BAR, FIB-4, RPR and TPR compared to those with early fibrosis
(F3). Lowered PLT and Alb and elevated RDW, TBil, APRI, BAR, FIB-4,
RPR and TPR were seen in patients with cirrhosis (F4). Table I displays the demographic and
laboratory characteristics of the patients.
 | Table IDemographical and laboratory
parameters of the subjects with primary biliary cholangitis
(n=78). |
Table I
Demographical and laboratory
parameters of the subjects with primary biliary cholangitis
(n=78).
| Variable (normal
range) | Insignificant
fibrosis (F1; n=12) | Significant fibrosis
[F2+F3+F4; n=66 (86.4%)] | P-value | Early fibrosis
(n=54) | Advanced fibrosis
[F3+F4; n=24 (30.8%)] | P-value | Non cirrhosis
(n=66) | Cirrhosis [F4; n=12
(15.4%)] | P-value |
|---|
| Female sex | 10 (83.3) | 58 (87.9) | 0.665 | 46 (85.2) | 22 (91.7) | 0.429 | 57 (86.4) | 11 (91.7) | 0.613 |
| Age, years | 55.2±8.2 | 53.7±9.5 | 0.628 | 53.3±1.2 | 55.5±2.0 | 0.319 | 53.6±1.2 | 55.9±2.2 | 0.364 |
| PLT, 109/l
(125-350) | 187.0±141.8 | 118.0±117.5 | 0.259 | 145.5±127.0 | 96.0±92.0 | 0.019 | 145.5±122.5 | 61.0±49.8 | <0.001 |
| RDW, %
(12.3-14.8) | 13.6±2.5 | 13.7±3.5 | 0.961 | 13.5±2.0 | 14.5±3.7 | 0.109 | 13.5±2.1 | 15.8±2.7 | 0.029 |
| ALT, IU/l (7-40) | 71.0±53.3 | 54.0±48.0 | 0.724 | 59.0±39.5 | 45.5±62.8 | 0.346 | 59.0±41.8 | 45.5±61.8 | 0.648 |
| AST, IU/l
(13-35) | 47.0±59.5 | 62.0±49.8 | 0.372 | 54.0±43.3 | 82.5±68.3 | 0.211 | 54.0±44.8 | 85.0±51.3 | 0.205 |
| GGT, IU/l (7-45) | 176.5±294.3 | 292.5±409.5 | 0.083 | 282.0±379.8 | 242.5±411.3 | 0.991 | 282.0±392.0 | 197.5±238.3 | 0.430 |
| ALP, IU/l
(50-135) | 217.5±273.5 | 265.0±260.5 | 0.315 | 261.5±267.5 | 260.0±222.3 | 0.717 | 261.5±273.8 | 253.5±116.8 | 0.901 |
| Alb, g/l
(40-55) | 40.5±3.2 | 37.6±6.5 | 0.110 | 39.9±5.0 | 35.8±7.7 | 0.001 | 39.5±6.1 | 35.8±7.8 | 0.008 |
| TBil, mg/dl
(<1.35) | 0.6±0.2 | 1.1±1.7 | 0.004 | 0.8±1.0 | 1.6±3.1 | 0.001 | 0.9±1.1 | 1.8±3.5 | 0.011 |
| AAR | 1.03±0.42 | 1.06±0.53 | 0.497 | 0.97±0.39 | 1.41±0.88 | 0.002 | 1.04±0.42 | 1.19±1.31 | 0.114 |
| APRI | 0.71±2.00 | 1.37±1.64 | 0.250 | 1.10±1.60 | 2.03±2.91 | 0.035 | 1.07±1.64 | 2.52±1.74 | 0.002 |
| BAR | 0.16±0.04 | 0.30±0.40 | 0.007 | 0.21±0.26 | 0.47±0.72 | 0.001 | 0.23±0.33 | 0.51v1.39 | 0.006 |
| FIB-4 | 2.20±4.83 | 3.77±5.20 | 0.184 | 2.79±3.68 | 6.64±6.78 | 0.002 | 2.96±3.95 | 8.44±4.44 | <0.001 |
| GPR | 1.77±5.34 | 6.05±6.62 | 0.043 | 4.83±6.44 | 6.88±7.16 | 0.135 | 4.83±6.60 | 7.44±9.07 | 0.124 |
| RPR | 0.07±0.12 | 0.11±0.13 | 0.601 | 0.09±0.11 | 0.16±0.19 | 0.017 | 0.09±0.11 | 0.24±0.14 | <0.001 |
| TPR | 0.07±0.16 | 0.17±0.46 | 0.034 | 0.13±0.21 | 0.35±0.87 | 0.001 | 0.13±0.24 | 0.53±1.02 | 0.001 |
Logistic regression analysis
First, univariate analysis was used to evaluate
variables related to cirrhosis, advanced fibrosis and substantial
fibrosis. In the univariate analysis, none of the factors was
significantly linked with fibrosis (P≥0.05). PLT [odds ratio
(OR)=0.993, P=0.035], Alb (OR=0.802, P=0.001) and TBil (OR=1.414,
P=0.015) exhibited negative correlations with advanced fibrosis,
whereas the latter had a positive correlation. In the multivariate
analysis, only Alb (OR=0.833, P=0.010) was an independent negative
predictor of advanced fibrosis (Table
II). In addition, cirrhosis was inversely linked with PLT
(OR=0.975, P=0.006) and Alb (OR=0.823, P=0.004). Positive
correlations were found between RDW (OR=1.470, P=0.025) and TBil
(OR=1.275, P=0.040) and cirrhosis. In the multivariate analysis,
none of the factors was an independent predictor of cirrhosis
(Table III).
