Introduction
Globally, lung cancer is the most lethal cancer type
among men and women (1), with up to
1.8 million associated deaths, accounting for 18% of all
cancer-associated deaths in 2020(2). Treatment for the disease includes
surgery, chemotherapy, radiotherapy, targeted therapy and
immunotherapy, depending on the patient Tumor-Node-Metastasis (TNM)
stage and differentiation grade (3,4). If a
patient's TNM stage is localized, surgery is the best choice
(5). However, patients frequently
experience perioperative complications, such as cardiopulmonary
dysfunction and limited exercise capacity during lung resection
(6,7). These changes occur particularly in the
right ventricle, causing dysfunction, and affect left ventricular
(LV) systolic function due to pulmonary vascular bed loss (8). Few reports have described right
ventricular (RV) dysfunction and increased mortality rates after
lung resection (9-11).
This type of surgery also alters cardiopulmonary interactions.
Eventually, this can lead to changes in blood oxygen levels, which
can directly and indirectly affect heart function. A previous
cardiovascular magnetic resonance (CMR) study reported that RV
ejection fraction (EF) decreased by ~10% compared with that before
surgery (10). However, the CMR
method frequently requires the patient to breath-hold and requires
isolation of the patient in a small chamber, and as such, this tool
has limited extensive application. Therefore, a more convenient
method, such as bedside echocardiography, may be alternative.
Transthoracic echocardiography (TTE) is a
non-invasive, convenient and reliable tool for assessing heart
function (12). Multiple parameters
can easily measure RV and LV function (11). Other common echocardiographic
modalities are M-mode (13) and
two-dimensional or pulse-wave Doppler (14) due to the ventricular volume status
and rate of myocardial relaxation. In addition, these methods are
preload-dependent and have low accuracy (15). Cardiac magnetic resonance imaging
and computed tomography angiography have been used to visualize the
coronary arteries and detect blockages or narrowing (16,17).
The technique can also provide information regarding structure and
other cardiovascular conditions. However, these modalities only
provide approximate, not detailed, information regarding cardiac
functions. Recent studies have demonstrated that tissue Doppler
imaging (TDI) has greater capacity than two-dimensional Doppler
echocardiography for recording systolic and diastolic velocities in
the myocardium, as well as at the corner of the annulus (18-21).
TDI can record peak velocities for early (E) and late (A) diastolic
filling, deceleration time and isovolumic relaxation time, which
reflect mitral valve inflow and aortic valve outflow (22). For the lateral mitral annulus, TDI
can be used to record peak early (e') velocity of diastole.
Therefore, TDI is a better tool for assessing function of the
myocardium and mitral annulus after lung resection due to its less
preload-dependent (23).
In the present study, TDI was used to measure the
diameter of the ascending aorta, ascending aorta size,
anterior-posterior diameters of the left atrium and ventricle, and
widths of the ventricular septum and right ventricle. Heart
function indices, such as LVEF, pulmonary valve flow rate,
tricuspid annular or mitral valve E peak/A peak, tricuspid
regurgitation flow, lateral mitral annulus e' and E/e' ratio, were
also determined. The study assessed parameters of heart function
and recommends the more routine clinical application of a method to
measure heart function before and after lung resection
Materials and methods
Patients
The present study retrospectively analyzed data from
an observational cohort that underwent TDI to assess left and right
heart function after lung resection. In total, 43 patients with
non-small cell lung cancer (NSCLC) (n=37) and metastatic cancer in
the lungs (n=6) were enrolled. Patients diagnosed and admitted to
Peking University Cancer Hospital (Beijing, China) between October
2015 and January 2020, who fulfilled predefined enrolment and
exclusion criteria, were included. Inclusion criteria were as
follows: i) An age >18 years; ii) primary lung cancer and
metastatic lung cancer confirmed by a combination of clinical
features, imaging data and pathological diagnosis of tumour issue
or biopsy; and iii) all patients underwent selective lung resection
with lobectomy. Exclusion criteria were as follows: i)
Non-malignant tumour or other benign diseases in the lungs; and ii)
aberrant heart, liver and kidney functions before lobectomy.
Surgery mainly included a single lobar lung resection of the left
or right lung. The surgical method and anesthetic techniques were
standardized. To precisely address structural and functional
changes after lobectomy, all patients were selected from among
those with lung cancer and no history of chronic cardiopulmonary
disease. This study was reviewed and approved by the Ethics
Committee of Peking University Cancer Hospital and Institute
(Beijing, China; approval number, 20210915). Written informed
consent was obtained from all participants.
TDI
Compared with traditional Doppler echocardiography,
TDI is less affected by afterload alterations, valvular
regurgitations and changes in heart rate. Therefore, TDI is a
powerful device to detect diastolic ventricular function. TDI data
were collected preoperatively and at 3 months postoperatively using
an ultrasound device (EPIQ CVx5.0; Philips Healthcare). Images were
acquired according to a standardized protocol that incorporated all
aspects required for a comprehensive standard echocardiogram.
