1. Introduction
In the course of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) viral infection, clinical manifestations
mainly occur in the respiratory system. As of September 29, 2023,
according to World Health Organization estimates, there are
770,875,433 confirmed cases of coronavirus disease 2019 (COVID-19)
infection. In this regard, 6,959,316 deaths were recorded, which
corresponds to 0,9% of mortality (1). In the initial period of the pandemic,
the activities undertaken focused mainly on the treatment of acute
respiratory failure, which is the leading cause of death in
infected patients (2). This was the
third outbreak this century caused by coronaviruses. The two
previous outbreaks were caused by SARS-CoV/SARS-CoV-1, which caused
Severe Acute Respiratory Syndrome beginning in 2002, and MERS-CoV
causing Middle East Respiratory Syndrome, which was recognised in
2012. The aforementioned three viruses were detected in some
patients with neurological symptoms in the cerebrospinal fluid and
within the brain in post-mortem examinations (2,3). A
previously published systematic review and meta-analysis showed the
occurrence of nervous system symptoms in a number of patients. A
decrease in neurocognitive functions was found during SARS-CoV-2
viral infection. In the acute phase of the disease, memory
disorders, confusion, insomnia, depressed mood and anxiety were
observed. The aforementioned symptoms affected between 27.9 and
41.9% of respondents (3). In the
post-morbid phase, 32.2% of respondents experienced symptoms of
post-traumatic stress. A total of ~15% of the respondents were
diagnosed with depression and anxiety disorders with the
simultaneous occurrence of chronic fatigue syndrome and
fibromyalgia (3,4).
2. Etiology of cognitive dysfunction in
patients with COVID-19 infection
SARS-CoV-2 activation occurs with the participation
of proteases, such as transmembrane serine protease 2 (TMPRSS2). In
order to enter host cells, the expression of the
angiotensin-converting enzyme 2 (ACE-2) receptor is required
(5). According to a previous study,
the binding of ACE-2 to the spike (S) protein of SARS-CoV-2 is at
least 10-fold more potent than in the case of other SARS viruses,
which may significantly increase the incidence of infection
(6). However, Rombel-Bryzek et
al (7) analysed the differences
in ACE-2 binding by SARS-CoV-1 and SARS-CoV-2 S proteins using
isothermal titration calorimetry and showed that both
receptor-binding domains (SARS-CoV-1 and SARS-CoV-2) bind to the
hACE-2 with similar and high affinity but different
thermodynamics.
Potential coreceptors or other receptors for
infection have also been identified. These include the following:
Integrins, glucose-regulating protein 78 (GRP78), vimentin, sialic
acid, heparan sulphate, receptor tyrosine kinase (AXL),
asialoglycoprotein receptor-1 (ASGR1), kringle containing
transmembrane protein 1 (KREMEN1), furin, neuropilin-1,
cadherin-17, CD133, CD147, CD209 and CD26 (8-12).
Previous studies have demonstrated the widespread
expression of ACE-2 mRNA. Nevertheless, its level varies depending
on the location. Kidney and heart cells, lung epithelial cells and
vascular endothelium are characterised by high expression (13). Within the nervous system, these
include neurons, oligodendrocytes, macrophages/microglia,
astrocytes, ependymal cells, neural stem cells
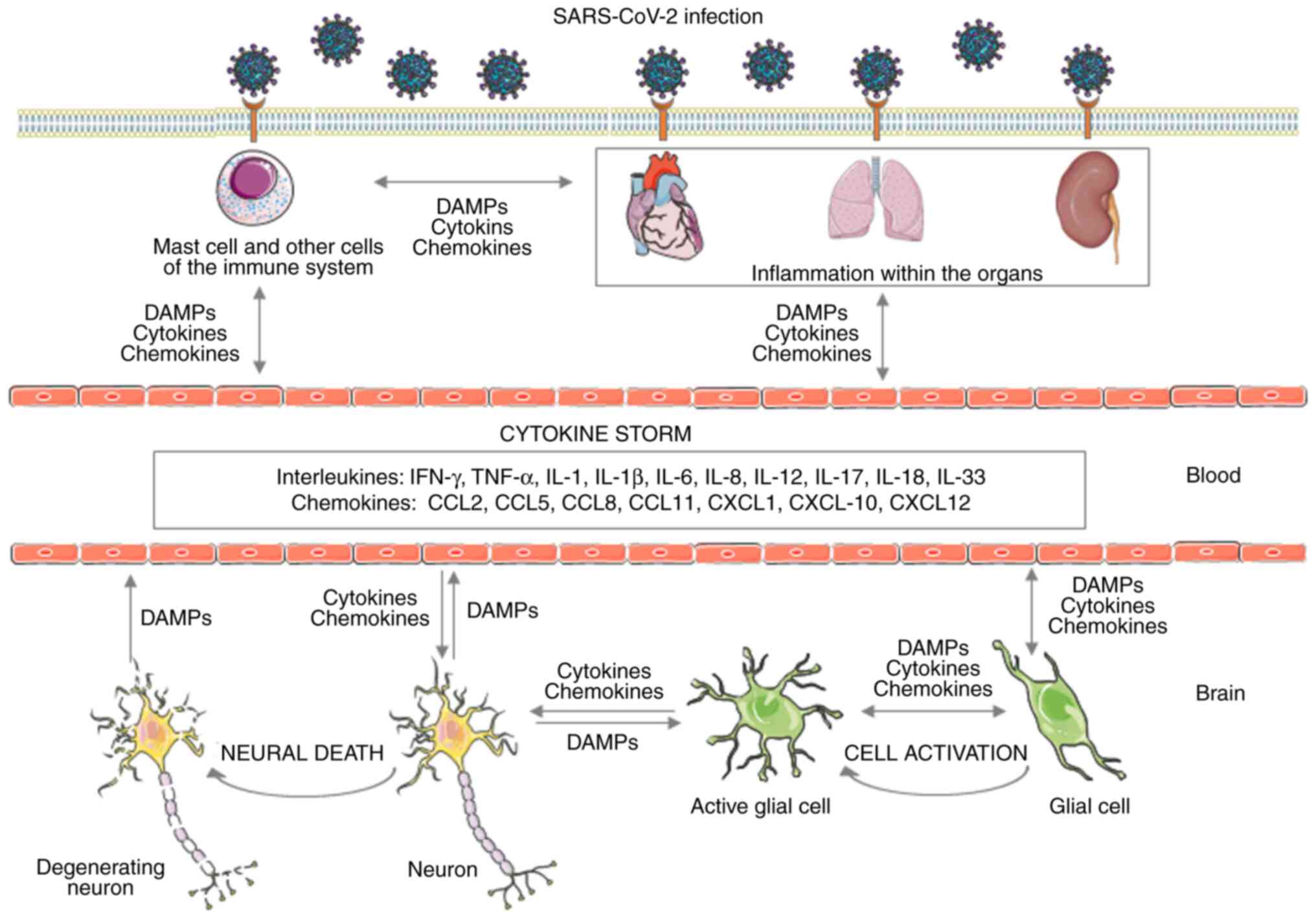
(NSCs)/non-parenchymal cells (NPCs) (Fig. 1). The aforementioned cells vary
depending on their function, shape, size, subtype and location
(11). Neuronal ACE-2 receptors are
located mainly in the structures of the brain stem, which are
responsible for the regulation of cardiac and respiratory functions
(14-17).
