Introduction
Pancreatic cancer (PC) is the 4th leading cause of
cancer-related death in male and female patients in developed
countries, with an incidence of 12.5/100,000 individuals in the
American continent (7% of all types of cancer) (1). PC is associated with poor prognosis,
and the mortality rate is similar to that of the incidence
(2). Long-term survival remains
poor, and the 5-year survival rate for patients with resectable
tumors is 15-20%, while patients with unresectable tumors at the
time of diagnosis have a 5-year survival of ~0% (3,4); it is
expected that by 2030, this tumor type will become the 2nd leading
cause of cancer-related death (4).
PC is a tumor that grows slowly and silently for ~10
years (5), and most cases occur in
patients with no evidence of hereditary family history (6). However, there are still no reliable
biomarkers used in the clinic. It is noteworthy that the serum
tumor biomarker CA 19-9 is a pentose related to the carbohydrate
antigen Sialyl Lewis located on erythrocyte surfaces, which is
absent in 10-15% of the population due to the lack of the enzyme
necessary for its synthesis (7-9).
Elevated levels of CA 19-9 (<1,000 IU/ml) are suggestive of
pancreatic adenocarcinoma (PAC); a decrease in values through
treatment suggests a good response, and at present, this is the
only serum tumor marker for PAC (10). The sensitivity (Se) of CA 19-9 is
69-98%, while the specificity (Sp) is 46-98% (11). This marker has also been found to be
increased in patients with other upper gastrointestinal tumors,
diseases that give rise to biliary obstruction, inflammatory
pathology and other benign conditions such as primary sclerosing
cholangitis (12), but it can also
be detected at normal levels in some patients with PAC (13). At present, CA 19-9 is mainly used as
a monitoring tool to assess patient response to treatment (10).
Another tumor biomarker is carcinoembryonic antigen
(CEA), a member of surface glycoproteins located at the apical pole
of erythrocytes; this molecule is the most commonly used tumor
marker in colon carcinoma (14).
However, CEA levels can also be increased in other pathological
conditions, such as gastric, pulmonary, pancreatic and breast
neoplasias, in the medullary carcinoma of the thyroids, as well as
in other conditions such as cirrhosis, ulcerative colitis,
pancreatitis and even in smokers. The totality of its functions
remains unknown (15).
In the clinic, CEA is associated with colorectal
cancer prognosis, staging, treatment response and recurrence;
however, it plays a notable role in other neoplasias too such as
PC. The positive predictive value (PPV) of CEA for PC is 78.6%, and
the negative predictive value (NPV) is 75% (16).
The tumor microenvironment of PC should be
investigated further, and biomarkers with high predictive potential
and Sp are urgently required.
Polyamines are aliphatic molecules consisted of
various amine groups (17). In
humans, polyamines are derived from two sources, including
endogenous biosynthesis by de novo biosynthesis and
interconversions among themselves, and also through digestive
secretions, especially intestinal, pancreatic and catabolism
products from intestinal cells. In addition, polyamines are also
produced by intestinal microorganisms using dietary intake, an
exogenous source of polyamines (18).
These small molecules are associated with numerous
cell processes, such as cell division, nucleic-acid packaging, DNA
replication and others. In mammals, polyamines are produced from
ornithine by ornithine decarboxylase (ODC), which produces
putrescine, which in turn gives rise to spermine and spermidine
through the spermidine and spermine synthases, respectively
(17).
One of the first events in cell proliferation is the
induction of polyamine biosynthesis; it is known that the
overexpression of ODC beyond the minimal threshold can induce cell
transformation and tumor promotion (19). However, ODC expression increases the
biosynthesis of putrescine and the subsequent biosynthesis of
spermine and spermidine. Thus, the activity of this enzyme is
sufficient for tumor promotion, making ODC a proto-oncogene
(20).
Consequently, high levels of polyamines were found
in the biological fluids of patients with cancer, and these have
been investigated as a biomarker in ovarian cancer (21), colorectal cancer (22,23),
breast cancer (23), lung cancer
(24), prostate cancer (25) and PC (26). These levels decrease after tumor
eradication and increase again in case of relapse (27); although these molecules are present
in some normal tissues, such as the bone marrow, pancreas,
intestinal mucosa and prostate, their expression is increased in
tumor tissues. Although it has been shown that blood or urine
polyamine concentrations are elevated in patients with cancer, it
is not known if ODC is expressed in peripheral blood mononuclear
cells (PBMCs) (28).
Asai et al (6) quantified polyamine levels in the
saliva of patients with PC, in patients with pancreatitis and in
healthy controls, showing that polyamines, especially spermine,
were notably increased in patients with PC compared with those in
the control group. A combination of the four metabolites spermine,
N1-aceylspermidine, N1-acetylspermine and 2-aminobutanoate also
exhibited marked increase in patients with cancer compared with
patients with benign pancreatic pathology (chronic pancreatitis) or
with healthy individuals, and could potentially be used for the
detection of PC.
In the present study, the potential of PBMCs to
exhibit ODC metabolism and their association with PC, was
investigated.
Materials and methods
Patients (cases)
The present case-control study involved patients who
were diagnosed with PAC. Mexican patients attending the Oncology
Service, High Specialty Medical Unit, Western National Medical
Center, Mexican Social Security Institute, Guadalajara, Mexico,
between November 2019 and February 2021 (≥18 years of age) with
histopathological diagnosis of PAC and without previous treatment
were invited to participate in the present study.
Controls
Mexican, ≥18-year-old female and male healthy
individuals or with controlled disease including hypertension,
hypothyroidism and diabetes, with no tumor-associated complications
were included in the control group.
The clinical and sociodemographic data of both
groups were collected in medical consultation through an interview
with the oncologist, and signed informed consent was obtained from
all study participants.
Inclusion criteria
For patients, the inclusion criteria were: i)
Histopathological diagnosis of PAC; and ii) no previous treatment.
For controls, the inclusion criteria were: i) Health or controlled
disease including hypertension, hypothyroidism and diabetes; ii)
smoking (<5 cigarettes/day for <5 years; and iii) no recorded
tumor-associated complications.
Exclusion criteria
The exclusion criteria for both groups were the
following: i) Inadequate sample; and ii) incomplete clinical and/or
laboratory data.
Samples
All samples were collected between November 2019 and
February 2021 by the clinical staff at the Oncology Service,
Specialty Hospital (Guadalajara, Unites Mexican States) before any
treatment. Samples were correctly labeled in closed containers with
double packaging and transported to the Division of Immunology.
