Introduction
In addition to affecting the respiratory system,
coronavirus disease 2019 (COVID-19) (1) frequently complicates itself by
involving multiple organs (2-5)
in its most severe forms, leading to various laboratory
abnormalities. Since the early onset of the pandemic, several key
laboratory characteristics have been identified in the acute phase,
facilitating the evaluation of disease severity (3-5).
The importance of certain laboratory abnormalities induced by
COVID-19, measured on the day of hospital admission, has been
extensively demonstrated in numerous articles (6-19).
However, during the period of hospitalization,
patients with severe COVID-19 experience a variety of changes in
clinical and laboratory measures. Routine hematological and
biochemical monitoring is essential for assessing the disease
severity, therapeutic options and treatment response (20). Notwithstanding, despite the
increased interest in laboratory abnormalities upon hospital
admission, studies exploring the evolution of laboratory parameters
over the course of the disease are limited and variable,
particularly in the setting of the intensive care unit (ICU). Some
of these studies include a small patient population or both
critically and non-critically ill patients (6,21-23),
whereas the limited number of large studies available (24-28)
on patients with COVID-19 admitted to the ICU have demonstrated
diverse results. Furthermore, the effects of dexamethasone,
recommended for the treatment of COVID-19(29), on the trajectory of various
laboratory values have not been fully investigated.
The aim of the present study was to investigate the
temporal trends in routine laboratory parameters characteristic of
COVID-19 according to the clinical outcome, as well as the
potential effects of dexamethasone treatment on patients admitted
to the ICU due to COVID-19, using a large database.
Patients and methods
The present study was a single-center retrospective
cohort study of prospectively collected data derived from the
COVID-19 dataset (formed in March, 2020) for all critically ill
patients admitted to the university ICU at ‘Evangelismos’ Hospital,
a tertiary care center in Athens, Greece, between March, 2020 and
December, 2021. All patients suffered from severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) infection, confirmed by a
real-time reverse transcriptase-polymerase chain reaction assay of
nasopharyngeal swab specimens.
Demographics, comorbidities, the severity of illness
upon admission to the ICU, mechanical ventilation, the length of
stay in the ICU, dexamethasone treatment and the ICU mortality
rates were recorded. The severity of illness was assessed using the
Acute Physiology and Chronic Health Evaluation (APACHE) II
(30) and Sequential Organ Failure
Assessment (SOFA) (31) scoring
systems. Routine laboratory tests for COVID-19, namely
hematological parameters, including white blood cell (WBC) count,
neutrophil count, lymphocyte count, neutrophil to lymphocyte ratio
(NLR), platelet (PLT) count, fibrinogen and D-dimer concentrations,
as well as the biochemical parameters, C-reactive protein (CRP),
lactate dehydrogenase (LDH) and albumin, were selected on the basis
of their routine use, on a daily basis. For analysis, laboratory
values upon admission to the ICU, and on days 3, 5, 10, 15 and 21
post-ICU admission were used. The determination of SARS-CoV-2
variants in the patients with ICU over course of the pandemic was
not performed routinely. However, the consecutive pandemic waves in
Greece followed the global pattern, e.g., prior to the current
Omicron wave, the predominant variants were Delta and Alpha. Taking
into consideration the study period, which was March, 2020 to
December, 2021, the vast majority of the cases in the present study
were attributed to the Alpha and Delta variants.
The collection of anonymized data for the study was
approved by the ‘Evangelismos’ Hospital Ethics Committee (Protocol
No. 116/2021). Informed was obtained from all included
patients.
Statistical analysis
All quantitative data are reported as the mean ± SD.
The diagrams in all figures represent the mean ± SEM values.
Qualitative variables are reported as number and percentage.
Comparisons between the two ICU outcome groups (survivors and
non-survivors) of quantitative variables were performed by using
the independent samples unpaired Student's t-test. Differences
between groups of patients of qualitative variables were assessed
using the Chi-squared or Fisher's exact tests when appropriate.
Repeated measurements were performed by using two-way repeated
measures ANOVA. Whenever sphericity assumption was violated, the
Greenhouse-Geisser test of within subjects effects was used. The
overall mean value of each variable per outcome group, the overall
mean differences of values across time, as well as the group x time
interaction were estimated. In both groups, post-hoc pairwise
within-subjects comparisons across time, as well as post-hoc
between-subjects comparisons at each one of the six time points
were performed. All P-values were adjusted for multiple comparisons
using the Bonferroni correction. The SPSS statistical program
(v.24; Dotmatics) was used for data analysis. A P-value <0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 733 patients (mean age, 65±13 years; 68%
males) were included in the present study. The mean APACHE II and
SOFA scores upon admission to the ICU were 15±6 and 6±3,
respectively. The crude ICU mortality rate was 45%. Of these
patients, 561 (76%) were treated with dexamethasone at a dose of 6
mg daily for up to 10 days. The characteristics of the patients the
whole cohort upon admission in, as well as according to clinical
outcomes, are presented in Table
I.
 | Table IBaseline characteristics of the
patients in the entire cohort and according to ICU outcomes. |
Table I
Baseline characteristics of the
patients in the entire cohort and according to ICU outcomes.
