Introduction
The clinical diagnosis of optic neuritis (ON) can be
achieved based on characteristic medical history, symptoms and
signs. However, with more advanced methods such as specific
antibody serology testing and magnetic resonance imaging (MRI)
being used, a more accurate diagnosis of ON can be made (1). In the pediatric population, due to the
difficulty in describing symptoms accurately (2), challenges in collecting physical signs
and limited number of cases, there are currently no guidelines or
results of large-scale studies specifically for pediatric ON.
It is necessary to explore and conduct in-depth
studies on pediatric optic neuritis, as it presents characteristics
distinct from optic neuritis in adults, such as a higher likelihood
of bilateral onset (3), potentially
better prognosis (4), lower
recurrence rates (5) and increased
susceptibility to medication side effects, as they are still in the
developmental stage. Therefore, pediatric optic neuritis has its
own characteristics in terms of predicting risk factors, choosing
and balancing long-term maintenance plans, monitoring complications
and determining follow-up frequency. It requires separate research
and evaluation.
The present case report aims to share experiences in
the diagnosis and treatment of pediatric ON to limitedly contribute
to future large-scale studies and relevant guidelines.
Case report
A male patient aged 7 years, came to the Department
of Ophthalmology at Peking University First Hospital (Beijing,
China) in April 2023 with the main complaint of ‘sudden blurred
vision in both eyes for about 2 days’. The patient had no obvious
triggering factor for the blurred vision that started 2 days ago.
The decrease in visual acuity was similar in both eyes. There were
no accompanying symptoms such as fever, vomiting, headache, eye
pain, redness or increased eye secretions. The patient had a normal
