Introduction
Bronchogenic cysts are rare congenital malformations
of the bronchial tree (a type of bronchopulmonary foregut
malformation) with an incidence of ~1 in 42,000 admissions at St.
Joseph Hospital between May 1975 and August 1986 (Houston, TX, USA)
(1) and are detected as a cystic
and/or mass lesion in the thoracic cavity, particularly in the
mediastinum or pulmonary parenchyma (2,3).
Bronchogenic cysts account for 6-15% of primary mediastinal masses
(4), while pulmonary parenchymal
bronchogenic cysts are ~20% of all bronchogenic cysts in the
thoracic cavity (5,6). Clinical manifestations of bronchogenic
cysts vary from asymptomatic incidental radiological findings to
large mass lesions with severe symptoms. The most common symptom is
chest pain, in particular, retrosternal or lateral chest pain,
depending on the lesion location. Other symptoms include cough,
dysphagia and fever, which are also dependent on compression to
other organs, rupture or complicating infection (5-7).
In pediatric patients, bronchogenic cysts often cause
life-threatening emergencies with airway obstruction resulting in
atelectasis, air trapping and respiratory distress as compressive
symptoms. Conversely, in adult cases, although some symptoms are
seen in 20-40% of patients, bronchogenic cysts often result in
incidental radiological findings with computed tomography (CT)
and/or magnetic resonance imaging (4,5).
Surgical therapy should be considered for symptomatic patients with
bronchogenic cysts as a primary treatment to prevent complications
and histopathologically determine a final diagnosis (3).
The key histopathological characteristic of
bronchogenic cysts is the pseudostratified ciliated columnar
epithelium (3). Govaerts et
al (8) defined the criteria for
bronchogenic cysts as the presence of pseudostratified, ciliated,
columnar epithelium in addition to the presence of at least one of
cartilage, smooth muscle or seromucous glands. Although
bronchogenic cysts are primarily located in the thoracic cavity,
however, they could ectopically occur anywhere along the
developmental pathway of the foregut. Other distant locations have
been reported, such as the skin, left ventricle and intraabdominal
and retroperitoneal regions (9-11).
However, it is unclear whether these distant lesions meet the
aforementioned criteria and express the phenotypes of the
respiratory epithelium. The present study retrospectively reviewed
cases diagnosed as bronchogenic cysts that were surgically
resected, including location of the bronchogenic cysts and how many
lesions in rare sites there were. For bronchogenic cysts in rare
sites, we reconfirmed the diagnoses based on the aforementioned
criteria and characterized the epithelial phenotypes.
Materials and methods
Samples
The present study reviewed 34 available bronchogenic
cysts from patients (age, 1 to 85-year-old, male/female: 22/12),
that were surgically resected at the Osaka Medical and
Pharmaceutical University Hospital (Takatsuki, Osaka, Japan) from
January 1998 to December, 2020. Representative hematoxylin and
eosin (H&E)-stained sections were selected and two pathologists
reexamined the pathological features and diagnoses according to
anatomopathological criteria (8).
Immunohistochemistry (IHC)
The phenotypes of the epithelium lining the cyst
were characterized using IHC. The tissue specimens were fixed in
10% neutral buffered formalin overnight at room temperature and
embedded in paraffin wax for H&E staining. IHC analysis was
performed using Vectastain Elite ABC kits (cat. no. PK-6100) from
Vector Laboratories, Inc. (Maravai LifeSciences) according to the
manufacturer's instructions for blocking, dilution of biotinylated
secondary antibody and labeling. Briefly, 4-µm-thick sections were
cut from the paraffin block. Following deparaffinization with
xylene and hydration with 100% ethanol, endogenous peroxidase
activity was quenched by incubation in 3% hydrogen peroxide
solution for 10 min at room temperature. Antigen retrieval was
performed using a preheated target retrieval solution (pH 6.0;
Dako; Agilent Technologies, Inc.) for 30 min in a boiling rice
cooker. The sections were incubated with primary antibodies and
biotinylated secondary antibody at room temperature for 60 and 30
min, respectively. 3,3-diaminobenzidine was freshly prepared from
tablets (Sigma-Aldrich; Merck KGaA) for chromogenic staining at
20˚C for 10 min. IHC-stained sections were evaluated using a light
microscope at x20 to x400 magnification.
Primary antibodies against CK7 (cat. no. OV-TL
12/30; 1:200), CK20 (cat. no. Ks20.8; 1:200), thyroid transcription
factor 1 (TTF-1, cat. no. 8G7G3/1; 1:50), synaptophysin (Syp, cat.
no. DAK-SYNAP; 1:50), and surfactant protein A (SP-A, cat. no.
PE10; 1:800) were obtained from Dako (Agilent Technologies).
Anti-p40 (cat. no. BC28; 1:100) antibody for detecting basal cells
was obtained from Nichirei Biosciences, Inc. Anti-α-smooth muscle
actin (α-SMA, cat. no. 1A4; 1:800) and napsin A (cat. no. IP64;
1:400) antibodies were obtained from Sigma-Aldrich (Merck KGaA) and
Leica Biosystems, respectively. Biotinylated secondary antibody
(dilution, 1:200) was included in Vectastain Elite ABC kit.
