Introduction
Non-occlusive mesenteric ischaemia (NOMI) refers to
irreversible intestinal ischemia and necrosis in the absence of
organic obstruction of the mesenteric blood vessels. In cases of
delayed diagnosis, the prognosis is poor and the mortality rate is
58-70%, being the highest among those with acute mesenteric
ischaemia (AMI) (1). The risk
factors for this condition include heart disease, sepsis and the
use of catecholamines and digitalis (2). The onset mechanism of NOMI involves a
reduction in intestinal blood flow due to factors such as decreased
cardiac output, reduced circulating blood volume, mesenteric
contraction, mesenteric vasospasm caused by sepsis or the use of
vasoconstrictive therapeutic agents. This pathological condition
leads to diminished intestinal blood flow, ultimately resulting in
intestinal ischaemia (3,4). In the early stages of onset, 20-30% of
the patients do not complain of abdominal pain. When abdominal pain
does occur, its location and severity can vary widely. However, as
ischaemia progresses, patients typically develop persistent
abdominal pain, melena and flatulence. With further disease
progression, symptoms of peritoneal irritation, such as muscular
guarding and Blumberg's sign, become evident (2,5).
Treatment with vasodilators is considered useful if there is no
intestinal necrosis; however, surgery is required if the intestinal
tract has reached an irreversible ischaemic state (2,5,6).
Therefore, early diagnosis of intestinal necrosis, which indicates
the need for surgery, is crucial. NOMI can manifest as a shock and
can be fatal, requiring immediate surgical treatment (2). The current study reported a case in
which early diagnosis of NOMI allowed for emergency surgery,
ultimately saving the patient's life.
Case report
