Introduction
Blackcurrant (Ribes nigrum L.) contains
polyphenols, particularly four anthocyanins: Cyanidin-3-glucoside,
cyanidin-3-rutinoside, delphinidin-3-glucoside and
delphinidin-3-rutinoside. Additionally, it contains large amounts
of vitamins A, C and E, as well as small amounts of each of the B
vitamins. Moreover, it contains abundant minerals, such as calcium,
iron, magnesium, phosphorus, potassium and zinc (1). These compounds elicit beneficial
health effects, including increased blood flow, cancer suppression
and prevention of glaucoma, eye strain and lifestyle-related
diseases, such as obesity and diabetes mellitus (2,3).
Phytoestrogens are plant-derived substances that
exhibit effects similar to those of endogenous estrogens. Multiple
phytoestrogens, including isoflavones and resveratrol, have been
identified (4,5). Decreased estrogen secretion associated
with increased age and menopause increases risk of developing
disorders, including decreased blood vessel function, dyslipidemia
and osteoporosis (6-8).
Our previous studies showed that blackcurrant extract (BCE) and its
anthocyanins exert phytoestrogenic activity via signaling through
estrogen receptors α and β (9,10) and
alleviate some menopausal symptoms, including arteriosclerosis,
hair loss, skin aging and dyslipidemia (11-14).
Bone is remodeled by constantly being resorbed and
formed by osteoblasts. Notably, imbalance between bone resorption
and formation causes a decrease in bone density, leading to
osteoporosis (15). Moreover, bone
remodeling is a complex process involving several hormones,
including estrogen (16,17). Osteoporosis refers to a condition in
which bone mass decreases and bone structure deteriorates,
weakening bone strength and increasing susceptibility to fractures
(18,19). Considering that estrogen regulates
bone metabolism, osteoporosis is more likely to occur following
menopause-associated decrease in estrogen, a condition called
postmenopausal osteoporosis (20).
During early stage osteoporosis, patients present
almost no symptoms, being difficult to diagnose this condition.
Thus, it is important to consume foods rich in calcium, such as
fish, dairy products and seaweed during menopause. The intake of
phytoestrogens, such as equol, which is produced by metabolism of
soy isoflavones contained in soybeans and soybean foods by
intestinal bacteria, is effective in preventing postmenopausal
osteoporosis (21,22). Blackcurrant has also been shown to
reduce the risk of osteoporosis and improve trabecular bone mass in
young mice (23,24). Additionally, blackcurrant alleviates
osteoporosis in humans, and clinical trials are currently ongoing
to validate its efficacy (25).
Estrogen acts on both osteoclasts and osteoblasts;
its deficiency in menopause is hypothesized to accelerate bone
resorption by osteoclasts and decrease bone mass (26). Although blackcurrant has been shown
to inhibit osteoclastogenesis, studies on its effects on
osteogenesis are lacking (27).
Additionally, the mechanism by which blackcurrant alleviates
osteoporosis remains unclear. As osteoblasts are sensitive to
estrogen, the present study aimed to investigate the
phytoestrogenic effects of BCE on osteoblast proliferation and
differentiation using mouse pre-osteoblastic MC3T3-E1 cells.
Notably, these cells produce large amounts of collagen,
differentiate into osteoblasts and ultimately form bone (28-30).
In osteoblasts, expression of differentiation
markers, such as collagen type I (Col-I), alkaline
phosphatase (Alp), bone γ-carboxyglutamate protein
(Bglap) and runt-related transcription factor 2
(Runx2), increase depending on the extent of differentiation
(31). Osteoblasts in the late
stage of differentiation produce mineralized deposits (calcified
nodules) that can be stained with Alizarin Red (32,33).
To the best of our knowledge, the present study is the first to
assess the health effects of BCE on osteoblast differentiation.
Materials and methods
Reagents and cell culture
BCE powdered extract was CaNZac-35 (Koyo Mercantile
Co., Ltd.), containing a high concentration of polyphenols and
anthocyanins (37.6 and 38.0% w/w, respectively) (10). 17β-estradiol (E2) was purchased from
Sigma-Aldrich (Merck KGaA). The mouse pre-osteoblast cell line
MC3T3-E1 was obtained from the Health Science Research Resources
Bank (Osaka, Japan). MC3T3-E1 cells were maintained in α-MEM
(FUJIFILM Wako Pure Chemical Corporation) supplemented with 10%
(v/v) FBS (Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin and 100
µg/ml streptomycin (FUJIFILM Wako Pure Chemical Corporation). Cell
culture experiments were conducted at 37˚C in a humidified
incubator under 5% CO2.
Cell treatment
MC3T3-E1 cells (1x104 cells/well) were
seeded in six replicates in 96-well plates and cultured overnight
in α-MEM supplemented with 10% (v/v) FBS. The medium was replaced
with phenol red-free α-MEM supplemented with 5% (v/v)
charcoal-stripped FBS (Thermo Fisher Scientific, Inc.). Cells were
cultured for 48 h at 37˚C in the presence or absence of 0.2, 1.0,
5.0 or 20.0 µg/ml BCE or 10 nM E2. E2 was used as a positive
control to examine the phytoestrogenic effect of BCE on MC3T3-E1
cells (32). Treatment duration and
the E2 concentration of 10 nM were selected based on previous
studies (29,33). The morphology of MC3T3-E1 cells was
analyzed under a phase-contrast microscope (CK40; Olympus
Corporation; magnification, x100) with an Anyty™ digital microscope
camera (3R-DKMCO4; Three R Solution Corp. Japan).
Proliferation assay
Quantification of cell proliferation was performed
using Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Inc.)
according to the manufacturer's instructions. CCK-8 solution was
added to the wells, and incubated for 30 min at 37˚C. Absorbance
was measured at 450 nm using a Benchmark microplate reader (Bio-Rad
Laboratories, Inc.).
Alp assay
MC3T3-E1 cells (5x104 cells/well) were
seeded in duplicate in 24-well plates and cultured overnight as
aforementioned. The growth medium was replaced with phenol red-free
α-MEM, supplemented with 5% (v/v) charcoal-stripped FBS. The cells
were cultured for 72 h with BCE or 10 nM E2 as aforementioned,
based on previous studies (30,34).