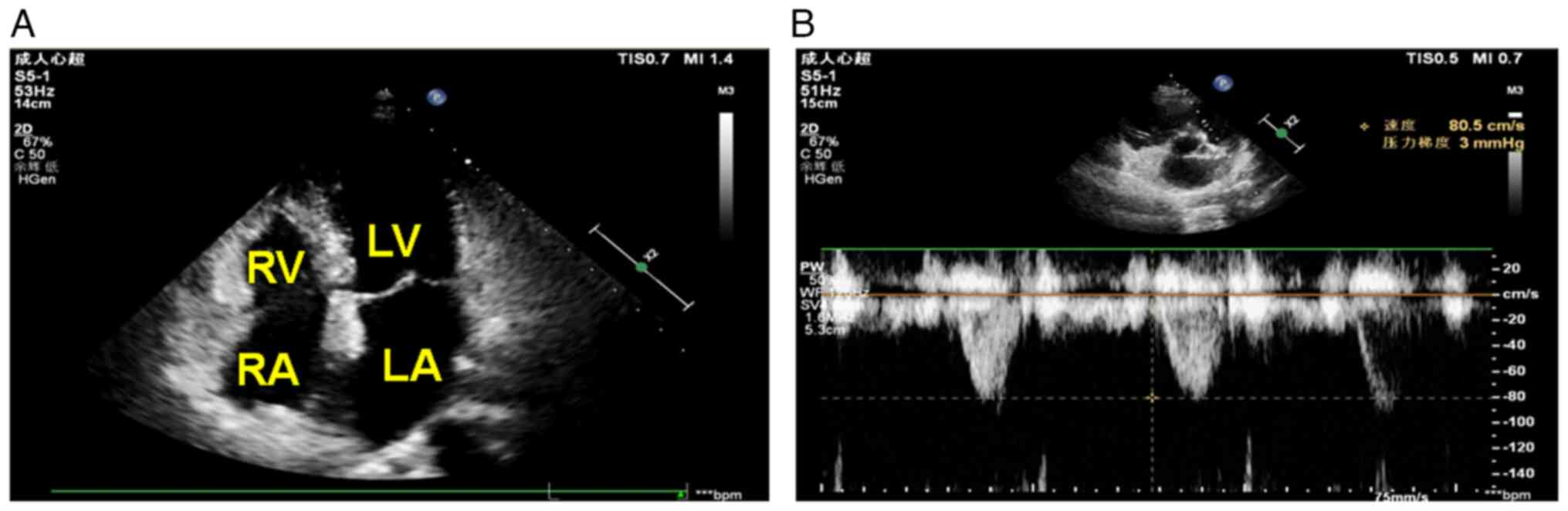
First, a standard four-chamber section was captured in the apical
view (Fig. 1A). Pulmonary arterial
blood flow was measured in sections along the parasternal short
axis (Fig. 1B).
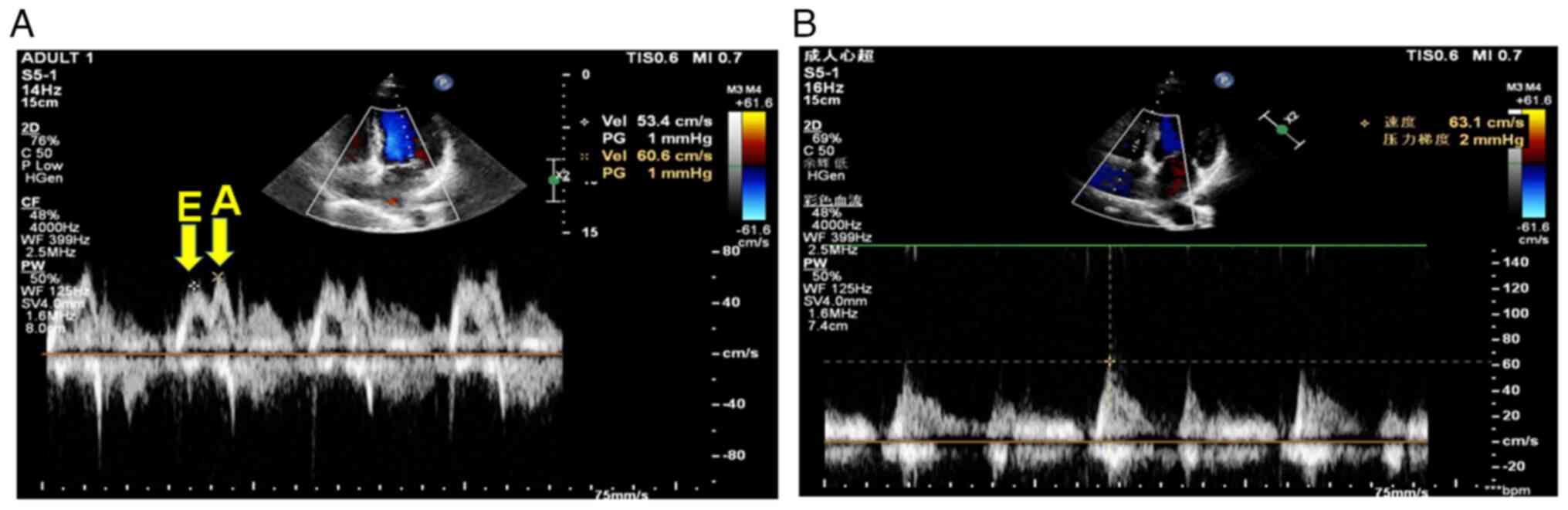
To assess left and right heart function, TDI was
used to capture changes in the mitral annulus (Fig. 2A) and blood flow through the
tricuspid valve (Fig. 2B). As shown
in Fig. 2A, the probe of the TDI
device was placed at the apex to record a bimodal narrowband
waveform. The first peak is the E peak of the mitral valve, which
is generated in the early diastolic phase of the left ventricle,
and the second peak is the A peak, which is generated by left
atrial contraction in the late diastolic phase of the left
ventricle. The E/A rate, which reflects LV relaxation capacity of
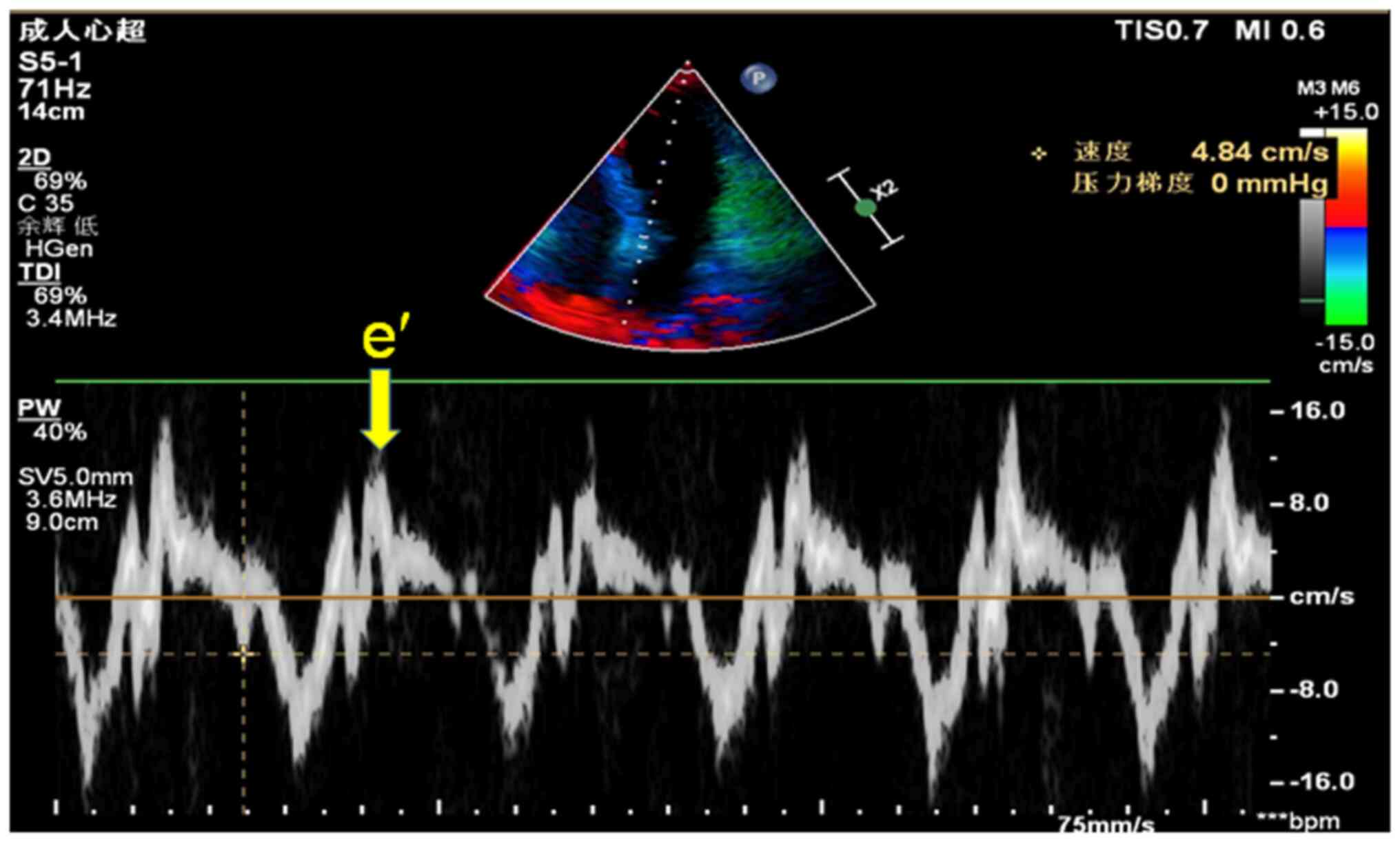
the entity, was then calculated. In addition, the lateral mitral
annulus e' peak was measured using a four-chamber section from the
apical view. The probe of the TDI device was placed at the mitral
annulus and images were captured during the diastolic and systolic
phases. In addition, a capture window was placed on the
intraventricular side of the mitral annulus to measure Doppler
images of the lateral mitral annulus to obtain the e' peak
(Fig. 3), which reflects the
movement of the myocardium. The E/e' ratio, which reflects the
movement capacity of the myocardium, was also calculated. To
