The coronavirus disease 2019 (COVID-19) pandemic has
caused social, economic, and political chaos worldwide (1). The effectiveness of antibody
treatments varies depending on the variation of the virus strain
(2). The World Health Organization
(WHO) has identified α, β, γ, δ and ο as severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) virus variants of concern
(3). These variants notably
decrease the effectiveness of vaccines, thus escalating the threat
to global public health. Nevertheless, vaccines continue to
demonstrate high efficacy in preventing severe illness,
hospitalization and death against these variants (3,4).
Without effective treatment, the virus can cause serious
respiratory disease, similar to SARS and Middle East respiratory
syndrome. Some cases of COVID-19 involve acute respiratory distress
syndrome or septic shock, which causes pressure and challenges to
the global medical and health system and consumes limited medical
resources (5). Countries worldwide
are actively responding to the COVID-19 pandemic and have
formulated policies, such as wearing masks in public, social
distancing and quarantining communities and cities. Despite these
measures, the COVID-19 pandemic continues. Certain studies suggest
vaccination may be the most effective measure to prevent COVID-19
infection (6,7). At present, most COVID-19 vaccines are
based on recombinant subunit proteins, virus-like particles,
messenger RNA, DNA and viral vectors (8).
Although COVID-19 infection primarily causes
respiratory symptoms, the virus may also damage other organs and
the kidney is one of the main sites of complications (9). Studies have shown that coronavirus
infection increases serum creatinine levels and acute tubular
necrosis, which may lead to impaired renal function (10,11).
Clinical studies have shown that patients with COVID-19 have acute
renal injury (10,12). At the same time, studies have shown
that chronic kidney disease (CKD) is a risk factor for COVID-19
death (13,14). The mortality rate among patients
with CKD and COVID-19 is significantly higher than that of
non-nephrotic patients and non-dialysis CKD patients with COVID-19.
Additionally, the severity of the disease is more pronounced in
elderly patients (15-17).
With the rapid mutation rate of the virus, there are still some
doubts about the effectiveness and safety of existing vaccines
(18-20).
The present study aimed to systematically analyze the efficacy and
safety of the immune response of patients with CKD receiving
hemodialysis (HD) and COVID-19 vaccine.
Inclusion criteria were as follows: i) Prospective
cohort study, randomized controlled trial or cross-sectional study;