mental state, appetite and sleep. There were no seizures or
abnormal emotional behavior. Approximately 3 weeks prior, the
patient had suffered a ‘common cold’ with symptoms of fever
(102.7˚F) and cough for ~1 week. The patient vomited 3 times during
that period but experienced relief after taking medication (the
guardian of the patient does not recall the specific medication,
but it was described as a generic over-the-counter cold symptom
relief medication). An ophthalmic examination conducted 1 month
previously showed 20/20 vision in both eyes.
The following examinations were completed during the
outpatient visit: Visual acuity was 20/400 in both eyes.
Non-contact intraocular pressure was 18 mmHg in the right eye (OD)
and 17 mmHg in the left eye (OS). There was no redness or swelling
of the eyelids and no conjunctival edema or congestion, and eye
movements were normal in all directions without any pain. The
cornea was transparent, the anterior chamber depth was clear and
both pupils were round with delayed light reflex. The lens was
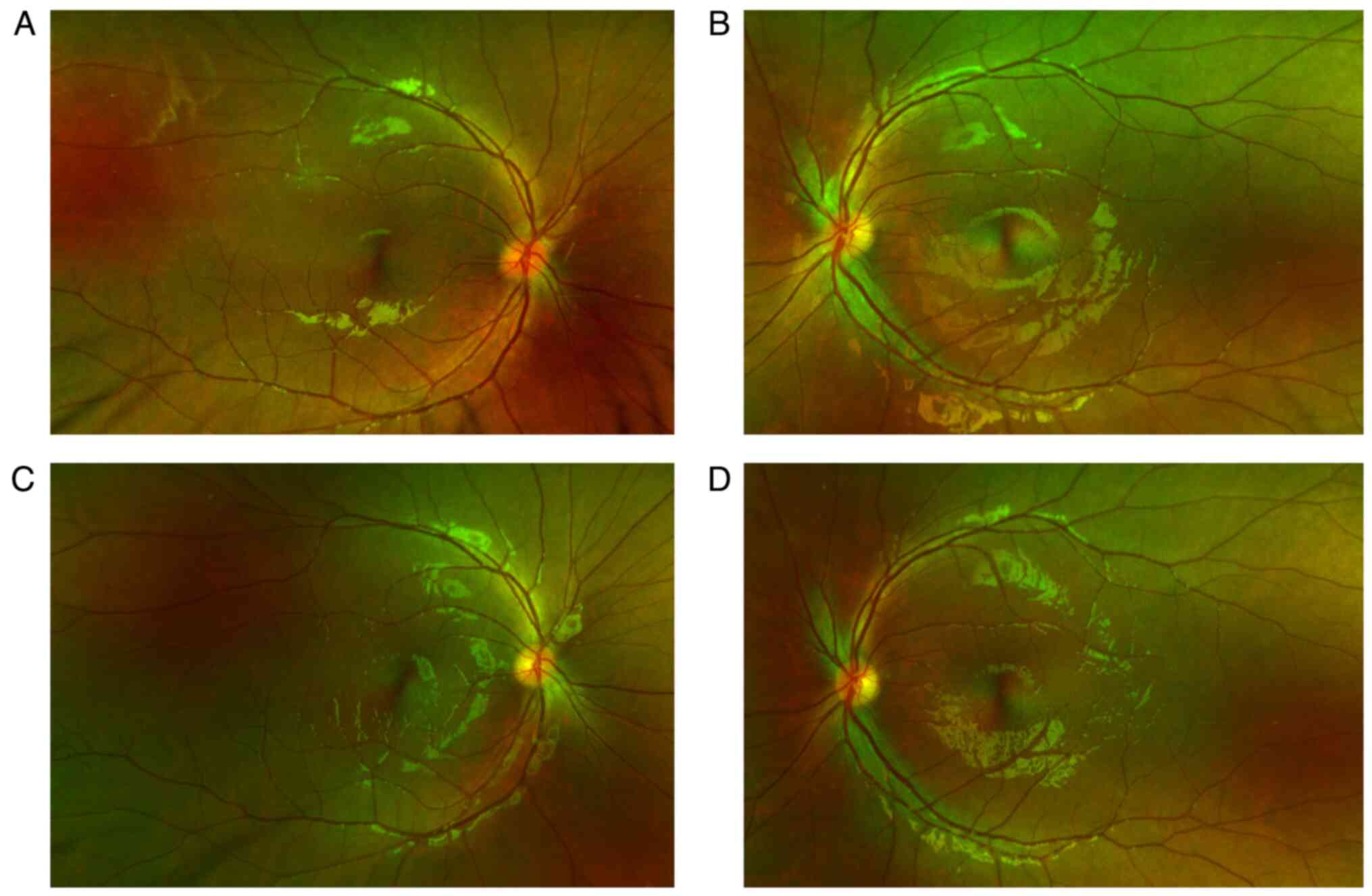
transparent. Fundus examination showed normal transparency of the
vitreous cavity in both eyes, indistinct and elevated optic disc
boundaries, redness and no obvious abnormalities in the retina and
blood vessels (Fig. 1). Goldman
visual field examination indicated a central scotoma in the OD and
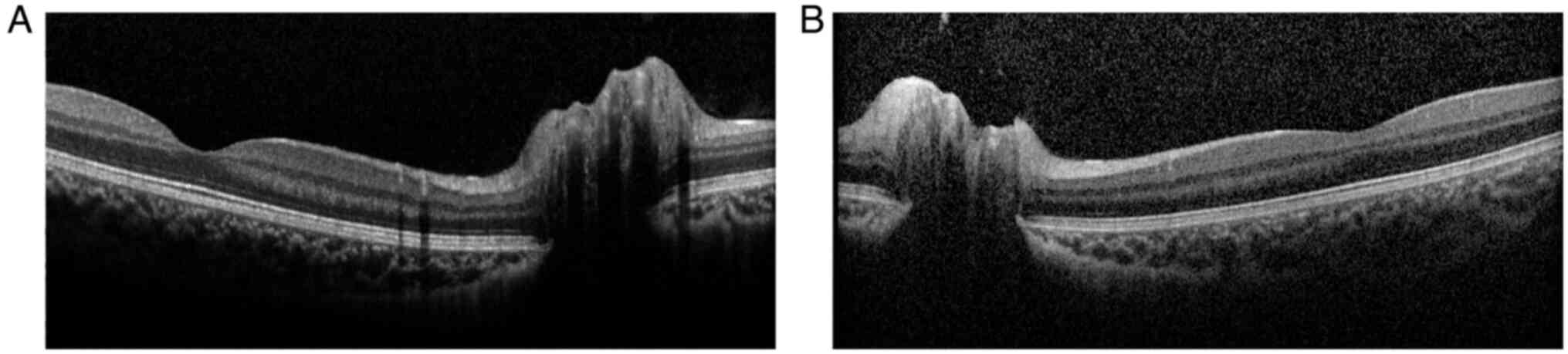
peripheral visual field defect in the upper half of the OS. OCT
revealed normal morphology of the macular central fovea and
significant optic disc elevation in both eyes (Fig. 2). The provisional diagnosis in the
ophthalmology clinic was ‘bilateral ON’ and it was recommended to
complete visual evoked potential (VEP) and optic nerve MRI as soon
as possible and to consult with the neurology department
promptly.