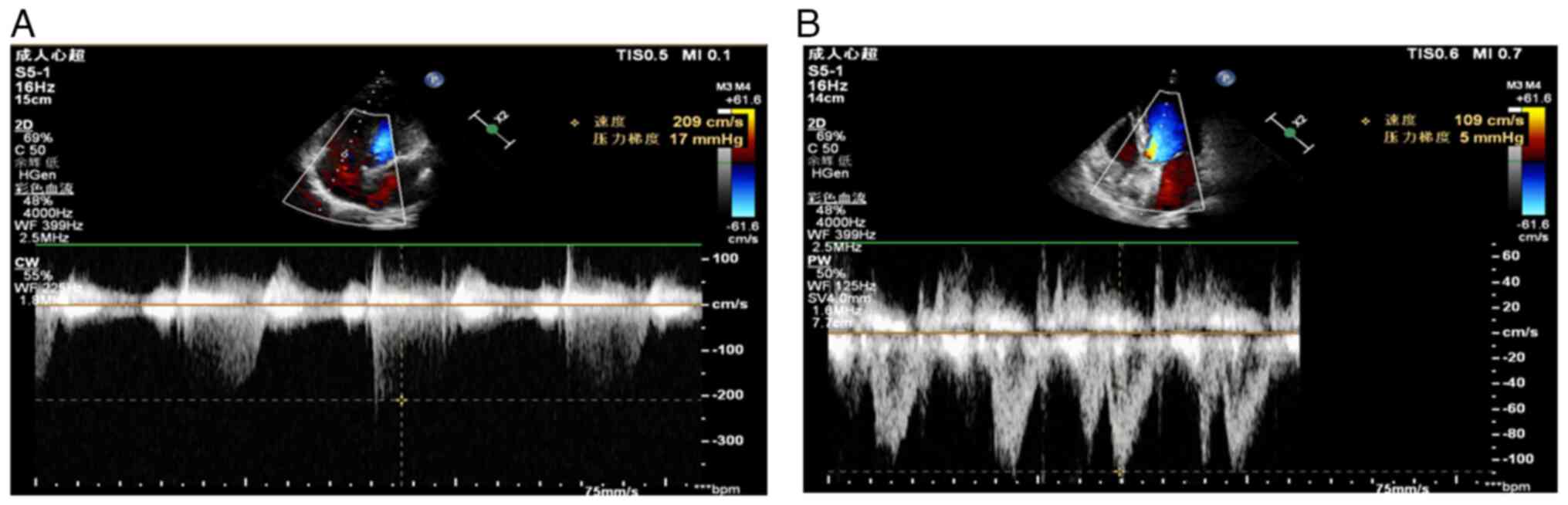
measure tricuspid valve blood flow, a capture window was placed in
the right atrium (RA) from the apical four-chamber section
(Fig. 4A). Aortic valve blood flow
was also measured from the apical view of the five-chamber section
(Fig. 4B).
To determine the clinical significance of these
measurements, the normal range of flow rate (m/sec) of the
pulmonary artery (PA) was defined as <1.0 m/sec. If the
pulmonary valve flow rate was >1.0 m/sec, PA stenosis was
suspected, with 3, 3-4 and >4 m/sec representing mild, moderate
and severe stenosis, respectively. By contrast, the normal blood
flow rate of the aortic artery is 0.7-1.7 m/sec. Flow rates of
2.6-2.9, 3.0-4.0 and >4 m/sec represented mild, middle, severe
aortic valve stenosis, respectively. In normal situations, the E/A
ratio for the mitral annulus is >1, indicating that the E peak
is higher than the A peak. An E/A value of <1 reflected low LV
relaxation capacity. In addition, the lateral mitral annulus e'
peak was calculated using TDI. This metric reflects the movement of
heart muscles. When the mitral e' peak was <8.5 cm/sec (side) or
<8 cm/sec (intraventricular), it indicated damage to the heart
muscle. When the E/e' value was calculated, <8 reflected normal
LV filling pressure. If the E/e' value was >15, the left
ventricle relaxation was damaged and indicated a poor prognosis.
Another measurement included the tricuspid valve E/A value, which