After washing with 0.1 M Tris-HCl buffer (pH, 9.8), 100 µl 0.1%
(v/v) Triton X-100 in 0.1 M Tris-HCl buffer (pH, 9.8) was added to
the medium and the plates were stored at -80˚C. The plates were
rapidly thawed at 37˚C and assayed using the LabAssay™ ALP kit
(FUJIFILM Wako Pure Chemical Corporation). Protein concentrations
were determined using a Takara BCA Protein Assay kit (Takara Bio,
Inc.). Absorbance relative to Alp activity and protein
concentrations were measured at wavelengths of 405 and 570 nm,
respectively, using a Benchmark microplate reader (Bio-Rad
Laboratories, Inc.).
Quantification of total collagen
MC3T3-E1 cells (1x105 cells/well) were
seeded in duplicate in 12-well plates and cultured overnight in
α-MEM supplemented with 10% (v/v) FBS. The growth medium was
replaced with differentiation medium [phenol red-free α-MEM
supplemented with 5% (v/v) charcoal-stripped FBS and
Osteoblast-Inducer Reagent (Takara Bio, Inc.)], according to the
manufacturer's protocol. Cells were cultured in the presence or
absence of BCE or 10 nM E2, as aforementioned, and the medium was
replaced every 3 days. Collagen staining was performed on day 14
using a Sirius Red/Fast Green Collagen Staining kit (Iwai Chemicals
Co. Ltd.), according to the manufacturer's instructions. Cells were
washed with PBS, followed by the addition of 0.5 ml Kahle fixative
at 22˚C for 10 min. Dye solution was added to culture plates and
incubated at 22˚C for 30 min. Cells were rinsed with 0.5 ml
distilled water until the solution was colorless. Digital images
were acquired using a phase-contrast microscope (CK40;
magnification x40) with an Anyty™ digital microscope camera.
Following microscopy, the dye was eluted. The optical density (OD)
of the eluted dye solution was measured at 540 and 605 nm using a
spectrophotometer (U-5100; Hitachi High-Technologies Corporation).
The calculation formulas followed the kit manufacturer's
instructions: Collagen
(µg/section)=OD540-(OD605 x 0.291)/0.0378;
non-collagen protein (µg/section)=OD605/0.00204.
Reverse transcription-quantitative PCR
(RT-qPCR)
MC3T3-E1 (2x105 cells/well) were seeded
in 6-well plates and cultured overnight as aforementioned until 80%
confluent. The medium was replaced with phenol red- and serum-free
α-MEM with or without BCE or 10 nM E2, as aforementioned. After
incubating at 37˚C for 24 h, cells were washed twice with PBS.
Total RNA was extracted using the RNeasy Mini kit (Qiagen GmbH),
according to the manufacturer's instructions. RNA was
reverse-transcribed into cDNA using PrimeScript RT Master Mix
(Takara Bio, Inc.), according to the manufacturer's protocol.
Col-I, Alp, Bglap and Runx2 mRNA
expression levels were quantified via RT-qPCR using TB Green Premix
Ex Taq II (Tli RNaseH Plus; Takara Bio, Inc.). Thermocycling
conditions were as follows: 30 sec at 95˚C, followed by 40 cycles
of 5 sec at 95˚C and 30 sec at 60˚C. Transcription levels were
normalized to β-actin (Actb). Primers (5'→3') were as
follows: Col-I forward, GAGCGGAGTACTGGATCG and reverse,
GCTTCTTTTCCTTGGGGTT (31);
Alp forward, GATCATTCCCACGTTTTCACATT and reverse,
TTCACCGTCCACCACCTTGT (31);
Bglap forward, GCGCTCTGTCTCTCTGACCT and reverse,
AAGCAGGGTCAAGCTCACAT (31);
Runx2 forward, AGCGGCAGAATGGATGAGTC and reverse,
ACCAGACAACACCTTTGACG (35) and
Actb forward, CATCCGTAAAGACCTCTATGCCAAC and reverse,
ATGGAGCCACCGATCCACA (36). PCR
specificity was determined using melting curve analysis. All
samples were analyzed in duplicate. Relative gene expression was
calculated using the 2-ΔΔCq method (37).
Mineralization assessment
Calcified nodule formation was assessed using
Calcification Evaluation set (PG Research), according to the
manufacturer's instructions. Briefly, 1x105 cells/well
were seeded in duplicate in 12-well plates containing
differentiation medium (Osteoblast-Inducer Reagent). Cells were
treated as aforementioned with BCE or 10 nM E2 on day 21 and the
medium was replaced every 3 days. At the end of treatment, medium
was removed and wells were washed with PBS. Cells were fixed with
neutral buffered 10% formalin at 22˚C for 10 min, followed by
washing with distilled water. The cells were stained with 1.0 ml
Alizarin Red solution at 22˚C for 30 min. Micrographs were acquired
using a fluorescence microscope (FSX100; Olympus Corporation;
magnification, x40). After removing the water, 0.5 ml calcified
nodule lysate [5% (v/v) formic acid] was added and the plates were
stirred for 10 min at 22˚C to elute the dye. The absorbance of the
eluate was measured at 450 nm using a spectrophotometer
(U-5100).
Animals and treatments
Ovariectomized (OVX) and sham-operated female
Sprague-Dawley rats (OVX, n=6; sham-operated rats, n=6; age;
weight, ~240 g; 12 weeks; CLEA Japan, Inc.) were housed in plastic
cages in air-conditioned rooms (23˚C; 50% humidity) under a 12/12-h
light/dark cycle at the Institute for Animal Experiments of
Hirosaki University Graduate School of Medicine (Hirosaki, Japan).
All experimental procedures were approved by the Animal Research
Committee of Hirosaki University (approval no. G18003). Our
previous study showed that 3% BCE has phytoestrogenic effects in
rats (10). All rats were fed
AIN-93M diet, with or without 3% BCE (Oriental Yeast Co., Ltd.) and
were divided into three groups (n=3/group): Sham, OVX and OVX + 3%
BCE. All rats had free access to food and water. After 3 months,
the animals were euthanized by anesthesia with isoflurane
(induction, 5%; maintenance, 3%), followed by decapitation, then
femurs were excised and fixed in 10% (v/v) formaldehyde at 22˚C for
1 week, demineralized in 10% (v/v) EDTA-2Na (pH, 7.2) at 4˚C for 3
weeks and embedded in paraffin. Femur sections (4 µm) were
routinely passed through xylene and descending ethanol series
before Alp staining.
