Introduction
The role of chemotherapy in the conservative
treatment of malignant tumors is pivotal, representing a
cornerstone in the therapeutic approach. The main objective in
molecular oncology is the exploration of the mechanisms underlying
chemotherapy-induced cellular changes and understanding the nature
of cell death (1). In the past
years, there has been an active exploration for agents and their
synergistic combinations tailored to selectively target the
pathways responsible for sustaining cancer resistance (2-5).
The efficacy of combining different modalities of conservative
therapy for breast cancer, especially the tandem use of
chemotherapy and hormonal therapy, remains a largely unresolved
question. While the impact of numerous chemotherapy drugs is linked
to DNA damage, the precise role of these alterations in potentially
influencing the estrogen receptor α (ERα) machinery and the
hormonal response of tumors remains unclear. Current research in
this domain heavily relies on the examination of clinical data,
particularly the analysis of combined chemotherapy and hormone
therapy effectiveness across diverse patient groups. However,
findings in this area are often conflicting and contradictory.
Specifically, evidence has revealed that incorporating tamoxifen
into chemotherapy cycles enhances outcomes for ERα-positive breast
cancer (6-9).
Likewise, the combination of hormonal and chemotherapy treatments
has been associated with improved survival among women aged over 60
years (10). Conversely, some
studies have reported that additional hormonal therapy fails to
yield a discernible impact on overall survival (8,11),
while supplementary chemotherapy does not demonstrate enhanced
outcomes when compared with hormonal therapy alone (12). Several studies have revealed changes
in ERα status during neoadjuvant chemotherapy for breast tumors
(13,14). Notably, a correlation has been
established between neoadjuvant chemotherapy and increased
expression of microRNA-18a, a member of the ERα suppressor family
(15). The potential role of DNA
damage in modulating ERα signaling was underscored in
investigations exploring the effects of radiation on breast cancer.
These studies revealed notable changes in hormonal signaling within
irradiated cells (16,17).
In the present study, it was revealed for the first
time that the treatment of MCF7 breast cancer cells with a single
dose of 5-fluorouracil (5-FU) induces significant DNA
fragmentation, which correlates with a transient suppression of
estrogen signaling. Notably, continuous 5-FU treatment leads to the
irreversible inhibition of ERα activity and the emergence of
partial resistance to the antiestrogen tamoxifen. The pivotal role
of DNA damage in altering estrogen signaling was further
corroborated through parallel experiments involving ultraviolet-C
(UVC)-irradiated cells. These irradiated cells exhibited a
pronounced inhibition of estrogen machinery, mirroring the effects
observed with 5-FU treatment. Chronic UVC irradiation, akin to
prolonged 5-FU exposure, resulted in irreversible changes to
estrogen receptor activity and a concomitant reduction in hormonal
sensitivity. These findings strongly support the role of DNA damage
in driving the progression of hormonal resistance.
Materials and methods
Cell cultures and reagents
Experiments were conducted on the MCF7 human breast
cancer cell line (18) (cat. no.
HTB-22™; American Type Culture Collection),
authenticated by morphology and STR profiling through ‘Gordiz’
(http://gordiz.ru/, accessed on February 1, 2022). The
cells were cultured at 37˚C with 5% CO2 in DMEM
containing 4.5 g/l glucose (cat. no. СC420-02; PanEco),
alanyl-glutamine (cat. no. Ф005; PanEco) and 7% fetal bovine serum
(FBS) (cat. no. SV30160.03; HyClone; Cytiva). The response of the
cells to tamoxifen (cat. no. 27190; Cayman Chemical Company) was
assessed by treating them with tamoxifen for 3 days, followed by
evaluating viability using the MTT assay
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
(cat. no. A2231; PanReac AppliChem) (19) modified as previously described
(20). Dimethyl sulfoxide (DMSO)
(cat. no. 191954; PanReac AppliChem) served as the solvent for the
assay. Ultrapure water for the experiments was prepared using a
Milli-Q water purification system (Merck KGaA).
Treatment of MCF7 cells with 5-FU and
the development of resistant clones
MCF7 breast cancer cells were seeded onto 24-well
culture plates at a density of 40,000 cells per well. To assess
cell sensitivity to 5-FU (cat. no. F6627; Sigma-Aldrich; Merck
KGaA), the cells were exposed to 15 µM 5-FU for a 3-day period,
followed by an analysis of the number of viable cells. To establish
a 5-FU-resistant subline, MCF7 cells (at a density of 150,000 cells
per well in 6-well plates) were cultured in DMEM medium with 7%
FBS. The cells were exposed to increasing concentrations of 5-FU
ranging from 5 to 30 µM over a span of 2 months, and this regimen
was maintained for at least 1 month after withdrawal of 5-FU.
UV irradiation and the selection of
UV-resistant cells
Irradiation was conducted using 6W UV-lamp, emitting
254 nm light (model VL-6.LC; Vilber Lourmat). MCF7 cells were
exposed to UVC irradiation (254 nm) at intensities of 50
J/m2. For the selection of UV-resistant cells, MCF7
cells were exposed to UVC once every three days for a duration of 4
weeks. Subsequently, cell growth was sustained for a minimum of 40
days following the conclusion of the last irradiation cycle.
Colony-forming test
MCF7 cells were initially plated on 60-mm culture
dishes at a density of 2 million cells per dish (Corning, Inc.).
The following day, the DMEM culture medium was removed, and the
seeded cells underwent UV irradiation (254 nm, 3 sec). After UV
exposure, varying cell quantities were immediately seeded onto a
6-well culture plate (Corning, Inc.) in DMEM culture medium, aiming
to establish 50-2,000 colonies per well. After a 14-day growth in a
cell culture incubator, the colonies were fixed and stained using a
solution of 20% methanol and 0.2% crystal violet at room
temperature for 10 min. Any colony comprising >50 cells was
identified and recorded as a viable surviving clone. Colonies were
counted manually.
Comet assay
The comet assay was conducted following established
procedures outlined in a previous study (21). MCF7 cells were subjected to varying
concentrations of 5-FU (15 and 30 µM) for a duration of 72 h or
exposed to UVC irradiation, and subsequently, embedded in agarose
on microscope slides. Following cell lysis and electrophoresis, the
slides were stained with a DNA dye (SYBR Gold) for 5 min at room
temperature. Observations were made using a Zeiss AxioVert 200
fluorescence microscope equipped with an EBQ isolated lamp at x10
magnification (Carl Zeiss AG). A minimum of 100 cells were captured
for each sample and analyzed using CometScore 2.0 software
(RexHoover) to quantify DNA damage.
Micronucleus assay
To inhibit microfilament assembly and cytokinesis,
cytochalasin B (cat. no. Х095; PanEco) was introduced into the
medium at a final concentration of 6 µg/ml, 28 h prior to fixation
across all experimental groups. Following cultivation, cells were
collected, centrifuged at 1,200 x g for 10 min, and exposed to
0.075 M KCl (cat. no. 60129-100; PanEco) for 2 min. Subsequently,
cells were fixed in ethanol-acetic acid (3:1), followed by another
centrifugation at 1,000 x g at 4˚C for 7 min. The fixed cells were
then transferred onto clean glass slides. All slides underwent
staining with Giemsa solution (cat. no. 0080; PanEco) for 1 min at
room temperature. Light microscopic analysis was performed on
encrypted preparations, studying 2,000 binuclear cells from each
group at a magnification of x400. The significance of differences
in cytogenetic damage levels between control and treated cells was
determined using Pearson's χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Transient transfection and the
measurement of reporter gene activity
The transcriptional activity of ERα was assessed
through reporter analysis, involving the transfection of ERE
plasmids (luciferase-expressing reporter construct ERE-tk-LUC,
which incorporates the estrogen response elements (EREs) from the
vitellogenin A2 gene upstream of the thymidine kinase promoter)
kindly provided by Professor George Reid from European Molecular
Biology Laboratory (Heidelberg, Germany) (22,23).
Transfection occurred under steroid-free conditions, utilizing DMEM
without phenol red supplemented with 2% charcoal/dextran-treated
fetal bovine serum (cat. no. SH30068.03; HyClone, Cytiva). This
process was carried out for 6 h at 37˚C using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.).
For the transfection of a single well in Costar® 24-well
clear TC-treated plate (cat. no. 3524; Corning, Inc.), 0.8 µl of
the transfection agent and 0.4 µg of plasmid DNA were employed.
Co-transfection with a plasmid carrying the β-galactosidase gene
served as a control to assess the efficiency and potential toxicity
of the transfection process. 17β-Estradiol (E2) (cat. no. 3301;
Sigma-Aldrich; Merck KGaA) in a concentration of 10 nM was used to
treat cells during 24 h before determination of the luciferase and
β-galactosidase activities. After 24 h post-transfection, cell
lysis was carried out in 1x lysis buffer (cat. no. E1531; Promega
Corporation), and luciferase activity was quantified using a Tecan
Infinite M200 Pro luminometer (Tecan Group), following the
manufacturer's protocol (Luciferase Assay System; cat. no. E1501;
Promega Corporation) (24,25). β-Galactosidase activity was
determined using ONPG (p-nitrophenyl β-D-galactopyranoside) (cat.
no. 34055; Thermo Fisher Scientific, Inc.), the substrate for
β-galactosidase. The cell lysates were combined with a phosphate
buffer (pH 7.5, 0.1 M) containing ONPG (3.3 mM), MgCl2
(1 mM), and β-mercaptoethanol (53 mM). Absorbance at 405 nm was
measured using the MultiScan FC reader (Thermo Fisher Scientific,
Inc.) ERE reporter activity was calculated in arbitrary units as
the luciferase/galactosidase activity ratio, following the method
outlined in previous studies (24,25).
Western blot analysis
To prepare samples for immunoblotting, cells were
lysed in a buffer (150 µl) comprising Tris-HCl pH 7.4 (50 mM),
Igepal CA-630 (1%), ethylenediamine tetraacetate (1 mM),
dithiothreitol (1 mM), aprotinin, pepstatin and leupeptin (1
µg/ml), as well as sodium fluoride and sodium orthovanadate (1 mM).