| Parameter | All patients
(n=733) | Survivors
(n=380) | Non-survivors
(n=353) | P-value |
|---|
| Sex, male, n
(%) | 498(68) | 256(67) | 242(68) | 0.75 |
| Age, years | 65±13 | 59±13 | 71±12 | 0.001 |
| Severity
scores | | | | |
|
Charlson
comorbidity index | 3±2 | 2±2 | 4±2 | 0.001 |
|
APACHE II
score | 15±6 | 11±4 | 19±6 | 0.001 |
|
SOFA
score | 6±3 | 4±2 | 8±3 | 0.001 |
| Comorbidities | | | | |
|
CKD, n
(%) | 58(7) | 12(3) | 44(12) | 0.001 |
|
Neoplasm, n
(%) | 68(8) | 17(4) | 45(12) | 0.001 |
|
COPD, n
(%) | 100(12) | 36(9) | 60(17) | 0.003 |
|
Coronary
disease, n (%) | 180(23) | 70(18) | 100(28) | 0.002 |
|
Obesity, n
(%) | 95(12) | 56(14) | 34(9) | 0.04 |
|
Diabetes, n
(%) | 191(24) | 92(24) | 94(26) | 0.5 |
|
Hypertension,
n (%) | 311(39) | 136(35) | 154(43) | 0.05 |
| Other patient
characteristics | | | | |
|
ICU LOS,
days | 21±20 | 22±21 | 19±18 | 0.036 |
|
MV duration,
days | 15±17 | 13±16 | 18±16 | 0.001 |
|
Pre-ICU
hospital stay, days | 4±7 | 3±5 | 5±7 | 0.001 |
|
Days before
hospital admission | 6±3 | 6±3 | 6±3 | 0.03 |
|
PaO2/FiO2
on admission, mmHg | 144±99 | 144±72 | 141±12 | 0.7 |
|
MV upon
admission, n (%) | 549±70 | 219±57 | 296±83 | 0.001 |
|
HFNO upon
admission, n (%) | 154±19 | 110±28 | 40±11 | 0.001 |
|
Shock
presence upon admission, n (%) | 251±32 | 131±34 | 109±30 | 0.2 |
|
CRRT need
during ICU stay, n (%) | 186±23 | 41±10 | 136±38 | 0.001 |
| Hematological
parameters | | | | |
|
WBC,
x103/µl | 11.5±6.4 | 10.3±5.4 | 12.8±7.2 | 0.001 |
|
Lymphocyte
count, x103/µl | 1.1±1.7 | 1.1±1.7 | 1.1±1.7 | 0.65 |
|
Neutrophil
count, x103/µl | 10.5±8.1 | 9.3±7.8 | 11.7±8.5 | 0.001 |
|
NLR | 14.6±12.1 | 12.2±8.9 | 17.4±14.7 | 0.001 |
|
Platelet
count, x103/µl | 258±110 | 269±106 | 242±11 | 0.002 |
|
Hemoglobin,
g/dl | 12.5±9.5 | 13.1±13.1 | 11.8±2.4 | 0.07 |
|
D-dimer,
µg/mla | 5.0±31.6 | 4.9±41.0 | 5.2±18.8 | 0.11 |
|
Fibrinogen,
mg/dl | 590±183 | 601±175 | 578±189 | 0.9 |
| Biochemical
parameters | | | | |
|
C-reactive
protein, mg/dlb | 13±9 | 12±8 | 14±9 | 0.003 |
|
Procalcitonin,
ng/mlc | 2.1±9.6 | 1.1±7.2 | 3.3±12.0 | 0.03 |
|
Troponin T,
pg/mld | 170±752 | 159±71 | 190±828 | 0.6 |
|
LDH,
IU/l | 552±488 | 477±279 | 642±646 | 0.001 |
|
ALT,
IU/l | 67±238 | 54±78 | 84±341 | 0.13 |
|
AST,
IU/l | 91±316 | 69±271 | 120±370 | 0.04 |
|
Creatinine,
mg/dl | 1.2±1.3 | 1.0±1.0 | 1.5±1.5 | 0.001 |
|
Na+,
mmol/l | 140±6 | 138±5 | 141±6 | 0.001 |
|
Ferritin,
µg/l | 1,255±2,022 | 984±1,461 | 1,589±2,518 | 0.004 |
|
Albumin,
g/dl | 3.2±0.5 | 3.3±0.4 | 3.0±0.5 | 0.001 |
|
Lactate,
mmol/l | 2.1±2.1 | 1.6±1.2 | 2.7±2.7 | 0.001 |
|
Dexamethasone
treatment, n (%) | 561±76 | 291±76 | 270±76 | 0.977 |
Temporal trends of hematological
parameters
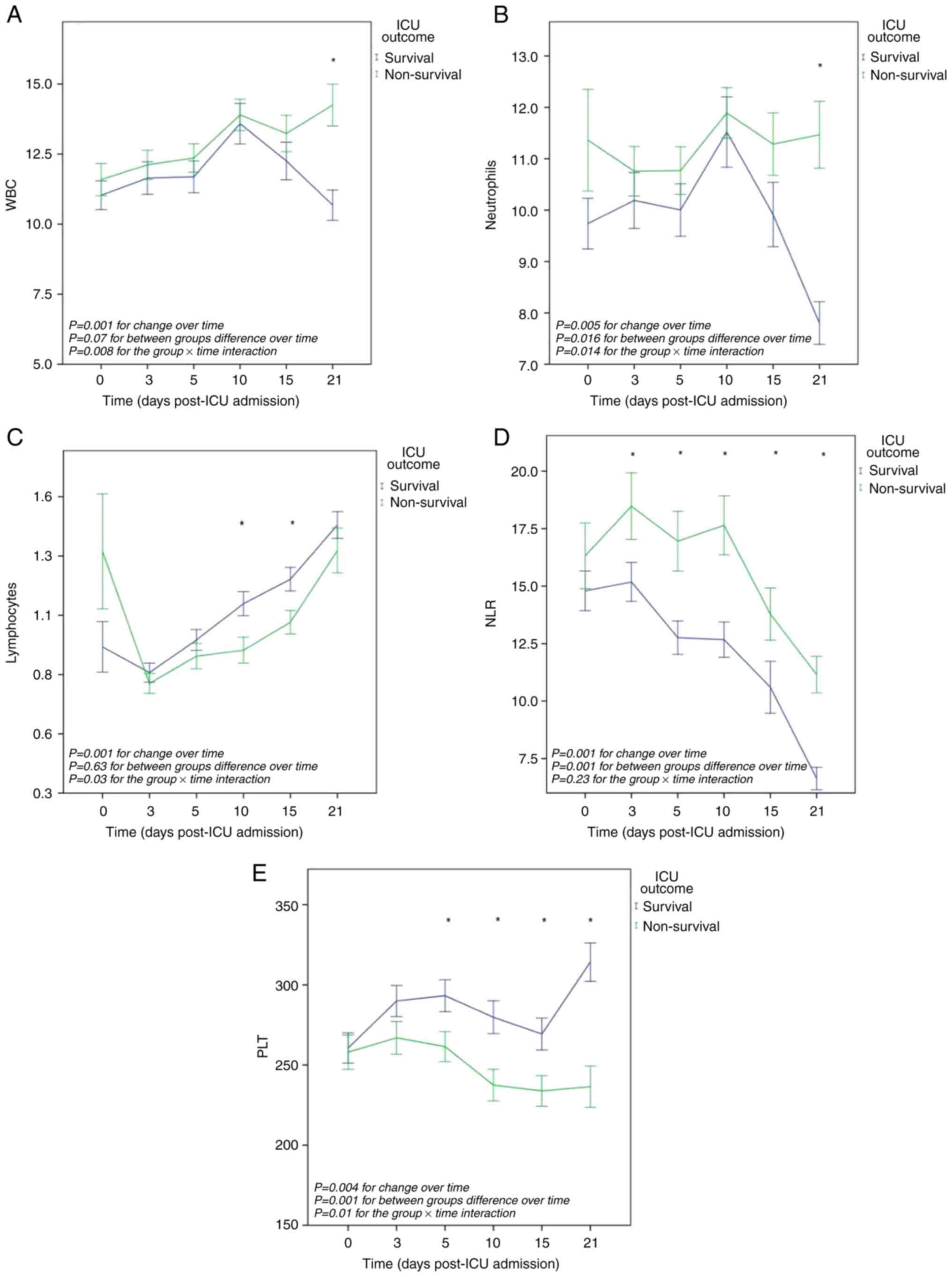
The overall mean value of WBC per outcome group did
not differ significantly. The overall mean differences in WBC
values over time were significant: F(3.8, 858)=6.64, P=0.001. The
group x time interaction was also significant: F(3.8, 858)=3.6,
P=0.008. Post hoc between-subject comparisons at each one of the
six time points revealed a significant difference in the mean WBC
between the two outcome groups (survival and non-survival) on day
21 (-3.5x103/µl, P=0.001) (Fig. 1A).