Blood samples (5 ml) were obtained by venipuncture
and collected in protease-free tubes with
ethylenediaminetetraacetic acid (EDTA) as anticoagulant and diluted
with PBS (1:1; cat. no. 10010023; Gibco; Thermo Fisher Scientific,
Inc.). This mix was gently placed in Ficoll-Hypaque (density,
1.077; cat. no. 10771; Sigma-Aldrich; Merck KGaA) to obtain
PBMCs.
Trypan blue was used to determine the number of
viable cells in a cell suspension. A total of 1.3x106
cells/ml were resuspended in fetal bovine serum (cat. no. 10437;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 5% of
dimethylsulphoxide (cat. no. D8418; Merck KGaA) for
cryopreservation until determination.
CA 19-9 and CEA determination using
enzyme-linked immunosorbent assay (ELISA)
Determination of CA 19-9 (CA 19-9 Accubind ELISA
kit; cat. no. 3925-300A; Monobind, Inc.) and CEA (CEA Next
Generation Accubind ELISA kit; cat. no. 4625-300A; Monobind, Inc.)
was carried out according to the manufacturer's instructions.
Briefly, 25 µl standard or samples from both study groups (controls
and patients) were placed in the appropriate well, and 100 µl
buffer solution was added, mixed and incubated at 37˚C for 90 min.
The plates were washed five times. A total of 100 µl either CA
19-9- or CEA-labeled antibody was added to each corresponding well,
mixed for 20-30 sec, covered and incubated for 60 min at room
temperature (RT). A total of 350 µl wash buffer was added twice,
and 100 µl working substrate solution was added, incubated again
for 15 min at RT, thereafter adding 50 µl stop solution, mixing and
reading in the multi-detection microplate reader (620-630 nm; Bio
Tek Synergy HTX Multimode Reader; Agilent Technologies, Inc.).
Results were expressed as U/ml.
Polyamine quantification by
high-performance liquid chromatography (HPLC). Sample collection
and treatment
All samples were collected by the clinical staff in
the Oncology Service prior to any treatments. Samples correctly
labeled in closed containers with double packaging were transported
to the Division of Immunology, Mexican Social Security Institute
(Guadalajara, United Mexican States) for research and stored at
-80˚C until polyamine determination.
Plasma. A total of 5-10 ml peripheral blood
with EDTA was collected, centrifuged at 1,368 x g, for 15 min at RT
(20-25˚C) and the plasma was separated and mixed with 5% perchloric
acid (cat. no. 244252; Sigma-Aldrich; Merck KGaA) at a 1:1 ratio.
In order to precipitate the proteins, a second centrifugation step
at 7,267 x g for 10 min at RT was carried out. The acidic extract
was recovered and stored at -80˚C until further analysis (29).
Urine. Urine was recovered in a sterile
flask; 0.5 ml urine was mixed with perchloric acid and treated as
plasma.
Saliva. Saliva was also recovered in a
sterile flask and processed as the other aforementioned
samples.
Polyamine determination
An analytical HPLC method for the quantitation of
polyamines was developed and validated in different samples,
including plasma, urine and saliva of patients with PAC. The
concentration of polyamines was calculated from three calibration
curves using a quaternary system (cat. no. G1310; Agilent series
1260; Agilent Technologies, Inc.), online degasser (cat. no.
G1379), autosampler (cat. no. G1329), fluorescence detector (cat.
no. G1365) and thermostat (cat. no. G1316).
Polyamine separation was carried out through a
Phenomenex Luna C18 column, (100x4.6 mm; 5 µm). Elution
was conducted through a gradient: Mobile phase consisted of a
gradient of solvent A circumvention, sodium phosphate buffer (40 mM
Na2HPO4)/acetonitrile (Tedia AS 1122001) at a
ratio of 5:95, and B (80:20) at a ratio A/B of 0:100 at time 0,
reversing the ratio in 10 min, with a running time of 12 min and a
flow of 1.0 ml/min, according to the description in Table I. Three polyamines were determined
simultaneously in the biofluids of patients with PAC at different
stages and compared with those of the controls.
 | Table IElution gradient for the
determination of polyamines. |
Table I
Elution gradient for the
determination of polyamines.
| Time, min | A, % | B, % | Flow, ml/min |
|---|
| 0 | 0 | 100 | 1.0 |
| 2 | 25 | 75 | 1.0 |
| 3 | 25 | 75 | 1.0 |
| 5 | 50 | 50 | 1.0 |
| 6 | 60 | 40 | 1.0 |
| 8 | 90 | 10 | 1.0 |
| 10 | 90 | 10 | 1.0 |
| 12 | 0 | 100 | 1.0 |
Detection of polyamines was carried out at an
excitation length of 360 nm and at an emission of 560 nm. All of
the elusion components were previously degassed, and the samples
were filtered using Durapore membranes (0.22-µm; cat. no. GWP01300;
MilliporeSigma).
PBMC recovery
Blood samples from patients and controls were
obtained by venipuncture in 6-ml EDTA tubes, and blood was gently
placed on Hystopaque®-1.077 (1.077-100 ml;
Sigma-Aldrich; Merch KGaA) at 1:1 ratio; next, samples were
centrifuged at 1,368 x g for 25 min at RT. The interface contained
PBMCs, which were collected, washed twice with PBS for complete
removal of Hystopaque and resuspended in 3 ml PBS. Cells were
counted using a hemocytometer and viability was determined with the
exclusion dye trypan blue.
Western blotting
Western blotting was performed as previously
described by Cruz-Gálvez et al (30). Briefly, 3x106 PBMCs from
patients with PAC were thawed for 10 min at room temperature; after
centrifugation (235 x g for 7 min at RT), the medium was gently
discarded. The cell pellet was resuspended, washed twice with PBS
at room temperature, lysed with radioimmunoprecipitation assay
(RIPA) buffer (0.5% deoxycholate, 1% NP-40, 0.1% sodium dodecyl
sulfate (SDS), 50 mM Tris-HCl, pH 8.0 and 150 mM NaCl; cat. no. sc
24948; Santa Cruz Biotechnology, Inc.), and maintained on ice with
protease/phosphatase inhibitors (cOmplete™, Mini, EDTA-free
Protease Inhibitor Cocktail; Sigma-Aldrich; Merck KGaA) for 30 min.
Cells were resuspended in RIPA buffer in an ice bath during 30 min.
Subsequently, cells were homogenized in a bio-disruptor by
hydrodynamic agitation (15 pulses, 50% amplitude), ensuring that
the temperature did not increase and cells were incubated on ice
for 30 min. The total number of lysed cells was transferred into
microcentrifuge tubes and centrifuged at 11,355 x g for 13 min at
4˚C.
Protein concentrations were determined using the DC
Protein Kit (Bio-Rad Laboratories, Inc.). A total of 60 µg protein
sample was subjected to electrophoresis using a 10% SDS/PAGE gel.