VEP performed 2 days after the initial presentation
revealed delayed latency of the P100 wave and decreased amplitudes
of the P100 wave at various spatial frequencies, as well as
decreased amplitudes of the P2 wave in both eyes. On the same day,
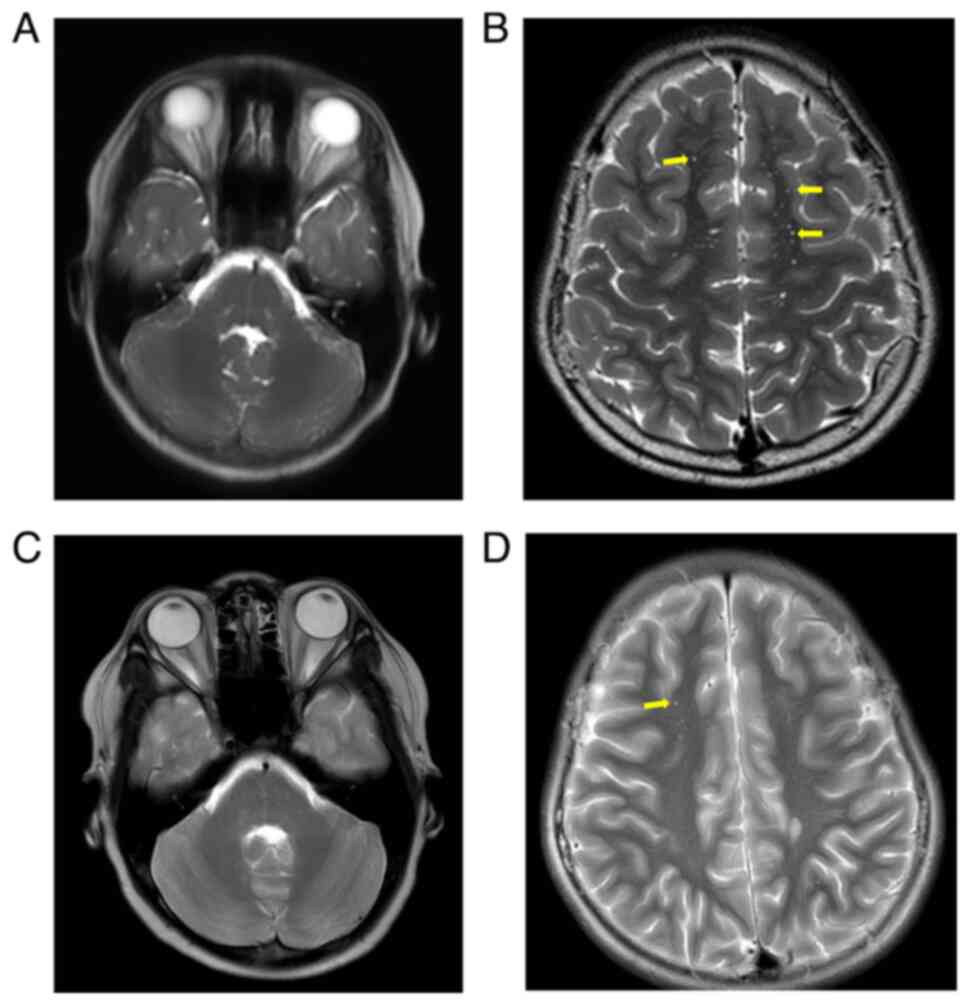
enhanced MRI (Fig. 3) showed slight
thickening of the posterior part of the intracranial segment of the
right optic nerve, bilateral optic canal segment and intracranial
segment, with significant enhancement visible. In addition,
multiple patchy T2-weighted imaging (T2WI) hyperintensity lesions
were observed in the bilateral frontal, temporal, occipital lobes,
pons, medulla oblongata and the right cerebellar hemisphere, with
multiple enhancements visible. Based on the above, the
ophthalmology department considered a diagnosis of ‘bilateral ON,
multiple sclerosis (MS)?’ and admitted the patient to the inpatient
department for further evaluation. On the same day, a consultation
was also carried out with the neurology department and a
provisional diagnosis of ‘acute disseminated encephalomyelitis
(ADEM)’ was made.
The patient was admitted to the ophthalmology ward 6
days after the initial presentation to receive comprehensive
screening for infectious diseases, tuberculosis skin test and other
systemic examinations. Pulse therapy of high-dose intravenous
methylprednisolone (IVMP) sodium succinate was administered, along
with a consultation with the pediatric neurology department. After
the consultation, the patient was transferred to the pediatric
neurology department for further diagnosis and treatment.
Subsequently, the patient underwent lumbar puncture, cranial and
spinal cord MRI and antibody testing for central nervous system
demyelinating diseases (conducted by the Beijing branch of Hangzhou
Oumengwei Medical Laboratory Co., Ltd.) [aquaporin (AQP)4, myelin
oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP)].
The results showed negative immunoglobulin G (IgG) and oligoclonal
bands in the cerebrospinal fluid, positive MOG-IgG in the serum (++