The overall mean value of neutrophils per outcome
group differed significantly: F(1.226)=5.9, P=0.016. The overall
mean differences in neutrophils values across time were
significant: F (3.7, 852)=3.86, P=0.005. The group x time
interaction was also significant: F(3.7, 873)=3.2, P=0.014.
Post-hoc between-subject comparisons at each one of the six time
points revealed a significant difference in the mean number of
neutrophils between the two outcome groups (survival and
non-survival) on day 21 (-3,6x103/µl, P=0.001) (Fig. 1B).
The overall mean value of lymphocytes per outcome
group did not differ significantly. The overall mean differences in
lymphocyte values over time were significant: F(1.7, 389)=12.3,
P=0.001. However, the group x time interaction was significant:
F(1.7, 389)=3.6, P=0.03. Post-hoc between-subject comparisons at
each one of the six time points revealed a significant difference
in the mean lymphocyte values between the two outcome groups
(survival and non-survival) on day 10 (0.19, P=0.01) and on day 15
(-0.18x103/µl, P=0.01) (Fig.
1C).
The overall mean value of NLR per outcome group
differed significantly: F(1,226)=15, P=0.001. The overall mean
differences in NLR values over time were significant: F(4.2,
952)=21.8, P=0.001. However, the group x time interaction did not
differ significantly. Both groups exhibited distinct trends in the
NLR over time, with a significant increase observed in
non-survivors compared to survivors. Post hoc between-subject
comparisons at each one of the six time points revealed significant
differences in the mean NLR between the two outcome groups
(survival and non-survival) on day 3 (-3.3, P=0.05), day 5 (-4.2,
P=0.004), day 10 (-4.9, P=0.001), day 15 (-3.1, P=0.05) and day 21
(-4.5, P=0.001) (Fig. 1D).
The overall mean value of PLT per outcome group
differed significantly F(1.228)=11.6, P=0.001). The overall mean
differences in PLT values over time were significant: F(3.4,
779)=4.12, P=0.004. The group x time interaction was also
significant: F(3.5, 779)=4.7, P=0.001. Both groups exhibited
distinct trends in PLT levels over time, with a significant
decrease in non-survivors compared to survivors. Post-hoc
between-subject comparisons at each one of the six time points
revealed significant mean PLT differences between the two outcome
groups (survival and non-survival) on day 5 (31x103/µl,
P=0.02), day 10 (42x103/µl, P=0.004), day 15
(35x103/µl, P=0.01) and day 21 (77x103/µl,
P=0.001) (Fig. 1E).
Temporal trends of biochemical
parameters
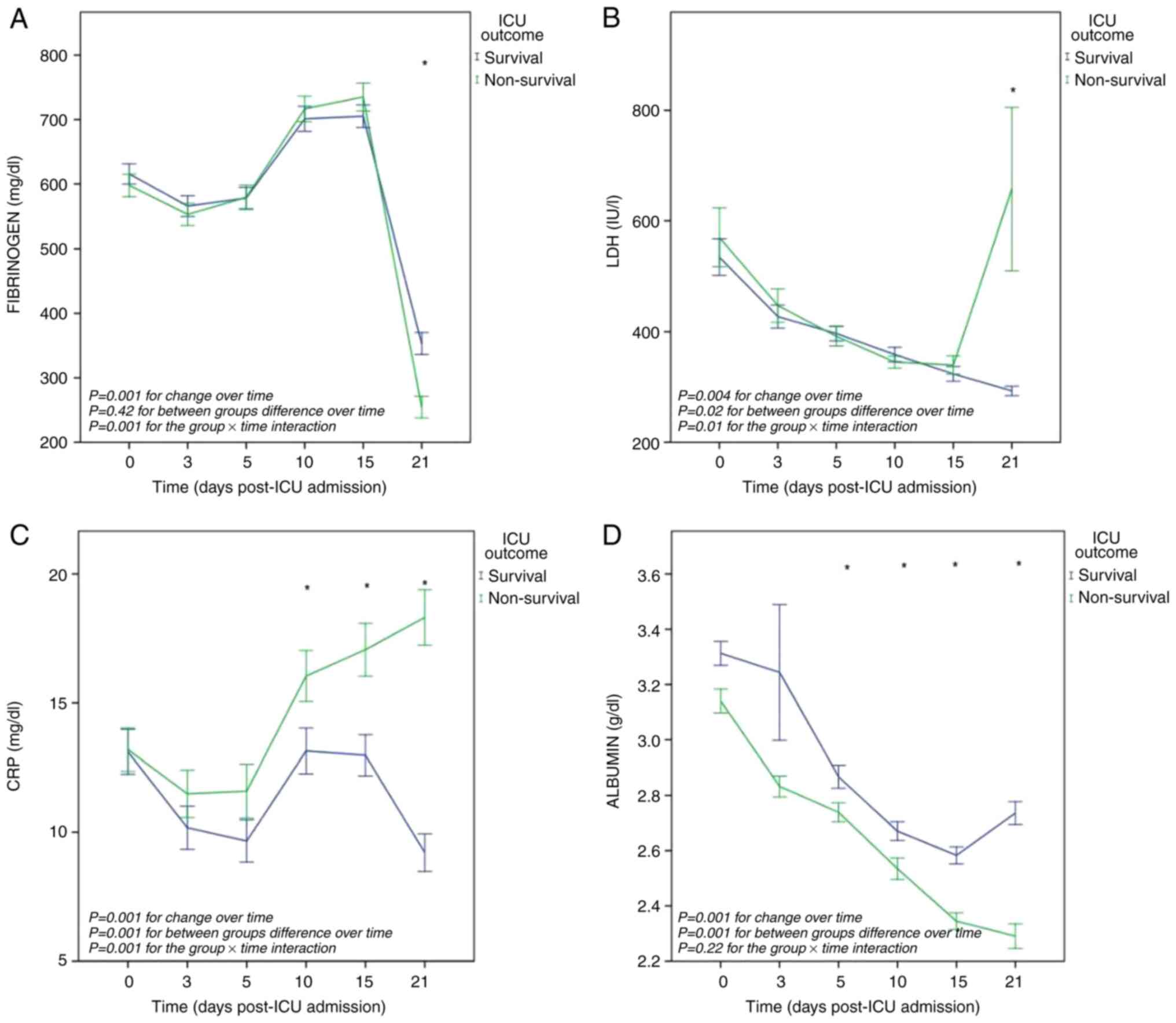
The overall mean value of fibrinogen per outcome
group did not differ significantly. The overall mean differences in
fibrinogen values over time was significant: F(3.9, 879)=218,
P<0.001. The group x time interaction was also significant:
F(3.9, 879)=4.9, P=0.001. Both groups exhibited parallel trends in
fibrinogen levels over time, apart from the values on day 21.