Results
Bronchogenic cysts are found in rare
sites
The locations of the 34 bronchogenic cysts are
listed in Table I; most were
located in the mediastinum or intrapulmonary region. Rare sites
included the retroperitoneum, skin, spinal cord and pericardial
cavity.
 | Table ILocation of bronchogenic cysts. |
Table I
Location of bronchogenic cysts.
| Site | Number |
|---|
| Mediastinum | 21 |
| Intrapulmonary | 6 |
| Neck | 1 |
|
Retroperitoneum | 2 |
| Skin | 2 |
| Spinal cord | 1 |
| Pericardial
cavity | 1 |
Retroperitoneal bronchogenic
cysts
A total of two cases of retroperitoneal bronchogenic
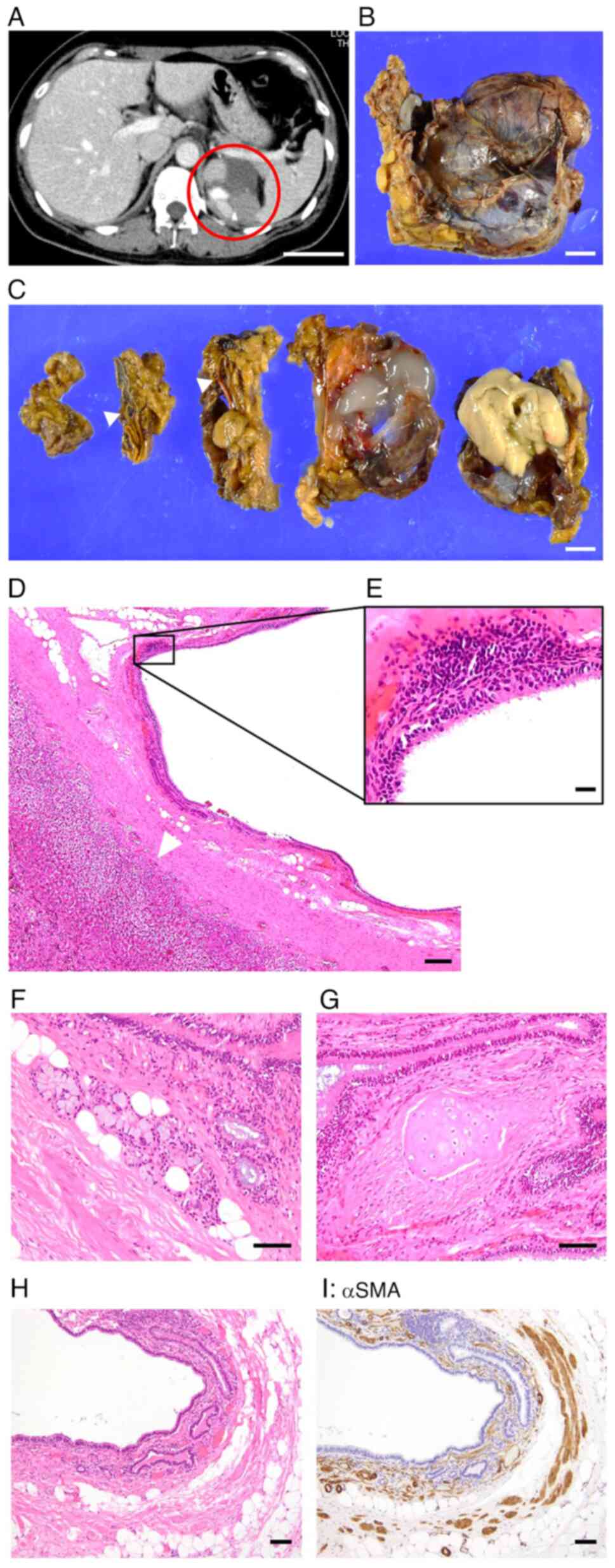
cyst were identified. One was a mass and cystic lesion on the left
side of the retroperitoneum in a 40-year-old female, which was
detected during a medical checkup (Fig.
1A). The patient was asymptomatic and medical and family
histories were unremarkable. Laboratory data, including hormone
levels (such as catecholamine), were within normal range. Based on
CT, neoplastic disease in the adrenal gland was a differential
diagnosis. Surgical excision of the lesion was performed to obtain
a pathological diagnosis. The specimen was a brownish, soft mass
lesion in the left adrenal gland and measured 5.5x5.0x3.5 cm
(Fig. 1B). The divided surfaces
after cross-sectioning revealed a multicystic lesion, including
atmospheric gelatinous or light-yellow contents. Macroscopically,
there was an intact adrenal gland (Fig.
1C), indicating that the lesion was not derived from it.
Microscopic examination revealed the cyst was lined
with ciliated epithelium and separated from the intact adrenal
gland (Fig. 1D and E). Furthermore, seromucous glands
(Fig. 1F), cartilage (Fig. 1G) and smooth muscle stained with
anti-αSMA antibody (Fig. 1H and
I) were detected near the cystic
lesion and met the criteria for bronchogenic cysts. Considering
these results, the diagnosis of retroperitoneal bronchogenic cyst
was confirmed.