ii) reported the effectiveness of COVID-19 vaccine response and
iii) studies reporting adverse events and COVID-19 vaccine
safety.
A total of two investigators independently performed
the literature search and data selection. Discrepancies were
resolved by the third reviewer. Extracted data included number of
participants, occurrence of endpoints of interest, mean age at
baseline, percentage of male participants, body mass index (BMI),
dialysis vintage and vaccine.
The study used a Risk Of Bias In Non-randomized
Studies - of Interventions (ROBINS-I) tool for bias detection
(21), which includes seven
component (bias due to confounding, bias in selection of
participants into the study, bias in classification of
Interventions, bias due to deviations from intended interventions,
bias due to missing data, bias in measurement of outcomes, and bias
in selection of the reported result. Overall bias was identified as
low, medium or serious bias.
The present study summarized the seropositivity rate
and adverse events of patients with CKD undergoing dialysis who
received the COVID-19 vaccine as outcome indicators. Heterogeneity
was evaluated using the I2 statistic as follows: 0-40,
no significant heterogeneity; 30-60, moderate heterogeneity; 50-90
substantial heterogeneity and 75-100%, considerable heterogeneity
(22). If I2 statistic
≥50%, the random benefit model was used; otherwise, a fixed-effects
model was used. Data reported as median and interquartile range
were converted to mean and SD as previously described (23). Data were analyzed using Review
Manager 5.1 (revman.cochrane.org/info) and STATA 18.0 (stata.com/). P<0.05 was considered to indicate a
statistically significant difference.
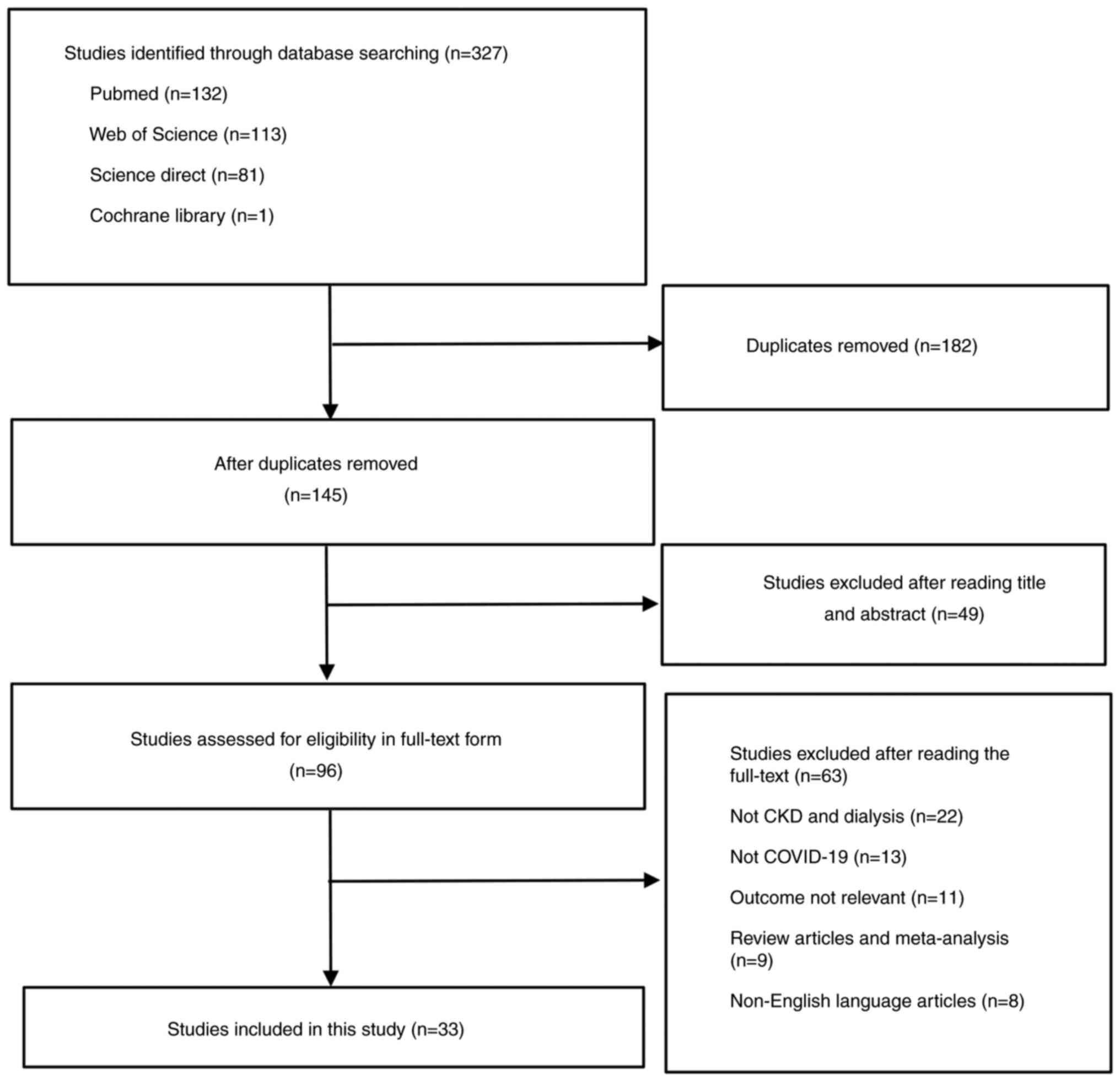
A total of 327 studies were initially retrieved. Of
these, 294 were excluded due to lack of relevant outcomes, patients
who did not have CKD or receive dialysis or COVID-19 vaccine and
articles published in a language other than English. Finally, 33
trials with a total of 81,348 patients were included in the
meta-analysis (24-56)
and mean dialysis vintage was 2.1 years. Details of age, BMI,
dialysis vintage, and vaccine name included in the analysis are
presented in Table I. At baseline,
the mean age was 64.9 years and the mean percentage of male
participants was 59.4%.
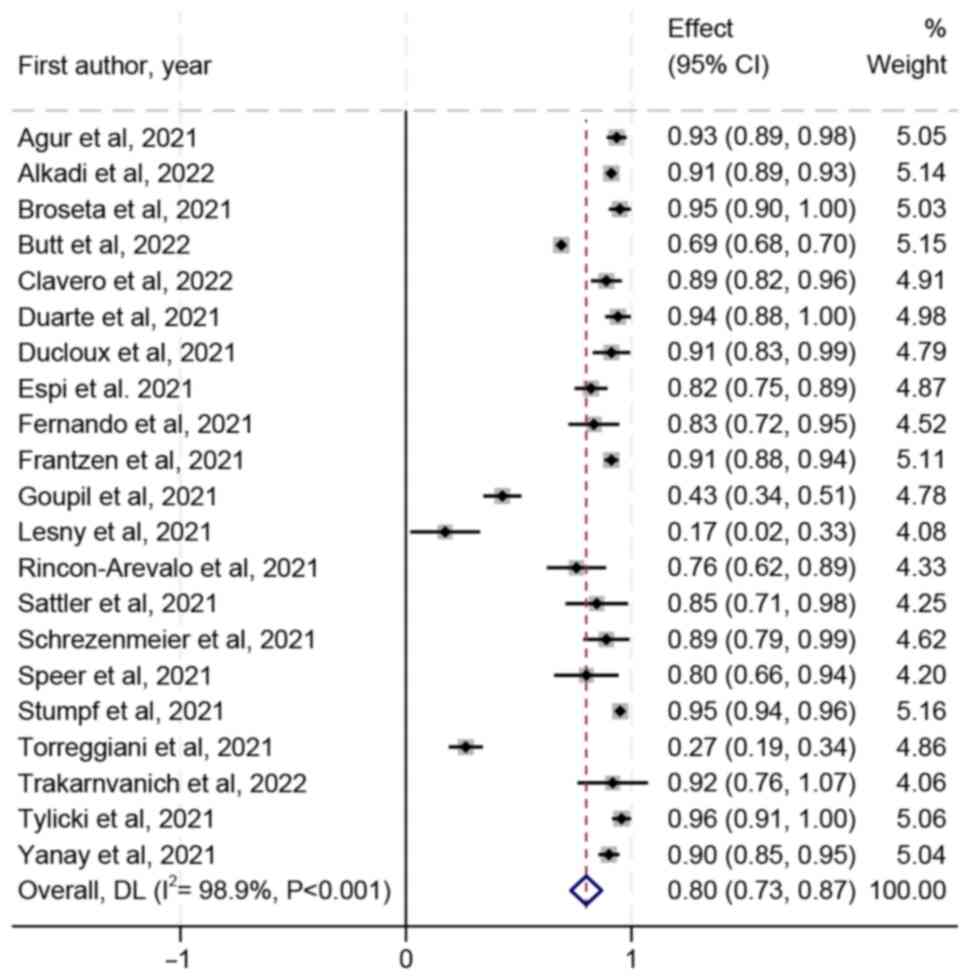
Seropositivity rate of patients with CKD and
dialysis after vaccination was 80% (95 CI, 73-87%) and the
heterogeneity was I2=98.9% (Fig. 2). After removing Bielopolski et
al (45) and Stumpf et
al (37) due to large sample
size, the sensitivity analysis found that the seropositivity rate
remained unchanged but the heterogeneity was reduced
(I2=96.4%; Fig. S1).