Alp staining
To measure femoral Alp activity, femurs were stained
with a TRAP/ALP kit (FUJIFILM Wako Pure Chemical Corporation),
according to the manufacturer's instructions. Nuclei were stained
with hematoxylin at 22˚C for 5 min. Specimens were examined and
photographed using a fluorescence microscope (FSX100; Olympus
Corporation; magnification, x40).
Statistical analysis
All data are expressed as mean ± SD from four
independent experiments. Graphs were generated using GraphPad Prism
(version 7.03; Dotmatics). Significant differences were determined
using Kruskal-Wallis analysis and Steel post hoc test via Bell
Curve in Excel software (version 3.2; Social Survey Research
Information Co., Ltd.). P<0.05 was considered to indicate a
statistically significant difference.
Results
BCE promotes MC3T3-E1 cell
proliferation
The present study investigated the effect of BCE on
MC3T3-E1 cell proliferation. Cells were treated with 0.2, 1.0, 5.0
or 20.0 µg/ml BCE, which showed phytoestrogenic effects in our
previous studies (12,13) or 10 nM E2 as a positive control.
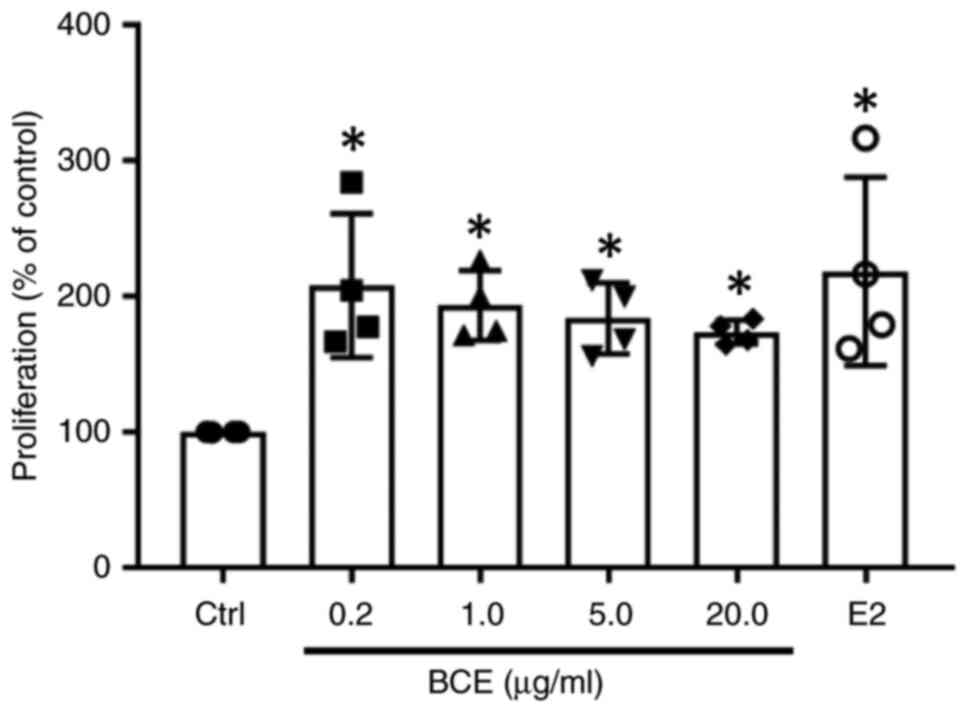
Cell proliferation increased significantly (P<0.05) after
treatment with all concentrations of BCE or 10 nM E2 (Fig. 1). However, there was no notable
difference in cell morphology following treatment with BCE or E2
(Fig. S1).
BCE enhances Alp activity in MC3T3-E1
cells
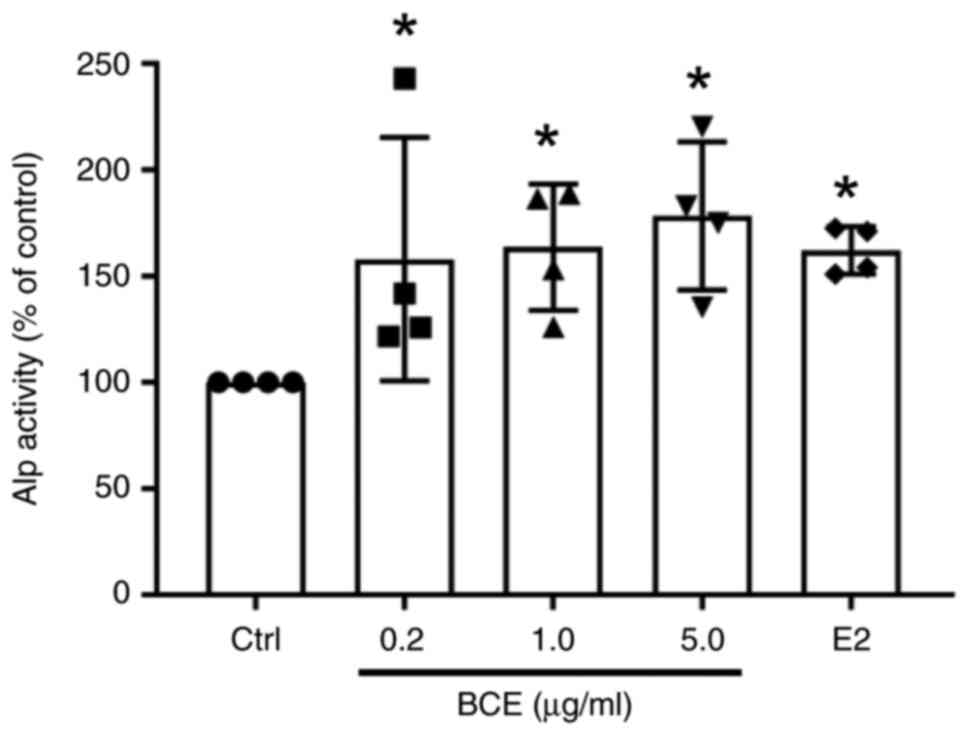
MC3T3-E1 cells were treated with 0.2, 1.0 or 5.0
µg/ml BCE or 10 nM E2 and cultured for 72 h. Alp activity increased
significantly (P<0.05) in a dose-dependent manner following
treatment with BCE and increased significantly (P<0.05) upon
treatment with E2 (Fig. 2).