Protein content was determined using the Bradford method. Prior to
centrifugation (10,000 x g, 10 min, 4˚C), the samples were
incubated on ice for 20 min. Electrophoresis was performed on a 10%
polyacrylamide gel, loaded with 60 µg of protein per lane, followed
by protein transfer to a nitrocellulose membrane (Santa Cruz
Biotechnology) and subsequent immunoblotting as described in our
previous study (25). The membranes
were immersed in a 5% non-fat milk solution (cat. no. A0830,0500;
PanReac AppliChem) in TBS buffer with pH 7.5, consisting of Tris
(20 mM) and NaCl (500 mM), supplemented with Tween-20 (0.1%) at
room temperature over a period of 30 min to prevent non-specific
absorption. Subsequently, the membranes were incubated with primary
antibodies overnight at 4˚C. The primary antibodies targeting
phosphorylated (p)-AKT (cat. no. 9271), AKT (cat. no. 9272),
p-Ribosomal Protein S6 Kinase B1(S6K) (cat. no. 9205), S6K (cat.
no. 2708) and ERα (cat. no. 8644) (all diluted at 1:1,000; all from
Cell Signaling Technology, Inc.) were employed, with antibodies
against α-tubulin (1:1,000; cat. no. 2144; Cell Signaling
Technology) serving as loading controls. Appropriate IgGs
(1:10,000; cat. no. 111-035-003; Jackson ImmunoResearch Europe)
conjugated with horseradish peroxidase at room temperature during
an hour were used as secondary antibodies. Signal detection was
achieved using ECL reagents prepared according to Mruk's protocol
(26) by ourselves, and the
ImageQuant LAS4000 system for chemiluminescence (GE HealthCare) was
utilized. Densitometry for the tested proteins/α-tubulin ratio was
carried out using ImageJ 1.53q software (National Institutes of
Health). The protocol for densitometry was provided by The
University of Queensland, with recommendations from the references
(27,28).
Statistical analysis
Each antiproliferative assay was independently
replicated three times, with each replication comprising three
technical replicates. Statistical analysis was performed using
Microsoft Excel 2019 (Microsoft Corp.) and GraphPad 9.0 software
(Dotmatics). The IC50 value was calculated to determine
the concentration of tamoxifen to produce 50% inhibition of cell
growth. The results were presented as the mean ± standard deviation
(S.D.), unless otherwise specified. P<0.05 was considered to
indicate a statistically significant difference.
Results
5-FU-induced DNA damage
The primary objective of this experiment was to
investigate the impact of DNA damage agents on estrogen signaling
and the subsequent sensitivity of breast tumors to hormonal
therapy. Specifically, the authors focused on 5-FU (29-31),
a cytostatic chemotherapeutic drug widely employed in breast cancer
treatment. The experiments were conducted on in
vitro-cultured MCF7 breast cancer cells. The effectiveness of
5-FU-induced DNA damage was assessed using the DNA fragmentation
test, specifically the Comet assay, and by measuring the
accumulation of micronuclei in cells as an outcome of DNA
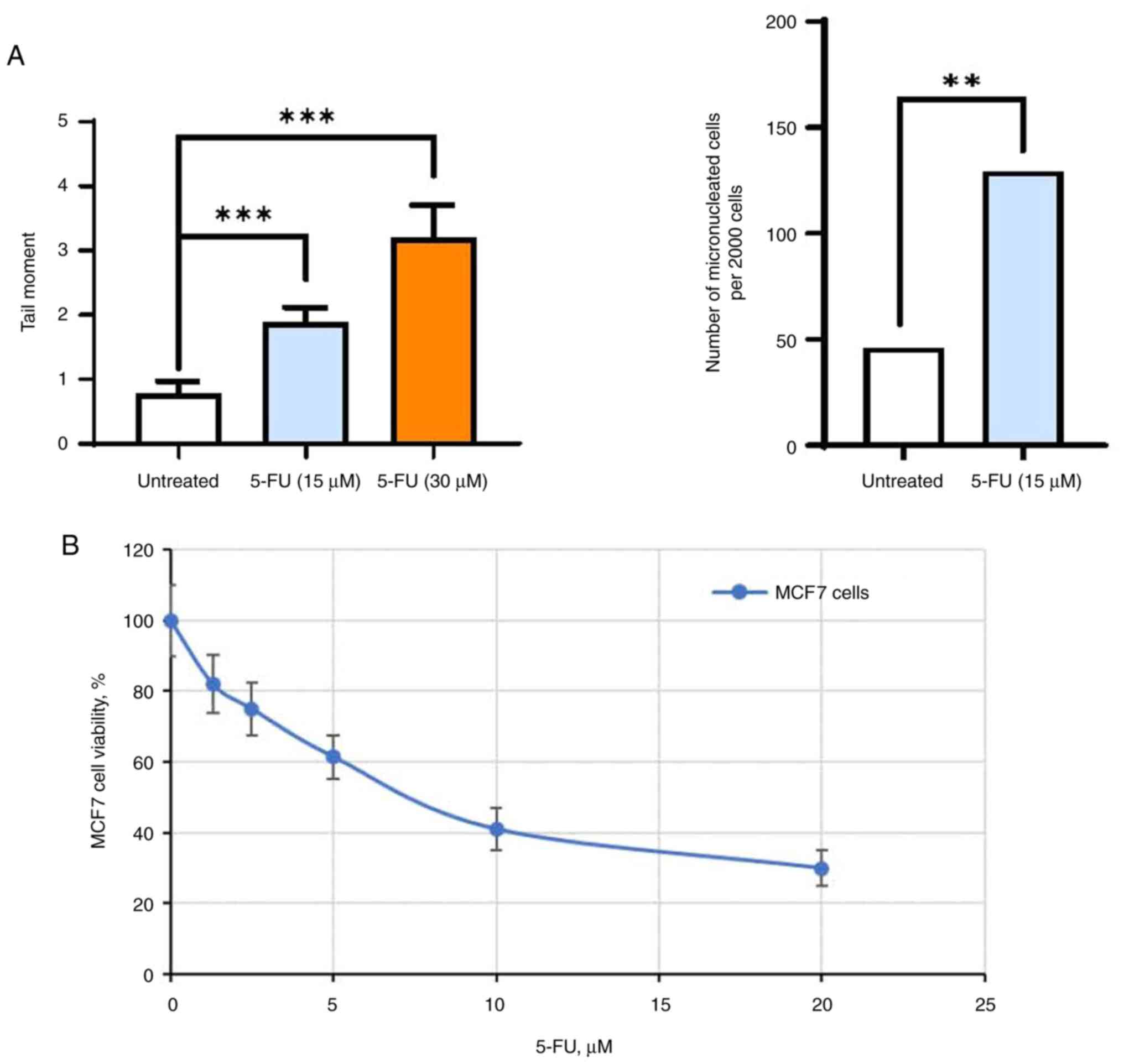
disruption. As demonstrated, a single exposure of MCF7 cells to
5-FU resulted in notable DNA fragmentation and the accumulation of
micronuclei within cells (Figs. 1A
and S1A and B), correlating with a substantial
decrease in the number of viable cells (Fig. 1B). To elucidate whether such DNA
damage can disrupt ERα signaling and to determine the duration of
such alterations, an in-depth analysis of estrogen signaling and
the responsiveness to hormone therapy in 5-FU-treated breast cancer
cells was conducted.
Influence of 5-FU on ERα signaling and
cell response to antiestrogen tamoxifen
MCF7 cells were subjected to a three-day treatment
with 5-FU, followed by the assessment of ERα expression and
activity. Western blot analysis revealed non-significant changes in
ERα expression in 5-FU-treated cells, while reporter analysis of
ERα transcriptional activity exhibited a significant suppression
following 5-FU exposure. Simultaneously, an activation of AKT, p85
S6K and p70 S6K phosphorylation in 5-FU-treated cells was observed,
suggesting a potential compensatory reaction to the inhibition of
ERα signaling (Fig. 2A and B). In parallel, the analysis of cell
sensitivity to the antiestrogen tamoxifen indicated a decrease in
cell sensitivity to the growth inhibitory effects of tamoxifen
(Fig. 2C). The IC50
values of tamoxifen were 7.2±0.9 and 12.1±1.3 µM for MCF7 and
MCF7/5-FU respectively. Upon withdrawal of 5-FU and the transfer of
cells to a standard medium for ten days, there was a notable
restoration of ERα transcriptional activity and cell sensitivity to
the antiestrogen tamoxifen (Fig. 2D
and E). This restoration was
concomitant with a reduction in AKT and p85 S6K and p70 S6K
phosphorylation levels (Fig.
2F).