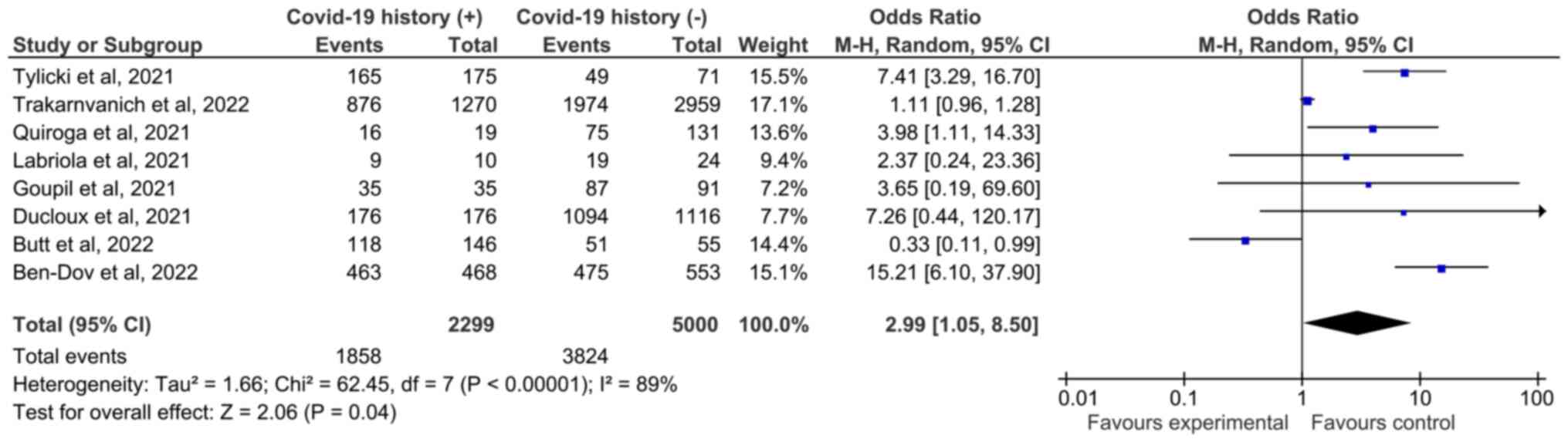
Seropositivity rate of patients without a history of COVID-19
infection was lower than that of patients with a history of
infection [Fig. 3; 76.48%
(3,824/5,000) of uninfected and 80.82% (1,858/2,299) of patients
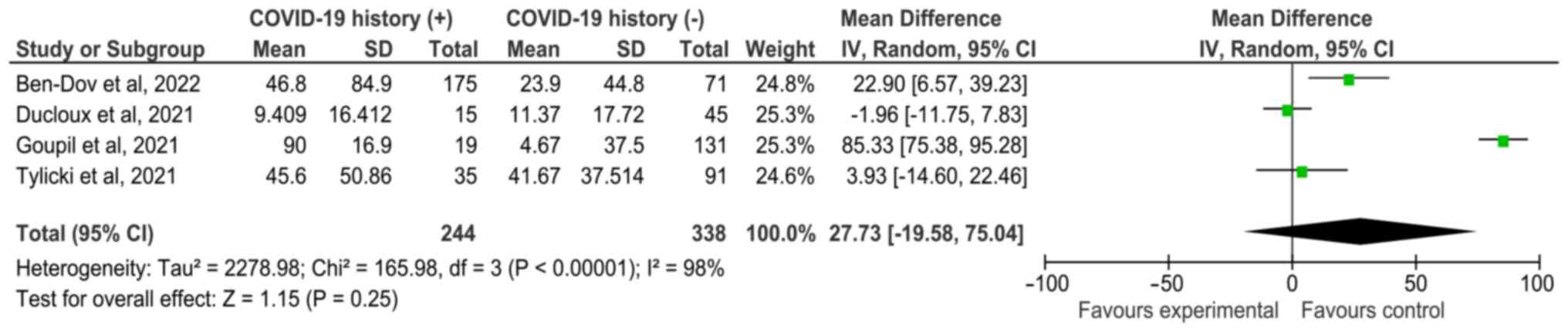
with a history of infection]. In addition, the antibody titers of
patients with CKD and dialysis who had a history of COVID-19
infection were compared; SMD was 27.73 (95% CI, -19.58-75.04),
indicating no significant difference (Fig. 4).
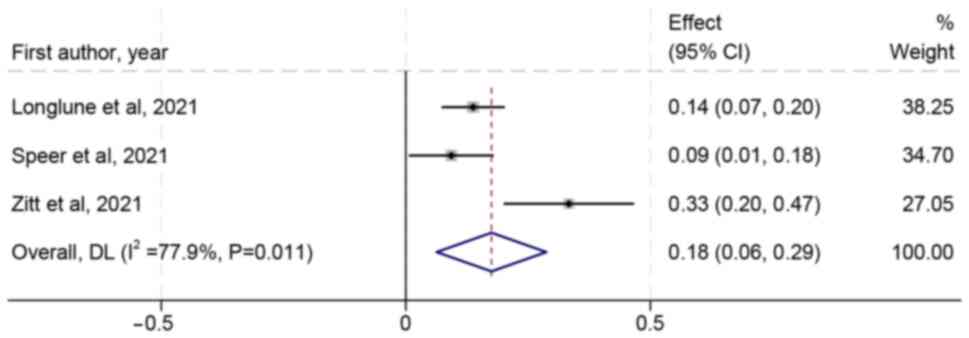
A total of nine trials reported adverse events.
Safety outcomes assessed were as follows: Pain at injection site,
redness, swelling, fatigue, headache and diarrhea (Table II). Pain at injection site was the
most common adverse event, accounting for 18% (95 CI, 6-29%;
Fig. 5). Other common adverse
events were fatigue and headache, accounting for 8 (95 CI, 4-13%)
and 6% (95 CI, 2-9%), respectively (Figs. S2 and S3).