 | Table IIFactors influencing progressive
fibrosis in subjects with primary biliary cholangitis. |
Table II
Factors influencing progressive
fibrosis in subjects with primary biliary cholangitis.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Female sex | 1.913
(0.375-9.770) | 0.436 | | |
| Age | 1.027
(0.975-1.083) | 0.316 | | |
| PLT | 0.993
(0.986-0.999) | 0.035 | 0.997
(0.990-1.005) | 0.482 |
| RDW | 1.266
(0.969-1.653) | 0.084 | | |
| ALT | 0.996
(0.984-1.009) | 0.584 | | |
| AST | 1.006
(0.997-1.016) | 0.183 | | |
| GGT | 1.000
(0.998-1.002) | 0.873 | | |
| ALP | 1.000
(0.998-1.002) | 0.960 | | |
| Alb | 0.802
(0.705-0.912) | 0.001 | 0.833
(0.725-0.957) | 0.010 |
| TBil | 1.414
(1.069-1.869) | 0.015 | 1.205
(0.892-1.627) | 0.225 |
 | Table IIIFactors connected to cirrhosis in
subjects with primary biliary cholangitis. |
Table III
Factors connected to cirrhosis in
subjects with primary biliary cholangitis.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Female sex | 1.737
(0.199-15.128) | 0.617 | | |
| Age | 1.028
(0.961-1.099) | 0.427 | | |
| PLT | 0.975
(0.958-0.993) | 0.006 | 0.979
(0.956-1.002) | 0.070 |
| RDW | 1.470
(1.050-2.059) | 0.025 | 1.042
(0.624-1.740) | 0.874 |
| ALT | 0.995
(0.978-1.012) | 0.573 | | |
| AST | 1.001
(0.991-1.012) | 0.811 | | |
| GGT | 0.999
(0.996-1.001) | 0.311 | | |
| ALP | 0.999
(0.995-1.002) | 0.487 | | |
| Alb | 0.823
(0.719-0.941) | 0.004 | 0.877
(0.755-1.019) | 0.086 |
| TBil | 1.275
(1.012-1.607) | 0.040 | 1.047
(0.784-1.398) | 0.755 |
Development of a novel index called
BAR x RPR (BARP)
From the above results, PLT, RDW, Alb and TBil were
statistically significant laboratory parameters among different
degrees of fibrosis. Consequently, a novel index was envisioned
that combined the BAR with RPR. The novel index called BARP was
conceived, calculated as BAR x RPR. The BARP was compared among
different fibrosis stages and it was found that there were
statistically significant differences between significant fibrosis
and insignificant fibrosis (P=0.032), advanced fibrosis and early
fibrosis (P<0.001), and cirrhosis and non-cirrhosis
(P<0.001), respectively (Table
IV).
 | Table IVAbility of each non-invasive marker
to diagnose cirrhosis, advanced fibrosis and severe fibrosis. |
Table IV
Ability of each non-invasive marker
to diagnose cirrhosis, advanced fibrosis and severe fibrosis.