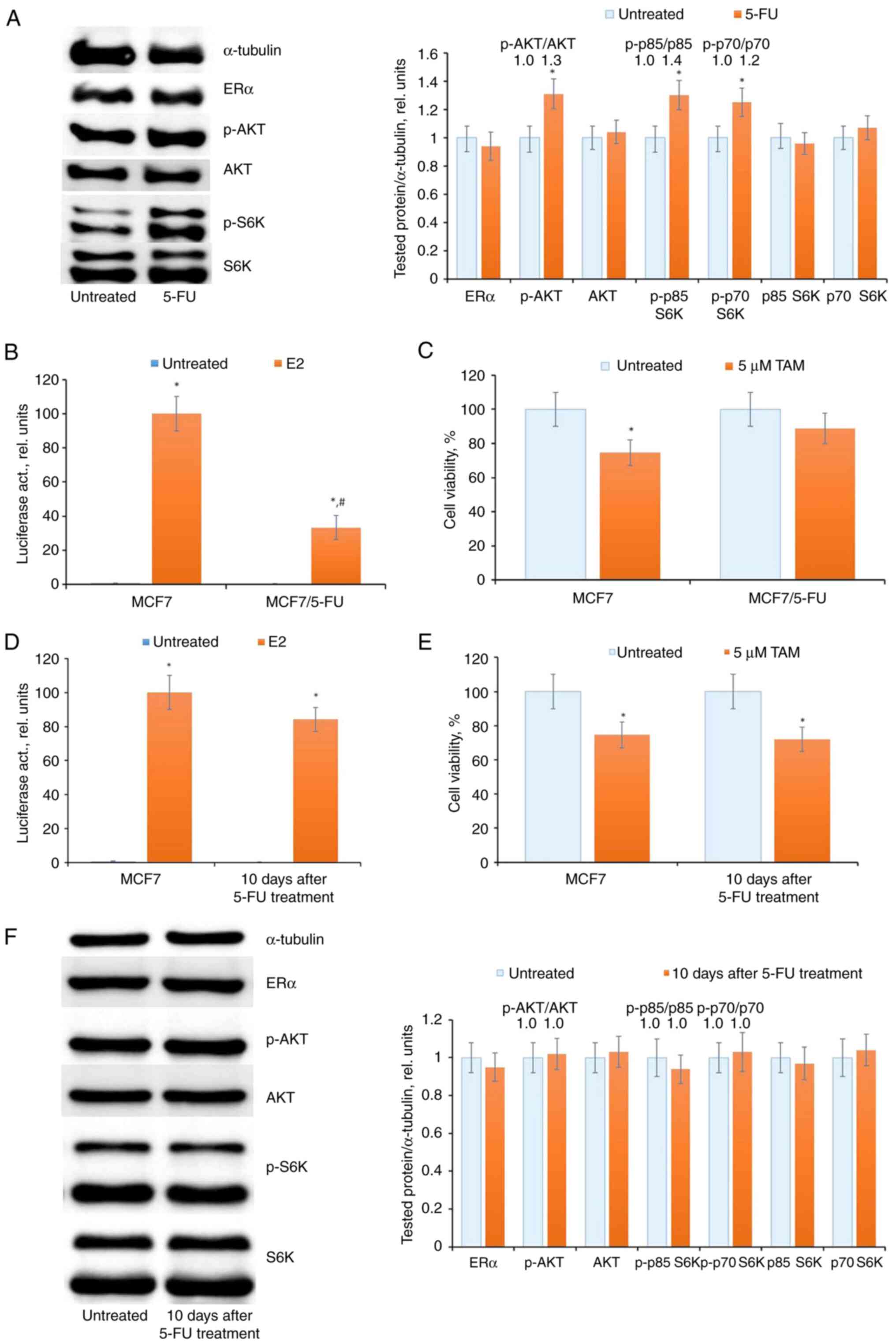 | Figure 25-FU treatment and ERα signaling in
MCF7 cells. (A) Western blot analysis of ERα, p-AKT, AKT,
p-p85/p-p70 S6K and p85/p70 S6K in cell extracts. The MCF7 cells
were treated with 15 µM 5-FU for three days and the cells were
subjected to western blotting. Protein loading was controlled by
membrane hybridization with α-tubulin antibodies. The blot
represents the results of one of three similar experiments.
Densitometry for the tested proteins/α-tubulin ratio was carried
out using ImageJ software (right diagram). *P<0.05.
(B) Reporter analysis of ERα transcriptional activity. The cells
were treated with 15 µM 5-FU for three days, then the cells were
transfected with the plasmid containing the luciferase reporter
gene under estrogen-responsive elements, and β-galactosidase
plasmid. The cells were treated with or without 10 nM 17β-estradiol
(E2) for 24 h, and the luciferase and β-galactosidase activities
were determined. The relative luciferase activity was calculated in
arbitrary units as the ratio of luciferase to the β-galactosidase
activity. A total of 100 relative units were set as the luciferase
activity in MCF7 cells treated with E2. Data represent the mean
values ± S.D. of three independent experiments:
*P<0.05 vs. untreated samples; #P<0.05
vs. E2-treated MCF7 cells. (C) Cell sensitivity to antiestrogen
tamoxifen. The cells were treated with 15 µM 5-FU for three days
following 5-FU withdrawal for three days. Then MCF7 cells were
treated with 5 µM tamoxifen for three days and the number of viable
cells was assessed by the MTT-test. Data represent the mean value ±
SD of three independent experiments. Percentage of 100% was set as
the viability of untreated cells. *P<0.05 vs.
untreated samples. (D) Analysis of luciferase activity in MCF7
cells after 5-FU withdrawal. The cells were treated with 15 µM 5-FU
for three days following 5-FU withdrawal for ten days:
*P<0.05 vs. untreated samples. (E) The cell response
to tamoxifen. *P<0.05 vs. untreated samples. (F)
Western blot analysis of ERα, p-AKT, AKT, p-S6K, and S6K in control
MCF7 cells and MCF7 cells 10 days after treatment. 5-FU,
5-fluorouracil; ERα, estrogen receptor α; 5-FU, 5-fluorouracil;
S6K, Ribosomal Protein S6 Kinase B1; p-, phosphorylated. |
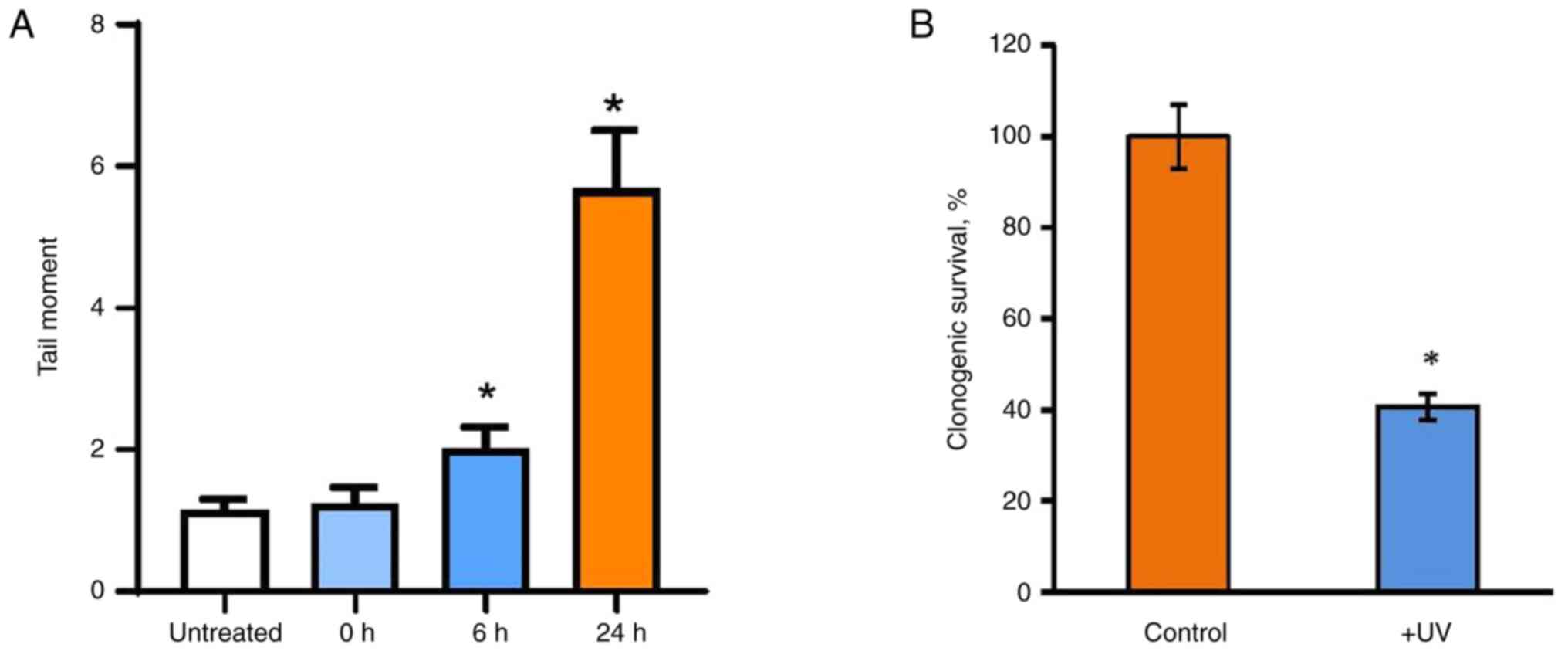
Effect of prolonged 5-FU treatment on
the ERα machinery
To explore the impact of repeated courses of
chemotherapy, the effect of prolonged 5-FU treatment was examined
on ERα signaling in MCF7 cells. These cells underwent a two-month
treatment with 5-FU, followed by withdrawal of 5-FU and cultivation
in standard medium for an additional month. The resulting cell
subline, designated as MCF7/FUR, exhibited a notable resistance to
5-FU (Fig. 3A), and significantly,
demonstrated marked resistance to antiestrogen tamoxifen (Fig. 3B). The comparative analysis of
5-FU-induced DNA damage revealed a decreased response in the
resistant subline to 5-FU treatment (Fig. 3C).