The infection routes of the SARS-CoV-2 virus within
the nervous system remain a topic of research. Potential ones
include retrograde axonal transport and transneuronal invasion,
with transmission through the intranasal and olfactory epithelium,
as well as through the oral cavity, gustatory nerves and trigeminal
nerve and lymphatic drainage. Additionally, the subject of research
is infection through the skin, peripheral nervous system and
intestinal vasculature. Hematogenous pathways include the route
through the ventricular system and choroid plexus, damaged
blood-brain barrier and infected immune cells (2,13). For
a holistic approach to the impact of SARS-CoV-2 on the nervous
system, both the mechanisms of infection, as well as the
neurological-psychiatric effects and direct and indirect cellular
consequences are important (13,18-23).
As a result of SARS-CoV-2 infection, anatomical and
functional changes in the nervous system may occur. As a result of
infection of the olfactory nerve, the virus can directly penetrate
the brain. A weakened sense of smell or its loss is observed in
patients with confirmed cognitive disorders and depressive
disorders (3,24,25).
There is also a known route through peripheral nerves using
intersynaptic transmission (26,27).
The results of studies on the presence of SARS-CoV-2 in the
cerebrospinal fluid of the examined patients are ambiguous
(28). The presence of the virus
was confirmed in NSCs, neurons and microglia. Infections also
involve ependymal cells, endothelial cells and astrocytes (27,29).
In numerous patients with symptoms of the nervous system, the
presence of SARS-CoV-2 was detected by reverse
transcription-quantitative PCR (RT-qPCR). However, there are
anecdotal studies of negative test results in this group of
patients (28,30-34).
The aforementioned differences may be related to the discrepancy in
the time of collection of test samples as well as the stage of the
patient's disease. An additional factor is the varying sensitivity
of RT-qPCR tests. The presence of neurological symptoms and the
presence of SARS-CoV-2 in the cerebrospinal fluid in some patients
is evidence of the possibility of the virus affecting the nervous
system. Nevertheless, this issue is a starting point for further
research (28,30-34).
3. Cytokine storm and neuroinflammation
The main role in the pathogenesis of cognitive
impairment in the course of COVID-19 is played by the cytokine
storm, which activates numerous leukocytes, mast cells, macrophages
and endothelial cells. As a consequence, significant amounts of
chemokines and pro-inflammatory cytokines are released (27,35,36).
The key released mediators that have a significant impact on the
course of the cytokine storm include interleukins: Tumour necrosis
factor-α (TNF-α), interferon-γ (IFN-γ), IL-6, IL-1, IL-1β, IL-2,
IL-3, IL-8, IL-12, IL-17, IL-18, IL-33 and chemokines [C-C motif
chemokine ligand 2 (CCL2), CCL5, CCL8, CCL11, C-X-C motif chemokine
ligand 1 (CXCL1), CXCL10 and CXCL12] as well as
granulocyte-macrophage colony-stimulating factor (GM-CSF) (35,37).
In particular, the pathogenesis of the aforementioned phenomenon is
related to the action of mast cells involved in the inflammatory
and neuroinflammatory response (38-43).
The peripheral immune reaction may intensify or
induce an acute or chronic neuroinflammatory response (44). SARS-CoV-2 viral infection may result
in the activation of mast cells residing in the respiratory tract
at the initial stage of the disease. At this level, they play the
role of the body's first line of defence against infection. Thus,
taking part in the direct elimination of the threat or in
supporting the immune system response. Mast cells, having a role in
inflammatory diseases, may also be associated with the genesis of
neuroinflammatory diseases, the occurrence of stress-related
disorders, and may also participate in post-traumatic brain damage
and the development of stroke. Mast cells occur in large numbers in
the nasal passage and the meninges. They are characterised by high
heterogeneity of morphology, varying degrees of response to
specific stimuli and performing different protective functions. In
the case of long-term protective effects, the nature of their
response may change to a harmful response (41).
The cytokine storm results in an increased level of
chemokines and pro-inflammatory cytokines secreted by mast cells,
mainly including TNF-α, IL-1, in particular IL1B, and IL-6
(35,45,46).
In addition, these cells secrete significant amounts of histamine,
tryptase, granulocyte growth factor, proteases, as well as CC
chemokine and ligand 2-CCL2 from the granules (35-37,45-47).
Additionally, they can release leukotriene C4 (LTC4) and
prostaglandin D2 (PGD2) at a significantly faster rate. Mast cells
are also capable of synthesising further inflammatory mediators in
the later stages of infection (48-50).