Subsequently, the proteins were transferred onto Immobilon-P PVDF
membranes (MilliporeSigma) and these were incubated with the
Odyssey® Blocking Buffer reagent for 2 h, at RT with
gentle agitation. Immunodetection of ODC was performed using a
mouse monoclonal anti-ODC antibody (243-272 aa; human anti-ODC
specific to the internal region of human ODC; cat. no. SC-398116;
Santa Cruz Biotechnology, Inc.) diluted at 1:500 in blocking buffer
and 0.1% Tween-20 at 4˚C overnight. β-actin (human anti-β actin;
cat. no. SC-47778; Santa Cruz Biotechnology, Inc.) was used as an
internal control. After incubation with a fluorescently-labeled
secondary antibody (IRDye® 680 Donkey Anti-Mouse IgG;
LI-COR Biosciences, Ltd; cat. no. 926-32212) diluted at 1:15,000 in
PBS + 0.1% Tween-20, and 0.1% SDS, the ODC protein was visualized
using the Odyssey® XF Imaging System (LI-COR
Biosciences, Ltd.). The results were normalized for all experiments
by the mean optical density of the gel background and zeroing with
a dark gel spot.
Statistical analysis
Clinical and sociodemographic data are expressed as
percentages and means were compared between the control and patient
groups using the non-parametric Mann-Whitney U test and the
Wilcoxon signed-rank test. Clinical parameters in patients were
expressed as frequencies and percentages. Polyamines are expressed
as mean ± standard deviation and compared using unpaired Student's
t-test. P<0.05 and P<0.01 were considered to indicate a
statistically significant difference. ODC expression was calculated
by relative expression, evaluated by an increase in fold change,
and analyzed by an unpaired Student's t-test. Survival of the
patients was analyzed using the Kaplan-Meier method. All
statistical analyses were performed using GraphPad Prism (version
9.5.0; Dotmatics).
Results
Patients
A total of 15 patients with a diagnosis of PAC
attended an appointment for the first time at the Gastrointestinal
Tumor Clinic of the Medical Oncology Service at the Western
National Medical Center, IMSS, between December 2019 and December
2020. Patients who accepted to voluntarily participate in the
present study (n=11) proceeded to sign an informed consent.
Clinical and sociodemographic data, and blood samples were then
collected.
Control group
The control group (n=11) included male volunteers
(36.4%) and female volunteers (63.6%) who accepted to participate
in the current study. They all signed an informed consent and
donated a blood sample. Controls of similar age to that of the
patient group were selected; some controls also had some risk
factors, and were individuals with no recorded tumor complication,
and with only one well-controlled chronic disease as follows: Two
individuals had type 2 diabetes mellitus (DM2; 18.18%), two
individuals had arterial hypertension (18.18%), one individual had
hyperthyroidism (9.09%) and one individual had pancreatitis
(9.09%). All controls provided their sociodemographic
information.
Clinical and sociodemographic
data
Patients (n=15) had a mean ± SD age of 54.80±8.54
years (range, 42-73 years) compared with that of the controls, who
had a mean age of 47.40±10.24 years (range, 27-65 years). The
patient group was consisted of 46.7 and 53.3% males and females,
respectively, while the control group was consisted of 36.4 and
63.6% males and females, respectively (Table II).
 | Table IIDemographical characteristics of
patients (n=15) and controls (n=11). |
Table II
Demographical characteristics of
patients (n=15) and controls (n=11).
| Demographical
characteristics | Patients | Controls | P-value |
|---|
| Age, years | 54.80±8.54 | 47.40±10.24 | 0.60 |
|
Range | 42-73 | 27-65 | 0.04a |
| Sex, % | | | |
|
Male | 46.7 | 36.4 | 0.60 |
|
Female | 53.3 | 63.6 | 0.60 |
| Risk factors,
% | | | |
|
DM2 | 37.3 | 9.1 | 0.78 |
|
DM2 recent
diagnosis | 6.6 | 0.0 | 0.43 |
| Smoking | 27.0 | 40.0 | 0.61 |
| Heavy smoking | 20.0 | 0.0 | 0.03a |
| Alcohol
consumption | 26.7 | 36.4 | 0.16 |
| Obesity | 18.2 | 34.4 | 0.27 |
| Hereditary-family
history of cancer | 37.3 | 54.5 | 0.34 |
| Chronic
pancreatitis | 0.0 | 6.6 | 0.35 |
When the risk factors of the studied groups were
compared, the following was observed: Although the presence of DM2
did not reveal significant differences, 9.1 and 37.3% of the
control and patient groups, respectively, were stratified by time
of disease evolution, and 6.7% of the patients had >10 years of
disease evolution compared with 13.3% of patients with a recent
diagnosis (<1 year).
Smoking was reported in 27.3 and 40% of controls and
patients, respectively, with 6.7% of patients being smokers for
>5 years. Regarding smoking intensity, 50% were moderate smokers
and 50% were heavy smokers. Smoking intensity appeared to be
significant risk factor (P<0.05).
Alcohol was consumed by 36.4 and 26.7% of controls
and patients, respectively. All controls who consumed alcohol
indicated light consumption, while 75% of patients indicated high
and excessive consumption according to the World Health
Organization degrees of intensity of alcohol consumption (31).
Hereditary-family history (HFH) of cancer was more
common in controls (54.5%), and it was homogenously distributed
between grade 1 and 2, compared with 37.3% of patients with a
predominant grade-1 antecedent.
Obesity was reported in 18.2 and 34.4% of patients
and controls, respectively. Pancreatitis was only reported in 6.6%
of controls. No significance was observed in these parameters when
they were compared between the control and patient groups.
The investigation of the symptoms of PAC (Table III) indicated that abdominal pain
was the principal symptom (46%), with all of the reported symptoms
fulfilling the chronicity criteria (>3 months with abdominal
pain that do not respond to treatment without pain control
evaluated in retrospective) (32).
All patients received symptomatic treatment and 10/11 patients had
>3 months of first-symptom evolution. Although the patients
presented secondary symptoms, imaging was not carried out.
 | Table IIIEarly signs and symptoms in patients
with pancreatic adenocarcinoma. |
Table III
Early signs and symptoms in patients
with pancreatic adenocarcinoma.
| Sign or
symptoms | n (%) |
|---|
| Abdominal pain | 7 (46.0) |
| Anorexia | 1 (6.6) |
| Reflux | 1 (6.6) |
| Dyspepsia | 1 (6.6) |
| Singultus | 1 (6.6) |
| Type 2 diabetes
mellitus | 1 (6.6) |
| Icterus | 10(66) |
| Weight loss | 3 (20.0) |
| Ascitis | 1 (6.6) |
General laboratory tests were requested by 4/11
patients. The most common symptoms of PAC included icteric skin,
weight loss and ascites in 66.6, 20.0 and 6.6% of patients.