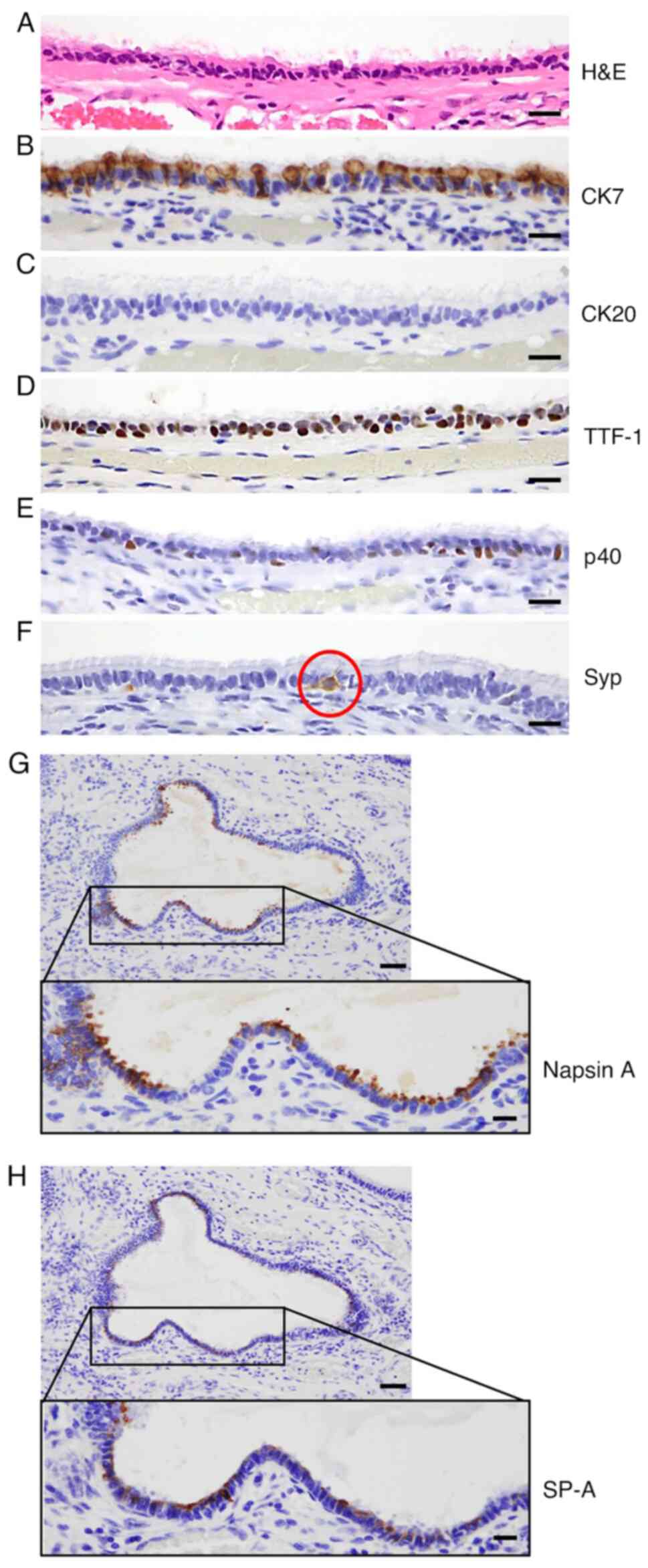
Epithelial phenotype was characterized by IHC. CK7
(a cytokeratin marker), TTF-1, p40, Syp (a neuroendocrine marker),
napsin A, which is preferentially expressed in bronchial and
alveolar epithelium, and SP-A (secreted by alveolar type II cells)
(12) showed positive staining in
the ciliated epithelium (Fig. 2A,
B and D-H), whereas, CK20 was negative (Fig. 2C). These IHC features were similar
to those of respiratory epithelium (CK7-positive and CK20-negative;
data not shown).
Another case involved a cystic lesion in the
retroperitoneum of a 65-year-old male (Fig. S1). On microscopic examination, the
cyst was lined with ciliated epithelium, and smooth muscle
(Fig. S1A), which met the criteria
for bronchogenic cyst. However, seromucous glands and cartilage
were not detected. IHC features were similar to those
aforementioned, with positive staining for CK7, TTF-1, p40, napsin
A and SP-A (Fig. S1B and D-G) and negative for CK20 (Fig. S1C) in the ciliated epithelium.
Retroperitoneal bronchogenic cysts showed phenotypes similar to
those of respiratory epithelium.
Bronchogenic cyst in the skin
There were two cases of bronchogenic cysts in the
skin. A 59-year-old male had a polyp-like lesion on the medial
anterior chest since childhood, which had grown with age (Fig. 3A). The patient did not complain of
any pain around the lesion; physical examination showed skin flare
and a fixed lesion on the sternal area. During radiological
examination, chest CT showed a cystic lesion measuring
14.7x12.0x8.0 cm (Fig. 3B), thus, a
median cyst was considered as a differential diagnosis. Surgical
excision of the lesion was performed to obtain a pathological
diagnosis. The cyst wall was lined with ciliated epithelium (blue),
which was located in the deep dermal tissue (Fig. 3C). Seromucous glands were detected
near the cyst wall (Fig. 3D).
Goblet cells were also found among the cyst-lining cells (Fig. 3E). Epithelial phenotypes of ciliated
epithelium were positive for CK7, TTF-1 and p40 (Fig. 3F-H) and negative for CK20 (Fig. 3J). Smooth muscle was found under the
cyst, confirmed by αSMA-positive staining (Fig. 3I). Although cartilage was not found,
a diagnosis of bronchogenic cyst was made based on the presence of
ciliated epithelium, seromucous glands and smooth muscle. However,
there was no evidence of atypical epithelium that indicated
malignant transformation. There has been no recurrence for four
years since the resection. For the giant skin bronchogenic cyst in
Fig. 3, we previously described the
treatment procedure (13).