is similar to the mitral E/A and reflects the blood flow rate at
the relaxation phase of the RA. The normal tricuspid valve E/A
value is 0.8-2.1. Tricuspid regurgitation indicates incomplete
tricuspid valve closure. The degree of damage was categorized as
mild (<20), medium (20-40%) and severe (>40%). Pulmonary
hypertension was determined based on blood flow rate during
tricuspid regurgitation.
Statistical analysis
Comparison of cardiac structure and function
parameters preoperatively and postoperatively was performed using
paired Student's t-test. Data are presented as the mean ± standard
deviation and was analyzed using GraphPad Prism version 9
(GraphPad; Dotmatics). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics of patients
with lung cancer
To analyze the specific effects of different types
of lobectomy on heart structure and function, 43 patients who had
undergone lobectomy only were selected for the study. The clinical
characteristics of the 43 patients [mean age, 59 years; age range,
41-75 years; 19 men (44.2%) and 24 women (55.8%)] included in this
study are summarized in Table I. A
total of 37 patients were diagnosed with NSCLC at stages T1-2, N0-2
and M0, whereas the other 6 patients had other tumor tissues that
had metastasized to the lungs. Among the 37 patients with NSCLC, 31
(72.1%) exhibited lung adenocarcinoma, lung squamous cell
carcinoma, or mixed lung cancer(s); the other 6 with metastatic
lung cancers comprised 5 (11.6%) and 1 (2.3%) cases, respectively.