BCE enhances total collagen production
in MC3T3-E1 cells
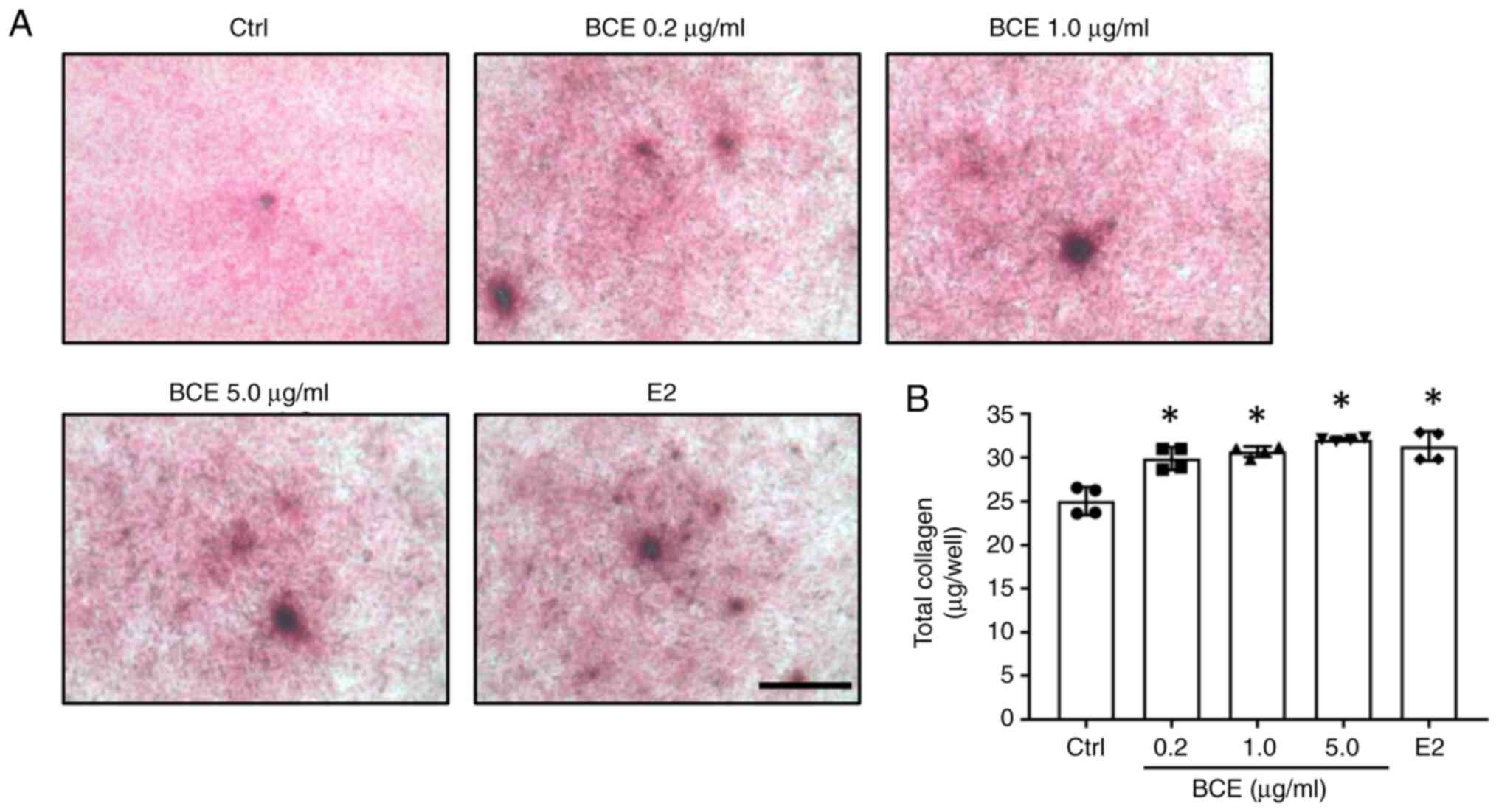
MC3T3-E1 cells were treated with 0.2, 1.0 or 5.0
µg/ml BCE or 10 nM E2 and cultured for 14 days. Total collagen was
quantified using Sirius Red staining. Total collagen increased
significantly (P<0.05) after treatment with all concentrations
of BCE and 10 nM E2 (Fig. 3).