Post-hoc between-subject comparisons at each one of the six time
points revealed significant mean fibrinogen difference between the
two outcome groups (survival and non-survival) on day 21 (98 mg/dl,
P=0.001) (Fig. 2A).
The overall mean value of LDH per outcome group
differed significantly: F(1,227)=5.3, P=0.02. The overall mean
differences in LDH values across time were significant: F(1.4,
323)=6.9, P=0.004. The group x time interaction was also
significant: F(1.4, 323)=5.4, P=0.01. Both groups exhibited
parallel trends in LDH levels across time, apart from the values on
day 21. Post hoc between-subject comparisons at each one of the six
time points revealed significant difference in the mean LDH values
between the two outcome groups (survival and non-survival) on day
21 (-364 IU/l, P=0.008) (Fig.
2B).
The overall mean value of CRP per outcome group
differed significantly: F(1,228)=18.9, P=0.001. The overall mean
differences in CRP values over time were significant:
F(4,936)=10.9, P=0.001. The group x time interaction was also
significant: F(4,936)=7.9, P=0.001. Both groups exhibited distinct
trends in CRP levels over time, with a significant increase in
non-survivors compared to survivors. Post hoc between-subject
comparisons at each one of the six time points revealed significant
mean CRP differences between the two outcome groups (survival and
non-survival) on day 10 (-2.9 mg/dl, P=0.03), day 15 (-4 mg/dl,
P=0.002) and day 21 (-9 mg/dl, P=0.001) (Fig. 2C).
The overall mean value of albumin per outcome group
differed significantly: F(1,272=20, P=0.001. The overall mean
differences in albumin values across time were significant:
F(1.2,287)=28.8, P=0.001. The group x time interaction was not
significant. Both groups exhibited parallel, declining, though
distinct trends in albumin levels over time, with a significant
decrease in non-survivors compared to survivors. Post-hoc
between-subject comparisons at each one of the six time points
revealed significant mean albumin differences between the two
outcome groups (survival and non-survival) on day 5 (0.12 g/dl,
P=0.02), day 10 (0.13 g/dl, P=0.008), day 15 (0.23 g/dl, P=0.001)
and day 21 (0.44 g/dl, P=0.001) (Fig.
2D).
Sensitivity analysis. Effects of
dexamethasone on temporal trends of laboratory parameters in the
whole population
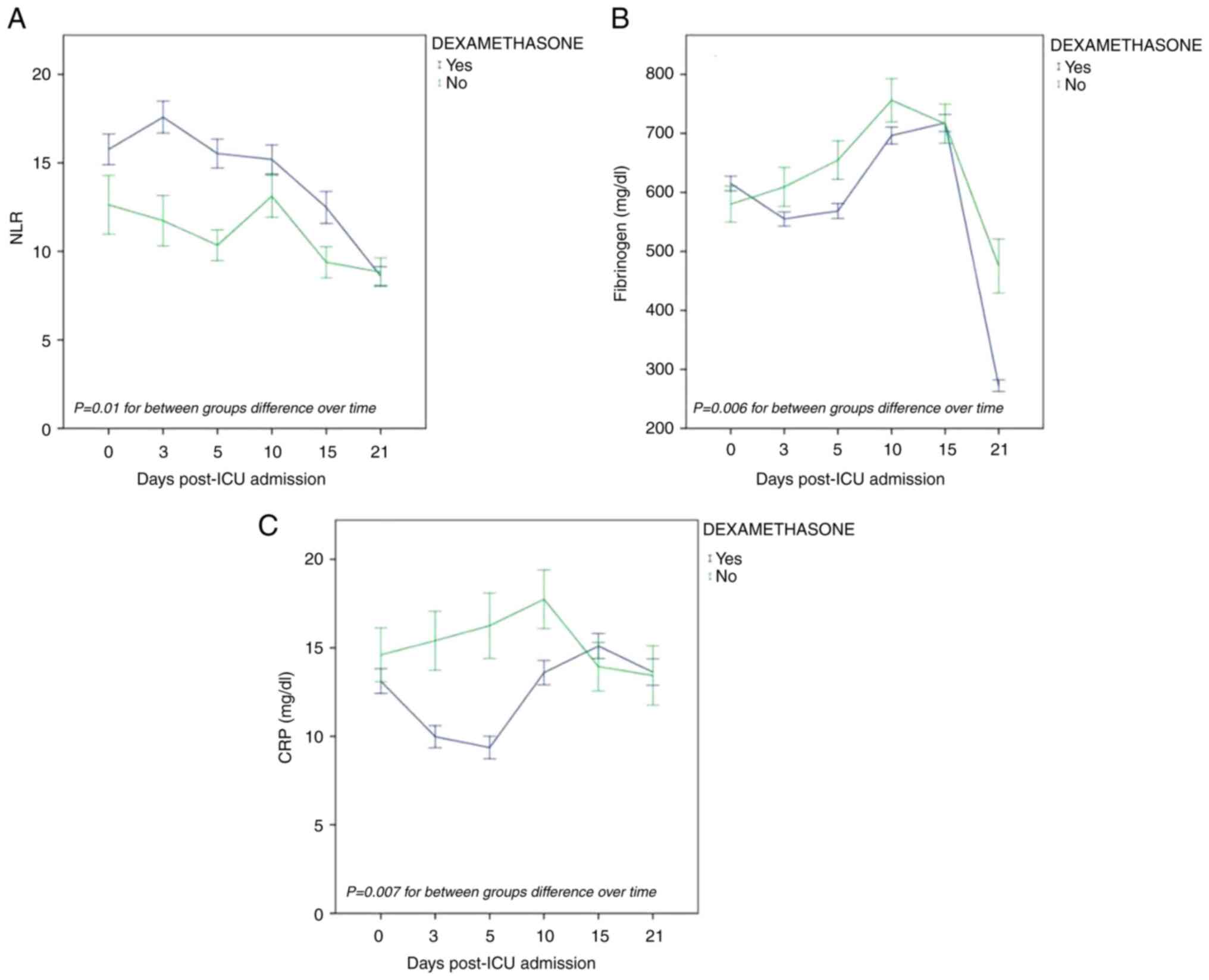
To determine the effects dexamethasone on the
laboratory data, two-way repeated measures analyses for the same
parameters that were analyzed above were performed. The patients
were categorized into two treatment groups, according to the
administration of dexamethasone or not. Only the overall mean
values of NLR, fibrinogen and CRP per treatment group differed
significantly: [F(1,237)=6.81, P=0.01], [F(1,234)=7.38, P=0.006]
and [F(1, 240)=7.37, P=0.007], respectively. More specifically,
both fibrinogen and CRP exhibited declining trends over time in the
dexamethasone group as compared to the group of patients who did
not receive dexamethasone. On the contrary, NLR exhibited an
increasing trend (Fig. 3).