These patients underwent resection of the following lobes: Left
upper lobe (n=12; 27.9%), left lower lobe (n=6; 14.0%), right upper
lobe (n=16; 37.2%), right middle lobe (n=2; 4.7%) and right lower
lobe (n=7; 16.3%). To further evaluate the effects of lobectomy on
structure and function, end-diastolic diameter (mm), posterior wall
thickness, interventricular thickness (mm), ejection fraction (EF),
E/A ratio and E/e' ratio of the left ventricle at pre- and
post-lobectomy were compared. The results showed that left upper
lobectomy was associated with a significantly decreased posterior
wall thickness, interventricular septum thickness and LVEF of the
left ventricle (Table II). The
right upper and right middle lobectomies were also associated with
a decreased interventricular septum thickness and LVEF of the left
ventricle respectively.
 | Table IClinical characteristics, demographics
and surgery styles of 43 patients. |
Table I
Clinical characteristics, demographics
and surgery styles of 43 patients.
| Demographics | Value |
|---|
| Mean age (range),
years | 59 (41-75) |
| Sex, n (%) | |
|
Male | 19 (44.2) |
|
Female | 24 (55.8) |
| TNM stage, n (%) | |
|
T1N0M0 | 26 (60.5) |
|
T1N1M0 | 4 (9.3) |
|
T2N0M0 | 5 (11.6) |
|
T2N2M0 | 1 (2.3) |
|
T3N2M0 | 2 (4.7) |
|
Metastatic | 5 (11.6) |
| Pathological type, n
(%) | |
|
Lung
adenocarcinoma | 31 (72.1) |
|
Lung
squamous cell carcinoma | 5 (11.6) |
|
Carcinoid | 1 (2.3) |
|
Other
metastatic cancer types | 6 (14.0) |
| Lung resections, n
(%) | |
|
Left upper
lobe | 12 (27.9) |
|
Left lower
lobe | 6 (14.0) |
|
Right upper
lobe | 16 (37.2) |
|
Right middle
lobe | 2 (4.7) |
|
Right lower
lobe | 7 (16.3) |
 | Table IIComparison of left ventricle structure
and function parameters (mean ± SD) pre- and post-lobectomy. |
Table II
Comparison of left ventricle structure
and function parameters (mean ± SD) pre- and post-lobectomy.
| Variable | Pre-lobectomy | Post-lobectomy | P-value |
|---|
| End-diastolic
diameter, mm | | | |
|
Left
upper | 45.78±5.70 | 47.00±3.89 | 0.50 |
|
Left
lower | 46.00±4.34 | 48.00±4.34 | 0.23 |
|
Right
upper | 43.00± 5.57 | 45.44±5.33 | 0.07 |
|
Right
middle | 44.50±3.54 | 44.50±3.53 | 0.50 |
|
Right
lower | 42.43± 1.70 | 45.29±3.04 | 0.11 |
| Posterior wall
thickness, mm | | | |
|
Left
upper | 9.24±1.36 | 8.08±0.90 | 0.01a |
|
Left
lower | 9.17±2.79 | 9.50±1.26 | 0.76 |
|
Right
upper | 9.69±2.33 | 8.31±1.45 | 0.02a |
|
Right
meddle | 9.00±2.83 | 7.50±0.71 | 0.66 |
|
Right
lower | 8.86±1.35 | 8.00±1.16 | 0.27 |
| Interventricular
septum thickness, mm | | | |
|
Left
upper | 9.33±1.38 | 8.42±1.17 | 0.03a |
|
Left
lower | 9.50±2.88 | 9.50±1.05 | 0.93 |
|
Right
upper | 10.06±1.95 | 8.31±1.40 | 0.002a |
|
Right
middle | 9.00±2.83 | 7.50±0.71 | 0.71 |
|
Right
lower | 9.00±1.56 | 8.14±1.46 | 0.29 |
| LVEF, % | | | |
|
Left
upper | 66.58±4.12 | 61.17±6.33 | 0.04a |
|
Left
lower | 66.67±4.93 | 63.83±2.56 | 0.34 |
|
Right
upper | 67.00±4.87 | 65.19±5.54 | 0.31 |
|
Right
middle | 71.00±5.66 | 60.50±4.95 | 0.03a |
|
Right
lower | 67.00±2.02 | 64.29±6.08 | 0.44 |
| LV E/A ratio | | | |
|
Left
upper | 0.87±0.26 | 0.83±0.24 | 0.57 |
|
Left
lower | 0.76±0.22 | 0.76±0.18 | 0.98 |
|
Right
upper | 0.93±0.28 | 0.92±0.32 | 0.94 |
|
Right
middle | 0.81±0.09 | 0.83±0.01 | 0.89 |
|
Right
lower | 1.20±0.33 | 1.03±0.23 | 0.39 |
| LV E/e' ratio | | | |
|
Left
upper | 10.11±2.84 | 13.00±4.06 | 0.06 |
|
Left
lower | 11.10±1.41 | 10.93±±4.85 | 0.93 |
|
Right
upper | 10.16±2.30 | 10.33±2.52 | 0.86 |
|
Right
middle | 9.60±1.41 | 10.75±0.35 | 0.53 |
|
Right
lower | 12.21±4.33 | 8.23±1.42 | 0.08 |
Changes in cardiac structure after
lung resection
After lung resection, cardiac structure exhibited
significant hemodynamic changes. The results are summarized in
Table III. Ascending aorta
diameters before and after surgery were 28.7±4.04 vs. 30.88±2.81
(P<0.001). There was a significant increase in width after lung
resection. The left atrial anterior-posterior meridian was
30.88±5.77 vs. 33.10±4.24 (P<0.001), which was also longer after
lung resection. Intraventricular septum thickness was 9.56±1.84 vs.