Distribution by age
In terms of age distribution, in patients with PAC
aged between 42 and 73 years, the highest risk was found between
the age of 50 and 60 years (P<0.05).
Diagnosis and associated
complications
Histological diagnosis was performed using a
surgical piece of the tumor resection in three patients who were
programed for surgery with curative intention (20%). For the
remainder of the patients, evidence of malignancy was obtained by
open biopsy (40%), endoscopy retrograde cholangiopancreatography
(ERCP; 26.6%) and imagery-guided biopsy (6.6%), while there was a
sample obtained by endoscopy from one patient who had metastasis in
the gastric cavity and bleeding in the upper digestive tract.
ERCP was performed in 10/15 patients; in all cases,
this was required for the treatment of obstruction in the bile
ducts. However, the histopathological analysis of ductal aspiration
and brushing was conclusive for PAC in only two patients. On the
other hand, among six patients with open biopsy, three presented
associated complications, including bleeding (n=1) and infection of
the surgical wound (n=2). Finally, histology in all cases confirmed
ductal PAC diagnosis.
Clinical stage (CS)
The predominant CS at diagnosis was IV (40%),
conditioned by hepatic and peritoneal metastasis, followed by CS
IIIB (33.3%), IIB (6.6%), IB (13.3%) and IIIA (6.6%), according to
the American Joint Committee on Cancer version 8(33).
Treatment
The approaches used in the treatment of the majority
of cases (38.4%) included treatment with FOLFIRINOX oxaliplatin (85
mg/m2), irinotecan (150 mg/m2), leucovorin
(200 mg/m2) and 5-fluoracil infusion (2,400
mg/m2) on day 1 by continuous infusion over 46 h, every
14 days for 12 cycles. A total of 7.6% of patients received a
double treatment scheme such as gemcitabine (1,000
mg/m2)-cisplatin (25 mg/m2) on day 1 and 8 or
gemcitabine (1,000 mg/m2)-capecitabine (100 mg orally
for 14 days), while 23% of patients were treated with monotherapy
and either gemcitabine or capecitabine (1,600 mg/m2/day
in two doses, from day 1 to 5).
Survival
Treatments and treatment intentions were variable.
Three patients underwent tumor resection; at first, all patients
(n=3) received adjuvant chemotherapy; one of them was programmed
for radiotherapy due to compromised margins, while a change in the
initial treatment intention from adjuvant to palliative treatment
was planned for a second patient due to hepatic metastasis
documented prior to the initiation of systemic treatment. One
patient continued neoadjuvant treatment until the end of the study
(February 2021). Among the 11 patients with non-resectable and
metastatic disease, six were not candidates for cytotoxic treatment
and received supportive care, while five patients received the same
scheme of chemotherapy (FOLFIRINOX) with palliative intention. At
cutoff, the death of five patients was recorded due to PC-related
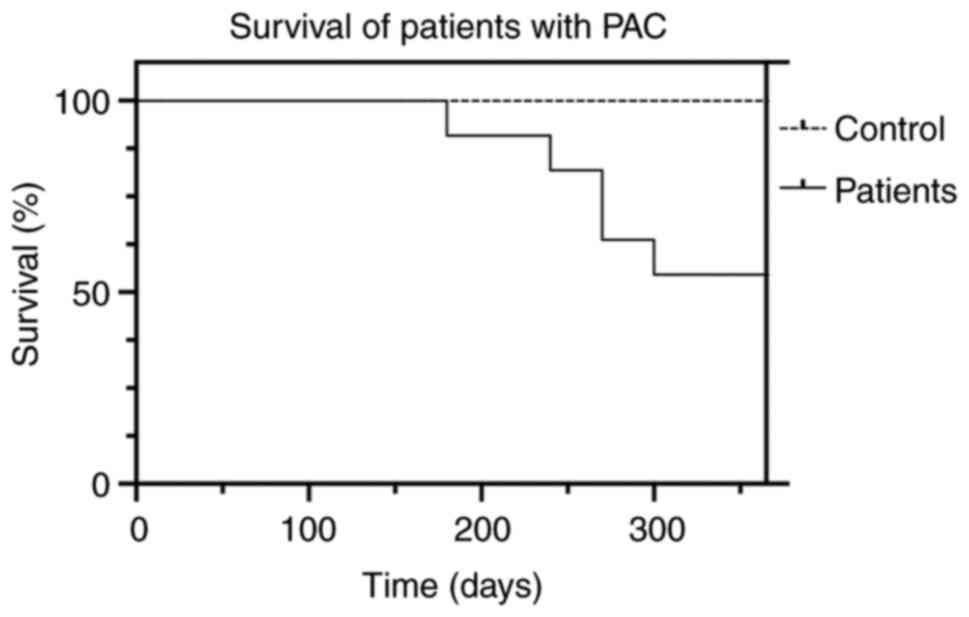
complications (n=4) and sepsis (n=1; Fig. 1).
Tumor markers CA 19-9 and CEA
As shown in Table
IV, the CA 19-9 levels in patients with PAC (1,273.32±1,258.25
U/ml) were significantly increased compared with those in the
control group (7.51±9.88 U/ml; P<0.01). Meanwhile, the levels of
CEA were also significantly increased in patients compared with
those in controls (69.81±59.50 vs. 1.35±0.32 U/ml, respectively;
*P<0.05).
 | Table IVTumor markers. |
Table IV
Tumor markers.