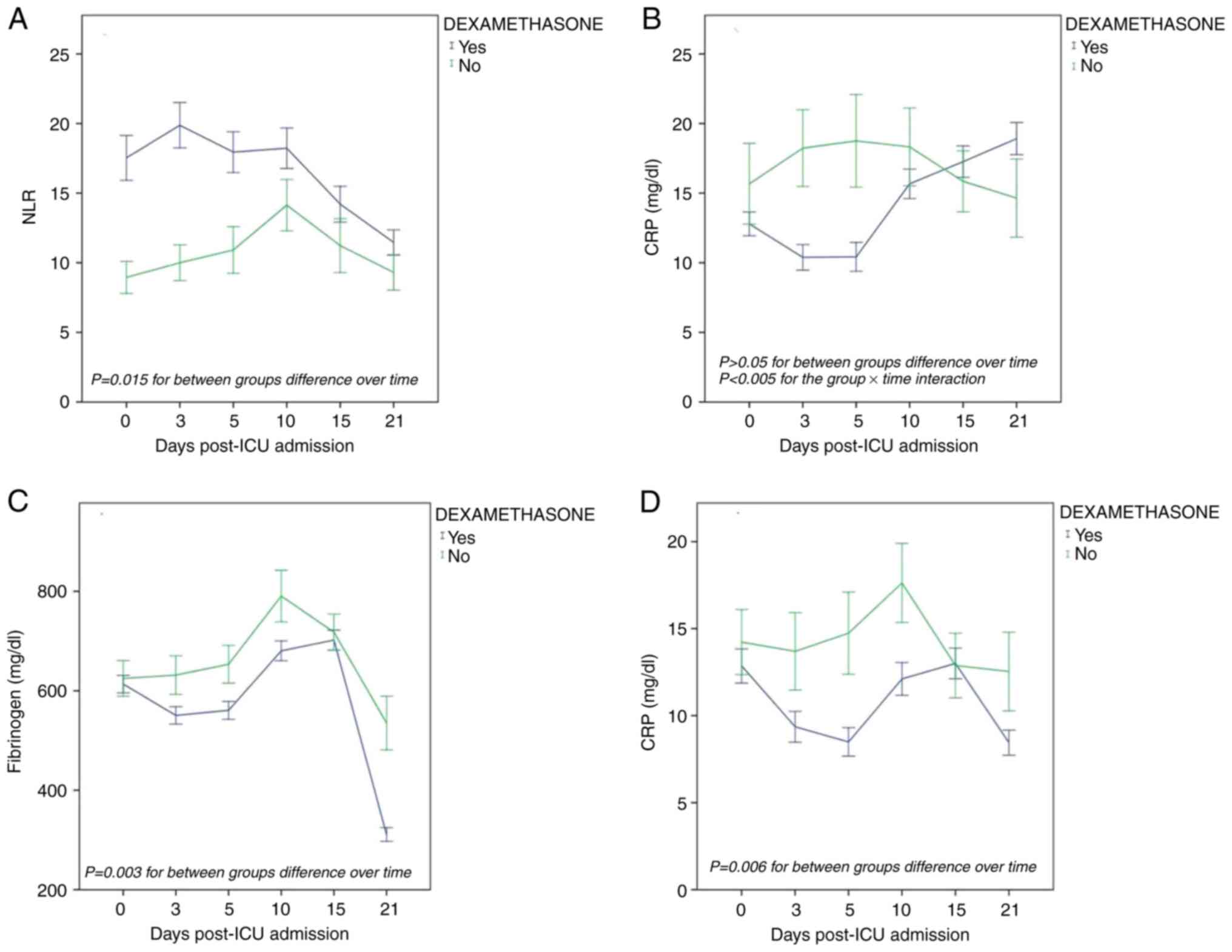
Effects of dexamethasone on the temporal trends
of laboratory parameters in non-survivors. Only the overall
mean value of NLR per treatment group differed significantly [F(1,
104)=6.17, P=0.015], exhibiting an increasing trend over time in
the dexamethasone group as compared to the group of patients who
did not receive dexamethasone (Fig.
4A). No significant difference was observed in the fibrinogen
and CRP levels between the two treatment groups. However, CRP x
dexamethasone interaction was significant [F(4,429)=3.75, P=0.005].
More specifically, CRP exhibited an initially decreasing trend
during the first 10 days in the dexamethasone group and thereafter,
an increasing trajectory (Fig.
4B).
Effects of dexamethasone on temporal trends of
laboratory parameters in survivors. Only the overall mean
values of fibrinogen and CRP per treatment group differed
significantly: [F(1,120)=9.32, P=0.003] and [F(1,121)=7.73,
P=0.006)], respectively. They both exhibited a decreasing
trajectory over time in the dexamethasone group as compared to the
group of patients who did not receive dexamethasone (Fig. 4C and D). There was no significant difference in
NLR between the two treatment groups.