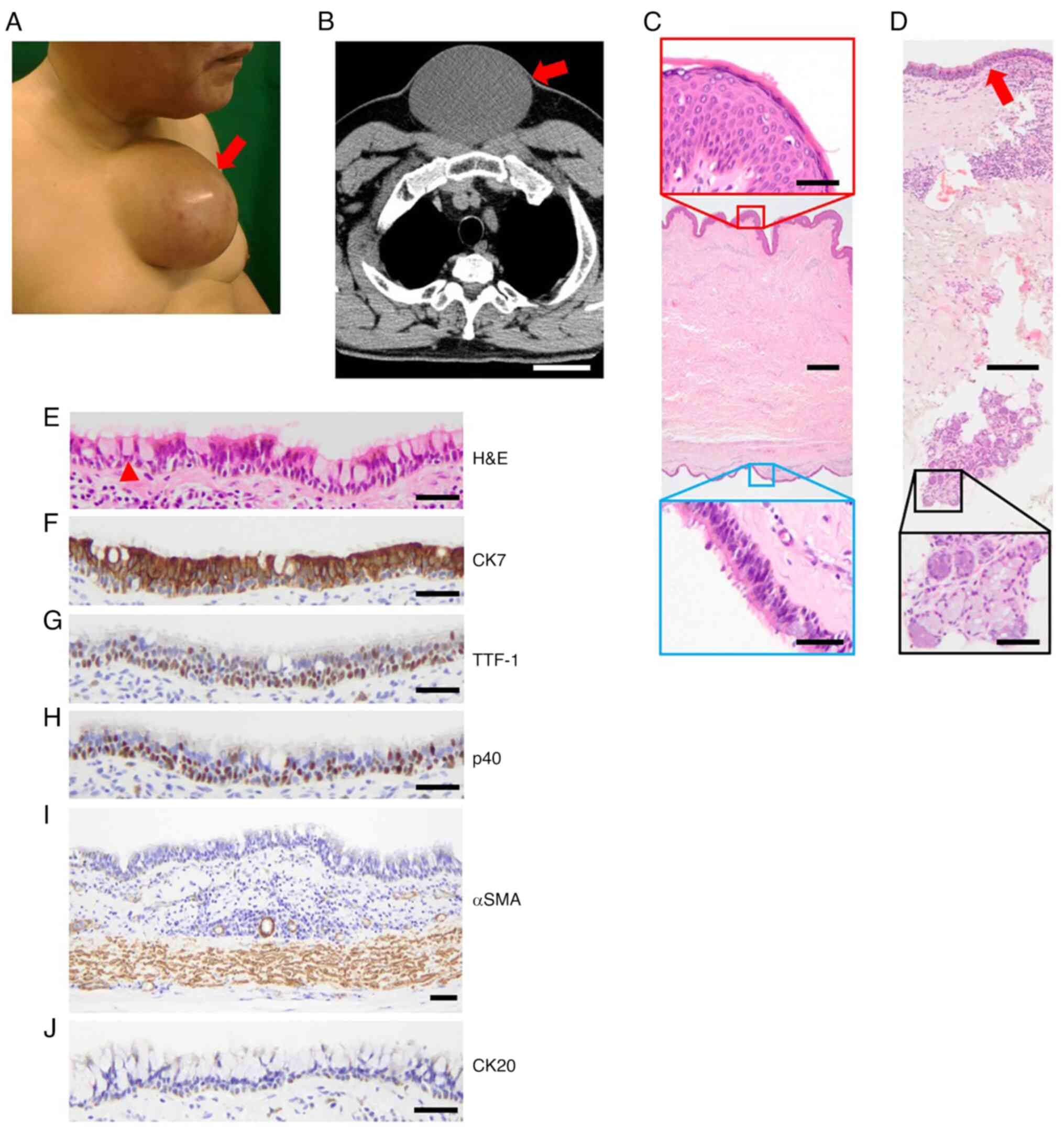 | Figure 3Bronchogenic cyst in the skin. (A)
Polyp-like lesion (arrow) on the chest. (B) Chest CT (transverse
section) showing a cystic lesion (arrow). Scale bar, 5 cm. (C)
H&E staining of the wall of the cyst. Scale bar, 500 µm. Red,
epidermis, scale bar, 50 µm; blue, ciliated epithelium, scale bar,
50 µm. (D) Seromucous glands. Arrow, ciliated epithelium. Scale
bar, 200 µm in lower magnification and 50 µm in black lined-higher
magnification. (E) Epithelial phenotypes of the ciliated
epithelium. Arrowhead, goblet cell. Immunohistochemical staining
for (F) CK7, (G) TTF-1, (H) p40, (I) αSMA and (J) CK20. Scale bar,
50 µm. CT, computed tomography; H&E, hematoxylin and eosin;
TTF-1, thyroid transcription factor 1; αSMA, α-smooth muscle
actin. |
Another case was a small polyp-like lesion on the
chest of a 1-year-old male (Fig.
S2A). The lesion was detected at birth and the differential
diagnosis was soft fibroma. The lesion did not show rapid growth or
infectious features and the diameter was ~10 mm, therefore,
surgical excision of the lesion was performed. Microscopic
examination revealed a multicystic lesion in the dermis (Fig. S2B). Ciliated epithelium with goblet
cells lined the cyst, in addition to seromucous glands and
cartilage (Fig. S2C and D) and showed positive staining for CK7,
TTF-1, and p40 (Fig. S2E-G) and
negative staining for CK20 (Fig.
S2H). In this case, smooth muscle was not detected. Based on
these findings, the diagnosis of bronchogenic cyst in the skin was
confirmed.