| | Significant
fibrosis | Advanced
fibrosis | Cirrhosis |
|---|
| Non-invasive
index | AUROC (95% CI) | Cut-off | Sensitivity | Specificity | P-value | AUROC (95% CI) | Cut-off | Sensitivity | Specificity | P-value | AUROC (95% CI) | Cut-off | Sensitivity | Specificity | P-value |
|---|
| AAR | 0.562
(0.399-0.724) | 1.469 | 22.7 | 100.0 | 0.497 | 0.726
(0.597-0.855) | 1.360 | 54.2 | 88.9 | 0.002 | 0.644
(0.466-0.822) | 1.716 | 41.7 | 89.4 | 0.114 |
| APRI | 0.604
(0.408-0.799) | 0.551 | 81.8 | 50.0 | 0.256 | 0.650
(0.508-0.792) | 1.818 | 62.5 | 70.4 | 0.035 | 0.776
(0.652-0.899) | 1.833 | 83.3 | 69.7 | 0.002 |
| BAR | 0.747
(0.598-0.897) | 0.176 | 75.8 | 83.3 | 0.007 | 0.742
(0.622-0.863) | 0.286 | 79.2 | 68.5 | 0.001 | 0.753
(0.601-0.904) | 0.341 | 83.3 | 65.2 | 0.006 |
| FIB-4 | 0.621
(0.430-0.812) | 2.536 | 69.7 | 66.7 | 0.184 | 0.716
(0.587-0.845) | 2.731 | 87.5 | 50.0 | 0.002 | 0.821
(0.720-0.921) | 3.598 | 100 | 57.6 | <0.001 |
| GPR | 0.684
(0.510-0.858) | 1.467 | 86.4 | 50.0 | 0.043 | 0.606
(0.473-0.740) | 1.963 | 87.5 | 31.5 | 0.135 | 0.640
(0.487-0.793) | 2.449 | 91.7 | 36.4 | 0.124 |
| RPR | 0.597
(0.406-0.787) | 0.077 | 71.2 | 58.3 | 0.289 | 0.670