The present meta-analysis showed that the immune
efficacy and safety of the COVID-19 vaccine in patients with CKD
undergoing dialysis was good and the seropositivity rate was 80%.
Individuals with a history of COVID-19 infection had higher
seropositivity rate. The primary adverse events were pain at the
injection site and fatigue after vaccination but the overall safety
was high. Therefore, administering the COVID-19 vaccine
significantly enhances protection against severe outcomes.
Injection site pain and local injection site
reactions are some of the most common reactions (93). Following COVID-19 vaccination, the
body exhibits an immune response and the muscles at the injection
site may become inflamed, leading to pain (94). In addition, some inactivated
vaccines use aluminum hydroxide as an adjuvant to strengthen
immunity. Certain scholars believe that aluminum hydroxide is
related to the pain at the injection site after inoculation
(95,96). The present results show that there
is a fatigue reaction following injection of the COVID-19 vaccine,
which may be due to brain hypoxia caused by excessive tension,
which leads to muscle weakness and fatigue. If the patient has
headaches after COVID-19 vaccination, the first thing to consider
is psychological factors such as fear, mistrust, and conspiracy
beliefs. Usually, after a few hours, the headache disappears when
the individual relaxes. This is consistent with previous studies on
major adverse events following COVID-19 vaccine injection (97,98).
The present study has limitations. First, most of
the studies included were inpatient cases, which may not fully
represent all infected people in the region, especially
asymptomatic cases. In addition, the included studies did not
obtain information about the characteristics, onset time, duration
and acute treatment response of side effects. Finally, further
research is needed to determine the protection level of naturally
acquired antibodies against mutant strains and the durability of
protection. Therefore, further research with a larger sample size
is required, considering the specific immune and physical
conditions of patients.
In conclusion, immune response of patients with CKD
undergoing dialysis was effective, which indicated that COVID-19
vaccine injection can reduce the incidence of COVID-19 in patients.
In addition, there were few common adverse events and there were no
potentially vaccine-related serious adverse events. Therefore, the
COVID-19 vaccine should be administered, considering the individual
immune levels of patients.
Not applicable.
Funding: No funding was received.
The data generated in the present study are included
in the figures and/or tables of this article.
KJ, YX and HY conceived and designed the study. XS
and BS designed the search strategy and performed quality
assessment. XS and BS confirm the authenticity of all the raw data
All authors analyzed data and wrote the manuscript. All authors
have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Vallée A: Geoepidemiological perspective
on COVID-19 pandemic review, an insight into the global impact.
Front Public Health. 11(1242891)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khoury DS, Docken SS, Subbarao K, Kent SJ,
Davenport MP and Cromer D: Predicting the efficacy of
variant-modified COVID-19 vaccine boosters. Nat Med. 29:574–578.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carabelli AM, Peacock TP, Thorne LG,
Harvey WT and Hughes J: COVID-19 Genomics UK Consortium. Peacock
SJ, Barclay WS, de Silva TI, Towers GJ and Robertson DL: SARS-CoV-2
variant biology: Immune escape, transmission and fitness. Nat Rev
Microbiol. 21:162–177. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bouhaddou M, Reuschl AK, Polacco BJ,
Thorne LG, Ummadi MR, Ye C, Rosales R, Pelin A, Batra J, Jang GM,
et al: SARS-CoV-2 variants evolve convergent strategies to remodel
the host response. Cell. 186:4597–4614.e26. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang S, Xia S, Ying T and Lu L: A novel
coronavirus (2019-nCoV) causing pneumonia-associated respiratory
syndrome. Cell Mol Immunol. 17(554)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Qualls N, Levitt A, Kanade N,
Wright-Jegede N, Dopson S, Biggerstaff M, Reed C and Uzicanin A:
CDC Community Mitigation Guidelines Work Group. Community
mitigation guidelines to prevent pandemic influenza-United States,
2017. MMWR Recomm Rep. 66:1–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Farsalinos K, Poulas K, Kouretas D,
Vantarakis A, Leotsinidis M, Kouvelas D, Docea AO, Kostoff R,
Gerotziafas GT, Antoniou MN, et al: Improved strategies to counter
the COVID-19 pandemic: Lockdowns vs. primary and community
healthcare. Toxicol Rep. 8:1–9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li H, Burm SW, Hong SH, Ghayda RA,
Kronbichler A, Smith L, Koyanagi A, Jacob L, Lee KH and Shin JI: A
comprehensive review of coronavirus disease 2019: Epidemiology,
transmission, risk factors, and international responses. Yonsei Med
J. 62:1–11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Perico L, Benigni A, Casiraghi F, Ng LFP,
Renia L and Remuzzi G: Immunity, endothelial injury and
complement-induced coagulopathy in COVID-19. Nat Rev Nephrol.
17:46–64. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu VC, Hsueh PR, Lin WC, Huang JW, Tsai
HB, Chen YM and Wu KD: SARS Research Group of the National Taiwan
University College of Medicine and National University Hospital.
Acute renal failure in SARS patients: More than rhabdomyolysis.
Nephrol Dial Transplant. 19:3180–3182. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cha RH, Joh JS, Jeong I, Lee JY, Shin HS,
Kim G and Kim Y: Critical Care Team of National Medical Center.
Renal complications and their prognosis in Korean patients with
Middle East respiratory syndrome-coronavirus from the central
MERS-CoV designated hospital. J Korean Med Sci. 30:1807–1814.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chan JFW, Yuan S, Kok KH, To KK, Chu H,
Yang J, Xing F, Liu J, Yip CC, Poon RW, et al: A familial cluster
of pneumonia associated with the 2019 novel coronavirus indicating
person-to-person transmission: A study of a family cluster. Lancet.
395:514–523. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gansevoort RT and Hilbrands LB: CKD is a
key risk factor for COVID-19 mortality. Nat Rev Nephrol.