| Marker, U/ml | Patients | Controls | P-value |
|---|
| CA 19-9 |
1273.32±1258.25 | 7.51±9.88 | 0.002b |
| CEA | 69.81±59.50 | 1.35±0.32 | 0.03a |
Polyamine quantification
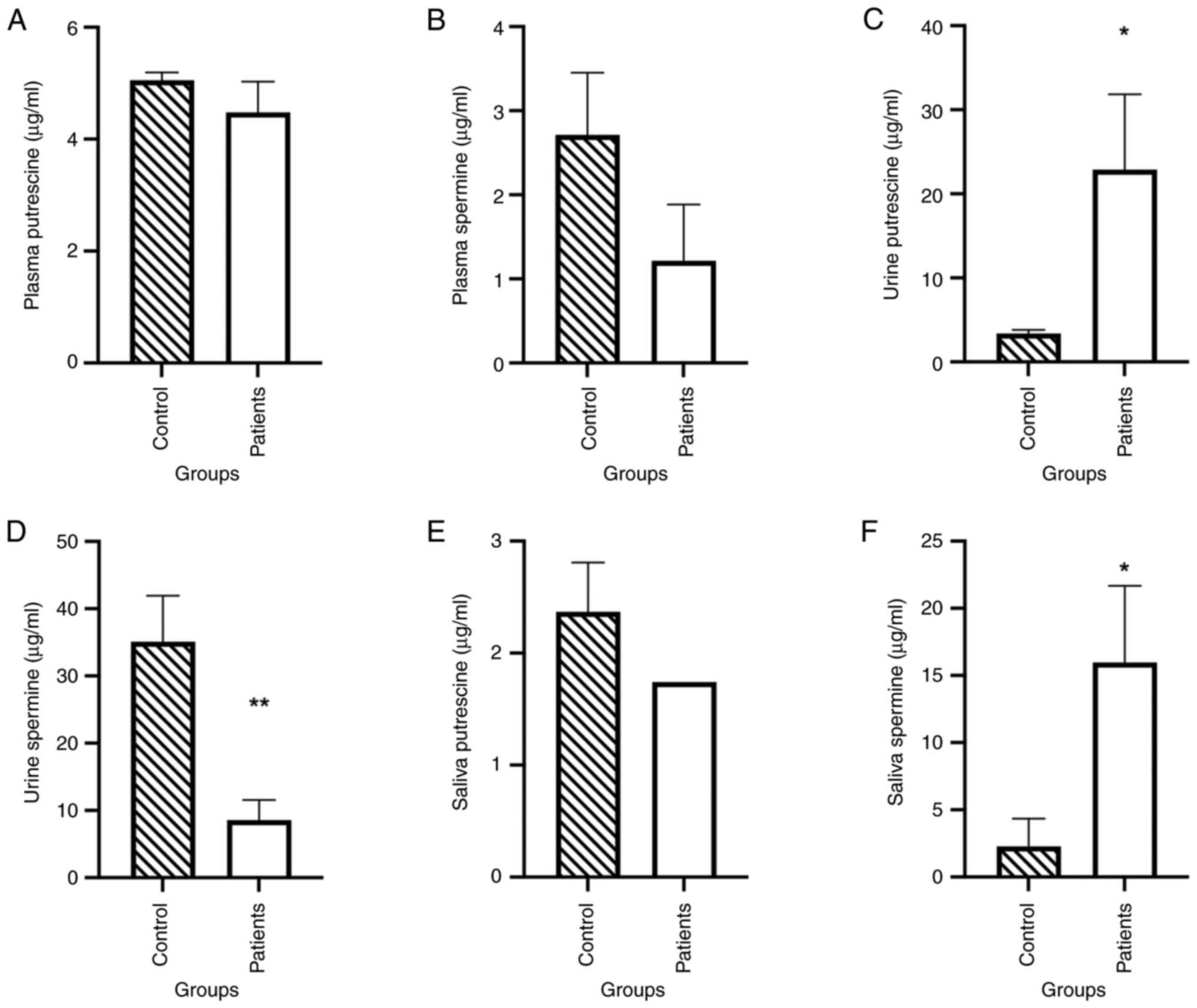
Regarding the levels of plasma polyamines, no
significant difference in putrescine concentration was observed
between the control group (4.475±1.7403 µg/ml) and the patient
group (5.0510±0.4494 µg/ml; Fig.
2A). The same observation was made for spermine concentration
between the control (2.7130±2.3371 µg/ml) and the patient group
(1.8520±1.4451 µg/ml; Fig. 2B).
Putrescine exhibited a significant increase in the
urine of patients compared with the control group
(*P<0.05; Fig. 2C),
while spermine was significantly decreased in the patient group
compared with the control group (**P<0.01; Fig. 2D).
Polyamine levels in the saliva of patients were also
compared with those in the controls (Fig. 2E). Putrescine did not vary
significantly between the two groups, while spermine increased
significantly in the patient group (*P<0.05; Fig. 2F). Spermidine levels were <0.001
µg/ml in the three fluids: Plasma, saliva and urine.
ODC expression
The ODC expression in PBMCs from patients with PAC
and in controls was investigated using western blotting. PBMC
recovery is depicted in Table V. A
total of 3.69±1.04x106 PBMCs were recovered from
patients, while in the control group, we recovered
6.25±2.36x106 PBMCs (**P<0.01).
 | Table VPBMC recovery. |
Table V
PBMC recovery.
| | Patients | Controls | P-value |
|---|
| x106
PBMCs/5 ml blood | 3.69±1.04 | 6.25±2.36 | 0.01a |
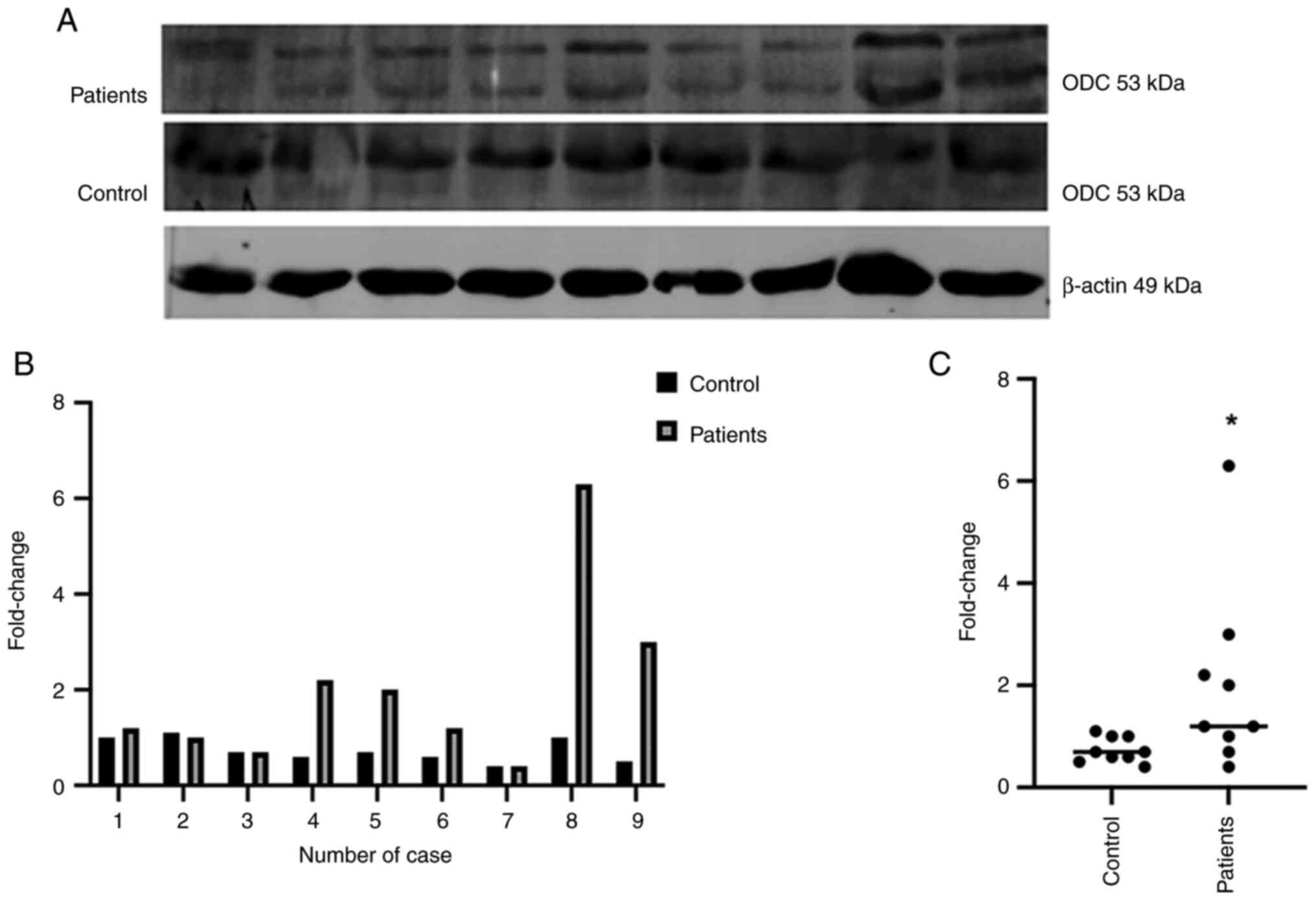
In Fig. 3A, the
expression of ODC (53 kDa) in patients with PAC (top) and in
controls (middle) was investigated using western blotting. Relative
expression was normalized using gel background, β-actin (bottom) is
shown as the internal control. In Fig.