(0.537-0.802) | 0.149 | 58.3 | 74.1 | 0.017 | 0.819
(0.721-0.918) | 0.136 | 91.7 | 66.7 | <0.001 |
| BARP | 0.696
(0.535-0.857) | 0.009 | 90.9 | 41.7 | 0.032 | 0.750
(0.635-0.865) | 0.031 | 79.2 | 57.4 | <0.001 | 0.832
(0.729-0.935) | 0.034 | 100 | 59.1 | <0.001 |
| TPR | 0.693
(0.530-0.857) | 0.097 | 66.7 | 66.7 | 0.034 | 0.735
(0.618-0.852) | 0.075 | 91.7 | 42.6 | 0.001 | 0.808
(0.699-0.917) | 0.141 | 100 | 56.1 | 0.001 |
Diagnostic performances of noninvasive
indices
The diagnostic performance of each noninvasive index
was estimated using ROC curves. Table
IV displays the AUROC, sensitivity, specificity and cutoff
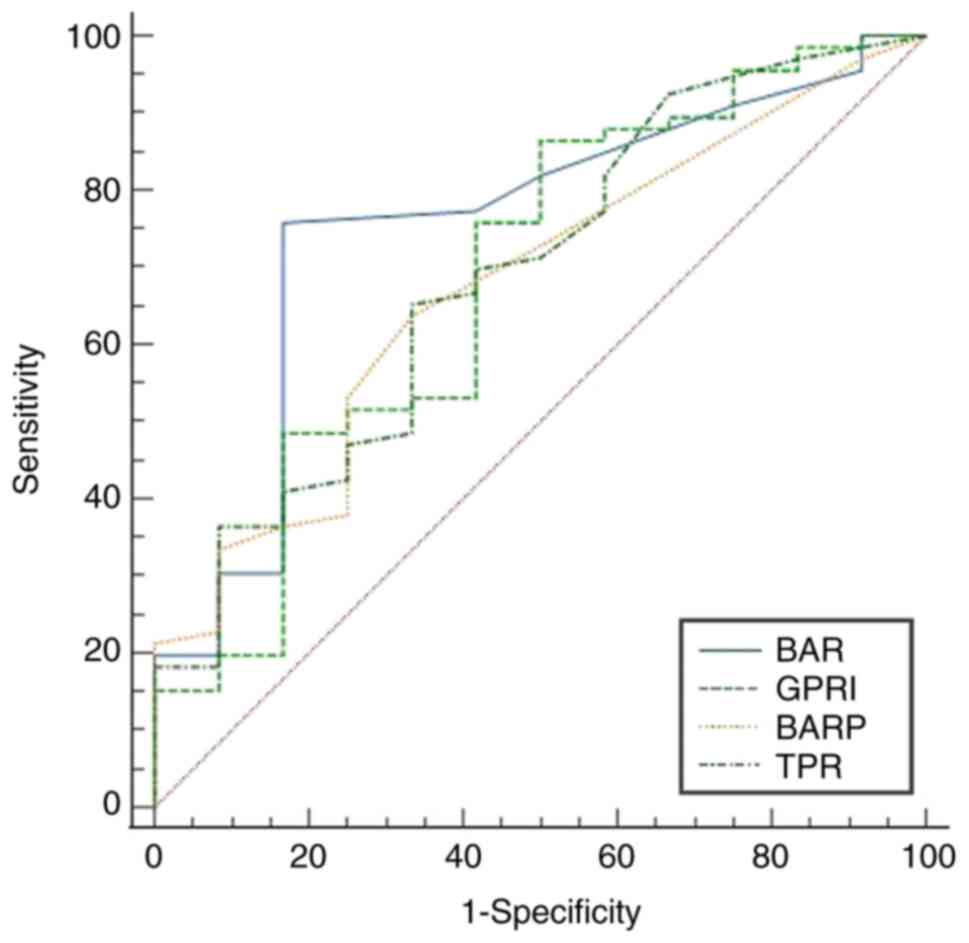
values. The AUROCs for BAR, GPR, TPR and BARP demonstrated
statistically significant coefficients for predicting substantial
liver fibrosis (≥F2) with P<0.05. Their respective values were
0.747 (95% CI: 0.598-0.897), 0.684 (95% CI: 0.510-0.858), 0.693
(95% CI: 0.530-0.857) and 0.696 (95% CI: 0.535-0.857), as
illustrated in Fig. 1. Except for
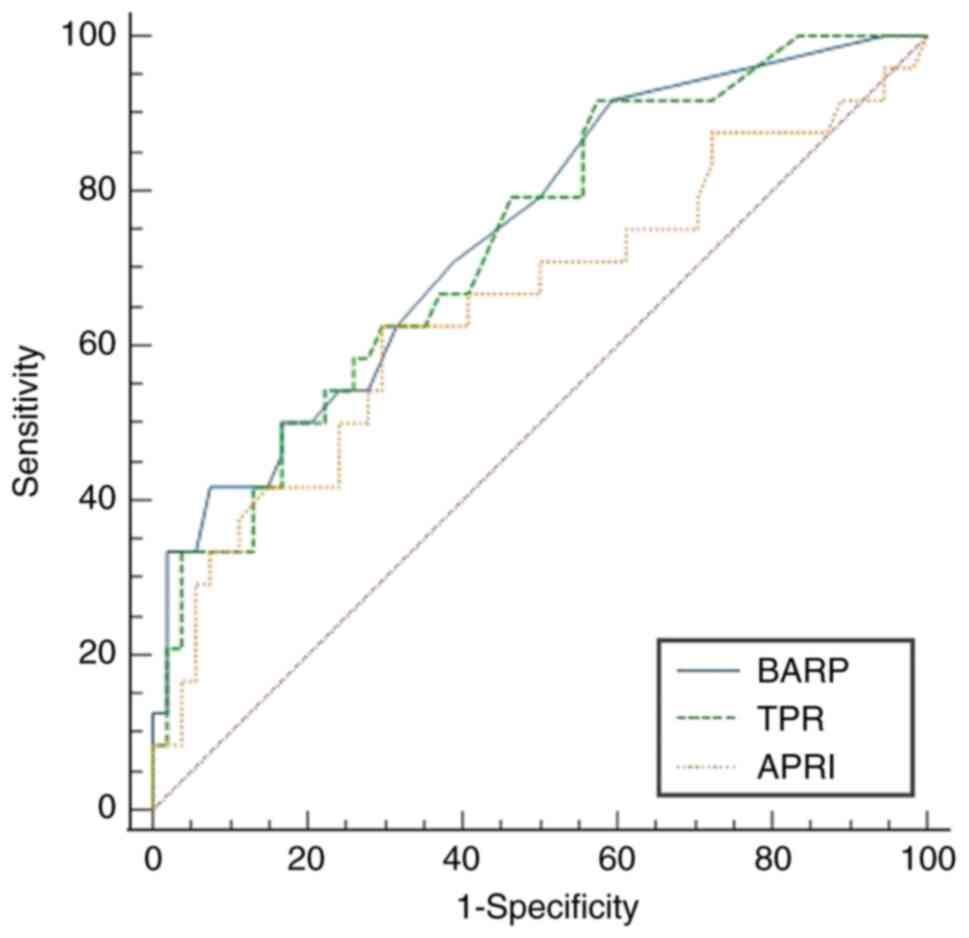
GPR (P=0.135), all of the noninvasive indicators' AUROCs for
differentiating advanced fibrosis (F3) exhibited statistically
noteworthy results (P<0.05; Fig.