BCE upregulates expression of
osteogenic markers in MC3T3-E1 cells
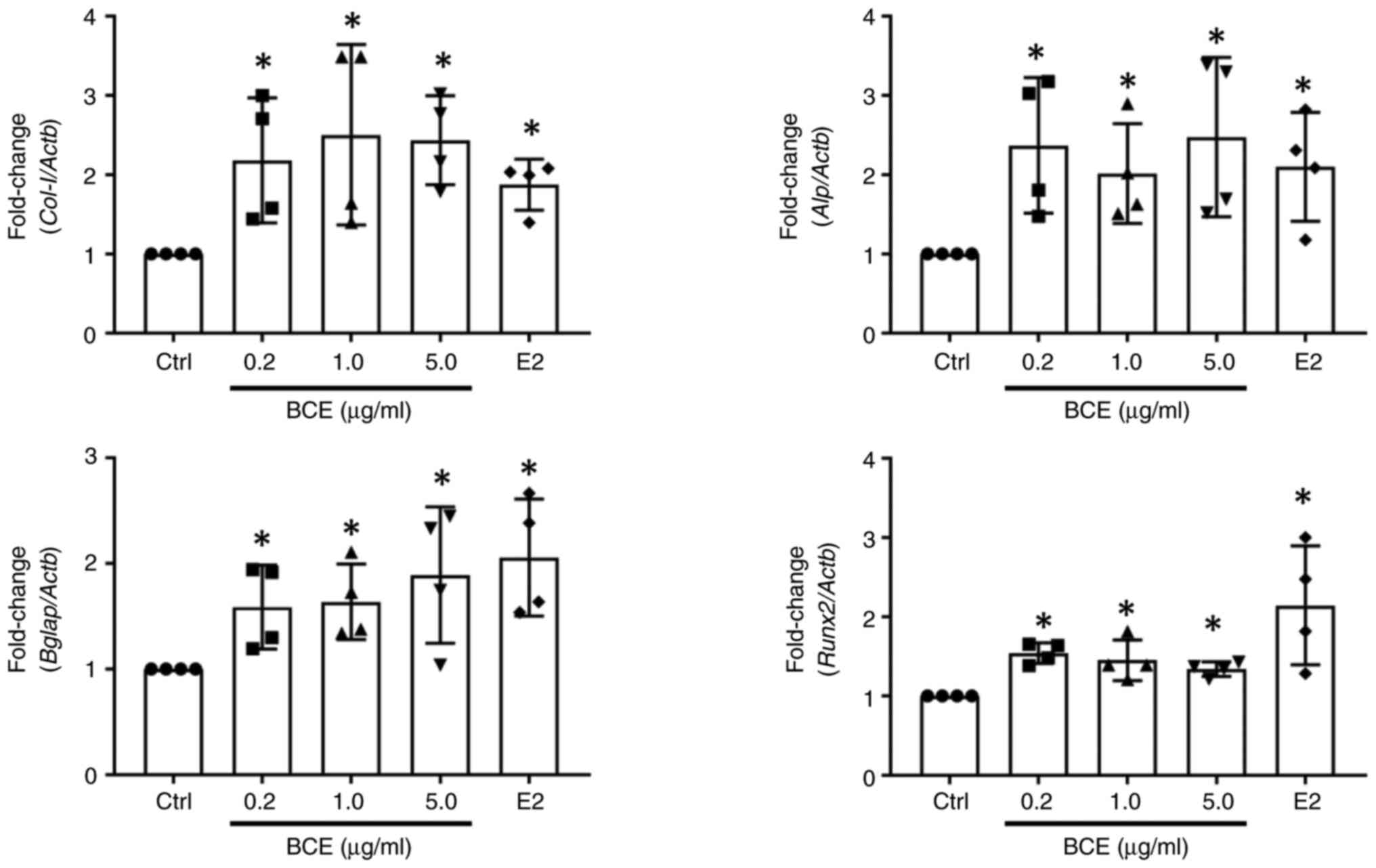
MC3T3-E1 cells were incubated with 0.2, 1.0 or 5.0
µg/ml BCE or 10 nM E2 for 24 h. Bone differentiation marker
expression was measured using RT-qPCR. Col-I, Alp,
Bglap and Runx2 expression increased significantly
(P<0.05) following treatment with all concentrations of BCE and
10 nM E2 (Fig. 4).
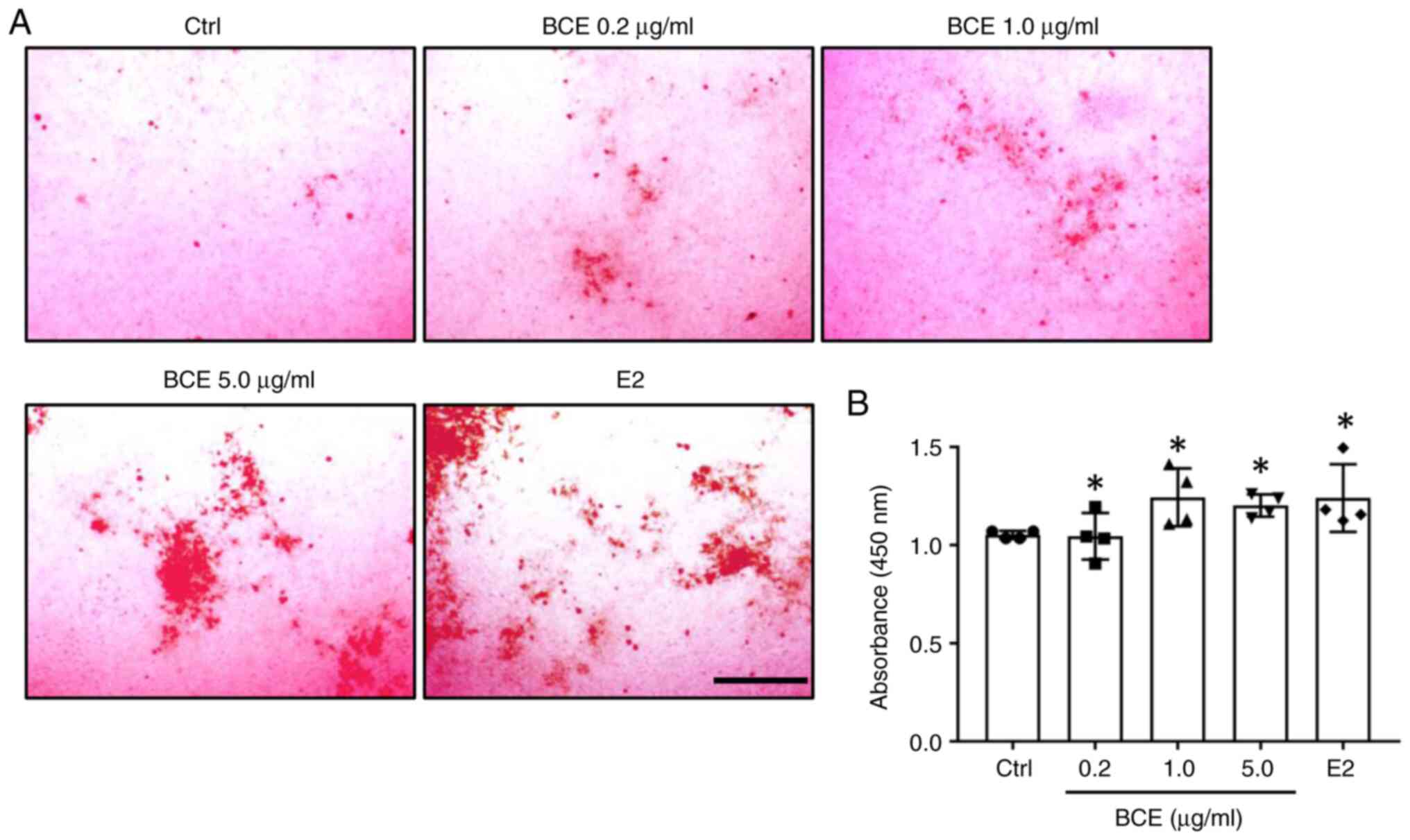
BCE promotes mineralization in
MC3T3-E1 cells
MC3T3-E1 cells were treated with 0.2, 1.0 or 5.0
µg/ml BCE or 10 nM E2 and cultured for 21 days. Cells were stained
with Alizarin Red to assess the extent of mineralization, which
revealed the presence of calcified nodules (Fig. 5A). Levels of calcified nodules
increased significantly (P<0.05) following treatment with all
concentrations of BCE and 10 nM E2 (Fig. 5B).