3B, a 2-6-fold increase in ODC expression was noted (range,
0.4-6.3) in patients compared with that in controls. In Fig. 3C, the mean of fold change is shown
in the control group (0.7333) compared with that in the patient
group (2.0000), with differences shown as mean ± standard error of
the mean (1.2670±0.6071), with a 95% confidence interval
(**P<0.0001).
In Table VI, the
area under the curve (AUC), Se, Sp, PPV, NPV, Youden's Index and
the cut-off value of every parameter are presented: CEA, CA 19-9,
polyamines in different biofluids and ODC expression.
 | Table VISensitivity and specificity of
biomarkers of pancreatic adenocarcinoma. |
Table VI
Sensitivity and specificity of
biomarkers of pancreatic adenocarcinoma.
| Biomarker | AUCa |
Sensitivitya, % |
Specificitya,% | PPV, % | NPV, % | YI | Cut-off value | ACC |
|---|
| CEA | 0.800 | 70.0 | 90.9 | 60.00 | 90.90 | 0.5090 | 1.91 | 22.66 |
| CA 19-9 | 0.800 | 50.0 | 100.0 | 50.00 | 100.00 | 0.5000 | 33.30 | 23.20 |
| PPut | 0.520 | 70.0 | 50.0 | 70.00 | 45.00 | 0.1540 | 4.81 | 19.71 |
| PSpm | 0.360 | 70.0 | 40 | 70.00 | 36.35 | 0.06300 | 1.11 | 20.57 |
| UPut | 0.764 | 70.0 | 81.6 | 70.00 | 81.81 | 0.5180 | 4.76 | 20.28 |
| USpm | 0.0810 | 11.1 | 18.2 | 80.00 | 100.00 | 0.8000 | 15.14 | 21.37 |
| SPut | 0.409 | 0.0 | 81.8 | 100.00 | 20.00 | 0.2000 | 2.85 | 20.20 |
| SSpm | 0.820 | 80.0 | 80 | 100.00 | 72.00 | 0.5272 | 1.08 | 21.00 |
| ODC | 0.735 | 67.0 | 77.8 | 66.66 | 87.50 | 0.5400 | 1.05 | 18.16 |
The cut-off value was obtained by means of the
minimal distance from the upper-left corner of the unit square of
the receiver operating characteristic curves. It was observed that
the most used biomarkers were CEA and CA 19-9, which demonstrated
an AUC of 0.80. In addition, urine putrescine, saliva spermine and
ODC expression were within the range 0.73-0.82. It was shown that
plasma putrescine and spermine showed 70% of Se with low Sp (50 and
40%, respectively), while putrescine in urine (70% Se; 81.6% Sp)
and spermine in saliva (80% Se; 80% Sp) exhibited higher
values.
The accuracy of all parameters was 18.16% (ODC) and
23.2% (CA 19-9).
Discussion
PAC is a condition with increased incidence,
characterized by late diagnosis due to the appearance of signs and
symptoms at advanced disease stages, and these are usually
non-specific contributing to a delay in diagnosis (34).
Despite notable advances in diagnostic imaging
techniques and the improvement in treatment options in the last
decades, at present, patients with PAC continue have a poor
prognosis, with an estimated 5-year survival of 10-15% for
resectable tumors and ~0% for locally advanced, non-resectable and
metastatic stages (3).
A marked window of opportunity for improving the
outcomes for these patients is the early diagnosis of PC, and the
investigation of useful biomarkers to achieve this is urgent.
Polyamines and ODC have been considered as possible biomarkers in
colon, breast, lung and PC (35,36).
To the best of our knowledge, no strategies to date have been
implemented to evaluate their diagnostic usefulness. The aim of the
present study was to evaluate the expression of ODC as a hallmark
of PC in patients attending an outpatient clinic of the Medical
Oncology Service at the CMNO.
Regarding risk factors, it is noteworthy that the
average age between the two groups with no statistical differences
indicated that both groups were homogeneous. A total of 6.6% of
patients with PAC were also recently diagnosed with DM2, but this
was not associated with the expression of ODC or polyamine levels
in biofluids. A difference in the intensity of smoking between the
groups was also investigated since it has been reported that heavy
smoking is a high risk factor: Other risk factors were consistent
with other published studies, such as smoking, obesity, alcohol
consumption and HFH cancer in the patient group (37).
The most commonly used biomarkers reported in
literature include CA 19-9 and CEA (3,38).
However, these biomarkers have some limitations, such as low Se (50
and 70%, respectively) but high Sp (100 and 90%, respectively) as
observed in the present study. It was observed that there were
patients with both high and normal levels of the aforementioned
markers in the current study. This observation highlighted the need
to identify novel biomarkers with higher Se and Sp. Polyamines and
ODC have been described as possessing important pleiotropic effects
on cancer cells, because they contributed to different processes
that drive the progression of PC and can be detected in tissue and
biofluids with good precision, which allows differentiation between
controls and patients with PAC (6).