2). The AUROCs of AAR, APRI, BAR, FIB-4, RPR, TPR and BARP were
0.726 (95%CI: 0.597-0.855), 0.650 (95%CI: 0.508-0.792), 0.742
(95%CI: 0.622-0.863), 0.716 (95%CI: 0.587-0.845), 0.670 (95%CI:
0.537-0.802), 0.735 (95%CI: 0.618-0.852) and 0.750 (95%CI:
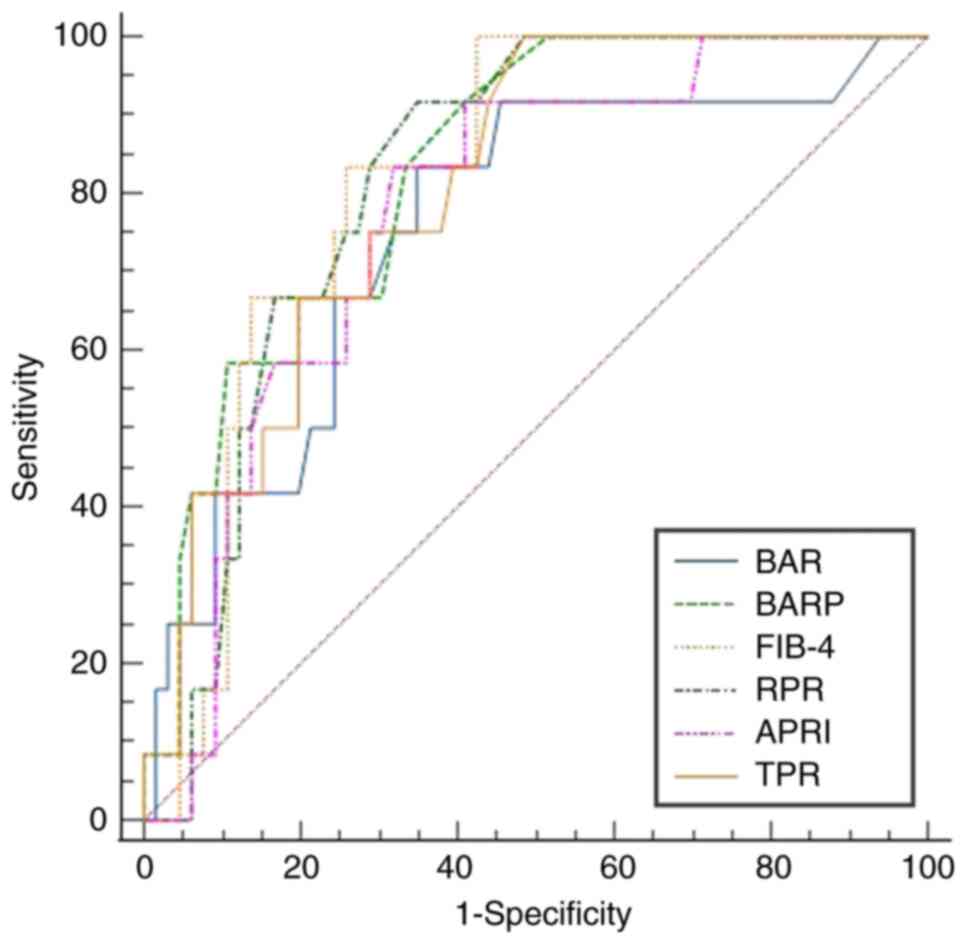
0.635-0.865), respectively. For the prediction of cirrhosis (=F4),
the AUROCs of APRI, BAR, FIB-4, RPR, TPR and BARP were 0.776
(95%CI: 0.652-0.899), 0.753 (95%CI: 0.601-0.904), 0.821 (95%CI:
0.720-0.921), 0.819 (95%CI: 0.721-0.918), 0.808 (95%CI:
0.699-0.917) and 0.832 (95%CI: 0.729-0.935), respectively, all of
which were statistically significant (P<0.05; Fig. 3). When predicting significant
fibrosis, there were no statistically significant differences among
the BAR, GPR, TPR and BARP (P≥0.05; Fig. 1). To diagnose advanced fibrosis, the
AUROCs of the BARP and TPR outperformed those of the APRI (P=0.021
and 0.044, respectively; Fig. 2).
However, when assessing cirrhosis, there were no statistically
significant differences between APRI, BAR, BARP, FIB-4, TPR and RPR
(P≥0.05; Fig. 3).
Discussion
PBC is an autoimmune cholestasis of the liver that
primarily affects females. Cholestatic biochemistry along with the
presence of AMA or other PBC-specific autoantibodies can typically
lead to a precise diagnosis of PBC in the majority of patients.
Liver biopsy is no longer recommended as a diagnostic technique,
with the exception of atypical cases, such as those with low
antibody levels or no PBC-specific autoantibodies, because of its
invasive nature, cost, potential for sampling errors, and the risk
of severe complications (1).