The patient first presented at Toyama University
Hospital (Toyama, Japan) in January 2022 with the chief complaint
of a painful tumour in the right cheek without an ulcer. The tumour
was elastic, firm and immobile, measuring 65x50 mm. Right maxillary
carcinoma (cT4bN1M0) was confirmed according to imaging and
histopathological examinations. The patient's medical history
included cerebral haemorrhage (left paresis), early gastric cancer,
colon polyps, hypertension and dementia. Since the tumour was
deemed unresectable, paclitaxel, carboplatin and cetuximab (PCE)
was administered as induction drug therapy. Although drug therapy
was successful and CT revealed the lesion was observed to shrink
after the first course of PCE therapy, the patient visited the
emergency department of our hospital with chief complaints of
melena and abdominal pain 4 days after day 1 of the second course
of PCE therapy. Upon transportation to our hospital, the patient's
Glasgow Coma Scale level of consciousness was E4V4M6 (Score: 14,
mild) (7), oxygen saturation was
90% (normal range, 96-99% under room air), blood pressure was 92/52
mmHg (normal value, 140/90 mmHg) and the heart rate was 111 bpm
(normal range, 60-100 bpm); furthermore, the patient experienced a
peripheral cold sensation. In addition, the abdomen was flat,
elastic and soft with no tenderness, and blood gas analysis
revealed pH of 7.385 (normal range, 7.35-7.45) and lactic acid
level of 4.8 mmol/l (normal range, 0.5-1.6 mmol/l). Based on these
findings, the patient was diagnosed with circulatory failure due to
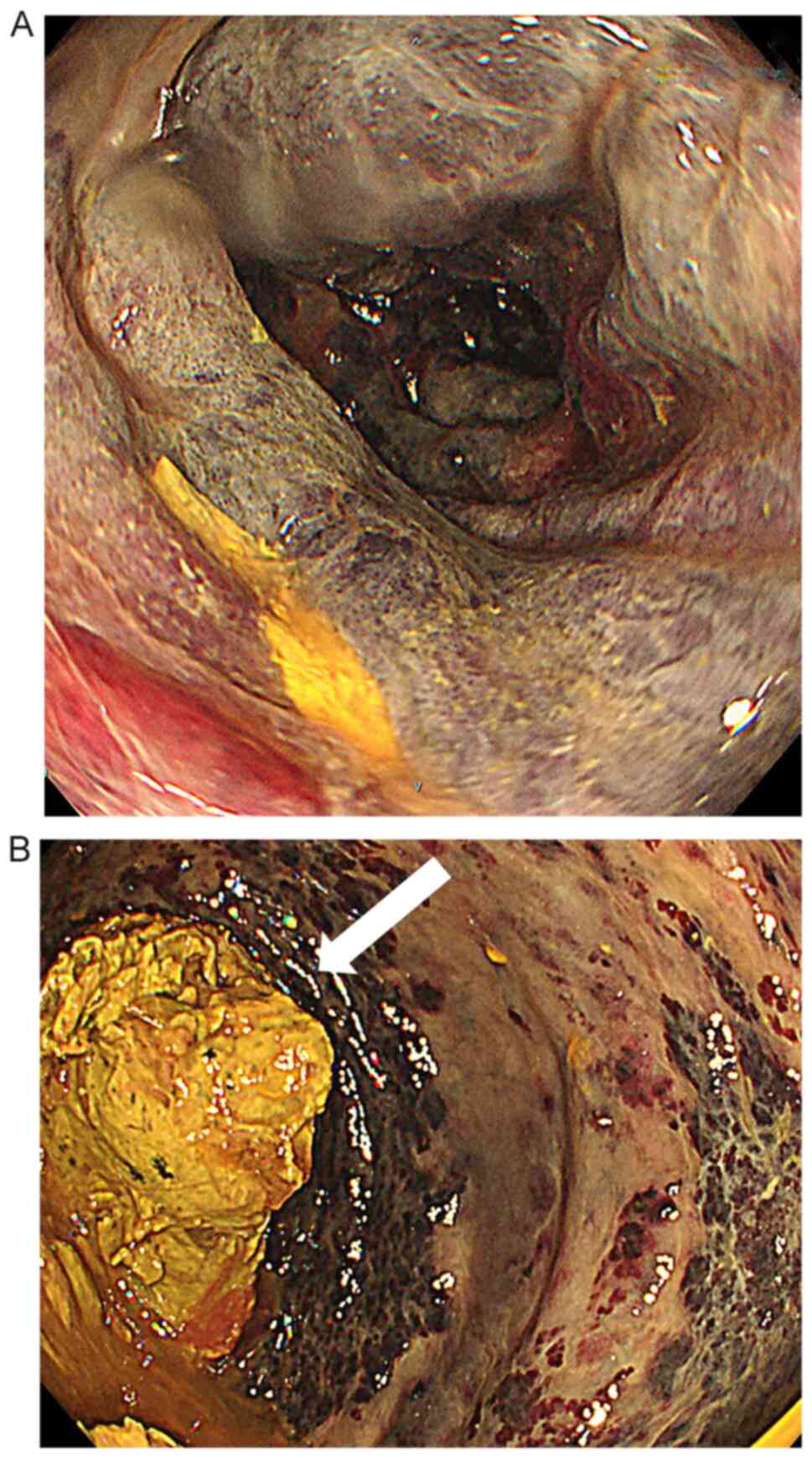
bleeding. Lower gastrointestinal endoscopy, performed to
investigate the bleeding points, revealed oedema and dark purple
discoloration of the intestinal mucosa, as well as a large amount
of faeces in the rectum (Fig. 1).