The protease tryptase that is released from mast cell granules may
also promote SARS-CoV-2 infection (49). An important aspect is that after
entry, viruses can activate mast cells by influencing toll-like
receptors and, as a result, increase the expression of inflammatory
mediators. Additionally, these cells have the ability to detect
damage-associated molecular patterns (DAMPs), which helps them
detect and respond to infection with the SARS-CoV-2 virus. The
infection is associated with increased levels of procoagulant
factors, D-dimers and prolongation of prothrombin time (27,36,51,52).
The occurrence of coagulation disorders, which may be associated
with the formation of clots and an increased risk of cerebral
bleeding, may be positively correlated with the occurrence of
ischemic and haemorrhagic stroke and cognitive impairment. This may
result in a deterioration of patients' condition, worse prognosis
and increased mortality (53-57)
(Fig. 1).
According to a previous study, the occurrence of
neuroinflammation in severe cases in some patients could correlate
with the occurrence of disorders such as cerebrovascular diseases,
as well as being a risk factor for stroke, encephalopathy or
epilepsy. The most common neurological symptoms include dizziness
and headaches, fever, disturbances of consciousness, neuralgia,
hyposmia and hypogeusia (58). A
number of studies confirm the relationship between the
aforementioned process and the occurrence of neuropsychiatric
diseases. They indicate the relationship between the severity of
central nervous system (CNS) dysfunction and the negative prognosis
of patients (58-60).
4. Nerve vs. glial cell
ACE-2 expression in neurons and glial cells makes
the CNS more susceptible to COVID-19 infection (61). Inflammatory molecules secreted as a
consequence by infected nerve cells can activate nearby immune
cells, mainly remaining mast cells and glial cells. Additionally,
other neurons and endothelial cells, pericytes and astrocytes are
susceptible to this process. Infection of endothelial cells by
SARS-CoV-2, which may be caused by the presence of the virus in the
microcirculation, may result in bleeding and blood-brain barrier
(BBB) dysfunction (62,63). This may result in the death of nerve
cells, deterioration of cognitive functions, and even in very
advanced cases, brain swelling, which poses a direct threat to
life. Inflammatory factors of the cytokine storm can activate
neurons, glial cells and subsequent mast cells, thereby
exacerbating or causing acute and chronic neuroinflammatory
reactions. Activated glial and immune cells as well as increased
activity of cytokines and chemokines positively associate with the
pathogenesis of neuroinflammatory and neurodegenerative diseases.
These include Alzheimer's disease and Parkinson's disease (64,65).
Infection of neurons by the SARS-CoV-2 virus may also directly
result in their death (66,67). Thereby causing a greater release of
PAMPs and DAMPs and contributing to the increased progression of
neuroinflammation.
The susceptibility of white matter to be damaged as
a result of ischemia is an element that reduces the mental
performance of patients. Some cases of COVID-19 showed decreased
cerebral perfusion, which correlated with decreased functioning of
the white matter (68). An
increasing number of studies suggested that the aforementioned may
be related to the pathology of TDP-43 and tau proteins.
Accumulation of amyloid beta has also been demonstrated. These
changes also concerned the area of the hippocampus responsible for
spatial and event memory. The observed memory deficits may be a
precursor to dementia disorders and, consequently, contribute to
the development of Alzheimer's disease (69-71).
The hippocampal system is a structure particularly
susceptible to stress. It has been revealed that as a result of
direct and indirect stress, the hypothalamic-pituitary-adrenal
(HPA) axis is disturbed among the studied patients. Increased
release of steroid hormones and the stress response of the body
disturb the functioning of the hippocampal system. Excessive
secretion of corticosteroids in stressful situations also
negatively affects the amygdala area. Due to the decreased function
of the prefrontal cortex, neurocognitive functions are limited
(71,72). Patients who succumbed due to
SARS-CoV-2 infection were characterised by increased concentrations
of pro-inflammatory cytokines. The aforementioned relationship
could be another stressor affecting cognitive functions.
Studies using TUNNEL staining and caspase 3
immunostaining reported increased neuronal cell death in the course
of COVID-19 (73-76).
Significant NSC/NPC cell mortality was also demonstrated. For other
supporting cells within the nervous system, less data and
researches are available. However, infection of these cells is
associated with an immune response, inflammatory reaction and
weakening of the neuroprotective function. This may promote the
death of neighbouring uninfected cells (73,74).
Transcriptomic analysis revealed transcription
defects, particularly in genes related to the response to hypoxia
and cytokine storm (73-76).
Increased expression of hypoxia inducible factor 1 subunit alpha
(HIF1a) was observed in cells in a local hypoxia environment. The
studies also showed impaired expression of genes related to
intercellular connections, cell secretory function, and, as a
result, potential impairment of the BBB and blood cerebrospinal
fluid barrier (BCSFB). Furthermore, these studies indicated that
the expression of vGLUT1 protein, a marker of presynaptic
stimulation, was also reduced. In some cases, abnormal localization
of the tau protein in the neuron body was identified (72). This localization was specific to
pT231, correlating with cells producing caspase 3. Abnormal
phosphorylation within the tau protein was associated with more
frequent cell apoptosis. According to observations, this was more
related to the response via type II interferon (INF-γ) than type I
interferon (IFN-α/β) (73-76).
5. Neuro-SARS, cognitive dysfunction, and
COVID-19 infection
In order to define neurological deficits associated
with SARS-CoV-2 infection, the following terms were introduced:
Neuro-SARS and neuroCOVID (77). It
is estimated that in the acute phase of the disease, more than 1/3
of patients exhibit symptoms typical of neuroCOVID (78). These symptoms correlate with a more
severe clinical course of the infection and significantly increase
the risk of complications. The most common symptoms include loss of
taste and smell, recurrent headaches and dizziness, qualitative
disturbances of consciousness and depressive symptoms. It has been
revealed that the severity of the disease may correlate with the
number of lymphocytes in the blood and the current level of
antibodies (78,79). Rare occurrences of encephalopathy,
strokes and peripheral neuropathies have also been observed. They
mainly concern older individuals with predominant
immunodeficiencies (78).