Regarding Se, good values for plasma putrescine and
spermine, urine putrescine, and saliva spermine and ODC were
observed; in addition, good Sp was observed for urine putrescine,
and for saliva spermine and putrescine.
It is noteworthy that it was possible to establish
the cut-off points for both ODC expression in PBMCs as well as for
the levels of polyamines in the different biofluids. The
aforementioned observations would enable the identification of
patients at early stages of the disease through accessible and
minimally invasive tests.
In the current study, ODC expression in the PBMCs of
patients with PAC was increased by ≥2-fold compared with that of
controls (67% Se; 77.8% Sp). This finding could represent the first
important evidence in the search for biomarkers for diagnostic use,
and it is pertinent to highlight that to the best of our knowledge
this has not been reported before.
In the current study, the risk factors for PAC as
well as the early signs and symptoms of the disease, diagnosis and
treatment time, clinical stage at the time of diagnosis and
treatments received were investigated in a Mexican cohort. These
data could explain the poor prognosis of the disease and highlight
the importance of identifying novel diagnostic biomarkers.
It has been established that the following
combination of symptoms, including abdominal pain with poor
response to symptomatic treatment for >4 months, weight loss
and/or recently diagnosed DM with early evolution and
out-of-context metabolic syndrome, would be criteria for performing
the evaluation of polyamines and ODC.
An important limitation of the present study was
that the patients were enrolled from 2019 to 2020, and the total
number of included cases was <30. It is noteworthy that although
the current study is a pilot study and a larger cohort size is
necessary for future studies, it contributed to innovative basic
knowledge due to the investigation of ODC expression in the PBMCs
of patients with PAC, as well as its diagnostic use, which to the
best of our knowledge has not been investigated before in
literature.
Acknowledgements
The authors wish to thank to Dr Ramón Reynoso-Orozco
for the kind donation of polyamine standards, Miss María de Jesús
Delgado-Ávila for her kind assistance with the development of this
project, Dr Martha Patricia Gallegos-Arreola for her kind
assistance with the statistical analysis and Professor Acela
Villaseñor-García for her support in the graphical design of the
figures.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LRRB and TDPR carried out clinical diagnosis,
patient recruitment, consent letter application, treatments, and
biofluid sample collection and transport. GHF acquired and analyzed
data, wrote and reviewed the manuscript, and provided financial
support for the western blotting experiments. PCOL developed the
methodology used, wrote, reviewed and edited the manuscript, and
provided financial support. ABC wrote and edited the manuscript,
analyzed data and provided financial support. AMML determined
polyamine levels in biofluids and carried out data analysis. KJPS
determined polyamine levels in biofluids. LAPM carried out western
blotting of ODC in patients and controls. AAL conceptualized the
study, and wrote, reviewed and edited the manuscript. LFJS used
software, carried out data analysis, and wrote, reviewed and edited
the manuscript. MMVG conceptualized the study, developed
methodology, used software, carried out data analysis, and wrote,
reviewed and edited the manuscript. LRRB, AMLL, GHF and MMVG
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present work was registered with the Mexican
Social Security Institute (IMSS) National Scientific Research
Committee (approval no. R-2019-785-101), and was approved by the
Ethics Committee of Health Research of the IMSS, Mexico, United
Mexican States. All participants were kindly invited to participate
and all of them provided written informed consent. A total of 5/15
patients did not agree to donate their biological samples, but they
did allow the use of their clinical and sociodemographic data. The
present study was carried out in compliance with the guidelines of
the Declaration of Helsinki.
Patient consent for publication
All participants (controls and patients) agreed to
participate in the present study, and to donate blood, urine and
saliva, and they provided written informed consent.
Authors' information
ORCID: Ruiz-Barrios LR, 0009-0006-1211-5907;
Pineda-Razo TD, 0000-0002-6757-731X; Hernández-Flores G,
0000-0002-4861-0065; Ortiz-Lazareno PC, 0000-0001-9045-7052;
Bravo-Cuéllar A, 0000-0002-2945-4503; Macias-Lamas AM,
0000-0001-9787-0819; Parra-Saavedra KJ, 0000-0002-9042-8777;
Palafox-Mariscal LA, 0000-0002-1209-3617; Aguilar-Lemarroy A,
0000-0001-9288-4824; Jave-Suárez LF, 0000-0001-6209-5031;
Villaseñor-García MM, 0000-0002-9280-7893.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society. Key Statistics
for Pancreatic Cancer. https://www.cancer.org/content/dam/CRC/PDF/Public/8778.00.pdf.
Accessed February 20, 2022.
|
|
2
|
Zhang L, Sanagapalli S and Stoita A:
Challenges in diagnosis of pancreatic cancer. World J
Gastroenterol. 24:2047–2060. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Poruk KE, Firpo MA, Adler DG and Mulvihill
SJ: Screening for pancreatic cancer: Why, how, and who? Ann Surg.
257:17–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Globocan: Global Cancer Statistics in
Mexico. Journal 2021: 2020. https://gco.iarc.fr/today/data/factsheets/populations/484-mexico-fact-sheets.pdf.
Accessed December 2021.
|
|
5
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Asai Y, Itoi T, Sugimoto M, Sofuni A,
Tsuchiya T, Tanaka R, Tonozuka R, Honjo M, Mukai S, Fujita M, et
al: Elevated polyamines in saliva of pancreatic cancer. Cancers
(Basels). 10(43)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
O'Neill RS and Stoita A: Biomarkers in the
diagnosis of pancreatic cancer: Are we closer to finding the golden
ticket? World J Gastroenterol. 27:4045–4087. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chang CY, Huang SP, Chiu HM, Lee YC, Chen
MF and Lin JT: Low efficacy of serum levels of CA 19-9 in
prediction of malignant diseases in asymptomatic population in
Taiwan. Hepatogastroenterology. 53:1–4. 2006.PubMed/NCBI
|
|
10
|
Hartwig W, Strobel O, Hinz U, Fritz S,
Hackert T, Roth C, Büchler MW and Werner J: CA19-9 in potentially
resectable pancreatic cancer: Perspective to adjust surgical and
perioperative therapy. Ann Surg Oncol. 20:2188–2196.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wingren C, Sandström A, Segersvärd R,
Carlsson A, Andersson R, Löhr M and Borrebaeck CA: Identification
of serum biomarker signatures associated with pancreatic cancer.