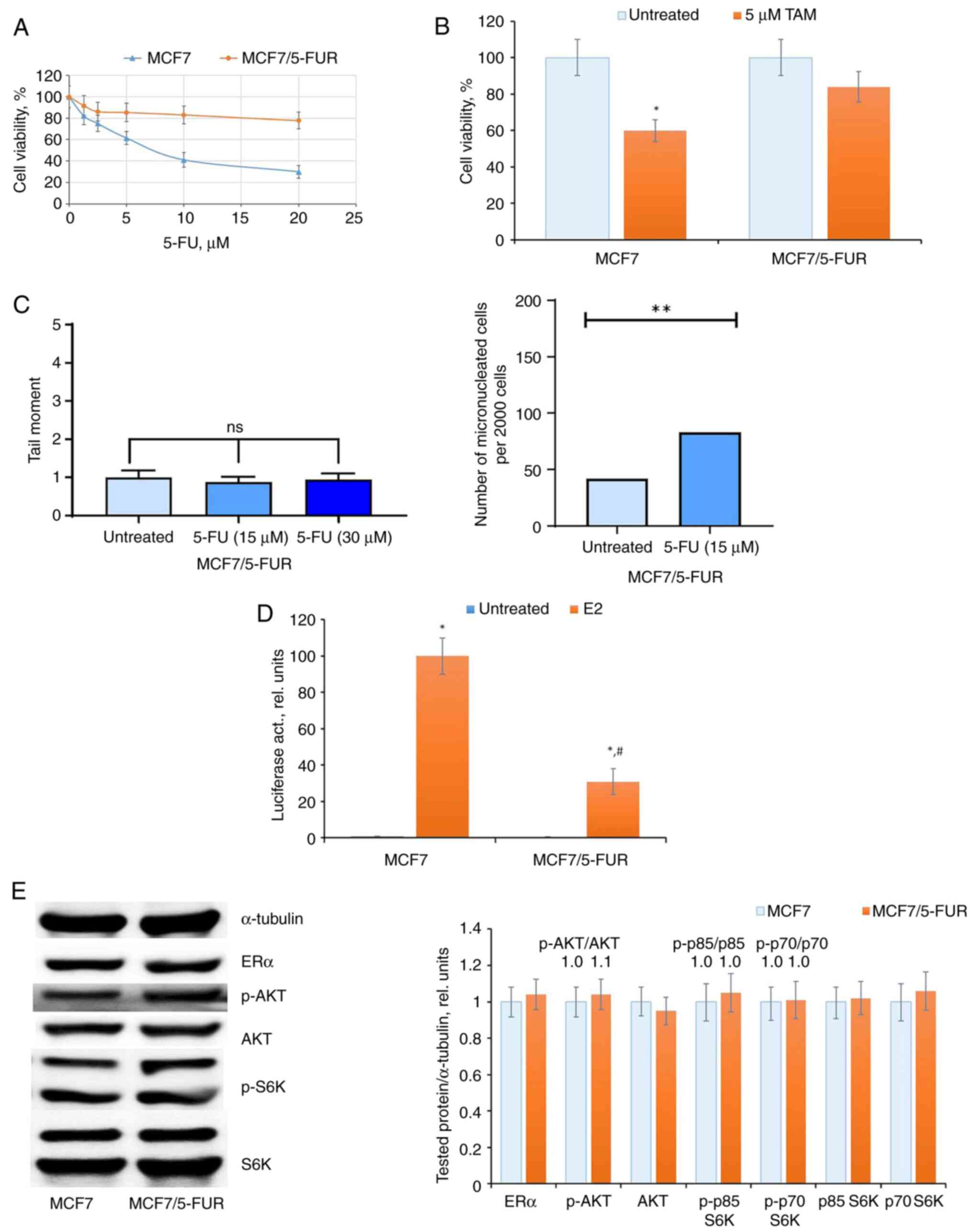 | Figure 3Prolonged 5-FU treatment and
selection of 5-FU-resistant cells. The MCF7 cells were treated with
15 µM 5-FU within two months with subsequent 5-FU withdrawal and
cell cultivation in medium without drug for the next one month. (A
and B) The sensitivity of the established MCF7/5-FUR cells to (A)
5-FU, (B) tamoxifen and (C) DNA damage tests (Comet assay and
accumulation of micronuclei). (D and E) ERα signaling in
5-FU-resistant MCF7/5-FUR cells. (D) Reporter analysis of ERα and
(E) western blot analysis of ERα, p-AKT, AKT, p-S6K, and S6K
expression in MCF7 and MCF7/5-FUR cells. *P<0.05 and
**P<0.01 vs. untreated samples; #P<0.05
vs. E2-treated MCF7 cells activity. ERα, estrogen receptor α; 5-FU,
5-fluorouracil; 5-FUR, 5-FU resistant; S6K, Ribosomal Protein S6
Kinase B1; TAM, tamoxifen; p-, phosphorylated. |
In the analysis of ERα machinery, a suppression of
ERα transcriptional activity was evident in 5-FU-resistant cells
(Fig. 3D). Subsequent examination
of growth-related signaling proteins indicated no significant
changes either in ERα expression or in the level of AKT and S6K
signaling in the resistant cells (Fig.
3E).
UVC irradiation and ERα signaling
The question of whether the 5-FU-induced suppression
of estrogen signaling is a shared event following DNA damage or if
these alterations are unique to 5-FU was addressed in the
subsequent experiments. The impact of UVC irradiation as a commonly
used DNA damage agent was examined on the estrogen signaling of
MCF7 cells. The results revealed that UVC irradiation leads to
pronounced DNA fragmentation and a reduction in the number of
viable cells (Figs. 4A and B and S2A
and B), albeit to a different
extent compared with the effects observed after 5-FU treatment.
The examination of ERα expression and
transcriptional activity in UV-exposed cells revealed a reduction,
coupled with the activation of AKT phosphorylation (Fig. 5A and B), no significant changes in the level of