Discussion
The present study describes the temporal progression
of routine laboratory markers, characteristic of COVID-19, in a
cohort of 733 patients critically ill with COVID-19. The main
findings were as follows: i) Persistently abnormal laboratory
values in both survivors and non-survivors; ii) significant
differences between survivors and non-survivors concerning the
dynamic changes of NLR, CRP, PLT and albumin, but not of WBC and
neutrophil count, fibrinogen and LDH over time; iii) significant
effects of dexamethasone treatment on the temporal progression of
fibrinogen and CRP values in survivors and that of NLR in
non-survivors.
Similar to previous studies (28,32,33),
WBC and neutrophil counts upon admission to the ICU, as well as
within the first 15 days post-admission were steadily elevated,
indicating persistent inflammatory activation; however, they did
not differ significantly between survivors and non-survivors.
Therefore, though a notable decrease in both counts was observed in
survivors on day 21 after admission, the temporal changes of these
variables cannot be used in prognostication. By contrast,
previously (17), WBC and
neutrophil counts were elevated over time only in non-survivors;
however, all hospitalized, and not exclusively patients with
COVID-19 in the ICU, were included in that study.
Both survivors and non-survivors had profound
lymphopenia upon admission, probably reflecting the severity of
COVID-19 in this critically ill population. Notably, during the
stay in the ICU, both groups had persistent lymphopenia, with a
nadir on day 3. Despite the gradual recovery observed thereafter,
consistent with that previously reported (26), the mean lymphocyte count did not
reach normal values within the 3 weeks of observation (remaining
<1,500/µl). Although significantly different mean lymphocyte
counts between survivors and non-survivors were observed only at
two time points (days 10 and 15), lower values in non-survivors
were steadily observed.
Even more impressive than the evolution of
lymphocyte values was the association between the temporal trends
of NLR values and survival. Highly elevated upon admission to the
ICU, NLR values did not differ significantly between survivors and
not survivors, in contrast to those previously reported by Zanella
et al (23) and Υe et
al (34). Subsequently, the
slopes of NLR were distinctly different between survivors and
non-survivors, with steadily higher values in non-survivors at each
time point. It should be noted that in survivors, although the mean
NLR values were gradually decreasing, apart from day 21, they
remained elevated, namely >10, indicating, along with the
non-resolving lymphopenia, a persistent inflammation. These
temporal changes in NLR are consistent with those of previous
studies (27,28,35),
confirming the superior role of NLR, as compared to the WBC count,
concerning risk stratification in the clinical context of
COVID-19.
Whereas the PLT count upon admission did not differ
between the two outcome groups, contrary to that reported elsewhere
(23,28), their temporal trend exhibited
significant differences between the two groups, with ICU survivors
presenting consistently higher counts. This is in accordance with
previous findings (23,28,36).
It should be noted that PLT counts in the patients in the present
study were maintained mostly within or higher than the normal
range, indicating a hypercoagulation state that persisted over
time. This finding is in accordance with that by Wendel Garcia
et al (24), although they
are in contrast to findings reported elsewhere showing
thrombocytopenia in patients critically ill with COVID-19 (27,31). The lower counts of PLT in
non-survivors relative to survivors may be attributed to their more
severe inflammatory status, leading to increased PLT destruction,
either mechanically through disseminated intravascular coagulation,
or immunologically.
Notable differences in temporal changes were also
found for CRP values. The high CRP values upon admission to the ICU
gradually decreased over the first 5 days in all patients. Such a
decrease has not been described in studies conducted during the
first wave of the pandemic (36).
This could be explained by the dexamethasone treatment which has
been recommended for severe COVID-19 after the first wave (29). Accordingly, Zacharias et al
(37) recently evaluated the
effects of dexamethasone on the trajectory of CRP values among
critically ill patients with COVID-19, demonstrating, similarly to
the findings of the present study, a significant reduction in CRP
in the first 3 days of treatment. Of note, in the present study,
following an initial decrease, a second increase in CRP values was
observed on days 10 and 15. A plausible explanation for this
increase may be the onset of nosocomial infections, usually
complicating the clinical course (38,39).
Indeed, in patients critically ill with COVID-19, ICU-acquired
bacteremia developed after a median time of 11 days after ICU
admission, as shown in a previous study from the ICU (40). Both survivors and non-survivors
exhibited similar trends up to and including day 10. After that
day, CRP values in non-survivors continued to increase, signaling a
more intense inflammatory response, whereas a tendency towards
normalization was observed in survivors.
A downward trend in LDH in both survivors and
non-survivors was observed in the present study. Although overall
significantly different over time, LDH values did not differ
between survivors and non-survivors, except for day 21. By
contrast, significant differences in temporal changes of LDH
between survivors and non-survivors were reported by Xie et
al (22) and by Wendel Garcia
et al (24), as well as by
two additional studies (23,41),
with LDH levels remaining elevated in patients with unfavorable
outcomes. Of note, the sharp increase of LDH on day 21 in
non-survivors in the present study is in accordance with a similar
increase of LDH on the day of or a day prior to death, in the study
by Chen et al (28). This,
combined with the sharp decrease in fibrinogen occurring on the
same day, could possibly indicate widespread tissue damage due to
ischemia, in the context of disseminated intravascular coagulation
secondary to late bacterial sepsis.
Finally, albumin kinetics exhibited a rapid decrease
following ICU admission, regardless of outcomes. Although initially
not different, serum albumin levels were persistently lower in
non-survivors compared to survivors. These findings are consistent
with those of previous studies (23,28,36).
Notably, following an initial deterioration phase, the albumin
levels improved after the 15th day in survivors, highlighting a
recovery phase. Notably, such a recovery time point occurred
earlier, at ~7 days after ICU admission, in the study by Su et
al (42). Thus far, additional
data reporting albumin kinetics in the context of COVID-19 are
lacking.
Information regarding the effects of dexamethasone
on the temporal progression of laboratory values in patients with
COVID-19 is limited (37). Since
dexamethasone is known to suppress the pro-inflammatory response,
the finding that, among survivors, both fibrinogen and CRP
exhibited decreasing values over time in patients treated with
dexamethasone as compared to those who did not, appears plausible.
On the other hand, the finding of an increasing NLR in
non-survivors who received dexamethasone, compared to those who did
not, could reflect their enhanced vulnerability caused be
dexamethasone to secondary bacterial infections and the subsequent
neutrophilia.