Bronchogenic cyst in the cervical
spinal cord and pericardial cavity
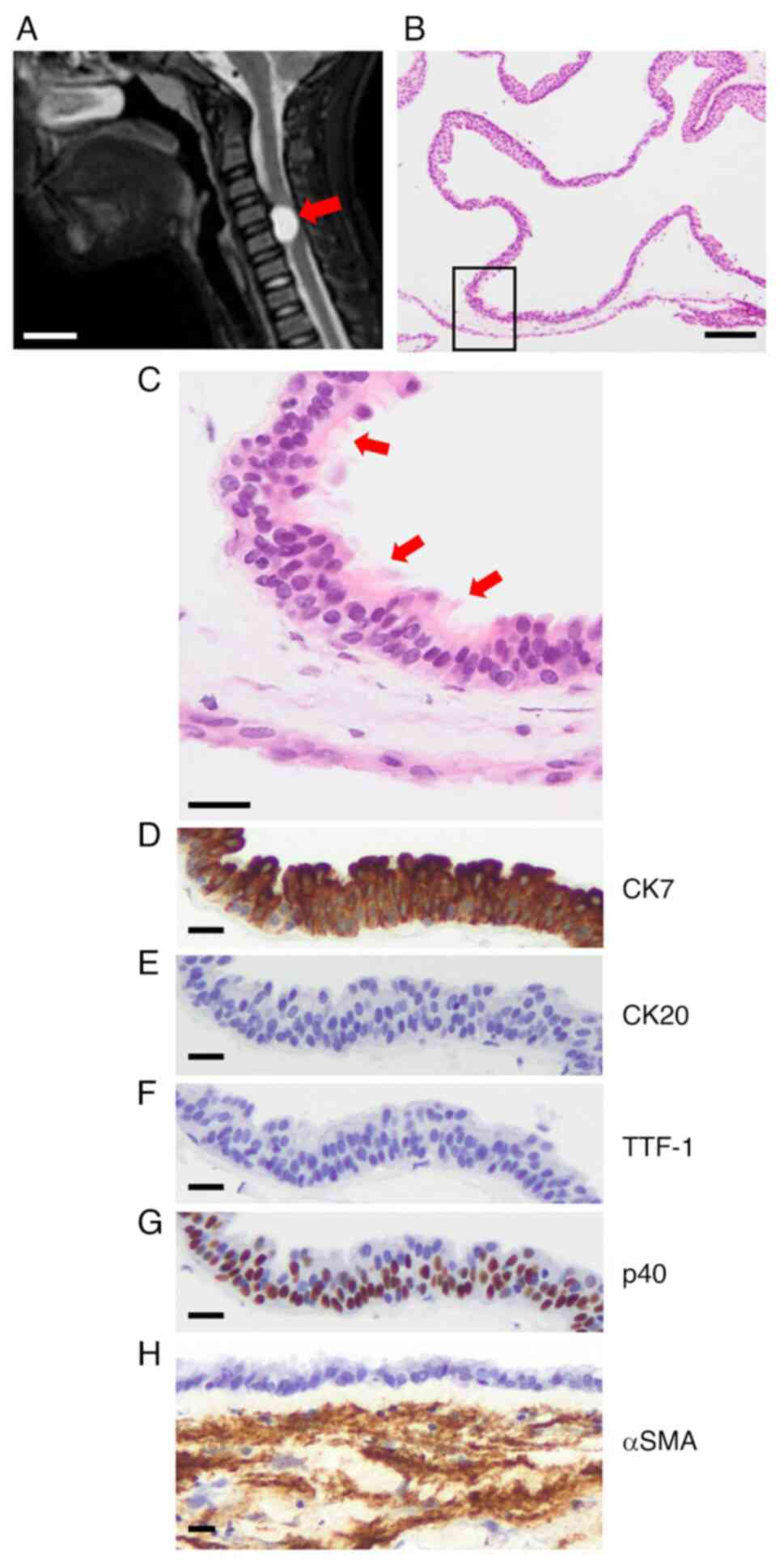
A nodular lesion (1.5x1.3x0.8 cm) was detected on
magnetic resonance imaging in the cervical spinal cord (C4-5) of a
12-year-old male (Fig. 4A). The
patient had pain and numbness in the left shoulder and upper arm,
which had persisted for 1 month before he visited an orthopedist.
Upon medical examination, he displayed loss of muscle strength in
the left upper and lower extremities. Laboratory, respiratory, and
cardiology tests showed no abnormalities. Therefore, bronchogenic
cyst or spinal cord neoplasm such as schwannoma was the
differential diagnosis and surgical excision of the lesion was
performed. H&E staining showed a multicystic lesion (Fig. 4B) lined with ciliated epithelium
(Fig. 4C). Epithelial cells were
positive for CK7 and p40 (Fig. 4D
and G) and negative for CK20 and
TTF-1 (Fig. 4E and F). Smooth muscle was found under the cyst,
which was confirmed by αSMA staining (Fig. 4H), resulting in the diagnosis of a
bronchogenic cyst. Seromucous glands and cartilage were not
detected.
A 56-year-old male underwent surgery for mitral
regurgitation that was detected during a medical checkup. He did
not experience discomfort. Physical examination revealed cardiac
murmur. However, changes in the electrocardiogram such as
arrhythmia were not observed. During surgery for the mitral
regurgitation, a cystic lesion was incidentally detected in the
pericardial cavity. On microscopic examination, it was lined with
ciliated epithelium, seromucous glands and cartilage (Fig. 5A and B). Epithelial phenotypes of ciliated
epithelium were positive for CK7, TTF-1, p40 and Syp (Fig. 5C and E-G) but were negative for CK20 (Fig. 5D). Smooth muscle was found under the
cyst, which was confirmed by αSMA-positive staining (Fig. 5H), resulting in diagnosis of a
bronchogenic cyst.