BCE induces osteoblast differentiation
in vivo
We investigated whether BCE induces osteoblast
differentiation in the femoral tissue of OVX rats, which were used
as a menopausal model. As Alp activity increases in the region
surrounding the epiphysis, where active osteoblast differentiation
occurs, rat femurs were stained to assess Alp activity. Compared
with that in the Sham group, Alp staining intensity was weak in the
epiphyses of rats in the OVX group. However, Alp staining intensity
was stronger in the epiphyses of OVX rats treated with 3% BCE than
in those of untreated OVX rats (Fig.
S2, arrows).
Discussion
BCE inhibits osteoclast differentiation (27); however, its effects on osteoblasts
are unknown. Therefore, the present study focused on the effect of
BCE on differentiation of MC3T3-E1 pre-osteoblasts into
osteoblasts.
Administration of phytoestrogens or 10 nM E2
significantly enhances MC3T3-E1 cell proliferation (28,38).
Here, BCE or E2 treatment increased the proliferation of MC3T3-E1
cells, which was consistent with previous findings (28,38).
Overall, the increase in MC3T3-E1 cell proliferation may be due to
the phytoestrogen activity of BCE.
Estrogen activity has been shown to increase Alp
activity and collagen secretion in MC3T3-E1 cells (31). In the present study, BCE increased
Alp activity and collagen secretion in MC3T3-E1 cells in a
concentration-dependent manner. Col-I, Alp,
Bglap and Runx2 are known osteoblast differentiation
markers (31). Here, BCE and E2
increased the expression of all four genes, which may be due to
phytoestrogen activity.
Consistent with the present findings, several
studies have shown that anthocyanin induces osteoblast
differentiation, with increased expression of Col-I,
Alp, Bglap and Runx2 (33,39,40).
Runx2 is a transcription factor required for osteoblast
differentiation and is expressed at an early stage of
differentiation (41). By contrast,
Col-I, Alp and Bglap are the earliest
biomarkers of mature osteoblast differentiation (42,43).
In addition to increased expression of these genes, there was an
increase in Alp activity and collagen protein secretion in the
present study. Furthermore, there was an increase in the levels of
calcified nodules. Collectively, these results suggested that BCE
induced osteogenesis and differentiation of pre-osteoblasts into
osteoblasts.
Our previous study revealed the half-maximal
inhibitory concentration for estrogen receptor α is ~10 nM for E2
and ~5 µg/ml for BCE; relative binding affinity of BCE is 0.06%
relative to E2(10). Therefore, BCE
may have significantly lower estrogenic activity in MC3T3-E1 cell
than 10 nM E2.
Although BCE has been shown to be effective in
animal models of osteoporosis (23,24,27),
to the best of our knowledge, there has been no study on the effect
of BCE on osteoblast differentiation in vivo. As the present
in vivo experiment was simple, more detailed studies using
microcomputed tomography, are required. Additionally, it is key to
isolate osteoblasts from BCE-treated and untreated OVX rats to
examine the effect of BCE on osteoblast differentiation. Moriwaki
et al (27) reported that
bilberry, BCE and their anthocyanins prevent osteoporosis by
inhibiting excessive osteoclastogenesis. Although osteoblasts were
also used in the aforementioned study, BCE treatment caused no
change in cell differentiation. Research findings indicate that
estrogen and phytoestrogen enhance osteoblast differentiation
(30,31,34);
however, results may differ depending on the estrogen sensitivity
of osteoblasts. In the present study, phytoestrogen effects were
observed in estrogen-sensitive MC3T3-E1 cells. Experiments using
estrogen receptor inhibitors were not performed in the present
study as our previous studies demonstrated that BCE has
phytoestrogenic effects (9,10). However, it is unclear which
molecules of BCE exhibit phytoestrogenic effects on MC3T3-E1 cells.
Therefore, detailed component analysis of BCE and experiments using
estrogen receptor inhibitors against its candidate molecules are
necessary in future. Additionally, it is important to verify the
effect of different compounds in BCE on osteoclasts. Polyphenols
include anthocyanin-rich plants and attenuate osteoporosis
(24,27). Notably, their mechanism is reported
to include the activation of signaling via the Wnt/β-catenin,
transforming growth factor-β/bone morphogenetic protein 2,
mitogen-activated protein kinase and PI3K/AKT pathways (44,45).
The present study focused on phytoestrogen activity. However, the
intracellular signaling mechanism is complex, as phytoestrogens
also activate PI3K/AKT, Src/ERK1/2, and nuclear factor κB via the
estrogen receptor (46,47). Moreover, BCE likely interacts with
these factors via estrogen receptors (10). A microarray analysis of breast
cancer MCF7 cells with high expression of estrogen receptors showed
that BCE activates multiple pathways (10).
Menopause is associated with increased risk of
osteoporosis owing to decreased estrogen secretion. Although
hormone replacement therapy is available for treatment of
osteoporosis, it is associated with complications, including
increased risk of breast cancer (48,49).