The present study had certain limitations, which
should be mentioned. First, some laboratory tests characteristic of
COVID-19, such as ferritin, D-dimer and various cytokines, were not
included in the present study as the selection of parameters was
based on their routine daily measurement; thus, the full laboratory
spectrum of COVID-19 was not analyzed. Second, age-related
differences in laboratory features, which may have played a role
(43) in the interpretation of the
results, were not considered. Third, as in almost all relevant
studies, comparisons with non-COVID-19 ICU patients were not
included. Therefore, it remains unclear whether laboratory trends
in COVID-19 differ from those in other causes of infection.
Nevertheless, the present large cohort study, based on
prospectively collected data, reveals the importance of
longitudinal data compared to a sole measurement on admission and
provides further information regarding the temporal trends in
laboratory parameters according to clinical outcomes, as well as
the effects of dexamethasone treatment on the laboratory values,
useful for prognostic models and risk stratification in this
critically ill subpopulation.
In conclusion, the present study demonstrates the
association between the temporal progression of routine laboratory
variables and clinical outcomes in patients admitted to the ICU due
to COVID-19. There were significant differences between survivors
and non-survivors concerning the dynamic changes over time of NLR,
CRP, PLT and albumin, but not of WBC and neutrophil count,
fibrinogen or LDH. The aforementioned differences may represent
distinct sub-phenotypes or endotypes of COVID-19, the further
elucidation of which may potentially have a profound effect on
treatment. Finally, dexamethasone treatment significantly affected
the temporal progression of fibrinogen and CRP values in survivors
and that of NLR and CRP in non-survivors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AKa, SKo and CR conceptualized the study. AKa, SKa,
CR, VEG, AG, DAS, DK, SKo, PK, TN, CG, MAK, GD, ET, SO and AKo made
a substantial contribution to data collection, interpretation and
analysis, and wrote and prepared the draft of the manuscript. SKo
and AKa analyzed the data and provided critical revisions. SK, AKo
and CR confirm the authenticity of all the data. All authors
contributed to manuscript revision, and have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in line with the
Declaration of Helsinki and obtained approval from the
‘Evangelismos’ Hospital Ethics Committee (protocol No. 116/2021).
Informed was obtained from all included patients.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO):
Coronavirus disease (COVID2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
Accessed October 22, 2020.
|
|
2
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grasselli G, Greco M, Zanella A, Albano G,
Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli
G, et al: Risk factors associated with mortality among patients
with COVID-19 in intensive care units in Lombardy, Italy. JAMA
Intern Med. 180:1345–1355. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li C, He Q, Qian H and Liu J: Overview of
the pathogenesis of COVID-19 (Review). Exp Ther Med.
22(1011)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng Z, Chen Y, Wu Y, Wang J, Zhang H and
Zhang W: Kidney involvement in coronavirus-associated diseases
(Review). Exp Ther Med. 21(361)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fukui S, Ikeda K, Kobayashi M, Nishida K,
Yamada K, Horie S, Shimada Y, Miki H, Goto H, Hayashi K, et al:
Predictive prognostic biomarkers in patients with COVID-19
infection. Mol Med Rep. 27(15)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Daher J: Endothelial dysfunction and
COVID-19 (Review). Biomed Rep. 15(102)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lippi G and Plebani M: Laboratory
abnormalities in patients with COVID-2019 infection. Clin Chem Lab
Med. 58:1131–1134. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ye J, Jiao Y, Zhang Y, Li Z, Zeng X, Deng
H and Yang M: Hematological changes in patients with COVID-19
(Review). Mol Med Rep. 22:4485–4491. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Henry BM, de Oliveira MHS, Benoit S,
Plebani M and Lippi G: Hematologic, biochemical and immune
biomarker abnormalities associated with severe illness and
mortality in coronavirus disease 2019 (COVID-19): A meta-analysis.
Clin Chem Lab Med. 58:1021–1028. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Skevaki C, Fragkou PC, Cheng C, Xie M and
Renz H: Laboratory characteristics of patients infected with the
novel SARS-CoV-2 virus. J Infect. 81:205–212. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang Y, Zhang Y and Ma L: Meta-analysis
of laboratory results in patients with severe coronavirus disease
2019. Exp Ther Med. 21(449)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bohn MK, Lippi G, Horvath A, Sethi S, Koch
D, Ferrari M, Wang CB, Mancini N, Steele S and Adeli K: Molecular,
serological, and biochemical diagnosis and monitoring of COVID-19:
IFCC taskforce evaluation of the latest evidence. Clin Chem Lab
Med. 58:1037–1052. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Georgakopoulou VE, Garmpis N, Damaskos C,
Valsami S, Dimitroulis D, Diamantis E, Farmaki P, Papageorgiou CV,
Makrodimitri S, Gravvanis N, et al: The impact of peripheral
eosinophil counts and eosinophil to lymphocyte ratio (ELR) in the
clinical course of COVID-19 patients: A retrospective study. In
Vivo. 35:641–648. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Georgakopoulou VE, Makrodimitri S,
Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A,
Papageorgiou CV, Spandidos DA, Gkoufa A, Papalexis P, et al:
Immature granulocytes: Innovative biomarker for SARS-CoV-2
infection. Mol Med Rep. 26(217)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bali T, Georgakopoulou VE, Kamiliou A,
Vergos I, Adamantou M, Vlachos S, Ermidis G, Sipsas NV, Samarkos M
and Cholongitas E: Abnormal liver function tests and coronavirus
disease 2019: A close relationship. J Viral Hepat. 30:79–80.
2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Georgakopoulou VE, Bali T, Adamantou M,
Asimakopoulou S, Makrodimitri S, Samara S, Triantafyllou M,
Voutsinas PM, Eliadi I, Karamanakos G, et al: Acute hepatitis and
liver injury in hospitalized patients with COVID-19 infection. Exp
Ther Med. 24(691)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Georgakopoulou VE, Lembessis P, Skarlis C,
Gkoufa A, Sipsas NV and Mavragani CP: Hematological abnormalities
in COVID-19 disease: Association with type I interferon pathway
activation and disease outcomes. Front Med (Lausanne).
9(850472)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cholongitas E, Bali T, Georgakopoulou VE,
Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D,
Basoulis D, Eliadi I, Karamanakos G, et al: Prevalence of abnormal
liver biochemistry and its impact on COVID-19 patients' outcomes: A
single-center Greek study. Ann Gastroenterol. 35:290–296.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ouyang SM, Zhu HQ, Xie YN, Zou ZS, Zuo HM,
Rao YW, Liu XY, Zhong B and Chen X: Temporal changes in laboratory
markers of survivors and non-survivors of adult inpatients with
COVID-19. BMC Infect Dis. 20(952)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Juneja GK, Castelo M, Yeh CH, Cerroni SE,
Hansen BE, Chessum JE, Abraham J, Cani E, Dwivedi DJ, Fraser DD, et
al: Biomarkers of coagulation, endothelial function, and
fibrinolysis in critically ill patients with COVID-19: A
single-center prospective longitudinal study. J Thromb Haemost.