16:705–706. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pakhchanian H, Raiker R, Mukherjee A, Khan
A, Singh S and Chatterjee A: Outcomes of COVID-19 in CKD patients:
A multicenter electronic medical record cohort study. Clin J Am Soc
Nephrol. 16:785–786. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
ERA-EDTA Council; ERACODA Working Group.
Chronic kidney disease is a key risk factor for severe COVID-19: A
call to action by the ERA-EDTA. Nephrol Dial Transplant. 36:87–94.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bajwa H, Riaz Y, Ammar M, Farooq S and
Yousaf A: The dilemma of renal involvement in COVID-19: A
systematic review. Cureus. 12(e8632)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Go AS, Chertow GM, Fan D, McCulloch CE and
Hsu C: Chronic kidney disease and the risks of death,
cardiovascular events, and hospitalization. N Engl J Med.
351:1296–1305. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
O'Callaghan KP, Blatz AM and Offit PA:
Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 324:437–438.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Calina D, Hartung T, Docea AO, Spandidos
DA, Egorov AM, Shtilman MI, Carvalho F and Tsatsakis A: COVID-19
vaccines: Ethical framework concerning human challenge studies.
Daru. 28:807–812. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sharpe HR, Gilbride C, Allen E,
Belij-Rammerstorfer S, Bissett C, Ewer K and Lambe T: The early
landscape of coronavirus disease 2019 vaccine development in the UK
and rest of the world. Immunology. 160:223–232. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sterne JA, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomised studies of interventions. BMJ.
355(i4919)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wan X, Wang W, Liu J and Tong T:
Estimating the sample mean and standard deviation from the sample
size, median, range and/or interquartile range. BMC Med Res
Methodol. 14(135)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Agur T, Ben-Dor N, Goldman S, Lichtenberg
S, Herman-Edelstein M, Yahav D, Rozen-Zvi B and Zingerman B:
Antibody response to mRNA SARS-CoV-2 vaccine among dialysis
patients-a prospectivecohort study. Nephrol Dial Transplant.
11(gfab155)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Broseta JJ, Rodríguez-Espinosa D, Soruco E
and Maduell F: Weekly seroconversion rate of the mRNA-1273
SARS-CoV-2 vaccine in haemodialysis patients. Nephrol Dial
Transplant. 36:1754–1755. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Labriola L, Scohy A, Van Regemorter E,
Robert A, Clerbaux G, Gillerot G, Pochet JM, Biller P, De
Schuiteneer M, Morelle J, et al: Immunogenicity of BNT162b2
SARS-CoV-2 vaccine in a multicenter cohort of nursing home
residents receiving maintenance hemodialysis. Am J Kidney Dis.
78:766–768. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lesny P, Anderson M, Cloherty G, Stec M,
Haase-Fielitz A, Haarhaus M, Santos C, Lucas C, Macario F and Haase
M: Immunogenicity of a first dose of mRNA- or vector-based
SARS-CoV-2 vaccination in dialysis patients: A multicenter
prospective observational pilot study. J Nephrol. 34:975–983.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Longlune N, Nogier MB, Miedougé M, Gabilan
C, Cartou C, Seigneuric B, Del Bello A, Marion O, Faguer S, Izopet
J and Kamar N: High immunogenicity of a messenger RNA-based vaccine
against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial
Transplant. 36:1704–1709. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rincon-Arevalo H, Choi M, Stefanski AL,
Halleck F, Weber U, Szelinski F, Jahrsdörfer B, Schrezenmeier H,
Ludwig C, Sattler A, et al: Impaired humoral immunity to SARS-CoV-2
BNT162b2 vaccine in kidney transplant recipients and dialysis
patients. Sci Immunol. 6(eabj1031)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sattler A, Schrezenmeier E, Weber UA,
Potekhin A, Bachmann F, Straub-Hohenbleicher H, Budde K, Storz E,
Proß V, Bergmann Y, et al: Impaired humoral and cellular immunity
after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in
kidney transplant recipients. J Clin Invest.
131(e150175)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schrezenmeier E, Bergfeld L, Hillus D,
Lippert JD, Weber U, Tober-Lau P, Landgraf I, Schwarz T, Kappert K,
Stefanski AL, et al: Immunogenicity of COVID-19 tozinameran
vaccination in patients on chronic dialysis. Front Immunol.
12(690698)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Simon B, Rubey H, Treipl A, Gromann M,
Hemedi B, Zehetmayer S and Kirsch B: Haemodialysis patients show a
highly diminished antibody response after COVID-19 mRNA vaccination
compared with healthy controls. Nephrol Dial Transplant.
36:1709–1716. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Speer C, Benning L, Töllner M, Nusshag C,
Kälble F, Reichel P, Schaier M, Bartenschlager M, Schnitzler P,
Zeier M, et al: Neutralizing antibody response against variants of
concern after vaccination of dialysis patients with BNT162b2.
Kidney Int. 100:700–702. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Speer C, Morath C, Töllner M, Buylaert M,
Göth D, Nusshag C, Kälble F, Schaier M, Grenz J, Kreysing M, et al:
Humoral responses to single-dose BNT162b2 mRNA vaccination in
dialysis patients previously infected with SARS-CoV-2. Front Med
(Lausanne). 8(721286)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Strengert M, Becker M, Ramos GM, Dulovic
A, Gruber J, Juengling J, Lürken K, Beigel A, Wrenger E, Lonnemann
G, et al: Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA
vaccine in patients on haemodialysis. EBioMedicine.
70(103524)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cserep G, Morrow D, Latchford K, Jesset R,
Dosa A and Kirmizis D: The effect of a single dose of BNT162b2
vaccine on the incidence of severe COVID-19 infection in patients
on chronic hemodialysis: A single-centre study. Clin Exp Nephrol.