Effective treatments can facilitate slower progression and better
prognosis (16). However, the
majority of individuals with PBC are often asymptomatic and the
condition is undetectable in the early stages. If no effective
treatment is available, the condition tends to progress in most
patients. PBC is a chronic autoimmune cholestatic liver disease
that can deteriorate rapidly in the end stage and culminate in
biliary cirrhosis and complications related to portal hypertension
over time (17). Therefore, a
condition with a lower overall survival rate than that of the
general population may substantially reduce the quality of life for
those affected and require long-term monitoring. Early diagnosis,
risk assessment, treatment and long-term management are vital for
patients with PBC (18).
Histological progression is closely related to prognosis in PBC.
Therefore, identifying liver fibrosis at an early stage is of
critical importance for preventing cirrhosis and improving the
prognosis of patients with PBC.
The present study aimed to explore new indices and
evaluate their diagnostic performance in predicting the stages of
liver fibrosis in patients with PBC. The results revealed that
individuals with severe fibrosis exhibited elevated levels of TBil
and GGT in their blood. Furthermore, advanced fibrosis was
associated with higher TBil levels and lower levels of PLT and Alb.
There were statistically significant differences in PLT, RDW, Alb
and TBil levels between the cirrhosis and non-cirrhosis groups.
Based on these results, it was hypothesized that a novel
noninvasive indicator called BARP, derived from BAR x RPR, could be
a valuable tool for predicting liver fibrosis in PBC. Alb was found
to be a distinct, unfavorable predictor of advanced fibrosis in the
multivariate analysis. Of note, unlike other noninvasive indices,
the BAR, TPR and BARP exhibited statistically significant
differences in their diagnostic performance for significant
fibrosis, advanced fibrosis and cirrhosis. The present result
suggested that BAR, with the highest AUROC value (0.747, 95%CI:
0.598-0.897), is a reasonable predictor of significant fibrosis.
Furthermore, the TPR and BAPR also showed definite advantages in
assessing advanced fibrosis (AUROC: 0.735, 0.750; 95%CI:
0.618-0.852, 0.635-0.865, respectively) and cirrhosis (AUROC:
0.808, 0.832; 95% CI: 0.699-0.917, 0.729-0.935, respectively). The
AUROCs for identifying advanced fibrosis were greater for BARP and
TPR compared to APRI (P=0.021, 0.044, respectively). The AAR, APRI,
FIB-4, GPR and RPR had AUROCs that were either slightly inferior to
or comparable to those of the BAR, TPR and BARP. To the best of our
knowledge, the present study was the first to use both the BAR
index and the new BARP index to predict the stages of PBC
fibrosis.
Various studies have reported on the value of
ultrasound elastography in predicting liver fibrosis (19,20).
Consistent with previous research, transient elastography has a
higher ability to diagnose the liver fibrosis stage than liver
stiffness measurement (21,22). The ideal cutoff value for each liver
condition varies depending on the etiology of the condition.
However, the accuracy of the results is influenced by factors such
as liver inflammation, cholestasis, congestion and the patient's
somatotype, and it is not suitable for patients with ascites around
the liver. Of note, it is difficult to distinguish the
differentiation between adjoining liver fibrosis stages because of
the extensive overlap of cutoff values. The clinical use of blood
biomarkers or indices in predicting the degree of liver fibrosis
has also been the subject of several investigations (23,24).
Serum indices such as RDW, RPR, AAR, APRI and GPR have a certain
utility for evaluating liver fibrosis. The TPR was found to more
reliably predict early liver fibrosis in PBC in the study by Jiang
et al (25), and its AUROC
was greater than that of the AAR, APRI, FIB-4 and RPR. The outcomes
of their study are comparable to those of the present study. The
histologic stage of individuals with PBC is associated with RDW,
RPR and the RDW to lymphocyte ratio, and these tests demonstrate
superior diagnostic capabilities compared to typical indices such
as APRI, FIB-4 and AAR (26-28).
In prior reports, the GPR (29) and
mean platelet volume (30) were
found to have certain utility for identifying advanced fibrosis in
PBC. However, the diagnostic capabilities of those studies could
not be fully examined due to their small size or absence of a 95%
CI for the AUROC. Compared to the AAR, FIB-4 and APRI, the current
investigation demonstrated that the growth arrest-specific gene 6
protein to Alb ratio was more likely to accurately diagnose
advanced fibrosis (31). The
diagnostic performances and cutoff values of these noninvasive
procedures and markers are multifarious. To date, no consensus has
been reached regarding the most effective noninvasive procedure,
serum index or the optimal cutoff value. Given the limitations of
liver biopsy, noninvasive measurement of hepatic fibrosis has
become the standard. This field of study is ongoing and the current
findings require further confirmation.