19:1546–1557. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xie J, Wu W, Li S, Hu Y, Hu M, Li J, Yang
Y, Huang T, Zheng K, Wang Y, et al: Clinical characteristics and
outcomes of critically ill patients with novel coronavirus
infectious disease (COVID-19) in China: A retrospective multicenter
study. Intensive Care Med. 46:1863–1872. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zanella A, Florio G, Antonelli M, Bellani
G, Berselli A, Bove T, Cabrini L, Carlesso E, Castelli GP, Cecconi
M, et al: Time course of risk factors associated with mortality of
1260 critically ill patients with COVID-19 admitted to 24 Italian
intensive care units. Intensive Care Med. 47:995–1008.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wendel Garcia PD, Fumeaux T, Guerci P,
Heuberger DM, Montomoli J, Roche-Campo F, Schuepbach RA and Hilty
MP: RISC-19-ICU Investigators. Prognostic factors associated with
mortality risk and disease progression in 639 critically ill
patients with COVID-19 in Europe: Initial report of the
international RISC-19-ICU prospective observational cohort.
EClinicalMedicine. 25(100449)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu
W, Xie J, Guan W, Liang W, Ni Z, et al: Longitudinal hematologic
and immunologic variations associated with the progression of
COVID-19 patients in China. J Allergy Clin Immunol. 146:89–100.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lim AYH, Goh JL, Chua MCW, Heng BH,
Abisheganaden JA and George PP: Temporal changes of haematological
and radiological findings of the COVID-19 infection-a review of
literature. BMC Pulm Med. 21(37)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kokkoris S, Kanavou A, Kremmydas P,
Katsaros D, Karageorgiou S, Gkoufa A, Georgakopoulou VE, Spandidos
DA, Giannopoulos C, Kardamitsi M and Routsi C: Temporal evolution
of laboratory characteristics in patients critically ill with
COVID-19 admitted to the intensive care unit (Review). Med Int
(Lond). 3(52)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen A, Zhao Z, Hou W, Singer AJ, Li H and
Duong TQ: Time-to-death longitudinal characterization of clinical
variables and longitudinal prediction of mortality in COVID-19
patients: A two-center study. Front Med (Lausanne).
8(661940)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
RECOVERY Collaborative Group. Horby P, Lim
WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N,
Brightling C, Ustianowski A, et al: Dexamethasone in hospitalized
patients with COVID-19. N Engl J Med. 384:693–704. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Knaus WA, Wagner DP, Draper EA and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985.PubMed/NCBI
|
|
31
|
Vincent JL, Moreno R, Takala J, Willatts
S, De Mendonça A, Bruining H, Reinhart CK, Suter PM and Thijs LG:
The SOFA (sepsis-related organ failure assessment) score to
describe organ dysfunction/failure. On behalf of the working group
on sepsis-related problems of the European society of intensive
care medicine. Intensive Care Med. 22:707–710. 1996.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng Y, Sun LJ, Xu M, Pan J, Zhang YT,
Fang XL, Fang Q and Caiet HL: Clinical characteristics of 34
COVID-19 patients admitted to intensive care unit in Hangzhou,
China. J Zhejiang Univ Sci B. 21:378–387. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel Coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ye W, Chen G, Li X, Lan X, Ji C, Hou M,
Zhang D, Zeng G, Wang Y, Xu C, et al: Dynamic changes of D-dimer
and neutrophil-lymphocyte count ratio as prognostic biomarkers in
COVID-19. Respir Res. 21(169)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhou S, Yang Y, Zhang X, Li Z, Liu X, Hu
C, Chen C, Wang D and Peng Z: Clinical course of 195 critically ill
COVID-19 patients: A retrospective multicenter study. Shock.
54:644–651. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
van Oers JAH, Kluiters Y, Bons JAP, de
Jongh M, Pouwels S, Ramnarain D, de Lange DW, de Grooth HJ and
Girbes ARJ: Endothelium-associated biomarkers mid-regional
proadrenomedullin and C-terminal proendothelin-1 have good ability
to predict 28-day mortality in critically ill patients with
SARS-CoV-2 pneumonia: A prospective cohort study. J Crit Care.
66:173–180. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zacharias H, Mungara R, Wilson AP, Singer
M and Arulkumaran N: The utility of CRP with the use of
dexamethasone and Tocilizumab in critically ill patients with
COVID-19. J Crit Care. 70(154053)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Du Q, Zhang D, Hu W, Li X, Xia Q, Wen T
and Jia H: Nosocomial infection of COVID-19: A new challenge for
healthcare professionals (Review). Int J Mol Med.
47(31)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Susan M, Susan R, Lazar V, Bagiu IC, Mihu
AG, Bagiu RV, Ionescu A, Iana AN, Dehelean CA, Lighezan D and Marti
DT: COVID-19 association with multidrug-resistant bacteria
superinfections: Lessons for future challenges. Exp Ther Med.
25(254)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kokkoris S, Papachatzakis I, Gavrielatou
E, Ntaidou T, Ischaki E, Malachias S, Vrettou C, Nichlos C, Kanavou
A, Zervakis D, et al: ICU-acquired bloodstream infections in
critically ill patients with COVID-19. J Hosp Infect. 107:95–97.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Montrucchio G, Sales G, Rumbolo F,
Palmesino F, Fanelli V, Urbino R, Filippini C, Mengozzi G and
Brazzi L: Effectiveness of mid-regional pro-adrenomedullin
(MR-proADM) as prognostic marker in COVID-19 critically ill
patients: An observational prospective study. PLoS One.
16(e0246771)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Su C, Hoffman KL, Xu Z, Sanchez E, Siempos
II, Harrington JS, Racanelli AC, Plataki M, Wang F and Schenck EJ:
Evaluation of albumin kinetics in critically ill patients with
coronavirus disease 2019 compared to those with sepsis-induced
acute respiratory distress syndrome. Crit Care Explor.
3(e0589)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vakili S, Savardashtaki A, Jamalnia S,
Tabrizi R, Nematollahi MH, Jafarinia M and Akbari H: Laboratory
findings of COVID-19 infection are conflicting in different age
groups and pregnant women: A literature review. Arch Med Res.
51:603–607. 2020.PubMed/NCBI View Article : Google Scholar
|