26:54–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stumpf J, Siepmann T, Lindner T, Karger C,
Schwöbel J, Anders L, Faulhaber-Walter R, Schewe J, Martin H,
Schirutschke H, et al: Humoral and cellular immunity to SARS-CoV-2
vaccination in renal transplant versus dialysis patients: A
prospective, multicenter observational study using mRNA-1273 or
BNT162b2 mRNA vaccine. Lancet Reg Heal Eur.
9(100178)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Torreggiani M, Blanchi S, Fois A, Fessi H
and Piccoli GB: Neutralizing SARS-CoV-2 antibody response in
dialysis patients after the first dose of the BNT162b2 mRNA
COVID-19 vaccine: The war is far from being won. Kidney Int.
99:1494–1496. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tylicki L, Biedunkiewicz B, Dąbrowska M,
Ślizień W, Tylicki P, Polewska K, Rosenberg I, Rodak S and
Dębska-Ślizień A: Humoral response to SARS-CoV-2 vaccination
promises to improve the catastrophic prognosis of hemodialysis
patients as a result of COVID-19: The COViNEPH project. Polish Arch
Intern Med. 131:797–801. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Weigert A, Bergman ML, Gonçalves LA,
Godinho I, Duarte N, Abrantes R, Borges P, Brennand A, Malheiro V,
Matoso P, et al: Longitudinal analysis of antibody responses to the
mRNA BNT162b2 vaccine in patients undergoing maintenance
hemodialysis: A 6-month follow-up. Front Med (Lausanne).
8(796676)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yanay NB, Freiman S, Shapira M, Wishahi S,
Hamze M, Elhaj M, Zaher M and Armaly Z: Experience with SARS-CoV-2
BNT162b2 mRNA vaccine in dialysis patients. Kidney Int.
99:1496–1498. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zitt E, Davidovic T, Schimpf J,
Abbassi-Nik A, Mutschlechner B, Ulmer H, Benda MA, Sprenger-Mähr H,
Winder T and Lhotta K: The safety and immunogenicity of the
mRNA-BNT162b2 SARS-CoV-2 vaccine in Hemodialysis patients. Front
Immunol. 12(704773)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Alkadi MM, Hamad A, Ghazouani H,
Elshirbeny M, Ali MY, Ghonimi T, Ibrahim R, Abuhelaiqa E,
Abou-Samra AB, Al-Malki H and Butt AA: Effectiveness of messenger
RNA vaccines against SARS-CoV-2 infection in hemodialysis patients:
A case-control study. Vaccines (Basel). 11(49)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ben-Dov IZ, Oster Y, Tzukert K, Alster T,
Bader R, Israeli R, Asayag H, Aharon M, Burstein I, Pri-Chen H, et
al: Impact of tozinameran (BNT162b2) mRNA vaccine on kidney
transplant and chronic dialysis patients: 3-5 months follow-up. J
Nephrol. 35:153–164. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bielopolski D, Libresco G, Barda N, Dagan
N, Steinmetz T, Yahav D, Charytan DM, Balicer RD and Rozen-Zvi B:
BNT162b2 vaccine effectiveness in chronic kidney disease
patients-an observational study. Clin Kidney J. 15:1838–1846.
2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Butt AA, Talisa VB, Yan P, Shaikh OS, Omer
SB and Mayr FB: Real-World effectiveness of the severe acute
respiratory syndrome Coronavirus 2 (SARS-CoV-2) mRNA vaccines in
preventing confirmed infection in patients on chronic hemodialysis.
Clin Infect Dis. 75:e617–e622. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Duarte R, Roldão M, Figueiredo C, Luz I,
Ferrer F, Gonçalves H, Sofia F and Lopes K: Humoral response to
BNT162b2 mRNA COVID-19 vaccine in peritoneal and hemodialysis
patients: A comparative study. Ther Apher Dial. 26:790–796.
2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Davidovic T, Schimpf J, Abbassi-Nik A,
Stockinger R, Sprenger-Mähr H, Lhotta K and Zitt E: Humoral and
cellular immune response after a 3-dose heterologous SARS-CoV-2
vaccination using the mRNA-BNT162b2 and viral vector Ad26COVS1
vaccine in hemodialysis patients. Front Immunol.
13(907615)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Quiroga B, Soler MJ, Ortiz A, Vaquera SM,
Mantecón CJ, Useche G, Márquez MG, Carnerero M, Rodríguez MT, Ramos
PM, et al: Safety and immediate humoral response of COVID-19
vaccines in chronic kidney disease patients: The SENCOVAC study.
Nephrol Dial Transplant. 37:1868–1878. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Trakarnvanich T, Ngamvichchukorn T,
Phumisantiphong U, Pholtawornkulchai K, Phochanasomboon K and
Manomaipiboon A: Immune response after COVID-19 vaccination among
patients with chronic kidney disease and kidney transplant.
Vaccine. 40:6499–6511. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ducloux D, Colladant M, Chabannes M,
Yannaraki M and Courivaud C: Humoral response after 3 doses of the
BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney
Int. 100:702–704. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Espi M, Charmetant X, Barba T, Koppe L,
Pelletier C, Kalbacher E, Chalencon E, Mathias V, Ovize A,
Cart-Tanneur E, et al: The ROMANOV study found impaired humoral and
cellular immune responses to SARS-CoV-2 mRNA vaccine in
virus-unexposed patients receiving maintenance hemodialysis. Kidney
Int. 100:928–936. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fernando E and Govindan S: Neutralizing
SARS-CoV-2 antibody response and protective effect of 2 Doses of
ChAdOx1 nCoV-19 and BBV152 vaccines in hemodialysis patients: A
preliminary report. Kidney Int Rep. 6:2521–2522. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Frantzen L, Cavaille G, Thibeaut S and
El-Haik Y: Efficacy of the BNT162b2 mRNA Covid-19 vaccine in a
hemodialysis cohort. Nephrol Dial Transplant. 36:1756–1757.