The present study has certain limitations. First, it
is a retrospective study without sampling and lacks a validation
cohort. Furthermore, certain patients included in the study had
received ursodeoxycholic acid treatments and therefore, the
accuracy of the results needs to be verified in further
investigations. Finally, the diagnostic performance of FibroScan
was not assessed, as only a limited number of individuals had
undergone the test. Larger, more diverse studies with robust
methodologies are needed to validate these markers and determine
their clinical relevance in managing PBC-related liver
fibrosis.
In conclusion, the present study demonstrated that
Alb is useful for early fibrosis prediction, while the BAR, BARP
and TPR offer benefits in assessing liver fibrosis in PBC. All of
these can be easily calculated using standard blood counts and
inexpensive biochemical markers. However, the accuracy of these
results needs to be substantiated through multicenter collaboration
and randomized trials.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the ‘Talent in
Kunlun High-end Innovative and Entrepreneurial Talents’ programme
of Qinghai Province (grant no. 2020-18) and the Natural Science
Foundation of Qinghai Province (grant no. 2022-ZJ-969Q).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Data curation: YL and MJZ. Formal analysis: YL. Data
analysis: YL, MJZ, XHW and SHL. Writing-original draft: YL.
Writing-review and editing: SL. All authors have read the final
version of the manuscript and checked and approved the authenticity
of the raw data.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Affiliated Hospital of Qinghai University
(Xining, China). All procedures followed were in accordance with
the declaration of Helsinki as revised in 2008. All participants
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
You H, Duan W, Li S, Lv T, Chen S, Lu L,
Ma X, Han Y, Nan Y, Xu X, et al: Guidelines on the diagnosis and
management of primary biliary cholangitis (2021). J Clin Transl
Hepatol. 11:736–746. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xu XY, Ding HG, Li WG, Xu JH, Han Y, Jia
JD, Wei L, Duan ZP, Ling-Hu EQ and Zhuang H: Chinese guidelines on
the management of liver cirrhosis (abbreviated version). World J
Gastroenterol. 26:7088–7103. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cui XW, Li KN, Yi AJ, Wang B, Wei Q, Wu GG
and Dietrich CF: Ultrasound elastography. Endosc ultrasound.
11:252–274. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chang Y, Guo C, Guo G, Yuan Z, Zhou X,
Wang J, Han Z, Chen Y, Jia G and Han Y: Erythrocyte count is
associated with prognosis in Chinese patients with primary biliary
cholangitis. Exp Ther Med. 19:2075–2082. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tarantino G, Barrea L, Capone D, Citro V,
Mosca T and Savastano S: Hematocrit values predict carotid
intimal-media thickness in obese patients with non-alcoholic fatty
liver disease: A cross-sectional study. Front Endocrinol
(Lausanne). 9(203)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sorokin A, Brown JL and Thompson PD:
Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic
risk: A systematic review. Atherosclerosis. 194:293–299.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schulz M, Wilde ACB, Demir M, Müller T,
Tacke F and Wree A: Shear wave elastography and shear wave
dispersion imaging in primary biliary cholangitis-a pilot study.
Quant Imaging Med Surg. 12:1235–1242. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cristoferi L, Calvaruso V, Overi D, Viganò
M, Rigamonti C, Degasperi E, Cardinale V, Labanca S, Zucchini N,
Fichera A, et al: Accuracy of transient elastography in assessing
fibrosis at diagnosis in naïve patients with primary biliary
cholangitis: A dual cut-off approach. Hepatology. 74:1496–1508.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meng Y, Liang Y and Liu M: The value of
MRI in the diagnosis of primary biliary cirrhosis and assessment of
liver fibrosis. PLoS One. 10(e0120110)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok ASF: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reinson T, Buchanan RM and Byrne CD:
Noninvasive serum biomarkers for liver fibrosis in NAFLD: current
and future. Clin Mol Hepatol. 29 (Suppl):S157–S170. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Z, Zhou Y, Yu P, Liu Y, Mei M, Bian
Z, Shao W, Lv J, Li X, Lu W and Xu L: Retrospective evaluation of
non-invasive assessment based on routine laboratory markers for
assessing advanced liver fibrosis in chronic hepatitis B patients.
Int J Gen Med. 15:5159–5171. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakano T, Inoue K, Hirohara J, Arita S,
Higuchi K, Omata M and Toda G: Long-term prognosis of primary
biliary cirrhosis (PBC) in Japan and analysis of the factors of
stage progression in asymptomatic PBC (a-PBC). Hepatol Res.