8.40±1.29 (P<0.001). Posterior wall thickness of the left
ventricle was 9.32±1.97 vs. 8.28±1.2 (P=0.001). Right ventricle
diameter was 20.33±3.03 vs. 19.74±1.90 (P=0.043). By contrast,
there were no significant differences in the ascending aortic valve
opening or LV anterior-posterior meridian.
 | Table IIIComparison of cardiac structure
before and after lung resection. |
Table III
Comparison of cardiac structure
before and after lung resection.
| Structure name | Before surgery | After surgery | P-value |
|---|
| Ascending aorta
diameter, mm | 28.70±4.04 | 30.88±2.81 | <0.01 |
| Ascending aorta
valve open, mm | 18.00±2.01 | 18.19±1.71 | 0.55 |
| Left atrium
anterior-posterior meridian, mm | 30.88±5.77 | 33.10±4.24 | <0.01 |
| Intraventricle
septum thickness, mm | 9.56±1.84 | 8.40±1.29 | <0.01 |
| Left ventricle
anterior-posterior meridian, mm | 44.18±5.35 | 46.02±4.41 | 0.09 |
| Posterior wall
thickness of the left ventricle, mm | 9.32±1.97 | 8.28±1.20 | <0.01 |
| Right ventricle
diameter, mm | 20.33±3.03 | 19.74±1.90 | 0.04 |
Functional changes after lung
resection
In addition to structural changes, changes in
cardiac function were also assessed, with results summarized in
Table IV. Changes in LVEF, flow
rate (m/sec) in the PA and aortic valve, tricuspid regurgitation
(m/sec) and tricuspid pressure (mmHg) were compared before and
after surgery. LVEF was 67.02±4.62 vs. 64.91±4.80 (P=0.04), showing
a significant decrease after lung resection. Tricuspid pressure was
20.58±5.85 vs. 24.31± 24.1±5.93 mmHg (P=0.02), which was
significantly elevated. By contrast, there were no significant
differences in the pulmonary value flow rate (0.87±0.17 vs.
0.92±0.15 m/sec, respectively; P=0.07) and tricuspid regurgitation
(2.32±0.13 vs. 2.44±0.29, respectively; P=0.16) before and after
surgery. These results indicate that lung resection damaged the EF
functions of the heart and that tricuspid pressure compensated for
the elevation.
 | Table IVComparison of cardiac function
parameters before and after lung resection. |
Table IV
Comparison of cardiac function
parameters before and after lung resection.
| Variables | Before surgery | After surgery | P-value |
|---|
| LVEF, % | 67.02±4.62 | 64.91±4.80 | 0.04 |
| Pulmonary valve
flow rate, m/sec | 0.87±0.17 | 0.92±0.15 | 0.07 |
| Tricuspid
regurgitation, m/sec | 2.32±0.13 | 2.44±0.29 | 0.16 |
| Tricuspid pressure,
mmHg | 20.58±5.85 | 24.31±5.93 | 0.02 |
E/A and E/e' ratio of the lateral
mitral annulus preoperatively and postoperatively
To further evaluate the entity LV relaxation and
movement capacity of the myocardium, the E/A and E/e' ratios of the
lateral mitral annulus were also measured. The results are
summarized in Table V. Preoperative
and postoperative mitral E/A ratios were 1.12±0.50 vs. 0.89±0.27
(P=0.28), respectively. Mitral E/e' ratio was 12 vs. 9 (P=0.32), 19
vs. 19 (P=0.88) and 12 vs. 15 (P=0.41) in the <8, 8-12 and
>12 groups, respectively. These results revealed no effects of
lung ventricle relaxation or the movement capacity of the
myocardium.
 | Table VComparison of mitral E/A and E/e'
before and after lung resection. |
Table V
Comparison of mitral E/A and E/e'
before and after lung resection.
| Variables | Before surgery | After surgery | P-value |
|---|
| Mitral E/A
ratio | 1.12±0.5 | 0.89±0.27 | 0.28 |
| Mitral E/e' | | | |
|
<8 | 12 | 9 | 0.32 |
|
8-12 | 19 | 19 | 0.88 |
|
>12 | 12 | 15 | 0.41 |
Discussion
With early detection and increased accuracy due to
technological advances, patients with NSCLC can be diagnosed at an
earlier stage and treated with the appropriate surgery. However,
cardiopulmonary function undergoes significant changes after lung
resection. The main findings of the present study revealed that the
widths of the ascending aorta and left atrium anterior-posterior
meridian after surgery were significantly wider and longer than
those before resection. By contrast, the intraventricular diameter,
posterior wall of the LV and RV diameter decreased after lung
resection. In addition, LVEF was significantly decreased, but
tricuspid pressure was significantly increased compared with
preoperative values.
Compared with traditional transthoracic
echocardiography, which is preferred to assess right-side functions
(12), TDI has many advantages, as
it can more accurately measure the functions of the left ventricle
as well as the right ventricle (24-26).
TDI can be also used to detect systolic and diastolic dysfunction
in both ventricles, as well as LV filling pressure (27). The present study exhibits clear
images for different functions. A total of 43 patients with
primitive and metastatic lung cancer, who underwent lung resection,
exhibited significant changes. The results revealed that the
ascending aortic diameter significantly widened after surgery. It
was also observed that the postoperative left atrial
anterior-posterior meridian was markedly longer than the
preoperative meridian. This indicates that the left atrium and left
ventricle have compensatory capacity due to the loss of oxygen
exchange after lung resection. These results show some differences
from a previous report (18), which
revealed that there were no significant differences in the left
atrial dimensions pre- and post-lung resection. This difference may
be caused by different genetic backgrounds. The intraventricular
diameter, posterior wall of the LV, and RV diameter were
significantly decreased post-resection. This may have resulted in
insufficient blood circulation after lung resection.