2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Goupil R, Benlarbi M, Beaubien-Souligny W,
Nadeau-Fredette AC, Chatterjee D, Goyette G, Gunaratnam L, Lamarche
C, Tom A, Finzi A, et al: Short-term antibody response after 1 dose
of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ.
193:E793–E800. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Clavero R, Parra-Lucares A, Méndez-Valdés
G, Villa E, Bravo K, Mondaca E, Aranda J, Brignardello R, Gajardo
C, Ordenes A, et al: Humoral immune response of BNT162b2 and
coronavac vaccinations in hemodialysis patients: A multicenter
prospective cohort. Vaccines (Basel). 10(1542)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu W, Zhou L, Yin W, Wang J and Zuo X:
Global, regional, and national burden of chronic kidney disease
attributable to high sodium intake from 1990 to 2019. Front Nutr.
10(1078371)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kovesdy CP: Epidemiology of chronic kidney
disease: An update 2022. Kidney Int Suppl (2011). 12:7–11.
2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Locatelli F, Nissenson AR, Barrett BJ,
Walker RG, Wheeler DC, Eckardt KU, Lameire NH and Eknoyan G:
Clinical practice guidelines for Anemia in chronic kidney disease:
Problems and solutions. A position statement from kidney disease:
Improving Global Outcomes (KDIGO). Kidney Int. 74:1237–1240.
2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zou X, Chen K, Zou J, Han P, Hao J and Han
Z: Single-cell RNA-seq data analysis on the receptor ACE2
expression reveals the potential risk of different human organs
vulnerable to 2019-nCoV infection. Front Med. 14:185–192.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Diao B, Wang C, Wang R, Feng Z, Zhang J,
Yang H, Tan Y, Wang H, Wang C, Liu L, et al: Human kidney is a
target for novel severe acute respiratory syndrome coronavirus 2
infection. Nat Commun. 12(2506)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Martinez-Rojas MA, Vega-Vega O and
Bobadilla NA: Is the kidney a target of SARS-CoV-2? Am J Physiol
Renal Physiol. 318:F1454–F1462. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Sanhueza ME, Martín PS, Brantes L, Caro S,
Carrasco G and Machuca E: Efficacy of vaccination against the
SARS-CoV-2 virus in patients with chronic kidney disease on
hemodialysis. Hum Vaccin Immunother. 19(2173904)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hou YC, Lu KC and Kuo KL: The efficacy of
COVID-19 vaccines in chronic kidney disease and kidney
transplantation patients: A narrative review. Vaccines (Basel).
9(885)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Rossi M, Pessolano G and Gambaro G: What
has vaccination against COVID-19 in CKD patients taught us? J
Nephrol. 36:1257–1266. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Martins MP and de Oliveira RB: COVID-19
and chronic kidney disease: A narrative review. COVID. 3:1092–1105.
2023.
|
|
67
|
Babel N, Hugo C and Westhoff TH:
Vaccination in patients with kidney failure: Lessons from COVID-19.
Nat Rev Nephrol. 18:708–723. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jiang Z, Liu J, Geng L, Zhong Z, Tan J,
Wen D, Zhou L, Tang Y and Qin W: The influences of COVID-19 on
patients with chronic kidney disease: A multicenter cross-sectional
study. Front Psychiatry. 12(754310)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Rozen-Zvi B, Yahav D, Agur T, Zingerman B,
Ben-Zvi H, Atamna A, Tau N, Mashraki T, Nesher E and Rahamimov R:
Antibody response to SARS-CoV-2 mRNA vaccine among kidney
transplant recipients: A prospective cohort study. Clin Microbiol
Infect. 27:1173.e1–1173.e4. 2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kunutsor SK and Laukkanen JA: Markers of
liver injury and clinical outcomes in COVID-19 patients: A
systematic review and meta-analysis. J Infect. 82:159–198.
2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Bohn MK, Loh TP, Wang CB, Mueller R, Koch
D, Sethi S, Rawlinson WD, Clementi M, Erasmus R, Leportier M, et
al: IFCC interim guidelines on serological testing of antibodies
against SARS-CoV-2. Clin Chem Lab Med. 58:2001–2008.
2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ma BM, Tam AR, Chan KW, Ma MKM, Hung IFN,
Yap DYH and Chan TM: Immunogenicity and safety of COVID-19 vaccines
in patients receiving renal replacement therapy: A systematic
review and meta-analysis. Front Med (Lausanne).
9(827859)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Javadinia SA, Alizadeh K, Mojadadi MS,
Nikbakht F, Dashti F, Joudi M, Harati H, Welsh JS, Farahmand SA and
Attarian F: COVID-19 vaccination in patients with malignancy; a
systematic review and meta-analysis of the efficacy and safety.
Front Endocrinol (Lausanne). 13(860238)2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Joudi M, Binabaj MM, Porouhan P,
PeyroShabany B, Tabasi M, Fazilat-Panah D, Khajeh M, Mehrabian A,
Dehghani M, Welsh JS, et al: A cohort study on the immunogenicity
and safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in
patients with breast cancer; does trastuzumab interfere with the
outcome? Front Endocrinol (Lausanne). 13(798975)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ciarambino T, Para O and Giordano M:
Immune system and COVID-19 by sex differences and age. Womens
Health (Lond). 17(17455065211022262)2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ho JQ, Sepand MR, Bigdelou B, Shekarian T,
Esfandyarpour R, Chauhan P, Serpooshan V, Beura LK, Hutter G and
Zanganeh S: The immune response to COVID-19: Does sex matter?
Immunology. 166:429–443. 2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Collier DA, Ferreira IATM, Kotagiri P,
Datir RP, Lim EY, Touizer E, Meng B and Abdullahi A: CITIID-NIHR
BioResource COVID-19 Collaboration. Elmer A, et al: Age-related
immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2.