at 1:32) and negative AQP4 and MBP. The MRI showed bilateral
occipital-temporal-parietal-occipital lobes, pons, medulla,
bilateral cerebellar hemisphere, right hippocampus with multiple
patchy T2WI hyperintensity, T2 fluid-attenuated inversion recovery
hyperintensities and T1WI hypointensities, some of which showed
ring-shaped diffusion-weighted imaging hyperintensities and
decreased apparent diffusion coefficient signals. Patchy T2WI
hyperintensities were also seen in C2, C5 and Th10. Therefore, the
patient was diagnosed with ‘MOG antibody-associated disease’ and
received three courses of high-dose methylprednisolone pulse
therapy combined with intravenous immunoglobulin (IVIG) therapy
(three days per course, a four-day IVIG therapy between the first
and second courses, and a four-day break between the second and
third courses), followed by oral prednisolone (initial dose of 40
mg/day, gradually tapered weekly). During the treatment process,
the patient's visual acuity initially continued to decline to
counting fingers at close range (7 days after initial
presentation), but gradually recovered to OD 20/16, OS 20/20 (15
weeks after initial presentation). Optic disc edema (Fig. 1) and central nervous system lesions
(Fig. 3) both improved. The patient
experienced mild to moderate elevation of intraocular pressure
after steroid pulse therapy, but it was controlled with topical
ocular hypotensive medications.
Discussion
Until now, the clinical diagnosis of ON has been
based on characteristic medical history, symptoms and physical
signs. The IVMP treatment for the acute phase of ON can be
initiated only based on the clinical diagnosis. However, in the
past decade, the understanding of ON has evolved and subtypes of
central nervous system (CNS) demyelinating diseases and relative ON
have expanded (6), emphasizing the
increasing importance of etiological diagnosis for precise
treatment, assessment of recurrence and prognosis and selection of
long-term maintenance strategies.