S6K phosphorylation were detected. Additionally, a concurrent
analysis of the cell response to the growth-inhibitory action of
tamoxifen revealed decreased tamoxifen sensitivity in UV-exposed
cells, substantiating the suppression of ERα signaling in these
cells (Fig. 5C).
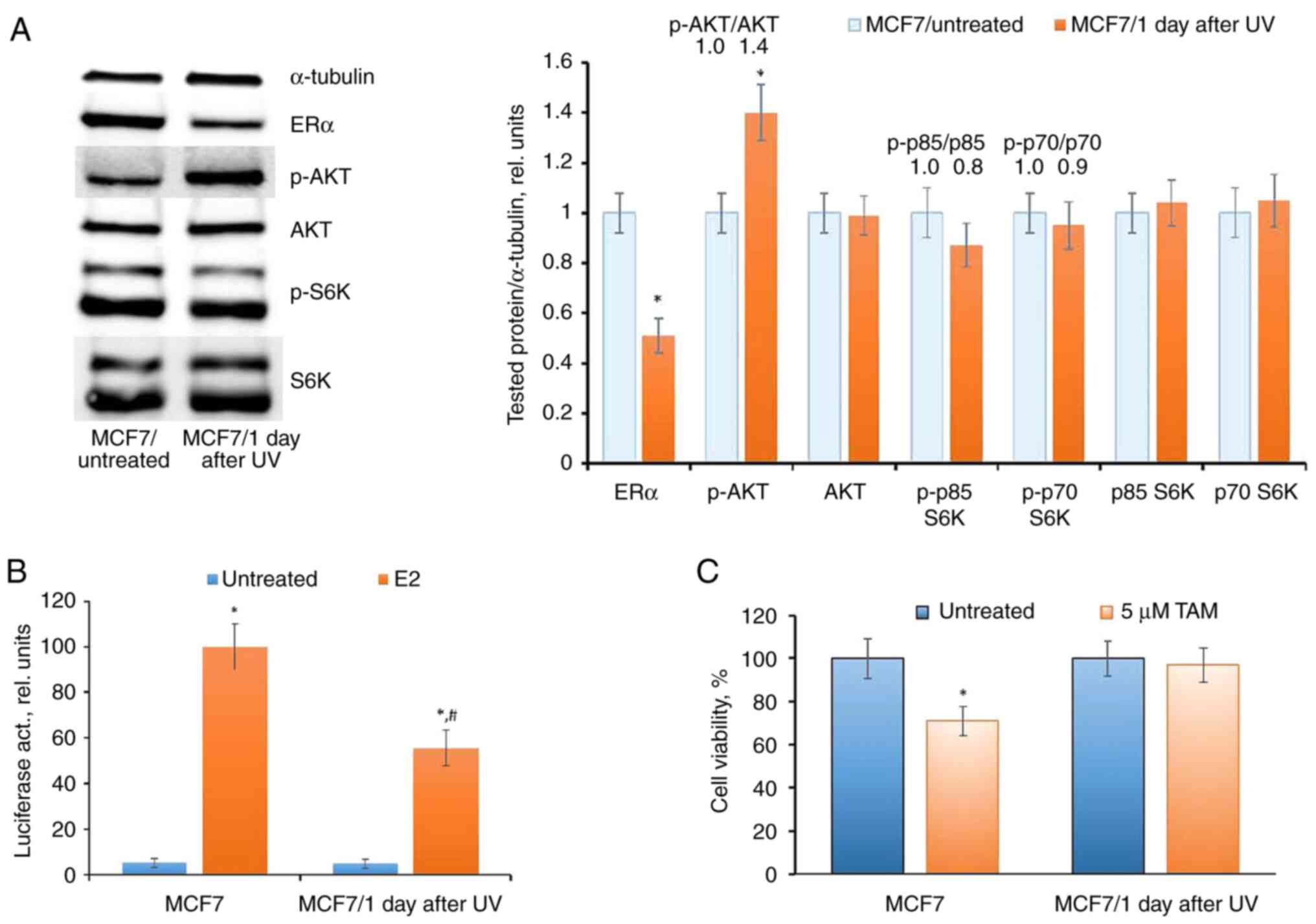 | Figure 5UVC influence on ERα signaling in
MCF7 cells. (A-C) The cells were exposed to a single UVC dose; (A)
western blot analysis of ERα, p-AKT, AKT, p-S6K and S6K (1 day
after treatment, *P<0.05 vs. MCF7/untreated), (B)
reporter analysis of ERα (*P<0.05 vs. untreated
samples; #P<0.05 vs. E2-treated MCF7 cells) and (C)
cell response to tamoxifen (3 days treatment with tamoxifen) were
performed. UV, ultraviolet; ERα, estrogen receptor α; S6K,
Ribosomal Protein S6 Kinase B; E2, 17β-estradiol; TAM, tamoxifen;
p-, phosphorylated. |
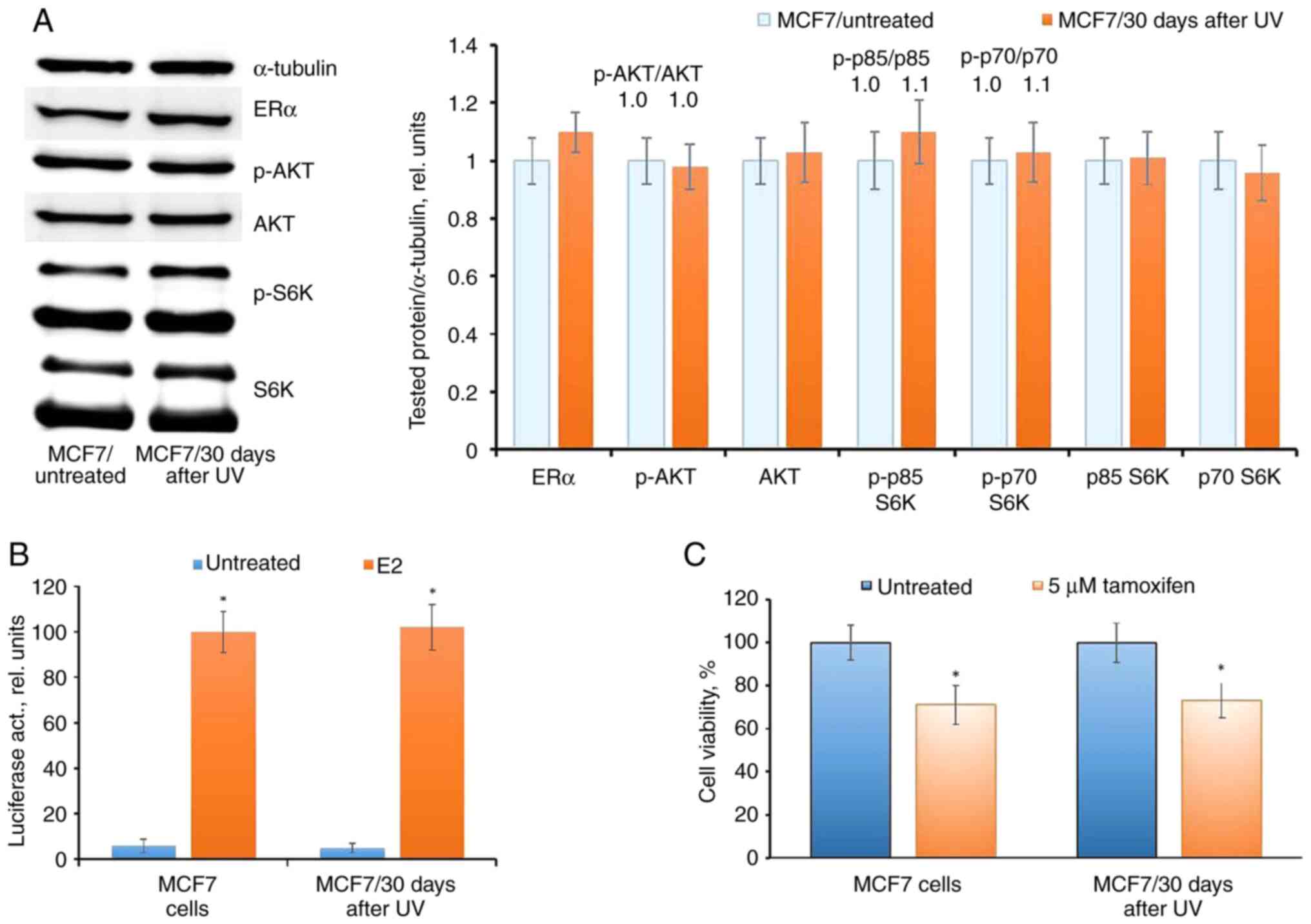
The subsequent analysis conducted 30 days after UV
irradiation demonstrated a complete restoration of ERα expression
and activity, alongside an unchanged level of AKT phosphorylation.
This restoration was correlated with the regained sensitivity of
cells to tamoxifen (Fig. 6A-C).
Selection and characterization of
UV-resistant clones
To explore the impact of continuous UV irradiation
on estrogen signaling, MCF7 cells underwent repeated UV exposure
once every three days for 4 weeks, followed by the maintenance of
cell growth for at least 40 days after the last irradiation. The
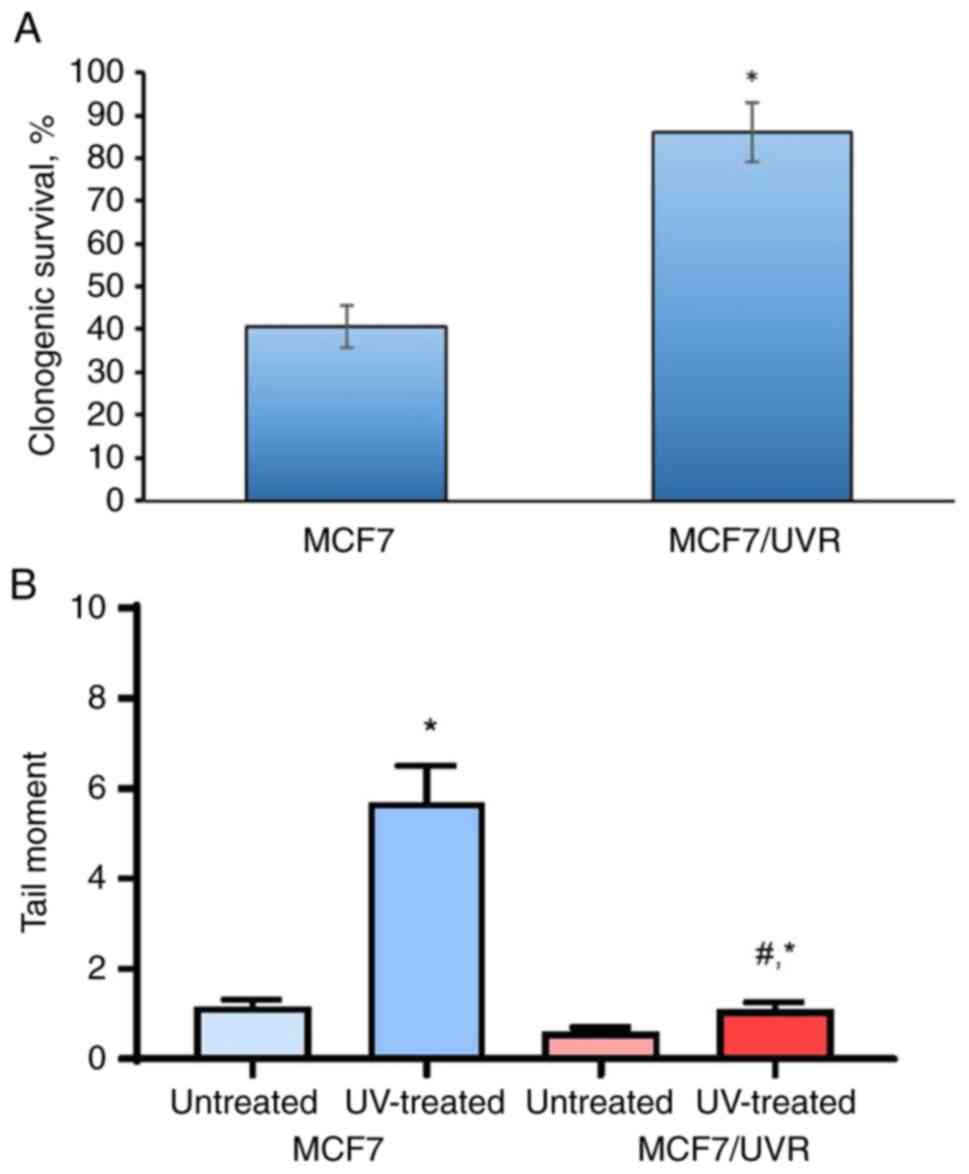
analysis of UV sensitivity in the selected cells, denoted as
MCF7/UVR, revealed a significant increase of cell survival under UV
compared with the UV-treated parent MCF7 cells (Fig. 7A). UVC irradiation of MCF7 induced
pronounced DNA fragmentation, while no significant difference in
DNA damage was observed in MCF7/UVR compared with the untreated
control (Fig. 7B).
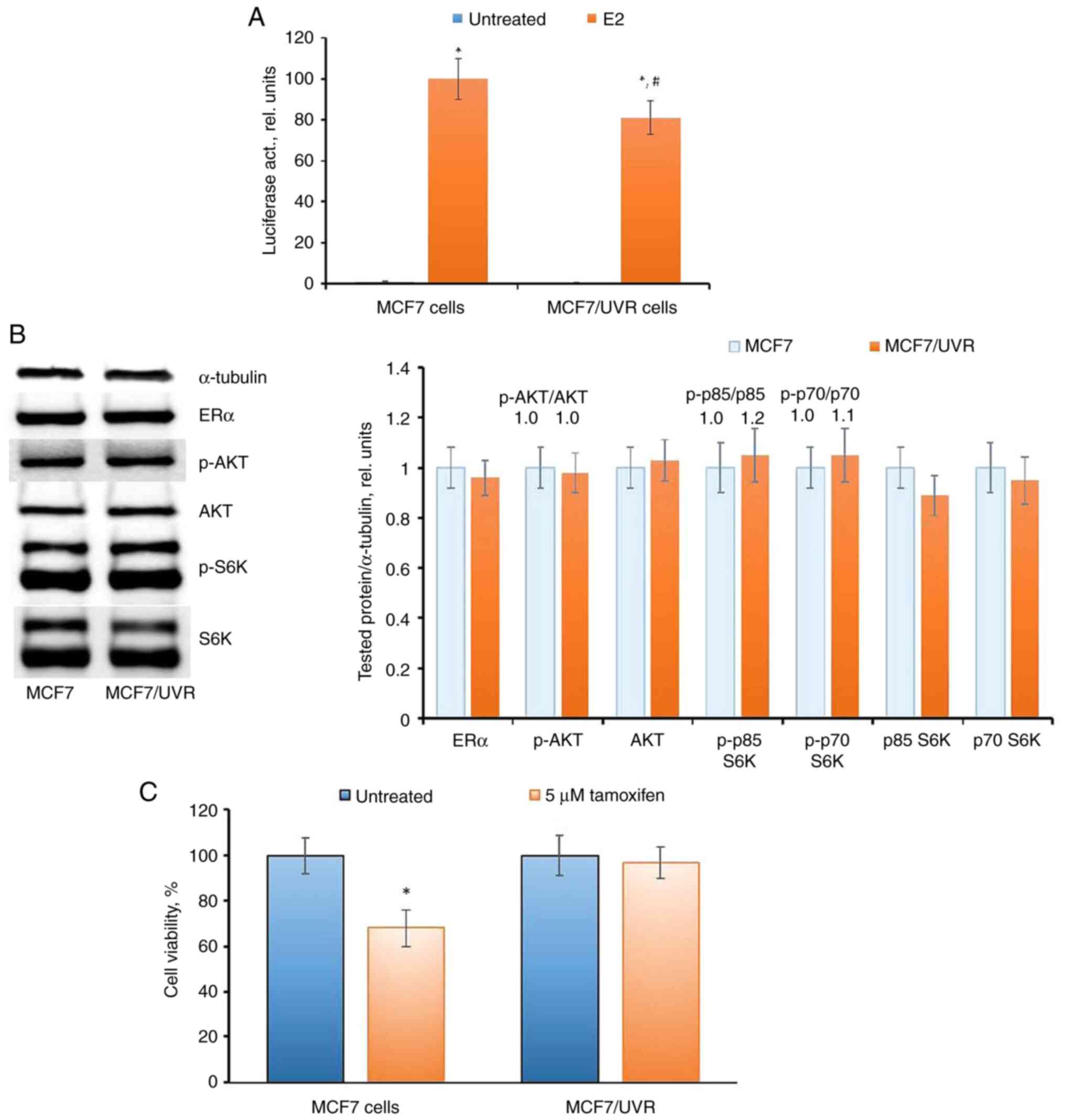
MCF7/UVR cells exhibited an irreversible reduction
in ERα transcriptional activity (Fig.