Nature. 596:417–422. 2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Chen Q, Zhao H, Yao X, Lin Z, Li J, Lin B,
Wang R, Huang Y, Su Y, Wu T, et al: Comparing immunogenicity of the
Escherichia coli-produced bivalent human papillomavirus vaccine in
females of different ages. Vaccine. 38:6096–6102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Fernandes M da CR, Vasconcelos GS, de Melo
ACL, Matsui TC, Caetano LF, de Carvalho Araújo FM and Fonseca MHG:
Influence of age, gender, previous SARS-CoV-2 infection, and
pre-existing diseases in antibody response after COVID-19
vaccination: A review. Mol Immunol. 156:148–155. 2023.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zheng YY, Ma YT, Zhang JY and Xie X:
COVID-19 and the cardiovascular system. Nat Rev Cardiol.
17:259–260. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Barnes E, Goodyear CS, Willicombe M,
Gaskell C, Siebert S, de Silva TI, Murray SM, Rea D, Snowden JA,
Carroll M, et al: SARS-CoV-2-specific immune responses and clinical
outcomes after COVID-19 vaccination in patients with
immune-suppressive disease. Nat Med. 29:1760–1774. 2023.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Letizia AG, Ge Y, Vangeti S, Goforth C,
Weir DL, Kuzmina NA, Balinsky CA, Chen HW, Ewing D,
Soares-Schanoski A, et al: SARS-CoV-2 seropositivity and subsequent
infection risk in healthy young adults: A prospective cohort study.
Lancet Respir Med. 9:712–720. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Brüssow H: COVID-19: Vaccination problems.
Environ Microbiol. 23:2878–2890. 2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Sáez-Peñataro J, Torres F, Bartra J,
Bascuas J, Vilella A, Tortajada M, Quesada S, González E,
López-Suñé E, Castells A, et al: Tolerability and reactogenicity
profile of mRNA SARS-Cov-2 vaccines from a mass vaccination
campaign in a tertiary hospital: Between-vaccine and
between-population prospective observational study (VigilVacCOVID
Study). BioDrugs. 36:509–520. 2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Al-Sadeq DW, Shurrab FM, Ismail A,
Amanullah FH, Thomas S, Aldewik N, Yassine HM, Rahim HF, Abu-Raddad
L and Nasralla GK: Comparison of antibody immune responses between
BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naïve and previously
infected individuals. J Travel Med. 28(taab190)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kitagawa H, Kaiki Y, Sugiyama A, Nagashima
S, Kurisu A, Nomura T, Omori K, Akita T, Shigemoto N, Tanaka J and
Ohge H: Adverse reactions to the BNT162b2 and mRNA-1273 mRNA
COVID-19 vaccines in Japan. J Infect Chemother. 28:576–581.
2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Hulme WJ, Horne EMF, Parker EPK, Keogh RH,
Williamson EJ, Walker V, Palmer TM, Curtis HJ, Walker AJ, Andrews
CD, et al: Comparative effectiveness of BNT162b2 versus mRNA-1273
covid-19 vaccine boosting in England: Matched cohort study in
OpenSAFELY-TPP. BMJ. 380(e072808)2023.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Ono S, Michihata N, Yamana H, Uemura K,
Ono Y, Jo T and Yasunaga H: Comparative effectiveness of BNT162b2
and mRNA-1273 booster dose after BNT162b2 primary vaccination
against the omicron variants: A retrospective cohort study using
large-scale population-based registries in Japan. Clin Infect Dis.
76:18–24. 2023.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Puspitasari M, Sattwika PD, Rahari DS,
Wijaya W, Hidayat ARP, Kertia N, Purwanto B and Thobari JA:
Immunogenicity and safety of inactivated SARS-CoV-2 vaccine in
haemodialysis patients: A prospective cohort study. Sci Rep.
13(11557)2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mehta N, Shah S, Paudel K, Chamlagain R
and Chhetri S: Safety and efficacy of coronavirus disease-19
vaccines in chronic kidney disease patients under maintenance
hemodialysis: A systematic review. Heal Sci Rep.
5(e700)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wang Y, Yang L and Xu G: New-onset acute
interstitial nephritis Post-SARS-CoV-2 infection and COVID-19
vaccination: A panoramic review. J Epidemiol Glob Health.
13:615–636. 2023.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhang S, He J, Tang B, Zhou Q, Hu Y, Yu Y,
Chen J, Liu Y, Li C, Ren H and Liao X: Cellular and humoral
responses to recombinant and inactivated SARS-CoV-2 vaccines in CKD
Patients: An observational study. J Clin Med.
12(1225)2023.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Qaderi K, Golezar MH, Mardani A, Mallah
MA, Moradi B, Kavoussi H, Shamsabadi A and Golezar S: Cutaneous
adverse reactions of COVID-19 vaccines: A systematic review.
Dermatol Ther. 35(e15391)2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Tan SW, Tam YC and Pang SM: Cutaneous
reactions to COVID-19 vaccines: A review. JAAD Int. 7:178–186.
2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Sharif N, Alzahrani KJ, Ahmed SN and Dey
SK: Efficacy, immunogenicity and safety of COVID-19 vaccines: A
systematic review and meta-analysis. Front Immunol.
12(714170)2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Xia S, Duan K, Zhang Y, Zhao D, Zhang H,
Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al: Effect of an
inactivated vaccine Against SARS-CoV-2 on safety and immunogenicity
outcomes: Interim analysis of 2 randomized clinical trials. JAMA.
324:951–960. 2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J,
Feng F, Zou H and Sun C: Safety of SARS-CoV-2 vaccines: A
systematic review and meta-analysis of randomized controlled
trials. Infect Dis Poverty. 10(94)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wu Q, Dudley MZ, Chen X, Bai X, Dong K,
Zhuang T, Salmon D and Yu H: Evaluation of the safety profile of
COVID-19 vaccines: A rapid review. BMC Med. 19(173)2021.PubMed/NCBI View Article : Google Scholar
|