The aforementioned group of patients is
characterised by a diverse set of cognitive disorders. They include
mild deficits, selective, specific and generalised changes with
significant symptom severity. The symptoms most frequently
mentioned in the literature include: Impaired concentration,
attention and executive functions, as well as short-term memory
disorders (77-80).
According to the authors of most studies, the assessment of
cognitive dysfunction in this group cannot be limited only to the
use of the so-called screening tests (78-81).
The next group of symptoms includes a syndrome of
cognitive dysfunction - frontal syndrome. Its features were
observed by Helms et al (82) and Zhou et al (83) in their research. To assess cognitive
functions, Zhou et al (83)
used the Continuous Performance Test and the Trail Making Test. In
both studies, symptoms of cognitive impairment syndrome were
observed in a significant group of patients. A correlation of the
obtained results with the concentration of pro-inflammatory
cytokines and C-reactive protein was also demonstrated (83). These inflammatory processes, which
are often chronic, may negatively affect the functioning of the
developing brain. Their influence on the development of
neurodegenerative diseases and the manifestation of some mental
diseases, mainly schizophrenia, is suggested. The symptoms include
decreased ability to think abstractly, self-control and assess the
own capabilities of the individual. Short-term memory disorders and
reduced effectiveness of attention-related processes are also
observed (82,83).
6. Long COVID-19
Prolonged COVID-19 infection may cause psychiatric
and neuropsychiatric disorders in patients and their environment
(3,60,84).
Stressful conditions during the disease worsen the prognosis of
patients. As a result, prolonged infection may result in
post-stress depression, anxiety and other disorders, including
post-traumatic stress disorder (27,36,85,86).
Mental stress caused by long COVID-19 results in increased
secretion of corticotropin-releasing hormone (CRH) as well as
activation of the HPA axis. This process is intensified by the
production of additional amounts of CRH and other neuropeptides by
mast cells, thereby contributing to the deepening of
neuroinflammation (40).
Numerous mediators of the immune response secreted
by mast cells play an important role in the pathogenesis of
stress-induced diseases, including neuroinflammatory and autoimmune
diseases. The presented pathologies related to uncontrolled
cytokine storm and neuroinflammation may contribute to
neurodegeneration and related disorders (40,41,87).
Cytokine storm increases the influx of inflammatory mediators into
the brain due to BBB disruption. A defect in this barrier may
facilitate the entry of the SARS-CoV-2 virus and, consequently, an
uncontrolled influx of immune cells into the brain. In a prolonged
immune response in the CNS, activated immune cells, including mast
cells and glial cells, release significant amounts of additional
inflammatory chemokines and cytokines, increasing inflammation
(41,65). A number of studies showed that mast
cells may play a significant role in acute and chronic diseases
caused by SARS-CoV-2 viral infection. Prolonged infection, in
addition to neurological symptoms, may result in other systemic
disorders, including the previously mentioned coagulation disorders
(88,89). Additionally, it may worsen existing
respiratory diseases, including asthma and obstructive pulmonary
disease (2,9,49,88).
Acute and chronic stress caused by the aforementioned infection may
worsen neuroinflammatory disorders, including nerve injuries,
traumatic brain injury and stroke (89).
According to studies, a total of 36% of patients
infected with COVID-19 experienced neurological symptoms, of which
25% had CNS-related symptoms (64,78,82).
In this group, 88% of patients reported taste disturbances and
85.6% of smell disturbances (90).
In 11% of patients, anosmia preceded other clinical disorders
(90). In total, 5.7% of patients
with neurological symptoms experienced ischemic stroke (78) (Table
I).
 | Table ISelected neurological disorders
occurring in patients in the course of COVID-19. |
Table I
Selected neurological disorders
occurring in patients in the course of COVID-19.
| Author | Percentage (%) | Neurological
symptom | (Refs.) |
|---|
| Mao et al,
Helms et al | 36 | All patients
infected with the SARS CoV-2 virus | (78,82) |
| Mao et
al | 25 | Direct involvement
of the CNS | (78) |
| Lechien et
al | 88 | Gustatory
dysfunctions | (90) |
| Lechien et
al | 85.6 | Olfactory
dysfunctions | (90) |
| Lechien et
al | 11 | Anosmia before
other clinical symptoms | (90) |
| Mao et
al | 5.7 | Ischemic stroke in
severe cases | (78) |
7. Prevention and treatment of cognitive
dysfunction in the course of COVID-19
COVID-19 infection may result in both short- and
long-term complications and symptoms related to inflammatory
processes correlating with the activation of immune cells and the
cytokine storm (49,91). Due to the confirmed relationship
between neuropsychiatric diseases and infections, especially viral
ones, primary and secondary prevention is recommended. The first
one involves health education and the use of recommended
vaccinations. In the case of secondary prevention, activities aimed
at early detection of the disease and implementation of appropriate
treatment are involved. In the next stage, activities are based on
limiting the medium- and long-term negative effects of the disease
process on the patient's health (91). Research on the impact of
inflammatory processes in the course of SARS-CoV-2 on the
functioning of the CNS suggests enhancing neuroprotective effects.
Knowledge about the pathophysiological processes in the course of
the aforementioned infection is still developing. The presented
research results suggest actions aimed at stabilising proper
perfusion and, as a result, adequate oxygenation and nutrition of
nerve cells (92,93). A significant part of research deals
with the impact of inflammation and immunological factors on the
aforementioned processes. Modifying their action is a potential way
to reduce the short- and long-term negative consequences of
SARS-CoV-2 infection. Conducting neurological and psychiatric
diagnostics and psychological tests is necessary in the event of
worsening neurological symptoms (94,95).