Research findings indicate that the intake of phytoestrogens, such
as soy isoflavones and equol, prevents and ameliorates osteoporosis
(21,22). However, as phytoestrogens are not
suitable for people who are allergic to soybeans, the
anti-osteoporotic effect of blackcurrant is key. Pre-clinical and
clinical trials are required to validate the present findings in
humans.
In conclusion, BCE and E2 promoted cell
proliferation, increased Alp activity and collagen production,
upregulated expression of osteoblast differentiation markers, and
enhanced mineralization and differentiation of pre-osteoblasts into
osteoblasts. Overall, although BCE has phytoestrogenic effects, the
present data suggest that it also affected osteoblast
differentiation. Collectively, the results suggested that BCE may
ameliorate osteoporosis during menopause.
Supplementary Material
Morphology of MC3T3-E1 cells. The
morphology did not change after treatment with BCE and 10 nM E2 for
48 h. Scale bar, 500 μm. Ctrl, control; BCE, blackcurrant
extract; E2, 17β-estradiol.
Light micrographs of alkaline
phosphatase staining of the femur. Representative alkaline
phosphatase staining images of the femur sections of rats in (A)
sham, (B) OVX and (C) OVX + 3% BCE groups. Arrows indicate
epiphyses. Scale bar, 200 μm. OVX, ovariectomized; BCE,
blackcurrant extract.
Acknowledgements
Not applicable.
Funding
Funding: The present study was partially supported by the Japan
Society for the Promotion of Science KAKENHI (grant no.
20K02402).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NN, IO and KH designed the study, performed the
experiments and analyzed the data. NN wrote the manuscript. IO
edited the manuscript. All authors have read and approved the final
manuscript. NN and KH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Animal
Research Committee of Hiroaki University (Hirosaki, Japan; approval
no. G18003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Golovinskaia O and Wang CK: Review of
functional and pharmacological activities of berries. Molecules.
26(3904)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cortez RE and Gonzalez de Mejia E:
Blackcurrants (Ribes nigrum): A review on chemistry,
processing, and health benefits. J Food Sci. 84:2387–2401.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gopalan A, Reuben SC, Ahmed S, Darvesh AS,
Hohmann J and Bishayee A: The health benefits of blackcurrants.
Food Funct. 3:795–809. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lephart ED: Phytoestrogens (resveratrol
and equol) for estrogen-deficient skin-controversies/misinformation
versus anti-aging in vitro and clinical evidence via
nutraceutical-cosmetics. Int J Mol Sci. 22(11218)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nguyen M and Osipo C: Targeting breast
cancer stem cells using naturally occurring phytoestrogens. Int J
Mol Sci. 23(6813)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jia M, Dahlman-Wright K and Gustafsson JA:
Estrogen receptor alpha and beta in health and disease. Best Pract
Res Clin Endocrinol Metab. 29:557–568. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lobo RA: Metabolic syndrome after
menopause and the role of hormones. Maturitas. 60:10–18.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Prisby RD: Mechanical, hormonal and
metabolic influences on blood vessels, blood flow and bone. J
Endocrinol. 235:R77–R100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nanashima N, Horie K and Maeda H:
Phytoestrogenic activity of blackcurrant anthocyanins is partially
mediated through estrogen receptor beta. Molecules.
23(74)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nanashima N, Horie K, Tomisawa T, Chiba M,
Nakano M, Fujita T, Maeda H, Kitajima M, Takamagi S, Uchiyama D, et
al: Phytoestrogenic activity of blackcurrant (Ribes nigrum)
anthocyanins is mediated through estrogen receptor alpha. Mol Nutr
Food Res. 59:2419–2431. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Horie K, Nanashima N and Maeda H:
Phytoestrogenic effects of blackcurrant anthocyanins increased
endothelial nitric oxide synthase (eNOS) expression in human
endothelial cells and ovariectomized rats. Molecules.
24(1259)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nanashima N and Horie K: Blackcurrant
extract with phytoestrogen activity alleviates hair loss in
ovariectomized rats. Molecules. 24(1272)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nanashima N, Horie K, Maeda H, Tomisawa T,
Kitajima M and Nakamura T: Blackcurrant anthocyanins increase the
levels of collagen, elastin, and hyaluronic acid in human skin
fibroblasts and ovariectomized rats. Nutrients.
10(495)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nanashima N, Horie K, Yamanouchi K,
Tomisawa T, Kitajima M, Oey I and Maeda H: Blackcurrant (Ribes
nigrum) extract prevents dyslipidemia and hepatic steatosis in
ovariectomized rats. Nutrients. 12(1541)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang Y, Liang J, Liu P, Wang Q, Liu L and
Zhao H: The RANK/RANKL/OPG system and tumor bone metastasis:
Potential mechanisms and therapeutic strategies. Front Endocrinol
(Lausanne). 13(1063815)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheng CH, Chen LR and Chen KH:
Osteoporosis due to hormone imbalance: An overview of the effects
of estrogen deficiency and glucocorticoid overuse on bone turnover.
Int J Mol Sci. 23(1376)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tunheim EG, Skallevold HE and Rokaya D:
Role of hormones in bone remodeling in the craniofacial complex: A
review. J Oral Biol Craniofac Res. 13:210–217. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Okagu IU, Aham EC, Ezeorba TPC, Ndefo JC,
Aguchem RN and Udenigwe CC: Osteo-modulatory dietary proteins and
peptides: A concise review. J Food Biochem.
46(e14365)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sfeir JG, Drake MT, Khosla S and Farr JN:
Skeletal aging. Mayo Clin Proc. 97:1194–1208. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou T, Gai Z, Gao X and Li L: The
potential mechanism of exercise combined with natural extracts to
prevent and treat postmenopausal osteoporosis. J Healthc Eng.
2021(2852661)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Harahap IA and Suliburska J: Probiotics
and isoflavones as a promising therapeutic for calcium status and
bone health: A narrative review. Foods. 10(2685)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mayo B, Vázquez L and Flórez AB: Equol: A
bacterial metabolite from the daidzein isoflavone and its presumed
beneficial health effects. Nutrients. 11(2231)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sakaki J, Melough M, Lee SG, Kalinowski J,
Koo SI, Lee SK and Chun OK: Blackcurrant supplementation improves
trabecular bone mass in young but not aged mice. Nutrients.