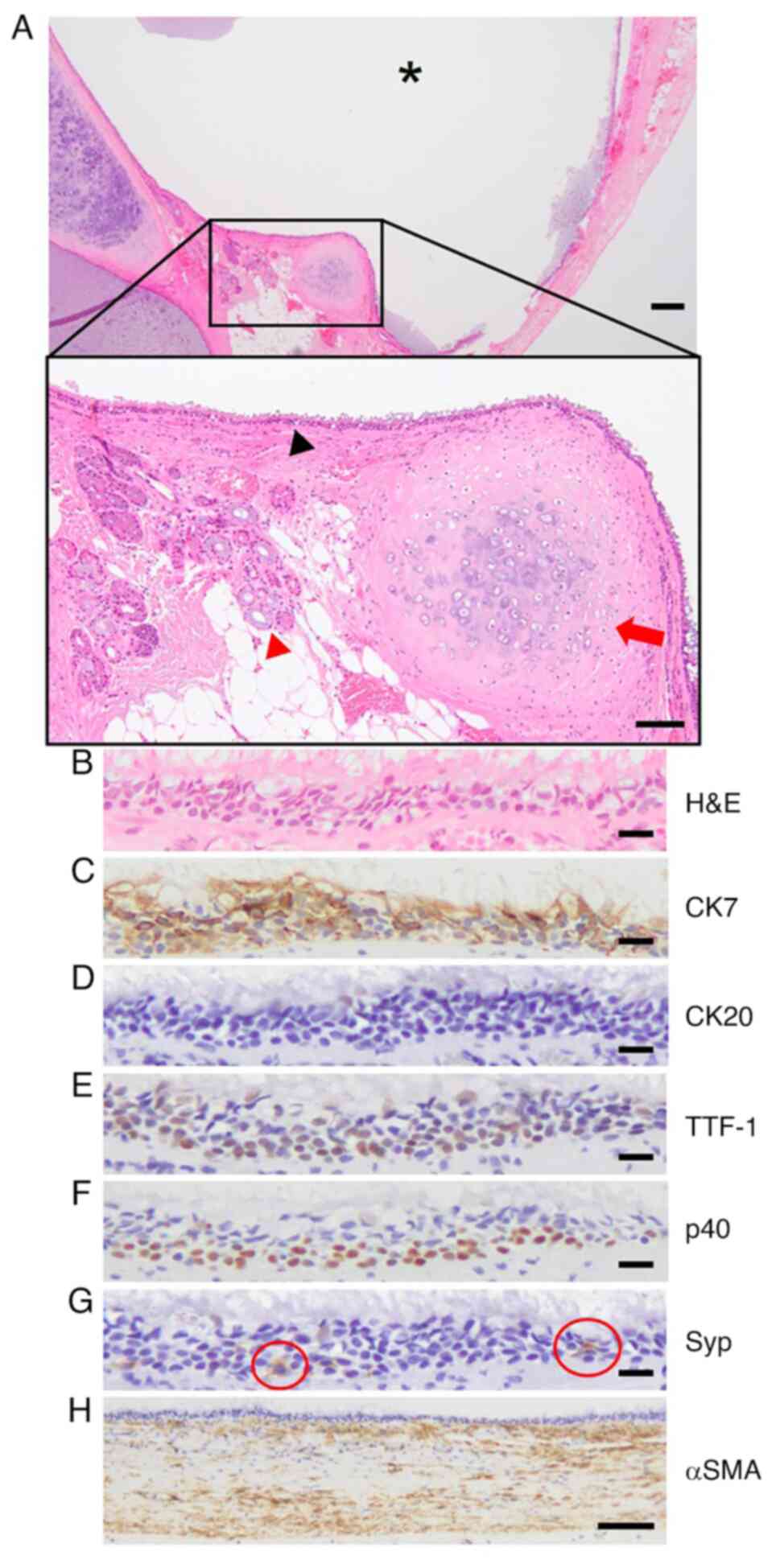 | Figure 5Bronchogenic cyst in the pericardial
cavity. (A) H&E staining of a cystic lesion (asterisk) with
ciliated epithelium (black arrowhead), seromucous glands (red
arrowhead) and cartilage (red arrow). Scale bar, 200 µm in lower
magnification and 100 µm in black lined-higher magnification. (B)
Ciliated epithelium was assessed for (C) CK7, (D) CK20, (E) TTF-1,
(F) p40 and (G) Syp (a positive cell in red circle), scale bar, 20
µm. (H) αSMA. Scale bar, 100 µm. H&E, hematoxylin and eosin;
TTF-1, thyroid transcription factor 1; Syp, synaptophysin; αSMA,
α-smooth muscle actin. |
Discussion
The present study confirmed the diagnoses of
bronchogenic cysts in rare sites, such as the retroperitoneum,
skin, spinal cord or pericardial cavity according to the diagnostic
criteria (8). Two cases each in the
retroperitoneum and pericardial cavity showed bronchogenic cysts
lined with ciliated epithelium with cartilage, smooth muscle and
seromucous glands. All cases in rare sites except spinal cord
bronchogenic cysts showed CK7-, TTF-1- and p40-positive and
CK20-negative cyst-lining epithelium. These findings indicated that
the aforementioned criteria were useful for diagnosis of remotely
located bronchogenic cysts and that the epithelial phenotypes of
bronchogenic cysts were similar to those of the respiratory
epithelium.
Bronchogenic cysts of the central nervous system are
also called neurenteric, enterogenous, or endodermal cysts, account
for 0.3-1.3% of all spinal canal tumors and are more common in the
lower cervical and upper thoracic spine (14). A total of ~90% of neurenteric cysts
are located in the intradural/extramedullary compartment (15). Embryologically, the cyst is
considered to be derived from the congenital maldevelopment of
endodermal tissue displaced into the spinal canal through the
interposed mesodermal layer that forms vertebral bodies (16,17).
The histological classification of endodermal cysts of the central
nervous system is as follows: Type A cysts contain columnar or
cuboidal cells, with ciliated and non-ciliated components mimicking
the respiratory or gastrointestinal epithelium; type B, type A
features in addition to any of glands producing mucinous or serous
fluid, smooth muscle, striated muscle, fat, cartilage, bone,
elastic fibers, lymphoid tissue, nerve fiber or ganglion cells and
type C, type B features in addition to ependymal or glial tissue
(14,15). The treatment for cysts is total
surgical resection; however, recurrence rates as of 37% have been
reported with incomplete resection (15). The present spinal cord bronchogenic
cyst was located in the lower cervical spinal cord (C4-5) and
intradural/extramedullary compartment. Histologically, the cyst
contained ciliated epithelium with smooth muscle but no other
components such as seromucous glands and cartilage, indicative of
type B cysts.
Recently, Kalfas and Scudieri (14) reported a case of intracranial
supratentorial endodermal cysts and showed that the cells were
positive for CK7. Here, epithelial cells of the spinal cord
bronchogenic cyst were positive for CK7 and p40, consistent with
the aforementioned study. Generally, the intestinal epithelium is
CK7-negative and CK20-positive (18), whereas the respiratory epithelium is
CK7-positive and CK20-negative. Although TTF-1 was negative in the
present case of spinal cord bronchogenic cyst, based on the
staining pattern of CK7 and CK20, an endodermal cyst in the central
nervous system may have phenotypes of respiratory rather than
intestinal epithelium.