Research indicates that inhibiting the activation of
mast cells and their subsequent degranulation may contribute to
reducing the intensity of the inflammatory process. The use of
antiviral and anti-inflammatory drugs, or those with a
neuroprotective effect, may support the therapeutic process of
patients and at the same time positively correlate with an improved
prognosis (41,64,96).
8. Conclusions
The presented studies demonstrated the impact of
SARS-CoV-2 on cognitive dysfunctions of various nature and
severity. A significant impact of immunological factors related to
the response against SARS-CoV-2 on impaired perfusion,
neuroprotective effect and functioning of nerve cells has been
proven. Particular attention is paid to the cytokine storm and the
related disproportion between pro- and anti-inflammatory effects,
oxidative stress, disturbances in the regulation of the HPA axis
and the body's stress response. An additional element are changes
in the expression of genes related to the response to hypoxia and
cytokine storm, such as: HIF1a, pT231, vGLUT1, genes related to
intercellular connections within the BBB and BCSFB barriers, as
well as IFN-γ and IFN-α/β (69-72).
These mechanisms directly affect the metabolism of endothelial
cells, nerve cells and BBB dysfunction. These processes may be the
starting point for cognitive function disorders, including mild,
selective, specific and generalised deficits with significant
symptom severity. Primary and secondary prevention related to
neurological and psychological diagnostics, as well as symptomatic
treatment is recommended. The processes occurring as a result of
SARS-CoV-2 infection require further research and analysis in order
to improve understanding of the pathophysiological mechanisms and
expand the possibilities of prevention and treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Institute of
Medical Sciences, University of Opole (Opole, Poland).
Availability of data and materials
Not applicable.
Authors' contributions
JS and TK conceived and designed the study, wrote
and prepared the draft of the manuscript, collected the data and
created the table. JS and ARB completed the study design and
carried out data interpretation. ARB verified the contents, revised
the manuscript and prepared the figure. RJB verified the contents
and critically revised and edited the manuscript. All authors
contributed to manuscript revision, and read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO Coronavirus (COVID-19) Dashboard.
|
|
2
|
Bodnar B, Patel K, Ho W, Luo JJ and Hu W:
Cellular mechanisms underlying neurological/neuropsychiatric
manifestations of COVID-19. J Med Virol. 93:1983–1998.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rogers JP, Chesney E, Oliver D, Pollak TA,
McGuire P, Fusar-Poli P, Zandi MS, Lewis G and David AS:
Psychiatric and neuropsychiatric presentations associated with
severe coronavirus infections: A systematic review and
meta-analysis with comparison to the COVID-19 pandemic. Lancet
Psychiatry. 7:611–627. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moldofsky H and Patcai J: Chronic
widespread musculoskeletal pain, fatigue, depression and disordered
sleep in chronic post-SARS syndrome; a case-controlled study. BMC
Neurol. 11(37)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wrapp D, Wang N, Corbett KS, Goldsmith JA,
Hsieh CL, Abiona O, Graham BS and McLellan JS: Cryo-EM structure of
the 2019-nCoV spike in the prefusion conformation. Science.
367:1260–1263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rombel-Bryzek A, Miller A and Witkowska D:
Thermodynamic analysis of the interactions between human ACE2 and
spike RBD of Betacoronaviruses (SARS-CoV-1 and SARS-CoV-2). FEBS
Open Bio. 13:174–184. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eslami N, Aghbash PS, Shamekh A,
Entezari-Maleki T, Nahand JS, Sales AJ and Baghi HB: SARS-CoV-2:
Receptor and co-receptor Tropism Probability. Curr Microbiol.
79(133)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lim S, Zhang M and Chang TL:
ACE2-independent alternative receptors for SARS-CoV-2. Viruses.
14(2535)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Strollo R and Pozzilli P: DPP4 inhibition:
Preventing SARS-CoV-2 infection and/or progression of COVID-19?
Diabetes Metab Res Rev. 36(e3330)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Raha AA, Chakraborty S, Henderson J,
Mukaetova-Ladinska E, Zaman S, Trowsdale J and Raha-Chowdhury R:
Investigation of CD26, a potential SARS-CoV-2 receptor, as a
biomarker of age and pathology. Biosci Rep.
40(BSR20203092)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kotani N and Nakano T: Candidate screening
of host cell membrane proteins involved in SARS-CoV-2 entry.
bioRxiv: 2020.09.09.289488, 2020.
|
|
13
|
Parma V, Ohla K, Veldhuizen MG, Niv MY,
Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M,
et al: More than smell-COVID-19 is associated with severe
impairment of smell, taste, and chemesthesis. Chem Senses.
45:609–622. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cure E and Cumhur Cure M:
Angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers may be harmful in patients with diabetes during COVID-19
pandemic. Diabetes Metab Syndr. 14:349–350. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Long B, Brady WJ, Koyfman A and Gottlieb
M: Cardiovascular complications in COVID-19. Am J Emerg Med.
38:1504–1507. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mankad K, Perry MD, Mirsky DM and Rossi A:
COVID-19: A primer for neuroradiologists. Neuroradiology.
62:647–648. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Saleki K, Banazadeh M, Saghazadeh A and
Rezaei N: The involvement of the central nervous system in patients
with COVID-19. Rev Neurosci. 31:453–456. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Torabi A, Mohammadbagheri E, Akbari
Dilmaghani N, Bayat AH, Fathi M, Vakili K, Alizadeh R,
Rezaeimirghaed O, Hajiesmaeili M, Ramezani M, et al:
Proinflammatory cytokines in the olfactory mucosa result in
COVID-19 induced anosmia. ACS Chem Neurosci. 11:1909–1913.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Samaranayake LP, Fakhruddin KS and
Panduwawala C: Sudden onset, acute loss of taste and smell in
coronavirus disease 2019 (COVID-19): A systematic review. Acta
Odontol Scand. 78:467–473. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shahbazi R, Yasavoli-Sharahi H, Alsadi N,
Ismail N and Matar C: Probiotics in treatment of viral respiratory
infections and neuroinflammatory disorders. Molecules.