8A), despite the restored level of ERα expression (Fig. 8B). Examination of the AKT pathway
did not reveal changes in the corresponding signaling proteins. The
analysis of the cell response to tamoxifen indicated that MCF7/UVR
cells retained partial resistance to tamoxifen for at least 40 days
after irradiation (Fig. 8C), in
contrast to the parent MCF7 cells after a single UV dose.
Discussion
Hormone therapy (32-36)
is extensively employed in the treatment of hormone-dependent
breast tumors, either as a monotherapy or more frequently in
combination with chemotherapy or radiotherapy. While the action of
most chemotherapy drugs is linked to DNA damage, the exact role of
DNA damage in influencing the estrogen receptor machinery in tumor
cells remains unclear.
Several studies have highlighted alterations in the
estrogen receptor status of breast tumors following neoadjuvant
chemotherapy (13). Additionally,
there has been evidence of the overexpression of miRNAs targeting
the estrogen receptor after neoadjuvant chemotherapy (15). Furthermore, a correlation has been
described between the decreased expression of DNA repair genes and
the emergence of hormone resistance in breast cancer cells
(37).
UV irradiation (38-41)
serves as a widely utilized experimental model for investigating
cellular responses to DNA damage treatment. Evidence has been
accumulated regarding the influence of UV irradiation on the
activity of various cell signaling proteins, including but not
limited to p38 MAPK, Jun N-terminal kinase, extracellular
signal-regulated kinase 1/2, NF-κB (42,43),
eIF2α (44), Toll-like receptors
(45), HER2/neu (46), death domain-associated protein
(DAXX) (47), and others. In
further studies, potential p42/44 ERKs-, AKT- and p38-mediated
phosphorylation of ERα in UVC-treated cells is to be investigated.
UV irradiation has been indicated to stimulate the bystander effect
(48), with corresponding events
such as apoptosis, premature senescence, single and double DNA
strand breaks, and reduced clonogenic survival described in
bystander cells (49).
However, contradictory findings exist, regarding the
relationship between DNA damage and hormonal resistance. Various
data suggest that radiation-induced DNA damage either does not lead
to or is associated with only a marginal increase in overall
survival for patients with ERα-negative breast cancer (50,51).
In patients with ERα-positive breast cancer, no significant trend
in this regard has been consistently identified (52-54).
In studies involving in vitro-cultured breast cancer cells,
previous studies have revealed a correlation between radiation
exposure and disruptions in hormonal cell signaling. These
disruptions include a partial loss of ERα and the development of
resistance to antiestrogen (16,17).
Furthermore, a relationship has been identified between the
development of acquired radioresistance and hormonal resistance in
breast cancer cells, providing general support for the possibility
of impairment in hormonal signaling during irradiation (55-58).
The primary objective of the present study was to
explore the impact of DNA damage agents on estrogen signaling and
the sensitivity of breast cancer cells to hormonal drugs. The
findings of the present study indicated that the response of MCF7
breast cancer cells to 5-FU was linked to alterations in estrogen
signaling and the activation of the bypass AKT signaling pathway. A
single treatment with 5-FU induces temporary changes in AKT
signaling pathways, whereas chronic 5-FU exposure leads to the
selection of 5-FU-resistant cells exhibiting irreversible
alterations in ERα signaling, correlated with partial hormonal
resistance. Similar alterations were observed in cells subjected to
UV-induced DNA damage, emphasizing the pivotal role of DNA damage
in modifying ERα signaling in breast cancer cells. These observed
changes persist in cells for several months after drug treatment,
suggesting the potential involvement of (epi)genetic machinery in
maintaining the resistant phenotype. ERα and AKT kinase are among
the key regulators of breast cancer cell proliferation. Significant
efforts of researchers are directed towards the development of
novel inhibitors of these targets. The development of such
inhibitors also takes into account the significant overlap between
signaling pathways. The signaling between ERα and AKT axis largely
determines the formation of resistance to targeted and hormonal
therapies, and the assessment of these parameters is important for
disease prognosis and, in some cases, for changing treatment
protocols (59-61).
Interestingly, AKT overexpression leads to upregulation of
estrogen-regulated pS2 gene, Bcl-2, and macrophage inhibitory
cytokine 1(62). Moreover, AKT
protects breast cancer cells from tamoxifen-induced apoptosis. The
AKT-mediated activation of ERα in 5-FU treated cells has not been
described in detail in the present study, and is of great interest
for further study, including by means of CRISPR/Cas9
technology.
Additional investigations are required to elucidate
the mechanism by which DNA damage agents deactivate estrogen
receptors. The present findings suggested that the inhibition of
ERα transcriptional activity induced by drugs/UV is not correlated
with corresponding changes in ERα expression. This underscores the
crucial role of post-translational modifications in the regulation
of ERα. The reduction in ERα transcriptional activity may stem from
an imbalance between ERα co-activators and corepressors induced by
DNA damage agents. Evidence supporting this includes the observed
suppression of ERα co-activator CBP/p300 in response to 5-FU
(63) and the modulation of ERα
coregulator MDC1 (mediator of DNA damage checkpoint 1) in response
to DNA damage (64). Similarly,
several studies have highlighted the involvement of ERα
coregulators (65) and ERα-binding
chaperones (66) in the cellular
response to irradiation-induced DNA damage agents. Significantly,
MCF7 cells are characterized as p53-positive tumor cells,
suggesting potential interactions between p53 and ERα signaling.
Currently, only few studies describe the interrelation between p53
and ERα, highlighting changes in p53 activity under estrogen
stimulation (67,68). MCF7 cells are wtERα and wtp53
positive (69) but the interplay
between the two transcription factors in 5-FU and UVC-treated cells
has not been investigated in the present study. Additionally, a
series of observations underscore the involvement of growth-related
pathways, including PI3K/AKT and MAP cascades, in the regulation of
ERα activity. Moreover, the role of ERα itself has been implicated
in the regulation of cellular radioresistance (58,70,71).
Further studies are required for the explanation of the mechanism
of the inactivation of ERα by DNA damage agents and how DNA damage
inhibits ERα transcription without affecting its expression. In
addition, an extension of the study is possible with the use of a
tamoxifen gradient, as it is known that the effects of tamoxifen
vary greatly depending on the dose used.
In conclusion, the present findings suggested that
the treatment of cancer cells with DNA damage agents may lead to
the irreversible suppression of estrogen signaling and the
progression of partial hormonal resistance, thus limiting the
efficiency of combined or subsequent chemo- and hormone
therapy.
Supplementary Material
Effects of 5-FU on MCF7 cells. (A)
Light microscopy images demonstrating morphology of MCF7 cells
after 5-FU treatment [Inverted microscope Diavert (Leitz),
phase-contrast objective Phaco x20, camera DP-70 with software
DP-controller (Olympus Corporation)]. (B) Comet assay indicating
DNA damage in 5-FU-treated MCF7 cells. The slides were observed
with a Zeiss AxioVert 200 (Carl Zeiss AG) fluorescence microscope
with an EBQ isolated lamp at x10 magnification. 5-FU,
5-fluorouracil.
Effects of UVC on MCF7 cells. (A)
Light microscopy images demonstrating morphology of MCF7 cells
after UVC exposure. (B) Comet assay indicating DNA damage in MCF7
cells after UVC exposure. The slides were observed with a Zeiss
AxioVert 200 fluorescence microscope with an EBQ isolated lamp at
x10 magnification. UV, ultraviolet.
Acknowledgements
The plasmids used in the present study were kindly
provided by Professor George Reid from European Molecular Biology
Laboratory (Heidelberg, Germany). The authors would like to thank
Ms. Alexandra A. Drobysheva (Department of Experimental Tumor
Biology), Mr. Fedor B. Bogdanov (Department of Experimental Tumor
Biology) and Dr Antonina Yu. Alexandrova (Laboratory of
Carcinogenesis Mechanisms; all of three are from Institute of
Carcinogenesis, N.N. Blokhin National Medical Research Center of
Oncology, the Ministry of Health of Russia, Moscow, Russian
Federation) for their kind assistance in cell experiments. The
authors would also like to thank Ms. Alvina I. Khamidullina
(Laboratory of Molecular Oncobiology, Institute of Gene Biology)
and the Center for Precision Genome Editing and Genetic
Technologies for Biomedicine, Institute of Gene Biology, Russian
Academy of Sciences, for providing the equipment of research
facilities (electrophoresis unit).