To the best of our knowledge, the components and
phenotypes in the epithelium of a bronchogenic cyst have been
rarely evaluated (14). The present
cases had two major components: Cyst-lining cells positive for CK7,
such as ciliated epithelium and basal cells, as shown by
p40-positive staining in the basal layer. Although there are some
reports that show TTF-1-positive staining in the epithelium of
extrapulmonary bronchogenic cysts in rare sites, such as the
retroperitoneum, skin and spinal cord (8-11,17),
few studies (10,14) have assessed other phenotypes for
bronchial and alveolar epithelium. The present study confirmed the
expression of napsin A and SP-A, which are primarily expressed in
bronchial and alveolar epithelium. TTF-1, napsin A and SP-A
staining demonstrated that the epithelium of bronchogenic cysts had
the same structure as respiratory epithelium. A bronchogenic cyst
of the retroperitoneum or pericardial cavity results from the
abnormal budding of the respiratory diverticulum (8). However, the mechanism by which a
bronchogenic cyst of the skin occurs is unclear. Moreover, a
diagnosis of teratoma should be excluded before a diagnosis of skin
bronchogenic cyst as teratoma is valid only when a tridermic
lineage is present (9). Here,
macroscopic and microscopic features representing germinal layers
were not found, which ruled out teratoma. Additionally, goblet
cells were observed among cyst lining-cells, which supported the
diagnosis of skin bronchogenic cysts because these cells are
components of the respiratory epithelium. Although the presence of
goblet cells is not included in the Govaerts et al (8) anatomopathological criteria, detection
of these cells is helpful for the diagnosis of bronchogenic
cyst.
To the best of our knowledge, few studies have
reported genetic analysis of bronchogenic cysts: Shiferaw et
al reported an incidental intramyocardial bronchogenic cyst
with p.H558R variant (a common polymorphism) in sodium
voltage-gated channel alpha subunit 5 (SCN5A) gene, which is
a human cardiac sodium channel gene (19). Mutations in the SCN5A gene
are involved in pathophysiology of cardiac arrhythmia and
cardiomyopathy. Moreover, the p.H558R variant in the SCN5A
gene variant may result in ion channel mutation, leading to
arrhythmias (20,21). The present case of the bronchogenic
cyst in the pericardial cavity was incidentally found during
surgery for mitral regurgitation and was not located in any
intramyocardial site. The patient did not show any change in
electrocardiogram to suggest arrhythmia. Therefore, genetic
analyses for SCN5A variants were not performed. Murakami
et al (22) reported an
abdominal bronchogenic cyst with a low-grade mucinous neoplasm
harboring a GNAS mutation; GNAS mutation (p.R201C) in
the atypical epithelium of the bronchogenic cyst indicated
potential mechanism for tumorigenesis and malignant transformation.
However, the present study did not find any atypical epithelium
that suggested a neoplasm or malignant transformation. Nonetheless,
genetic analyses may be warranted in some cases of symptomatic
and/or malignant transformed bronchogenic cysts.
In conclusion, the present study evaluated
bronchogenic cysts in rare sites, including the retroperitoneum,
skin, spinal cord and pericardial cavity, and confirmed the
diagnoses, epithelial components and phenotypes, which
histopathologically suggested that the epithelium of bronchogenic
cysts had the same structure as respiratory epithelium. None of the
cases had recurrence since complete resection. Although most cases
of bronchogenic cyst are asymptomatic and found incidentally during
medical checkups or detailed examinations of other diseases,
surgical treatment should be considered due to a risk of malignant
transformation (2,22,23).
The present study demonstrated that bronchogenic cysts can occur in
rare sites and should be considered as a differential diagnosis
before and after surgical resection to recognize a risk of
malignant transformation and implement relevant management
modalities such as treatment procedure and follow-up time.
Supplementary Material
Characterization of epithelial
phenotypes of retroperitoneal bronchogenic cyst. (A) Hematoxylin
and eosin staining of the wall of the cystic lesion. Scale bar, 200
μm in lower magnification; Red, ciliated epithelium, scale
bar, 20 μm; blue, α-smooth muscle actin staining,
scale bar, 50 μm. Ciliated epithelium was assessed for (B)
CK7, (C) CK20, (D) thyroid transcription factor 1, (E) p40, (F)
napsin A and (G) surfactant protein A. Scale bar, 20
μm.
Bronchogenic cyst in the skin. (A)
Polyp-like lesion on the chest (red arrow). (B) Hematoxylin and
eosin staining of cystic lesions (asterisks) with seromucous glands
(black-lined higher magnification, scale bar, 20 μm). Scale
bar, 500 μm in lower magnification. (C) Anatomopathological
features for the diagnosis of bronchogenic cyst. Scale bar, 50
μm. Seromucous glands (red arrowhead) and cartilage (arrow)
were detected near the ciliated epithelium (black arrowhead). (D)
Ciliated epithelium. Arrowhead, goblet cell. (E) CK7, (F) thyroid
transcription factor 1, (G) p40 and (H) CK20 immunohistochemical
analyses. Scale bar, 20 μm.
Acknowledgements
The authors would like to thank Ms Megumi Miyauchi
(Department of Pathology, Faculty of Medicine, Osaka Medical and
Pharmaceutical University; Takatsuki, Osaka, Japan) for technical
support with the IHC analyses, and Mr Kentaro Morimoto (Faculty of
Medicine, Osaka Medical and Pharmaceutical University; Takatsuki,
Osaka, Japan) for comments about pathological diagnostic approaches
in this study.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YO collected and analyzed data. SK and EY designed
the study and evaluated the pathological findings. NI, HA, YuH, KU,
MN and MD contributed to clinical data acquisition and
interpretation. YK and YoH performed pathological diagnosis. SK
wrote the manuscript. All authors have read and approved the final
manuscript. SK and EY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Osaka Medical and Pharmaceutical University
(approval no. 2020-124; Takatsuki, Osaka, Japan). A total of four
patients provided written informed consent to participate; two
patients participated through an opt-out approach to them and their
guardians.