25(4891)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ferguson AV: Circumventricular organs:
integrators of circulating signals controlling hydration, energy
balance, and immune function. In: Neurobiology of Body Fluid
Homeostasis: Transduction and Integration. De Luca LA, Menani JV
and Johnson AK (eds.) CRC Press/Taylor & Francis, Boca Raton
(FL), 2014.
|
|
22
|
Kim J, Alejandro B, Hetman M, Hattab EM,
Joiner J, Schroten H, Ishikawa H and Chung DH: Zika virus infects
pericytes in the choroid plexus and enters the central nervous
system through the blood-cerebrospinal fluid barrier. PLoS Pathog.
16(e1008204)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alam SB, Willows S, Kulka M and Sandhu JK:
Severe acute respiratory syndrome coronavirus 2 may be an
underappreciated pathogen of the central nervous system. Eur J
Neurol. 27:2348–2360. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang F, Wu X, Gao J, Li Y, Zhu Y and Fang
Y: The relationship of olfactory function and clinical traits in
major depressive disorder. Behav Brain Res.
386(112594)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yahiaoui-Doktor M, Luck T, Riedel-Heller
SG, Loeffler M, Wirkner K and Engel C: Olfactory function is
associated with cognitive performance: Results from the
population-based LIFE-adult-study. Alzheimers Res Ther.
11(43)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li YC, Bai WZ and Hashikawa T: The
neuroinvasive potential of SARS-CoV2 may play a role in the
respiratory failure of COVID-19 patients. J Med Virol. 92:552–555.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li H, Xue Q and Xu X: Involvement of the
nervous system in SARS-CoV-2 infection. Neurotox Res. 38:1–7.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Andriuta D, Roger PA, Thibault W, Toublanc
B, Sauzay C, Castelain S, Godefroy O and Brochot E: COVID-19
encephalopathy: Detection of antibodies against SARS-CoV-2 in CSF.
J Neurol. 267:2810–2811. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sinanović O, Muftić M and Sinanović S:
COVID-19 pandemia: Neuropsychiatric comorbidity and consequences.
Psychiatr Danub. 32:236–244. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xiang P, Xu X, Lu X, Gao L, Wang H, Li Z,
Xiong H, Li R, Xiong Y, Pu L, et al: Case report: Identification of
SARS-CoV-2 in cerebrospinal fluid by ultrahigh-depth sequencing in
a patient with coronavirus disease 2019 and neurological
dysfunction. Front Med (Lausanne). 8(629828)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cebrián J, Gonzalez-Martinez A,
García-Blanco MJ, Celdrán-Vivancos D, Palacios EL, Reig-Roselló G,
Casado-Fernández L, Vivancos J and Gago-Veiga AB: Headache and
impaired consciousness level associated with SARS-CoV-2 in CSF: A
case report. Neurology. 95:266–268. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fadakar N, Ghaemmaghami S, Masoompour SM,
Shirazi Yeganeh B, Akbari A, Hooshmandi S and Ostovan VR: A first
case of acute cerebellitis associated with coronavirus disease
(COVID-19): A case report and literature review. Cerebellum.
19:911–914. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kremer S, Lersy F, de Sèze J, Ferré JC,
Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G,
Martin-Blondel G, et al: Brain MRI findings in severe COVID-19: A
retrospective observational study. Radiology. 297:E242–E251.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Virhammar J, Kumlien E, Fällmar D,
Frithiof R, Jackmann S, Sköld MK, Kadir M, Frick J, Lindeberg J,
Olivero-Reinius H, et al: Acute necrotizing encephalopathy with
SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology.
95:445–449. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Azkur AK, Akdis M, Azkur D, Sokolowska M,
van de Veen W, Brüggen MC, O'Mahony L, Gao Y, Nadeau K and Akdis
CA: Immune response to SARS-CoV-2 and mechanisms of
immunopathological changes in COVID-19. Allergy. 75:1564–1581.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tang SW, Helmeste D and Leonard B:
Inflammatory neuropsychiatric disorders and COVID-19
neuroinflammation. Acta Neuropsychiatr. 33:165–177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Elieh Ali Komi D, Wöhrl S and Bielory L:
Mast cell biology at molecular level: A comprehensive review. Clin
Rev Allergy Immunol. 58:342–365. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cabrera-Pastor A, Llansola M, Montoliu C,
Malaguarnera M, Balzano T, Taoro-Gonzalez L, García-García R,
Mangas-Losada A, Izquierdo-Altarejos P, Arenas YM, et al:
Peripheral inflammation induces neuroinflammation that alters
neurotransmission and cognitive and motor function in hepatic
encephalopathy: Underlying mechanisms and therapeutic implications.
Acta Physiol (Oxf). 226(e13270)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kempuraj D, Ahmed ME, Selvakumar GP,
Thangavel R, Dhaliwal AS, Dubova I, Mentor S, Premkumar K, Saeed D,
Zahoor H, et al: Brain injury-mediated neuroinflammatory response
and Alzheimer's disease. Neuroscientist. 26:134–155.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kempuraj D, Mentor S, Thangavel R, Ahmed
ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS and
Zaheer A: Mast cells in stress, pain, blood-brain barrier,
neuroinflammation and Alzheimer's disease. Front Cell Neurosci.
13(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kempuraj D, Selvakumar GP, Ahmed ME,
Raikwar SP, Thangavel R, Khan A, Zaheer SA, Iyer SS, Burton C,
James D and Zaheer A: COVID-19, mast cells, cytokine storm,
psychological stress, and neuroinflammation. Neuroscientist.