10(1671)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zheng X, Mun S, Lee SG, Vance TM, Hubert
P, Koo SI, Lee SK and Chun OK: Anthocyanin-rich blackcurrant
extract attenuates ovariectomy-induced bone loss in mice. J Med
Food. 19:390–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nosal BM, Sakaki JR, Macdonald Z, Mahoney
K, Kim K, Madore M, Thornton S, Tran TDB, Weinstock G, Lee ECH and
Chun O: Blackcurrants reduce the risk of postmenopausal
osteoporosis: A pilot double-blind, randomized, placebo-controlled
clinical trial. Nutrients. 14(4971)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Oursler MJ, Landers JP, Riggs BL and
Spelsberg TC: Oestrogen effects on osteoblasts and osteoclasts. Ann
Med. 25:361–371. 1993.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moriwaki S, Suzuki K, Muramatsu M, Nomura
A, Inoue F, Into T, Yoshiko Y and Niida S: Delphinidin, one of the
major anthocyanidins, prevents bone loss through the inhibition of
excessive osteoclastogenesis in osteoporosis model mice. PLoS One.
9(e97177)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahmad Hairi H, Jamal JA, Aladdin NA,
Husain K, Mohd Sofi NS, Mohamed N, Mohamed IN and Shuid AN:
Demethylbelamcandaquinone B (Dmcq B) is the active compound of
marantodes pumilum var. Alata (Blume) kuntze with osteoanabolic
activities. Molecules. 23(1686)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim M, Lim J, Lee JH, Lee KM, Kim S, Park
KW, Nho CW and Cho YS: Understanding the functional role of
genistein in the bone differentiation in mouse osteoblastic cell
line MC3T3-E1 by RNA-seq analysis. Sci Rep. 8(3257)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo D, Kang L, Ma Y, Chen H, Kuang H,
Huang Q, He M and Peng W: Effects and mechanisms of
8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells
RAW264.7. Food Sci Nutr. 2:341–350. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jiang X, Chen W, Shen F, Xiao W, Guo H, Su
H, Xiu J and Sun W: Pinoresinol promotes MC3T3-E1 cell
proliferation and differentiation via the cyclic AMP/protein kinase
A signaling pathway. Mol Med Rep. 20:2143–2150. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Czekanska EM, Stoddart MJ, Richards RG and
Hayes JS: In search of an osteoblast cell model for in vitro
research. Eur Cell Mater. 24:1–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mao W, Huang G, Chen H, Xu L, Qin S and Li
A: Research progress of the role of anthocyanins on bone
regeneration. Front Pharmacol. 12(773660)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim H, Tabata A, Tomoyasu T, Ueno T,
Uchiyama S, Yuasa K, Tsuji A and Nagamune H: Estrogen stimuli
promote osteoblastic differentiation via the subtilisin-like
proprotein convertase PACE4 in MC3T3-E1 cells. J Bone Miner Metab.
33:30–39. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cai W, Sun B, Song C, Liu F, Wu Z and Liu
Z: Resveratrol induces proliferation and differentiation of mouse
pre-osteoblast MC3T3-E1 by promoting autophagy. BMC Complement Med
Ther. 23(121)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Norikura T, Sasaki Y, Kojima-Yuasa A and
Kon A: Glyoxylic Acid, an α-keto acid metabolite derived from
glycine, promotes myogenesis in C2C12 cells. Nutrients.
15(1763)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiao HH, Fung CY, Mok SK, Wong KC, Ho MX,
Wang XL, Yao XS and Wong MS: Flavonoids from Herba epimedii
selectively activate estrogen receptor alpha (ERα) and stimulate
ER-dependent osteoblastic functions in UMR-106 cells. J Steroid
Biochem Mol Biol. 143:141–151. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu B, Chen L, Chen Y, Zhang Z, Wang X and
Zhou B: Cyanidin-3-glucoside regulates osteoblast differentiation
via the ERK1/2 signaling pathway. ACS Omega. 6:4759–4766.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Park KH, Gu DR, So HS, Kim KJ and Lee SH:
Dual role of cyanidin-3-glucoside on the differentiation of bone
cells. J Dent Res. 94:1676–1683. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Matsushita Y, Ono W and Ono N: Growth
plate skeletal stem cells and their transition from cartilage to
bone. Bone. 136(115359)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fusaro M, Crepaldi G, Maggi S, D'Angelo A,
Calo L, Miozzo D, Fornasieri A and Gallieni M: Bleeding, vertebral
fractures and vascular calcifications in patients treated with
warfarin: Hope for lower risks with alternative therapies. Curr
Vasc Pharmacol. 9:763–769. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shao X, Cao X, Song G, Zhao Y and Shi B:
Metformin rescues the MG63 osteoblasts against the effect of high
glucose on proliferation. J Diabetes Res.
2014(453940)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Setchell KDR and Lydeking-Olsen E: Dietary
phytoestrogens and their effect on bone: Evidence from in vitro and
in vivo, human observational, and dietary intervention studies. Am
J Clin Nutr. 78 (3 Suppl):593S–609S. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Torre E: Molecular signaling mechanisms
behind polyphenol-induced bone anabolism. Phytochem Rev.
16:1183–1226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Arnal JF, Lenfant F, Metivier R, Flouriot
G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P,
Katzenellenbogen B and Katzenellenbogen J: Membrane and nuclear
estrogen receptor alpha actions: From tissue specificity to medical
implications. Physiol Rev. 97:1045–1087. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Canivenc-Lavier MC and Bennetau-Pelissero
C: Phytoestrogens and health effects. Nutrients.
15(317)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dinger J, Bardenheuer K and Heinemann K:
Drospirenone plus estradiol and the risk of serious cardiovascular
events in postmenopausal women. Climacteric. 19:349–356.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dinger J, Do Minh T and Heinemann K:
Impact of estrogen type on cardiovascular safety of combined oral
contraceptives. Contraception. 94:328–339. 2016.PubMed/NCBI View Article : Google Scholar
|