Patient consent for publication
Written informed consent was obtained from four
patients for publication of this case report. For the other two
patients, the consent was obtained through the opt-out approach to
them and their guardians.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coselli MP, de Ipolyi P, Bloss RS, Diaz RF
and Fitzgerald JB: Bronchogenic cysts above and below the
diaphragm: Report of eight aases. Ann Thorac Surg. 44:491–494.
1987.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cuypers P, De Leyn P, Cappelle L,
Verougstraete L, Demedts M and Deneffe G: Bronchogenic cysts: A
review of 20 cases. Eur J Cardiothorac Surg. 10:393–396.
1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gross DJ, Briski LM, Wherley EM and Nguyen
DM: Bronchogenic cysts: A narrative review. Mediastinum.
7(26)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ribet ME, Copin MC and Gosselin B:
Bronchogenic cysts of the mediastinum. J Thorac Cardiovasc Surg.
109:1003–1010. 1995.PubMed/NCBI View Article : Google Scholar
|
|
5
|
St-Georges R, Deslauriers J, Duranceau A,
Vaillancourt R, Deschamps C, Beauchamp G, Pagé A and Brisson J:
Clinical spectrum of bronchogenic cysts of the mediastinum and lung
in the adult. Ann Thorac Surg. 52:6–13. 1991.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Suen HC, Mathisen DJ, Grillo HC, LeBlanc
J, McLoud TC, Moncure AC and Hilgenberg AD: Surgical management and
radiological characteristics of bronchogenic cysts. Ann Thorac
Surg. 55:476–481. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee DH, Park CK, Kum DY, Kim JB and Hwang
I: Clinical characteristics and management of intrathoracic
bronchogenic cysts: A single center experience. Korean J Thorac
Cardiovasc Surg. 44:279–284. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Govaerts K, Van Eyken P, Verswijvel G and
Van der Speeten K: A bronchogenic cyst, presenting as a
retroperitoneal cystic mass. Rare Tumors. 4(e13)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sun J, Yuan T and Deng H: Cutaneous
bronchogenic cyst in the left scapular region of a boy. World J
Pediatr. 10:365–367. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nakagawa A, Nagase K, Inoue T, Miura Y and
Fujisaki A: Upper arm bronchogenic cyst: A rare case presentation.
Eur J Dermatology. 33:321–322. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wen Y, Chen W, Chen J and He X:
Retroperitoneal bronchogenic cyst resembling an adrenal tumor: two
case reports and literature review. J Int Med Res.
48(300060520925673)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kalhor N and Moran CA: Immunohistology of
Pulmonary and Pleural Neoplasms. In: Diagnostic
Immunohistochemistry. 6th edition. Dabbs DJ (ed). Elsevier, Inc.,
Philadelphia PH, pp434-497, 2021.
|
|
13
|
Nakao T, Katayama M, Kino H, Hanaoka N and
Ueda K: A case of giant subcutaneous bronchogenic cyst. Arch Clin
Med Case Rep. 4:590–595. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kalfas F and Scudieri C: Endodermal cysts
of the central nervous system: Review of the literature and a case
report. Asian J Neurosurg. 15:989–996. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Savage JJ, Casey JN, McNeill IT and
Sherman JH: Neurenteric cysts of the spine. J Craniovertebr
Junction Spine. 1:58–63. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen IH, Kao KP, Penn IW and Ho DM: Double
intraspinal enterogenous cysts. J Neurol Neurosurg Psychiatry.
58:110–111. 1995.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Baek WK, Lachkar S, Iwanaga J, Oskouian
RJ, Loukas M, Oakes WJ and Tubbs RS: Comprehensive review of spinal
neurenteric cysts with a focus on histopathological findings.
Cureus. 10(e3379)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Krasinskas AM and Zheng W: Immunohistology
of the Gastrointestinal Tract. In: Diagnostic Immunohistochemistry,
6th edition. Dabbs DJ (ed). Elsevier, Inc., Philadelphia PH,
pp528-560, 2021.
|
|
19
|
Shiferaw K, Lobrinus AJ, Grabherr S,
Michaud K, Mangin P and Schrag B: One case, 3 rare simultaneous
findings: Intramyocardial bronchogenic cyst, P. H558R variant of
SCN5A gene, and granular cell tumor of the esophagus. Am J Forensic
Med Pathol. 33:335–338. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gouas L, Nicaud V, Berthet M, Forhan A,
Tiret L, Balkau B and Guicheney P: D.E.S.I.R. Study Group.
Association of KCNQ1, KCNE1, KCNH2 and SCN5A polymorphisms with QTc
interval length in a healthy population. Eur J Hum Genet.
13:1213–1222. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shan L, Makita N, Xing Y, Watanabe S,
Futatani T, Ye F, Saito K, Ibuki K, Watanabe K, Hirono K, et al:
SCN5A variants in Japanese patients with left ventricular
noncompaction and arrhythmia. Mol Genet Metab. 93:468–474.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Murakami T, Shimizu H, Yamazaki K, Nojima
H, Usui A, Kosugi C, Shuto K, Obi S, Sato T, Yamazaki M and Koda K:
Intra-abdominal ectopic bronchogenic cyst with a mucinous neoplasm
harboring a GNAS mutation : A case report. World J Clin Cases.
10:8709–8717. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taira N, Kawasaki H, Atsumi E, Ichi T,
Kawabata T, Saio M and Yoshimi N: Mucoepidermoid carcinoma of
arising from a bronchogenic cyst of the diaphragm. Ann Thorac
Cardiovasc Surg. 24:247–250. 2018.PubMed/NCBI View Article : Google Scholar
|