26:402–414. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kustrimovic N, Marino F and Cosentino M:
Peripheral immunity, immunoaging and neuroinflammation in
Parkinson's disease. Curr Med Chem. 26:3719–3753. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Magrone T, Magrone M, Russo MA and Jirillo
E: Peripheral immunosenescence and central neuroinflammation: A
dangerous liaison-A dietary approach. Endocr Metab Immune Disord
Drug Targets. 20:1391–1411. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Theoharides TC: Effect of stress on
neuroimmune processes. Clin Ther. 42:1007–1014. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Debuc B and Smadja DM: Is COVID-19 a new
hematologic disease? Stem Cell Rev Rep. 17:4–8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wilson MP and Jack AS: Coronavirus disease
2019 (COVID-19) in neurology and neurosurgery: A scoping review of
the early literature. Clin Neurol Neurosurg.
193(105866)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nile SH, Nile A, Qiu J, Li L, Jia X and
Kai G: COVID-19: Pathogenesis, cytokine storm and therapeutic
potential of interferons. Cytokine Growth Factor Rev. 53:66–70.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Karamloo F and König R: SARS-CoV-2
immunogenicity at the crossroads. Allergy. 75:1822–1824.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Theoharides TC: COVID-19, pulmonary mast
cells, cytokine storms, and beneficial actions of luteolin.
Biofactors. 46:306–308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kritas SK, Ronconi G, Caraffa A, Gallenga
CE, Ross R and Conti P: Mast cells contribute to
coronavirus-induced inflammation: New anti-inflammatory strategy. J
Biol Regul Homeost Agents. 34:9–14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Connell NT, Battinelli EM and Connors JM:
Coagulopathy of COVID-19 and antiphospholipid antibodies. J Thromb
Haemost. 18:E1–E2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Markus HS and Brainin M: COVID-19 and
stroke-A global world stroke organization perspective. Int J
Stroke. 15:361–364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Khalili N, Haseli S, Bahrami-Motlagh H,
Keshavarz E, Khalili N, Langroudi TF, Khameneh A and Taheri MS:
Neurologic involvement in COVID-19: Radiologists' perspective. Acad
Radiol. 27:1051–1053. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Qiu H, Wu J, Hong L, Luo Y, Song Q and
Chen D: Clinical and epidemiological features of 36 children with
coronavirus disease 2019 (COVID-19) in Zhejiang, China: An
observational cohort study. Lancet Infect Dis. 20:689–696.
2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhang J, Xie B and Hashimoto K: Current
status of potential therapeutic candidates for the COVID-19 crisis.
Brain Behav Immun. 87:59–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z
and Zhang Z: D-dimer levels on admission to predict in-hospital
mortality in patients with Covid-19. J Thromb Haemost.
18:1324–1329. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Connors JM and Levy JH: COVID-19 and its
implications for thrombosis and anticoagulation. Blood.
135:2033–2040. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Dixon L, Varley J, Gontsarova A, Mallon D,
Tona F, Muir D, Luqmani A, Jenkins IH, Nicholas R, Jones B and
Everitt A: COVID-19-related acute necrotizing encephalopathy with
brain stem involvement in a patient with aplastic anemia. Neurol
Neuroimmunol Neuroinflammation. 7(e789)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ogier M, Andéol G, Sagui E and Dal Bo G:
How to detect and track chronic neurologic sequelae of COVID-19?
Use of auditory brainstem responses and neuroimaging for long-term
patient follow-up. Brain Behav Immun Health.
5(100081)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Park CL, Russell BS, Fendrich M,
Finkelstein-Fox L, Hutchison M and Becker J: Americans' COVID-19
stress, coping, and adherence to CDC guidelines. J Gen Intern Med.
35:2296–2303. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Baig AM, Khaleeq A, Ali U and Syeda H:
Evidence of the COVID-19 virus targeting the CNS: Tissue
distribution, host-virus interaction, and proposed neurotropic
mechanisms. ACS Chem Neurosci. 11:995–998. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pineton de Chambrun M, Frere C, Miyara M,
Amoura Z, Martin-Toutain I, Mathian A, Hekimian G and Combes A:
High frequency of antiphospholipid antibodies in critically ill
COVID-19 patients: a link with hypercoagulability? J Intern Med.
289:422–424. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Finsterer J and Stollberger C: Update on
the neurology of COVID-19. J Med Virol. 92:2316–2318.
2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Heneka MT, Golenbock D, Latz E, Morgan D
and Brown R: Immediate and long-term consequences of COVID-19
infections for the development of neurological disease. Alzheimers
Res Ther. 12(69)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Leonardi M, Padovani A and McArthur JC:
Neurological manifestations associated with COVID-19: A review and
a call for action. J Neurol. 267:1573–1576. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Serrano-Castro PJ, Estivill-Torrús G,
Cabezudo-García P, Reyes-Bueno JA, Ciano Petersen N,
Aguilar-Castillo MJ, Suárez-Pérez J, Jiménez-Hernández MD,
Moya-Molina MÁ, Oliver-Martos B, et al: Impact of SARS-CoV-2
infection on neurodegenerative and neuropsychiatric diseases: A
delayed pandemic? Neurologia (Engl Ed). 35:245–251. 2020.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
67
|
Parrella E, Porrini V, Benarese M and
Pizzi M: The role of mast cells in stroke. Cells.
8(437)2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Bougakov D, Podell K and Goldberg E:
Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol.
58:564–575. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ritchie K, Chan D and Watermeyer T: The
cognitive consequences of the COVID-19 epidemic: Collateral damage?
Brain Commun. 2(fcaa069)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Forkel SJ, Friedrich P, Thiebaut de
Schotten M and Howells H: White matter variability, cognition, and
disorders: A systematic review. Brain Struct Funct. 227:529–544.
2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Krause-Utz A, Oei NYL, Niedtfeld I, Bohus
M, Spinhoven P, Schmahl C and Elzinga BM: Influence of emotional
distraction on working memory performance in borderline personality
disorder. Psychol Med. 42:2181–2192. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Dinakaran D, Manjunatha N, Naveen Kumar C
and Suresh BM: Neuropsychiatric aspects of COVID-19 pandemic: A
selective review. Asian J Psychiatr. 53(102188)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Song E, Zhang C, Israelow B, Lu-Culligan
A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, et al:
Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med.