The study also measured LVEF, pulmonary valve flow
rate, tricuspid regurgitation and tricuspid pressure, and found
that LVEF significantly declined after lung resection. By contrast,
tricuspid pressure exhibited robust elevation. This further
confirmed the compensatory capacity in both ventricles in response
to reduced postoperative oxygen exchange.
Previous studies have revealed that lung resection
affects LV expansion (28,29). If this is true, then the left
atrium, PA and pulmonary capillary pressures should increase. In
fact, the present study showed that the ascending aorta exhibited a
significantly increased width and confirmed this concept. In
addition, mitral E/A and E/e' are sensitive indicators for left
ventricle relaxation capacity and myocardial movement. Lung
resection results in pulmonary vascular reduction, which decreases
LV expansion. The present results revealed that in the mitral E/e'
<8 normal, 8-12 and >12 groups, there were no significant
differences before and after surgery. This may have been due to the
small sample size. If the E/e' value is >15, the left ventricle
relaxes and the patient has a poor prognosis (30). Another measurement taken was the
tricuspid valve E/A value, which is similar to the mitral E/A value
and reflects the blood flow rate in the relaxation phase of the RA.
The E/A value for a normal tricuspid valve is within the range of
0.8-2.1(31). Tricuspid
regurgitation indicates incomplete tricuspid valve closure. In the
present study, the degree of damage was categorized as mild
(<20), medium (20-40%) and severe (>40%). The results
confirmed that the mitral E/e' is an excellent indictor for
evaluation of LV diastolic function. Pulmonary hypertension was
determined based on blood flow rate during tricuspid regurgitation.
However, the present results demonstrated no significant
differences for preoperative vs. postoperative comparisons.
The present study had a few limitations. First, the
data demonstrated that lobectomy in patients with lung cancer can
affect cardiac structure and functions based on a limited number of
cases (i.e., n=43), which may be the result of sampling bias.
Larger-scale studies including more patients are required to
confirm these findings. Second, data from this investigation were
obtained from a single center, which may have generated selection
bias. Multicenter studies may eliminate this bias. Third, this
study did not include a healthy control group. The TDI reference
parameters used in this study were obtained from the literature,
which may have resulted in procedural bias. As such, include
healthy controls should be included in future studies. In addition,
parameters in this study were only from TDI measurements, and other
traditional echocardiographic data such as two-dimensional Doppler
echocardiography were not compared. Therefore, to confirm TDI is
superior to other echocardiography techniques, data comparison
between TDI and other machines will be required in later
studies.
In conclusion, in the present study, lung resection
significantly affected LVEF, ascending aorta width, tricuspid
pressure, left atrial anterior meridian and intraventricular
diameter, but did not affect the mitral E/e' ratios, indicating
that the LV and RV dimensions were affected, but that the LV
filling pressure was preserved after lobectomy. These results
highlight the need to devote more attention to postoperative
changes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC conceived and designed the study. DS, ZS, and YZ
performed the experiments and analyzed the data. JC and LZ
performed the statistical analysis. JC, DS and ZS drafted the
manuscript, and all authors read and approved the final manuscript.
JC and DS confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was conducted in accordance with the
principles of the Declaration of Helsinki. Study approval was
granted by the Ethics Committee of Peking University Cancer
Hospital and Institute (Beijing, China; approval number, 20210915).
Written informed consent was obtained from all participants before
surgery.
Patient consent for publication
Written informed consent was obtained from the
patients for publication of anonymized case details and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): Cancer.
WHO, Geneva, 2017.
|
|
2
|
Thandra KC and Barsouk A, Saginala K,
Aluru JS and Barsouk A: Epidemiology of lung cancer. Contemp Oncol
(Pozn). 25:45–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: Diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
4
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
5
|
Rueth NM and Andrade RS: Is VATS lobectomy
better: Perioperatively, biologically and oncologically? Ann Thorac
Surg. 89:S2107–S2111. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sarna L, Evangelista L, Tashkin D, Padilla
G, Holmes C, Brecht ML and Grannis F: Impact of respiratory
symptoms and pulmonary function on quality of life of long-term
survivors of non-small cell lung cancer. Chest. 125:439–445.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Win T, Groves AM, Ritchie AJ, Wells FC,
Cafferty F and Laroche CM: The effect of lung resection on
pulmonary function and exercise capacity in lung cancer patients.
Respir Care. 52:720–726. 2007.PubMed/NCBI
|
|
8
|
Vainshelboim B, Fox BD, Saute M, Sagie A,
Yehoshua L, Fuks L, Schneer S and Kramer MR: Limitations in
exercise and functional capacity in long-term postpneumonectomy
patients. J Cardiopulm Rehabil Prev. 35:56–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matyal R, Mahmood F, Hess P, Zhao X,
Mitchell J, Maslow A, Gangadharan S and Decamp M: Right ventricular
echocardiographic predictors of postoperative supraventricular
arrhythmias after thoracic surgery: A pilot study. Ann Thorac Surg.