22:250–260. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vardar G, Okan MA, Karadag N, Topcuoglu S,
Ozalkaya E, Karatepe HO and Karatekin G: Intravenous immunoglobulin
in hemolytic disease of the newborn: A moving target in time. Niger
J Clin Pract. 25:1262–1268. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Y, Liu H, Chen K, Wu X, Wu J, Yang Z,
Yao L, Wen G, Zhang C, Chen X, et al: Pathological significance and
prognostic roles of indirect bilirubin/albumin ratio in hepatic
encephalopathy. Front Med (Lausanne). 8(706407)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang L, Sun K, Tian A, Liu Y, Zhang M,
Zhou X and Han Y: Fenofibrate improves GLOBE and UK-PBC scores and
histological features in primary biliary cholangitis. Minerva Med.
113:974–982. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Warnes TW, Roberts SA, Smith A, Cope VM,
Vales P, Haboubi NY and McMahon RF: Portal hypertension in primary
biliary cholangitis: Prevalence, natural history and histological
correlates. Eur J Gastroenterol Hepatol. 33:1595–1602.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hirschfield GM, Dyson JK, Alexander GJM,
Chapman MH, Collier J, Hübscher S, Patanwala I, Pereira SP, Thain
C, Thorburn D, et al: The British society of
gastroenterology/UK-PBC primary biliary cholangitis treatment and
management guidelines. Gut. 67:1568–1594. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gherlan GS: Liver ultrasound elastography:
More than staging the disease. World J Hepatol. 7:1595–1600.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yu JH, Lee HA and Kim SU: Noninvasive
imaging biomarkers for liver fibrosis in nonalcoholic fatty liver
disease: Current and future. Clin Mol Hepatol. 29 (Suppl
1):S136–S149. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Branchi F, Conti CB, Baccarin A,
Lampertico P, Conte D and Fraquelli M: Non-invasive assessment of
liver fibrosis in chronic hepatitis B. World J Gastroenterol.
20:14568–14580. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Florea M, Serban T, Tirpe GR, Tirpe A and
Lupsor-Platon M: Noninvasive assessment of hepatitis C virus
infected patients using vibration-controlled transient
elastography. J Clin Med. 10(2575)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Castera L, Friedrich-Rust M and Loomba R:
Noninvasive assessment of liver disease in patients with
nonalcoholic fatty liver disease. Gastroenterology.
156:1264–1281.e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Michalak A, Guz M, Kozicka J, Cybulski M,
Jeleniewicz W, Lach T and Cichoż-Lach H: Red blood cell
distribution width derivatives in alcohol-related liver cirrhosis
and metabolic-associated fatty liver disease. World J
Gastroenterol. 28:5636–5647. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang M, Yan X, Song X, Yan Q, Zhao Y,
Wang L and Gao P: Total bile acid to platelet ratio: A noninvasive
index for predicting liver fibrosis in primary biliary cholangitis.
Medicine (Baltimore). 99(e20502)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jiang X, Wang Y, Su Z, Yang F, Lv H, Lin L
and Sun C: Red blood cell distribution width to platelet ratio
levels in assessment of histologic severity in patients with
primary biliary cholangitis. Scand J Clin Lab Invest. 78:258–263.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang H, Xu H, Wang X, Wu R, Gao X, Jin Q
and Niu J: Red blood cell distribution width to platelet ratio is
related to histologic severity of primary biliary cirrhosis.
Medicine (Baltimore). 95(e3114)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Meng J, Xu H, Liu X, Wu R and Niu J:
Increased red cell width distribution to lymphocyte ratio is a
predictor of histologic severity in primary biliary cholangitis.
Medicine (Baltimore). 97(e13431)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Avcioğlu U, Eruzun H and Ustaoğlu M: The
gamma-glutamyl transferase to platelet ratio for noninvasive
evaluation of liver fibrosis in patients with primary biliary
cholangitis. Medicine (Baltimore). 101(e30626)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tahtaci M, Yurekli OT, Bolat AD, Balci S,
Akin FE, Buyukasik NS and Ersoy O: Increased mean platelet volume
is related to histologic severity of primary biliary cirrhosis. Eur
J Gastroenterol Hepatol. 27:1382–1385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hayashi M, Abe K, Fujita M, Takahashi A,
Hashimoto Y and Ohira H: Serum Gas6 and Axl as non-invasive
biomarkers of advanced histological stage in primary biliary
cholangitis. Hepatol Res. 50:1337–1346. 2020.PubMed/NCBI View Article : Google Scholar
|