218(e20202135)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Jacob F, Pather SR, Huang WK, Zhang F,
Wong SZH, Zhou H, Cubitt B, Fan W, Chen CZ, Xu M, et al: Human
pluripotent stem cell-derived neural cells and brain organoids
reveal SARS-CoV-2 neurotropism predominates in choroid plexus
epithelium. Cell Stem Cell. 27:937–950.e9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mesci P, Macia A, Saleh A, Martin-Sancho
L, Yin X, Snethlage C, Avansini S, Chanda SK and Muotri A:
Sofosbuvir protects human brain organoids against SARS-CoV-2.
bioRxiv: 2020.05.30.125856, 2020.
|
|
76
|
Ramani A, Müller L, Ostermann PN, Gabriel
E, Abida-Islam P, Müller-Schiffmann A, Mariappan A, Goureau O,
Gruell H, Walker A, et al: SARS-CoV-2 targets neurons of 3D human
brain organoids. EMBO J. 39(e106230)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Oliveira R, Sotero FD and Teodoro T:
NeuroCOVID: Critical review of neuropsychiatric manifestations of
SARS-CoV-2 infection. Ir J Med Sci. 190:851–852. 2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q,
Chang J, Hong C, Zhou Y, Wang D, et al: Neurologic manifestations
of hospitalized patients with coronavirus disease 2019 in Wuhan,
China. JAMA Neurol. 77:683–690. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Banerjee D and Viswanath B:
Neuropsychiatric manifestations of COVID-19 and possible pathogenic
mechanisms: Insights from other coronaviruses. Asian J Psychiatry.
54(102350)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li J, Long X, Zhang Q, Fang X, Fang F, Lv
X, Zhang D, Sun Y, Li N, Hu S, et al: Emerging evidence for
neuropsycho-consequences of COVID-19. Curr Neuropharmacol.
19:92–96. 2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Sozzi M, Algeri L, Corsano M, Crivelli D,
Daga MA, Fumagalli F, Gemignani P, Granieri MC, Inzaghi MG, Pala F,
et al: Neuropsychology in the times of COVID-19 the role of the
psychologist in taking charge of patients with alterations of
cognitive functions. Front Neurol. 11(573207)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Helms J, Kremer S, Merdji H, Clere-Jehl R,
Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana
M, et al: Neurologic features in severe SARS-CoV-2 infection. N
Engl J Med. 382:2268–2270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu
H, Shi C and Hu S: The landscape of cognitive function in recovered
COVID-19 patients. J Psychiatr Res. 129:98–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Vinkers CH, van Amelsvoort T, Bisson JI,
Branchi I, Cryan JF, Domschke K, Howes OD, Manchia M, Pinto L, de
Quervain D, et al: Stress resilience during the coronavirus
pandemic. Eur Neuropsychopharmacol. 35:12–16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Steenblock C, Todorov V, Kanczkowski W,
Eisenhofer G, Schedl A, Wong ML, Licinio J, Bauer M, Young AH,
Gainetdinov RR and Bornstein SR: Severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) and the neuroendocrine stress axis. Mol
Psychiatry. 25:1611–1617. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ye Q, Wang B and Mao J: The pathogenesis
and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect.
80:607–613. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Kempuraj D, Ahmed ME, Selvakumar GP,
Thangavel R, Raikwar SP, Zaheer SA, Iyer SS, Burton C, James D and
Zaheer A: Psychological stress-induced immune response and risk of
Alzheimer's disease in veterans from operation enduring freedom and
operation iraqi freedom. Clin Ther. 42:974–982. 2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
De Felice FG, Tovar-Moll F, Moll J, Munoz
DP and Ferreira ST: Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) and the central nervous system. Trends Neurosci.
43:355–357. 2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Sheraton M, Deo N, Kashyap R and Surani S:
A review of neurological complications of COVID-19. Cureus.
12(e8192)2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lechien JR, Chiesa-Estomba CM, De Siati
DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia
F, Distinguin L, et al: Olfactory and gustatory dysfunctions as a
clinical presentation of mild-to-moderate forms of the coronavirus
disease (COVID-19): A multicenter European study. Eur Arch
Otorhinolaryngol. 277:2251–2261. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Barlow A, Landolf KM, Barlow B, Yeung SYA,
Heavner JJ, Claassen CW and Heavner MS: Review of emerging
pharmacotherapy for the treatment of coronavirus disease 2019.
Pharmacotherapy. 40:416–437. 2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Gigante A, Aquili A, Farinelli L, Caraffa
A, Ronconi G, Enrica Gallenga C, Tetè G, Kritas SK and Conti P:
Sodium chromo-glycate and palmitoylethanolamide: A possible
strategy to treat mast cell-induced lung inflammation in COVID-19.
Med Hypotheses. 143(109856)2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Conti P, Gallenga CE, Tetè G, Caraffa A,
Ronconi G, Younes A, Toniato E, Ross R and Kritas SK: How to reduce
the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection
and lung inflammation mediated by IL-1. J Biol Regul Homeost
Agents. 34:333–338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Negrini F, Ferrario I, Mazziotti D,
Berchicci M, Bonazzi M, de Sire A, Negrini S and Zapparoli L:
Neuropsychological features of severe hospitalized coronavirus
disease 2019 patients at clinical stability and clues for postacute
rehabilitation. Arch Phys Med Rehabil. 102:155–158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Verkhratsky A, Li Q, Melino S, Melino G
and Shi Y: Can COVID-19 pandemic boost the epidemic of
neurodegenerative diseases? Biol Direct. 15(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Minihan E, Gavin B, Kelly BD and
McNicholas F: COVID-19, mental health and psychological first aid.
Ir J Psychol Med. 37:259–263. 2020.PubMed/NCBI View Article : Google Scholar
|