Funding
Funding: The present study was supported (grant no. 22-25-00368)
by the Russian Scientific Foundation, (M.V.G.,
https://rscf.ru/project/22-25-00368/, accessed on November 24,
2023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MAK and MVG conceived and designed experiments. MVG
managed the project. MAK and AMS wrote original draft. AMS prepared
the manuscript and figures for submission. MAK and AMS confirm the
authenticity of all the raw data. AMS, MVG, KIK and MAK contributed
reagents/materials/analysis tools, analyzed data and critically
revised manuscript. DVS, VER, YYS, OEA, DIS, TIF, OAV and KIK
performed the experiments, analyzed all data, and amended the
revised version. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tiezzi DG, De Andrade JM, Cândido dos Reis
FJ, Marana HR, Ribeiro-Silva A, Tiezzi MG and Pereira AP: Apoptosis
induced by neoadjuvant chemotherapy in breast cancer. Pathology.
38:21–27. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khan M, Rasul A, Yi F, Zhong L and Ma T:
Jaceosidin induces p53-dependent G2/M phase arrest in U87
glioblastoma cells. Asian Pac J Cancer Prev. 12:3235–3238.
2011.PubMed/NCBI
|
|
3
|
Rasul A, Khan M, Yu B, Ma T and Yang H:
Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in
human gastric adenocarcinoma SGC-7901 cells. Asian Pac J Cancer
Prev. 12:1219–1223. 2011.PubMed/NCBI
|
|
4
|
Ding M, Shao Y, Sun D, Meng S, Zang Y,
Zhou Y, Li J, Lu W and Zhu S: Design, synthesis, and biological
evaluation of BRD4 degraders. Bioorg Med Chem.
78(117134)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chrienova Z, Rysanek D, Oleksak P, Stary
D, Bajda M, Reinis M, Mikyskova R, Novotny O, Andrys R, Skarka A,
et al: Discovery of small molecule mechanistic target of rapamycin
inhibitors as anti-aging and anti-cancer therapeutics. Front Aging
Neurosci. 14(1048260)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Davidson NE, O'Neill AM, Vukov AM, Osborne
CK, Martino S, White DR and Abeloff MD: Chemoendocrine therapy for
premenopausal women with axillary lymph node-positive, steroid
hormone receptor-positive breast cancer: Results from INT 0101
(E5188). J Clin Oncol. 23:5973–5982. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
International Breast Cancer Study Group.
Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M,
Price KN, Coates AS and Gelber RD: Tamoxifen after adjuvant
chemotherapy for premenopausal women with lymph node-positive
breast cancer: International breast cancer study group trial 13-93.
J Clin Oncol. 24:1332–1341. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morales L, Canney P, Dyczka J, Rutgers E,
Coleman R, Cufer T, Welnicka-Jaskiewicz M, Nortier J, Bogaerts J,
Therasse P, et al: Postoperative adjuvant chemotherapy followed by
adjuvant tamoxifen versus nil for patients with operable breast
cancer: A randomised phase III trial of the European organisation
for research and treatment of cancer breast group. Eur J Cancer.
43:331–340. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fukuda M, Yamaguchi S, Ohta T, Nakayama Y,
Ogata H, Shimizu K, Nishikawa T, Adachi Y and Fukuma E: Combination
therapy for advanced breast cancer: Cyclophosphamide, doxorubicin,
UFT, and tamoxifen. Oncology (Williston Park). 13 (7 Suppl
3):S77–S81. 1999.PubMed/NCBI
|
|
10
|
Hupperets PS, Wils JA, Volovics L,
Schouten LJ, Fickers MM, Bron HN, Jager JJ, de Jong JM and Blijham
GH: Adjuvant chemo-hormonal therapy with cyclophosphamide,
doxorubicin and 5-fluorouracil (CAF) with or without
medroxyprogesterone acetate (MPA) for node-positive cancer
patients, update at 12 years follow up. Breast. 10:35–37.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gordon NH, Silverman P, Lasheen W, Meinert
J and Siminoff LA: Thirty-year follow-up of chemo/hormonal therapy
in node-positive breast cancer. Breast Cancer Res Treat.
102:301–312. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alramadhan M, Ryu JM, Rayzah M, Nam SJ,
Kim SW, Yu J, Lee SK, Bae SY, Park S, Paik HJ and Lee JE: Goserelin
plus tamoxifen compared to chemotherapy followed by tamoxifen in
premenopausal patients with early stage-, lymph node-negative
breast cancer of luminal A subtype. Breast. 30:111–117.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De La Cruz LM, Harhay MO, Zhang P and
Ugras S: Impact of neoadjuvant chemotherapy on breast cancer
subtype: Does subtype change and, if so, how?: IHC profile and
neoadjuvant chemotherapy. Ann Surg Oncol. 25:3535–3540.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang N, Moran MS, Huo Q, Haffty BG and
Yang Q: The hormonal receptor status in breast cancer can be
altered by neoadjuvant chemotherapy: a meta-analysis. Cancer
Invest. 29:594–598. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luengo-Gil G, García-Martínez E,
Chaves-Benito A, Conesa-Zamora P, Navarro-Manzano E,
González-Billalabeitia E, García-Garre E, Martínez-Carrasco A,
Vicente V and Ayala de la Peña F: Clinical and biological impact of
miR-18a expression in breast cancer after neoadjuvant chemotherapy.
Cell Oncol (Dordr). 42:627–644. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Paulsen GH, Strickert T, Marthinsen AB and
Lundgren S: Changes in radiation sensitivity and steroid receptor
content induced by hormonal agents and ionizing radiation in breast
cancer cells in vitro. Acta Oncol. 35:1011–1019. 1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bravatà V, Cava C, Minafra L, Cammarata
FP, Russo G, Gilardi MC, Castiglioni I and Forte GI:
Radiation-induced gene expression changes in high and low grade
breast cancer cell types. Int J Mol Sci. 19(1084)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rose HN and McGrath CM: Alpha-lactalbumin
production in human mammary carcinoma. Science. 190:673–675.
1975.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iselt M, Holtei W and Hilgard P: The
tetrazolium dye assay for rapid in vitro assessment of
cytotoxicity. Arzneimittelforschung. 39:747–749. 1989.PubMed/NCBI
|
|
20
|
Volkova YA, Antonov YS, Komkov AV,
Scherbakov AM, Shashkov AS, Menchikov LG, Chernoburova LI and
Zavarzin IV: Access to steroidal pyridazines via modified
thiohydrazides. RSC Adv. 6:42863–42868. 2016.
|
|
21
|
Singh NP, McCoy MT, Tice RR and Schneider
EL: A simple technique for quantitation of low levels of DNA damage
in individual cells. Exp Cell Res. 175:184–191. 1988.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Reid G, Hübner MR, Métivier R, Brand H,
Denger S, Manu D, Beaudouin J, Ellenberg J and Gannon F: Cyclic,
proteasome-mediated turnover of unliganded and liganded ERalpha on
responsive promoters is an integral feature of estrogen signaling.
Mol Cell. 11:695–707. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reid G, Métivier R, Lin CY, Denger S,
Ibberson D, Ivacevic T, Brand H, Benes V, Liu ET and Gannon F:
Multiple mechanisms induce transcriptional silencing of a subset of
genes, including oestrogen receptor alpha, in response to
deacetylase inhibition by valproic acid and trichostatin A.
Oncogene. 24:4894–4907. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Scherbakov AM, Komkov AV, Komendantova AS,
Yastrebova MA, Andreeva OE, Shirinian VZ, Hajra A, Zavarzin IV and
Volkova YA: Steroidal pyrimidines and dihydrotriazines as novel
classes of anticancer agents against hormone-dependent breast
cancer cells. Front Pharmacol. 8(979)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Semina SE, Scherbakov AM, Vnukova AA,
Bagrov DV, Evtushenko EG, Safronova VM, Golovina DA, Lyubchenko LN,
Gudkova MV and Krasil'nikov MA: Exosome-mediated transfer of cancer
cell resistance to antiestrogen drugs. Molecules.
23(829)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mruk DD and Cheng CY: Enhanced
chemiluminescence (ECL) for routine immunoblotting: An inexpensive
alternative to commercially available kits. Spermatogenesis.
1:121–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Taylor SC, Berkelman T, Yadav G and
Hammond M: A defined methodology for reliable quantification of
Western blot data. Mol Biotechnol. 55:217–226. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Butler TAJ, Paul JW, Chan EC, Smith R and
Tolosa JM: Misleading Westerns: Common quantification mistakes in
western blot densitometry and proposed corrective measures. Biomed
Res Int. 2019(5214821)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kho MC and Parsonnet V: Observations on
the use of 5-fluorouracil in metastatic carcinoma of the breast. J
Newark Beth Isr Hosp. 12:93–100. 1961.PubMed/NCBI
|
|
30
|
Curreri AR and Ansfield FJ: Comparison of
5-fluorouracil and 5-fluoro-2'-deoxyuridine in the treatment of
far-advanced breast and colon lesions. Cancer Chemother Rep.
16:387–388. 1962.PubMed/NCBI
|
|
31
|
Del Mastro L, Poggio F, Blondeaux E, De
Placido S, Giuliano M, Forestieri V, De Laurentiis M, Gravina A,
Bisagni G, Rimanti A, et al: Fluorouracil and dose-dense adjuvant
chemotherapy in patients with early-stage breast cancer (GIM2):
End-of-study results from a randomised, phase 3 trial. Lancet
Oncol. 23:1571–1582. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bogush TA, Polezhaev BB, Mamichev IA,
Bogush EA, Polotsky BE, Tjulandin SA and Ryabov AB: Tamoxifen never
ceases to amaze: New findings on non-estrogen receptor molecular
targets and mediated effects. Cancer Invest. 36:211–220.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Semiglazov VF, Semiglazov VV, Klemtsel AA,
Barash NIu, Zhil'tsova EK, Bozhok AA, Mel'nikova OA, Paltuev RM,
Dashian GA, Petrovskiĭ SG, et al: New results of endocrine therapy
of breast cancer (the role of Aromasin). Vopr Onkol. 50:729–736.