Cancer Res. 72:2481–2490. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hermida Lazcano I, Sánchez Tejero E, Nerín
Sánchez C, Cordero Bernabé R, Mora Escudero I and Pinar Sánchez J:
Tumor markers. Marcadores Tumorales. Rev Clín Med Fam. 9:31–42.
2016.
|
|
13
|
Goonetilleke KS and Siriwardena AK:
Systematic review of carbohydrate antigen (CA 19-9) as a
biochemical marker in the diagnosis of pancreatic cancer. Eur J
Surg Oncol. 33:266–270. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Menéndez-Sánchez P, Villarejo-Campos P,
Padilla-Valverde D, Menéndez-Rubio JM and Rodríguez-Montes JA:
Tumor markers in colorectal cancer. Cir Cir. 81:169–175.
2013.PubMed/NCBI(In Spanish).
|
|
15
|
Tellez-Avila F, García-Osogobio S and
Residente R: The carcinoembryonic antigen: by the way a well-known
friend. Rev Invest Clin. 57:814–819. 2005.(In Spanish).
|
|
16
|
Irigoyen Oyarzabal AM, Amiguet García JA,
López Vivanco G, Genollá Subirats J, Muñoz Villafranca MC,
Ojembarrena Martínez E and Liso Irurzun P: Tumoral markers and
acute-phase reactants in the diagnosis of pancreatic cancer.
Gastroenterol Hepatol. 26:624–629. 2003.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
17
|
Guasco Herrera C, Chávez Servín JL, Ferriz
Martínez RA, de la Torre Carbot K, Elton Puente E and García Gasca
T: Polyamines: Little Giants of Metabolic Regulation. REB.
33:51–57. 2014.(In Spanish).
|
|
18
|
Wallace HM, Fraser AV and Hughes A: A
perspective of polyamine metabolism. Biochem J. 376(Pt 1):1–14.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Medina MA, Quesada AR, Núñez de Castro I
and Sánchez-Jiménez F: Histamine, polyamines, and cancer. Biochem
Pharmacol. 57:1341–1344. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Madan M, Patel A, Skruber K, Geerts D,
Altomare DA and Iv OP: ATP13A3 and caveolin-1 as potential
biomarkers for difluoromethylornithine-based therapies in
pancreatic cancers. Am J Cancer Res. 6:1231–1252. 2016.PubMed/NCBI
|
|
21
|
Niemi RJ, Roine AN, Häkkinen MR,
Kumpulainen PS, Keinänen TA, Vepsäläinen JJ, Lehtimäki T, Oksala NK
and Mäenpää JU: Urinary polyamines as biomarkers for ovarian
cancer. Int J Gynecol Cancer. 27:1360–1366. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kawakita M, Hiramatsu K, Yanagiya M, Doi Y
and Kosaka M: Determination of N¹,N¹²-diacetylspermine in urine: A
novel tumor marker. Methods Mol Biol. 720:367–378. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Umemori Y, Ohe Y, Kuribayashi K, Tsuji N,
Nishidate T, Kameshima H, Hirata K and Watanabe N: Evaluating the
utility of N1,N12-diacetylspermine and N1,N8-diacetylspermidine in
urine as tumor markers for breast and colorectal cancers. Clin Chim
Acta. 411:1894–1899. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takahashi Y, Sakaguchi K, Horio H,
Hiramatsu K, Moriya S, Takahashi K and Kawakita M: Urinary N1,
N12-diacetylspermine is a non-invasive marker for the diagnosis and
prognosis of non-small-cell lung cancer. Br J Cancer.
113:1493–1501. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tsoi TH, Chan CF, Chan WL, Chiu KF, Wong
WT, Ng CF and Wong KL: Urinary Polyamines: A pilot study on their
roles as prostate cancer detection biomarkers. PLoS One.
11(e0162217)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sugimoto M, Wong DT, Hirayama A, Soga T
and Tomita M: Capillary electrophoresis mass spectrometry-based
saliva metabolomics identified oral, breast and pancreatic
cancer-specific profiles. Metabolomics. 6:78–95. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Soda K: The mechanisms by which polyamines
accelerate tumor spread. J Exp Clin Cancer Res.
30(95)2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Massaro C, Thomas J and Phanstiel Iv O:
Investigation of polyamine metabolism and homeostasis in pancreatic
cancers. Med Sci (Basel). 5(32)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Venza M, Visalli M, Cicciu D and Teti D:
Determination of polyamines in human saliva by high-performance
liquid chromatography with fluorescence detection. J Chromatogr B
Biomed Sci Appl. 757:111–117. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cruz-Galvez CC, Ortiz-Lazareno PC,
Pedraza-Brindis EJ, Villasenor-Garcia MM, Reyes-Uribe E,
Bravo-Hernandez A, Solis-Martinez RA, Cancino-Marentes M,
Rodriguez-Padilla C, Bravo-Cuellar A and Hernandez-Flores G:
Pentoxifylline enhances the apoptotic effect of carboplatin in Y79
retinoblastoma cells. In Vivo. 33:401–412. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shmulewitz D, Aharonovich E, Witkiewitz K,
Anton RF, Kranzler HR, Scodes J, Mann KF, Wall MM and Hasin D:
Alcohol Clinical Trials Initiative (ACTIVE Group). The World Health
Organization Risk Drinking Levels Measure of Alcohol Consumption:
Prevalence and Health Correlates in Nationally Representative
Surveys of U.S. Adults, 2001-2002 and 2012-2013. Am J Psychiatry.
178:548–559. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lukic S, Mijac D, Filipovic B,
Sokic-Milutinovic A, Tomasevic R, Krstic M and Milosavljevic T:
Chronic abdominal pain: Gastroenterologist approach. Dig Dis.
40:181–186. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rawla P, Sunkara T and Gaduputi V:
Epidemiology of pancreatic cancer: Global trends, etiology and risk
factors. World J Oncol. 10:10–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McNamara KM, Gobert AP and Wilson KT: The
role of polyamines in gastric cancer. Oncogene. 40:4399–4412.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Holbert CE, Cullen MT, Casero RA Jr and
Stewart TM: Polyamines in cancer: Integrating organismal metabolism
and antitumour immunity. Nat Rev Cancer. 22:467–480.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kenner BJ, Chari ST, Maitra A, Srivastava
S, Cleeter DF, Go VL, Rothschild LJ and Goldberg AE: Early
detection of pancreatic cancer-a defined future using lessons from
other cancers: A white paper. Pancreas. 45:1073–1079.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Y, Li Y and Huang C: Circulating miRNAs
Increasing the Risk of Cancer. In: Cancer and Noncoding RNAs.
Chakrabarti J and Mitra S (eds). Vol. 1. Academic Press, Boston,
pp79-94, 2018.
|