90:1080–1086. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McCall PJ, Arthur A, Glass A, Corcoran DS,
Kirk A, Macfie A, Payne J, Johnson M, Kinsella J and Shelley BG:
The right ventricular response to lung resection. J Thorac
Cardiovasc Surg. 158:556–565 e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McCall P, Soosay A, Kinsella J, Sonecki P
and Shelley B: The utility of transthoracic echocardiographic
measures of right ventricular systolic function in a lung resection
cohort. Echo Res Pract. 6:7–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wharton G, Steeds R, Allen J, Phillips H,
Jones R, Kanagala P, Lloyd G, Masani N, Mathew T and Oxborough D: ,
et al: A minimum dataset for a standard adult transthoracic
echocardiogram: A guideline protocol from the British Society of
Echocardiography. Echo Res Pract. 2:G9–G24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sahn DJ, DeMaria A, Kisslo J and Weyman A:
Recommendations regarding quantitation in M-mode echocardiography:
Results of a survey of echocardiographic measurements. Circulation.
58:1072–1083. 1978.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuo LC, Quinones MA, Rokey R, Sartori M,
Abinader EG and Zoghbi WA: Quantification of atrial contribution to
left ventricular filling by pulsed Doppler echocardiography and the
effect of age in normal and diseased hearts. Am J Cardiol.
59:1174–1178. 1987.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bilgi M, Yerdelen D, Colkesen Y and
Muderrisoglu H: Evaluation of left ventricular diastolic function
by tissue Doppler imaging in patients with newly diagnosed and
untreated primary generalized epilepsy. Seizure. 22:537–541.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nikolaou K, Alkadhi H, Bamberg F, Leschka
S and Wintersperger BJ: MRI and CT in the diagnosis of coronary
artery disease: Indications and applications. Insights Imaging.
2:9–24. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kwong RY and Yucel EK: Cardiology patient
pages. Computed tomography scan and magnetic resonance imaging.
Circulation. 108:e104–e106. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Colkesen Y, Acil T, Findikcioglu A, Tekin
A, Kilic D, Ozin B and Müderrisoğlu H: Tissue Doppler evaluation of
the effects of major lung resection on cardiac functions. Turk
Kardiyol Dern Ars. 37:317–320. 2009.PubMed/NCBI
|
|
19
|
He T, Tian Z, Liu YT, Li J, Zhou DB and
Fang Q: Evaluating heart function in patients with POEMS syndrome.
Echocardiography. 36:1997–2003. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kepez A, Akdogan A, Sade LE, Deniz A,
Kalyoncu U, Karadag O, Hayran M, Aytemir K, Ertenli I, Kiraz S, et
al: Detection of subclinical cardiac involvement in systemic
sclerosis by echocardiographic strain imaging. Echocardiography.
25:191–197. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chin JH, Kim S, Kim D, Nam JS, Kim K and
Choi IC: Peak systolic myocardial velocity in patients undergoing
surgical aortic valve replacement for severe aortic stenosis:
Prognostic value and natural course. J Clin Monit Comput.
37:327–336. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nagueh SF, Mikati I, Kopelen HA, Middleton
KJ, Quinones MA and Zoghbi WA: Doppler estimation of left
ventricular filling pressure in sinus tachycardia. A new
application of tissue doppler imaging. Circulation. 98:1644–1650.
1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Farias CA, Rodriguez L, Garcia MJ, Sun JP,
Klein AL and Thomas JD: Assessment of diastolic function by tissue
Doppler echocardiography: Comparison with standard transmitral and
pulmonary venous flow. J Am Soc Echocardiogr. 12:609–617.
1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Peverill RE, Cheng K, Cameron J, Donelan L
and Mottram PM: Relationships of global longitudinal strain with
s', long-axis systolic excursion, left ventricular length and heart
rate. PLoS One. 15(e0235791)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zawadka M, Marchel M and Andruszkiewicz P:
Diastolic dysfunction of the left ventricle-a practical approach
for an anaesthetist. Anaesthesiol Intensive Ther. 52:237–244.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cianciulli TF, Saccheri MC, Papantoniou A,
Mendez RJ, Gagliardi JA, Prado NG, Riarte AR, Morita LA, Clérici JE
and Lax JA: Use of tissue doppler imaging for the early detection
of myocardial dysfunction in patients with the indeterminate form
of Chagas disease. Rev Soc Bras Med Trop.
53(e20190457)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Medecins Sans Frontieres. Campaign for
Access to Essential Medicines: International meeting: New
diagnostic tests are urgently needed to treat patients with Chagas
disease. Rev Soc Bras Med Trop. 41:315–319. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tamura Y, Sawabata N, Susaki Y, Nakamura T
and Taniguchi S: Effect of cardiac expansion on postsurgical
pulmonary resection recovery. In Vivo. 33:1977–1984.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakamura T, Sawabata N, Susaki Y and Muro
S: Desaturation during the stair-climbing test for patients who
will undergo pulmonary resection: An indicator of postoperative
complications. Gen Thorac Cardiovasc Surg. 68:49–56.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Previtali M, Chieffo E, Ferrario M and
Klersy C: Is mitral E/E' ratio a reliable predictor of left
ventricular diastolic pressures in patients without heart failure?
Eur Heart J Cardiovasc Imaging. 13:588–595. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sugahara M, Mano T, Goda A, Masai K,
Soyama Y, Daimon A, Asakura M and Masuyama T: Prognostic value of
time interval between mitral and tricuspid valve opening in
patients with heart failure. Circ J. 83:401–409. 2019.PubMed/NCBI View Article : Google Scholar
|