2004.PubMed/NCBI(In Russian).
|
|
34
|
Lukina SS, Burdennyy AM, Zavarykina TM,
Riabchikov DA, Kazubskaya TP, Kruglova MP and Loginov VI: The role
of ESR1 gene polymorphic markers in the development of breast
cancer and resistance to tamoxifen therapy. Bull Exp Biol Med.
170:350–355. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jordan VC: 50th anniversary of the first
clinical trial with ICI 46,474 (tamoxifen): Then what happened?
Endocr Relat Cancer. 28:R11–R30. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Babyshkina N, Vtorushin S, Dronova T,
Patalyak S, Slonimskaya E, Kzhyshkowska J, Cherdyntseva N and
Choynzonov E: Impact of estrogen receptor α on the tamoxifen
response and prognosis in luminal-A-like and luminal-B-like breast
cancer. Clin Exp Med. 19:547–556. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Anurag M, Punturi N, Hoog J, Bainbridge
MN, Ellis MJ and Haricharan S: Comprehensive profiling of DNA
repair defects in breast cancer identifies a novel class of
endocrine therapy resistance drivers. Clin Cancer Res.
24:4887–4899. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang SC, Chang HS, Tang JY, Farooqi AA,
Kuo YT, Hsuuw YD, Lee JW and Chang HW: Combined treatment with
cryptocaryone and ultraviolet C promotes antiproliferation and
apoptosis of oral cancer cells. Int J Mol Sci.
23(2981)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen W, Tang Z, Hu S, Yi X and Wang J:
Ultraviolet C irradiation-induced dehybridization of
double-stranded oligonucleotides: mechanism investigation and
label-free measurement of the photodamage level. Langmuir.
38:15190–15197. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bangruwa N, Srivastava M and Mishra D:
CISS-based label-free novel electrochemical impedimetric detection
of UVC-induced DNA damage. ACS Omega. 7:37705–37713.
2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Potapovich AI, Kostyuk TV, Shman TV,
Ermilova TI, Shutava TG and Kostyuk VA: DNA repair activation and
cell death suppression by plant polyphenols in keratinocytes
exposed to ultraviolet irradiation. Rejuvenation Res. 26:1–8.
2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Muthusamy V and Piva TJ: The UV response
of the skin: A review of the MAPK, NFkappaB and TNFalpha signal
transduction pathways. Arch Dermatol Res. 302:5–17. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
László CF and Wu S: Mechanism of
UV-induced IkappaBalpha-independent activation of NF-kappaB.
Photochem Photobiol. 84:1564–1568. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lu W, László CF, Miao Z, Chen H and Wu S:
The role of nitric-oxide synthase in the regulation of UVB
light-induced phosphorylation of the alpha subunit of eukaryotic
initiation factor 2. J Biol Chem. 284:24281–24288. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fishelevich R, Zhao Y, Tuchinda P, Liu H,
Nakazono A, Tammaro A, Meng TC, Lee J and Gaspari AA:
Imiquimod-induced TLR7 signaling enhances repair of DNA damage
induced by ultraviolet light in bone marrow-derived cells. J
Immunol. 187:1664–1673. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Madson JG and Hansen LA: Multiple
mechanisms of Erbb2 action after ultraviolet irradiation of the
skin. Mol Carcinog. 46:624–628. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shan Z, Liu L, Shen J, Hao H, Zhang H, Lei
L, Liu F and Wang Z: Enhanced UV resistance role of death
domain-associated protein in human MDA-MB-231 breast cancer cells
by regulation of G2 DNA damage checkpoint. Cell Transplant.
29(963689720920277)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wu S, Jin C, Lu X, Yang J, Liu Q, Qi M, Lu
S, Zhang L and Cai Y: Bystander effect induced by UVC radiation in
Chinese hamster V79 cells. Photochem Photobiol. 90:837–844.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Krzywon A and Widel M: Bystander Me45
melanoma cells increase damaging effect in UVC-irradiated cells.
Photochem Photobiol. 95:1019–1028. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Eaton BR, Jiang R, Torres MA, Kahn ST,
Godette K, Lash TL and Ward KC: Benefit of adjuvant radiotherapy
after breast-conserving therapy among elderly women with T1-T2N0
estrogen receptor-negative breast cancer. Cancer. 122:3059–3068.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang PS, Chen CM, Liu MC, Jian JM, Horng
CF, Liu MJ, Yu BL, Lee MY and Chi CW: Radiotherapy can decrease
locoregional recurrence and increase survival in mastectomy
patients with T1 to T2 breast cancer and one to three positive
nodes with negative estrogen receptor and positive lymphovascular
invasion status. Int J Radiat Oncol Biol Phys. 77:516–522.
2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen JX, Zhang WW, Dong Y, Sun JY, He ZY
and Wu SG: The effects of postoperative radiotherapy on survival
outcomes in patients under 65 with estrogen receptor positive
tubular breast carcinoma. Radiat Oncol. 13(226)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hughes KS, Schnaper LA, Berry D,
Cirrincione C, McCormick B, Shank B, Wheeler J, Champion LA, Smith
TJ, Smith BL, et al: Lumpectomy plus tamoxifen with or without
irradiation in women 70 years of age or older with early breast
cancer. N Engl J Med. 351:971–977. 2004.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chesney TR, Yin JX, Rajaee N, Tricco AC,
Fyles AW, Acuna SA and Scheer AS: Tamoxifen with radiotherapy
compared with Tamoxifen alone in elderly women with early-stage
breast cancer treated with breast conserving surgery: A systematic
review and meta-analysis. Radiother Oncol. 123:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Steelman LS, Navolanic P, Chappell WH,
Abrams SL, Wong EW, Martelli AM, Cocco L, Stivala F, Libra M,
Nicoletti F, et al: Involvement of Akt and mTOR in
chemotherapeutic- and hormonal-based drug resistance and response
to radiation in breast cancer cells. Cell Cycle. 10:3003–3015.
2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Jang H, Baek J, Nam KS and Kim S:
Determination of the optimal time for tamoxifen treatment in
combination with radiotherapy. Int J Oncol. 49:2147–2154.
2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Luzhna L, Lykkesfeldt AE and Kovalchuk O:
Altered radiation responses of breast cancer cells resistant to
hormonal therapy. Oncotarget. 6:1678–1694. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Semina SE, Scherbakov AM, Kovalev SV,
Shevchenko VE and Krasil'nikov MA: Horizontal transfer of tamoxifen
resistance in MCF-7 cell derivates: Proteome study. Cancer Invest.
35:506–518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Khatpe AS, Adebayo AK, Herodotou CA, Kumar
B and Nakshatri H: Nexus between PI3K/AKT and estrogen receptor
signaling in breast cancer. Cancers (Basel). 13(369)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Golden E, Rashwan R, Woodward EA, Sgro A,
Wang E, Sorolla A, Waryah C, Tie WJ, Cuyàs E, Ratajska M, et al:
The oncogene AAMDC links PI3K-AKT-mTOR signaling with metabolic
reprograming in estrogen receptor-positive breast cancer. Nat
Commun. 12(1920)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
du Rusquec P, Blonz C, Frenel JS and
Campone M: Targeting the PI3K/Akt/mTOR pathway in estrogen-receptor
positive HER2 negative advanced breast cancer. Ther Adv Med Oncol.
12(1758835920940939)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Campbell RA, Bhat-Nakshatri P, Patel NM,
Constantinidou D, Ali S and Nakshatri H: Phosphatidylinositol
3-kinase/AKT-mediated activation of estrogen receptor alpha: A new
model for anti-estrogen resistance. J Biol Chem. 276:9817–9824.
2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Du C, Huang D, Peng Y, Yao Y, Zhao Y, Yang
Y, Wang H, Cao L, Zhu WG and Gu J: 5-Fluorouracil targets histone
acetyltransferases p300/CBP in the treatment of colorectal cancer.
Cancer Lett. 400:183–193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zou R, Zhong X, Wang C, Sun H, Wang S, Lin
L, Sun S, Tong C, Luo H, Gao P, et al: MDC1 enhances Estrogen
receptor-mediated transactivation and contributes to breast cancer
suppression. Int J Biol Sci. 11:992–1005. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chen J, Liu J, Zeng P, Zhao C, Liu X, Sun
J, Wang J, Fang P, Chen W and Ding J: Estrogen and BRCA1 deficiency
synergistically induce breast cancer mutation-related DNA damage.
Biochem Biophys Res Commun. 613:140–145. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ashrafizadeh M, Farhood B, Eleojo Musa A,
Taeb S and Najafi M: Damage-associated molecular patterns in tumor
radiotherapy. Int Immunopharmacol. 86(106761)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Thibodeau PA, Bissonnette N, Bédard SK,
Hunting D and Paquette B: Induction by estrogens of methotrexate
resistance in MCF-7 breast cancer cells. Carcinogenesis.
19:1545–1552. 1998.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Molinari AM, Bontempo P, Schiavone EM,
Tortora V, Verdicchio MA, Napolitano M, Nola E, Moncharmont B,
Medici N, Nigro V, et al: Estradiol induces functional inactivation
of p53 by intracellular redistribution. Cancer Res. 60:2594–2597.
2000.PubMed/NCBI
|
|
69
|
Pairawan S, Zhao M, Yuca E, Annis A, Evans
K, Sutton D, Carvajal L, Ren JG, Santiago S, Guerlavais V, et al:
First in class dual MDM2/MDMX inhibitor ALRN-6924 enhances
antitumor efficacy of chemotherapy in TP53 wild-type hormone
receptor-positive breast cancer models. Breast Cancer Res.
23(29)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Paramanantham A, Jung EJ, Go SI, Jeong BK,
Jung JM, Hong SC, Kim GS and Lee WS: Activated ERK signaling is one
of the major hub signals related to the acquisition of
radiotherapy-resistant MDA-MB-231 breast cancer cells. Int J Mol
Sci. 22(4940)2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sun XY, Li HZ, Xie DF, Gao SS, Huang X,
Guan H, Bai CJ and Zhou PK: LPAR5 confers radioresistance to cancer
cells associated with EMT activation via the ERK/Snail pathway. J
Transl Med. 20(456)2022.PubMed/NCBI View Article : Google Scholar
|