MS, neuromyelitis optica spectrum disorders, ADEM
and myelin oligodendrocyte glycoprotein antibody-associated disease
(MOGAD) can all lead to demyelinating lesions and ON, resulting in
significant clinical overlap (6).
This poses challenges in the etiological diagnosis of ON, as the
initial clinical diagnosis is often revised multiple times before
arriving at a final diagnosis. Based on medical history, symptoms
and signs, and MRI results, the patient of the current study was
initially suspected of having MS and ADEM by the ophthalmology and
neurology outpatient departments, respectively, before being
admitted to the hospital. The diagnosis of MOGAD was ultimately
confirmed based on immunological tests conducted after admission.
The diagnostic process of this case suggests that the value of MRI
and immunological tests is directly linked to the etiological
diagnosis of ON, and the completion of these two examinations is
important for evaluating the overall condition and establishing
etiological diagnoses. It is worth noting that, since relevant
immunological tests have only gradually matured in the past decade,
the establishment of reference values may require further
correction and improvement with a larger amount of data. For now,
the reference values for immunological tests may vary significantly
among institutions and it is recommended to refer to the normal
value range within the respective institution.
Currently, there have been no prospective
intervention studies specifically focusing on the pediatric
population in terms of ON treatment strategies. Based on published
cases and retrospective studies, the acute treatment of pediatric
ON still largely follows the strategy recommended by the ON
Treatment Trial (7), which involves
the use of IVMP followed by oral steroids to expedite visual
recovery. In severe cases or cases with suboptimal response to
treatment, steroid therapy is often combined with IVIG or
plasmapheresis (PLEX) (4), as in
the case of the present study. Among the cases reported in the
literature, a regimen of IVIG combined with PLEX without the use of
steroids was only employed in one case (8). In contrast to acute treatment, the
long-term maintenance treatment strategy for pediatric ON is more
varied and controversial. Using MOGAD as an example, the incidence
rate in children is higher than in adults (9), but existing studies have shown that
the risk of relapse is lower in children (10). This raises the question of whether
long-term maintenance therapy is necessary in the pediatric
population as it is in adults. This is particularly important
considering the potential side effects of medications such as
hormones and immunosuppressants on children who are still in the
developmental stage and have a longer life expectancy.
However, current statistics show that as many as 79%
of pediatric cases receive maintenance treatment (7). Common maintenance therapies include
oral corticosteroids for >4 weeks, oral immunosuppressive agents
(such as azathioprine, mycophenolate mofetil), monthly IVIG therapy
and monoclonal antibody-targeted therapy. Long-term maintenance
therapy is often used for cases of relapse, continuous positivity
for MOG-IgG and imaging findings suggesting new CNS lesions
(7). In the present case, due to
the typical CNS lesions during attacks, significant involvement of
the optic nerve and severe visual impairment, oral steroid
maintenance therapy was provided and gradually tapered off. At the
time of conclusion of the present study (December 2023, >30
weeks after the initial consultation), there has been no relapse in
this patient and vision has recovered to the pre-onset level.
Currently, due to the limited number of cases with sufficient
follow-up duration and clear etiological diagnosis, there is a lack
of high-level evidence-based research on risk prediction factors
for relapse of pediatric ON, the necessity of long-term maintenance
drug therapy and how to choose the appropriate treatment. Global
collaboration among peers is urgently needed to further investigate
these areas.
Going through the diagnostic process in the present
case, various problems or areas of improvement were identified.
First, although the diagnostic process was comprehensive, the
workflow was somewhat inefficient. This was due to two reasons: i)
The medical resources at the large medical center were crowded,
resulting in a delay in the timely completion of the crucial CNS
MRI examination, which delayed the subsequent diagnostic and
treatment steps; ii) considering the financial burden on the
patient, from the initial consultation in April 2023 to the
completion of infection screening and immunological examinations
related to CNS demyelinating diseases before IVMP therapy 6 days
later, we did indeed spend several days waiting for the MRI results
to confirm the next move. It may be more effective to conduct
infection screening and immunological examinations at the initial
consultation if the patient's financial condition allows, while
waiting for the MRI to be completed at the same time. This would
make the diagnosis and treatment process more compact and
efficient. Secondly, regarding the treatment strategy, in the
present case, the patient continued to take oral corticosteroids
for >4 weeks after symptom relief, which led to complications of
increased intraocular pressure, necessitating the addition of
medications to control the intraocular pressure. In ophthalmic
clinical practice, we have observed that children are highly
sensitive to corticosteroids in terms of intraocular pressure and
are prone to developing steroid-induced glaucoma. Therefore, when
selecting a maintenance treatment regimen, the necessity of
long-term corticosteroid treatment should have been questioned and
the possibility of combining immunosuppressive agents to reduce the
dosage and duration of corticosteroid use should have been
explored.
It is esteemed that the complete diagnostic and
therapeutic process, as well as the analysis, discussion and
reflection on this case, may contribute to the optimization of the
diagnosis and treatment of pediatric ON.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YC was a major contributor in writing the
manuscript. YC and YW both participated in the diagnosis and
treatment of this case. All authors have read and approved the
final manuscript. YC and YW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
This case report does not include any identifying
information. Consent for publication of case data/description and
images has been obtained from the patient's guardian.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Petzold A, Fraser CL, Abegg M, Alroughani
R, Alshowaeir D, Alvarenga R, Andris C, Asgari N, Barnett Y,
Battistella R, et al: Diagnosis and classification of optic
neuritis. Lancet Neurol. 21:1120–1134. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lehman SS and Lavrich JB: Pediatric optic
neuritis. Curr Opin Ophthalmol. 29:419–422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wilejto M, Shroff M, Buncic JR, Kennedy J,
Goia C and Banwell B: The clinical features, MRI findings, and
outcome of optic neuritis in children. Neurology. 67:258–262.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wan MJ, Adebona O, Benson LA, Gorman MP
and Heidary G: Visual outcomes in pediatric optic neuritis. Am J
Ophthalmol. 158:503–507.e2. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Waters P, Fadda G, Woodhall M, O'Mahony J,
Brown RA, Castro DA, Longoni G, Irani SR, Sun B, Yeh EA, et al:
Serial anti-myelin oligodendrocyte glycoprotein antibody analyses
and outcomes in children with demyelinating syndromes. JAMA Neurol.
77:82–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gise RA and Heidary G: Update on pediatric
optic neuritis. Curr Neurol Neurosci Rep. 20(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Klein da Costa B, Banwell BL and Sato DK:
Treatment of MOG-IgG associated disease in paediatric patients: A
systematic review. Mult Scler Relat Disord.
56(103216)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baumann M, Hennes EM, Schanda K, Karenfort
M, Kornek B, Seidl R, Diepold K, Lauffer H, Marquardt I,
Strautmanis J, et al: Children with multiphasic disseminated
encephalomyelitis and antibodies to the myelin oligodendrocyte
glycoprotein (MOG): Extending the spectrum of MOG antibody positive
diseases. Mult Scler. 22:1821–1829. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
de Mol CL, Wong Y, van Pelt ED, Wokke B,
Siepman T, Neuteboom RF, Hamann D and Hintzen RQ: The clinical
spectrum and incidence of anti-MOG-associated acquired
demyelinating syndromes in children and adults. Mult Scler.
26:806–814. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cobo-Calvo A, Ruiz A, Rollot F, Arrambide
G, Deschamps R, Maillart E, Papeix C, Audoin B, Lépine AF, Maurey
H, et al: Clinical features and risk of relapse in children and
adults with myelin oligodendrocyte glycoprotein antibody-associated
disease. Ann Neurol. 89:30–41. 2021.PubMed/NCBI View Article : Google Scholar
|