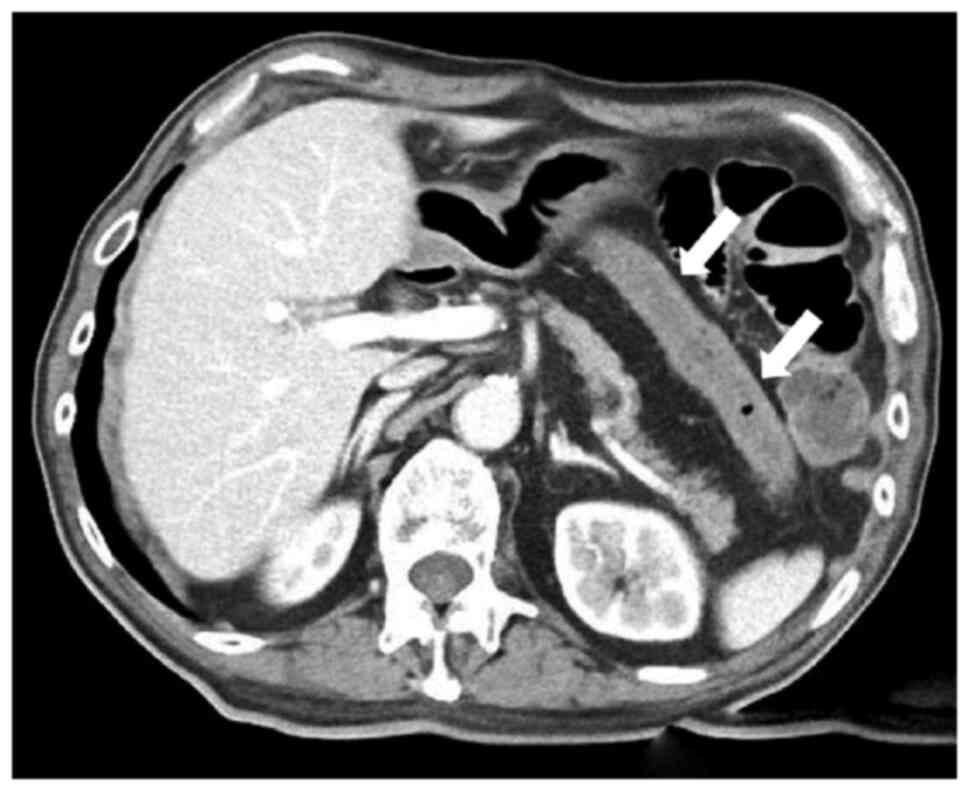
Acute intestinal ischaemia was diagnosed and contrast-enhanced CT
was performed to check for arterial occlusion due to the thrombus.
Although the images showed ischaemia from the transverse to
descending colon (Fig. 2), no
thrombus was found in the superior mesenteric artery. At that time,
the abdomen was slightly hard and tender. Based on these findings,
the patient was diagnosed with NOMI. Emergency surgery, including
colectomy (from the sigmoid colon to the sigmoid rectum) and
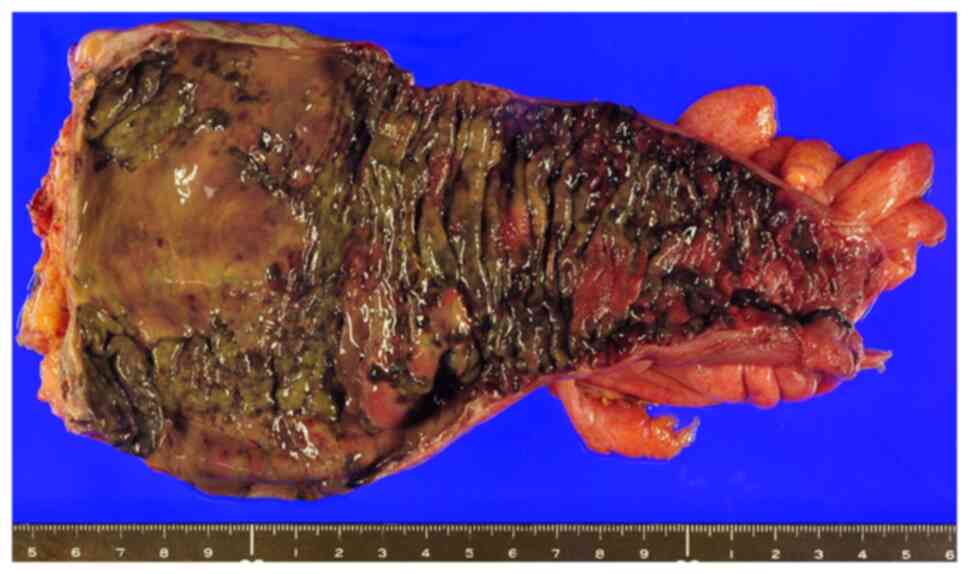
colostomy augmentation, was performed on the same day. The resected
specimen showed full-thickness necrosis of the sigmoid rectum
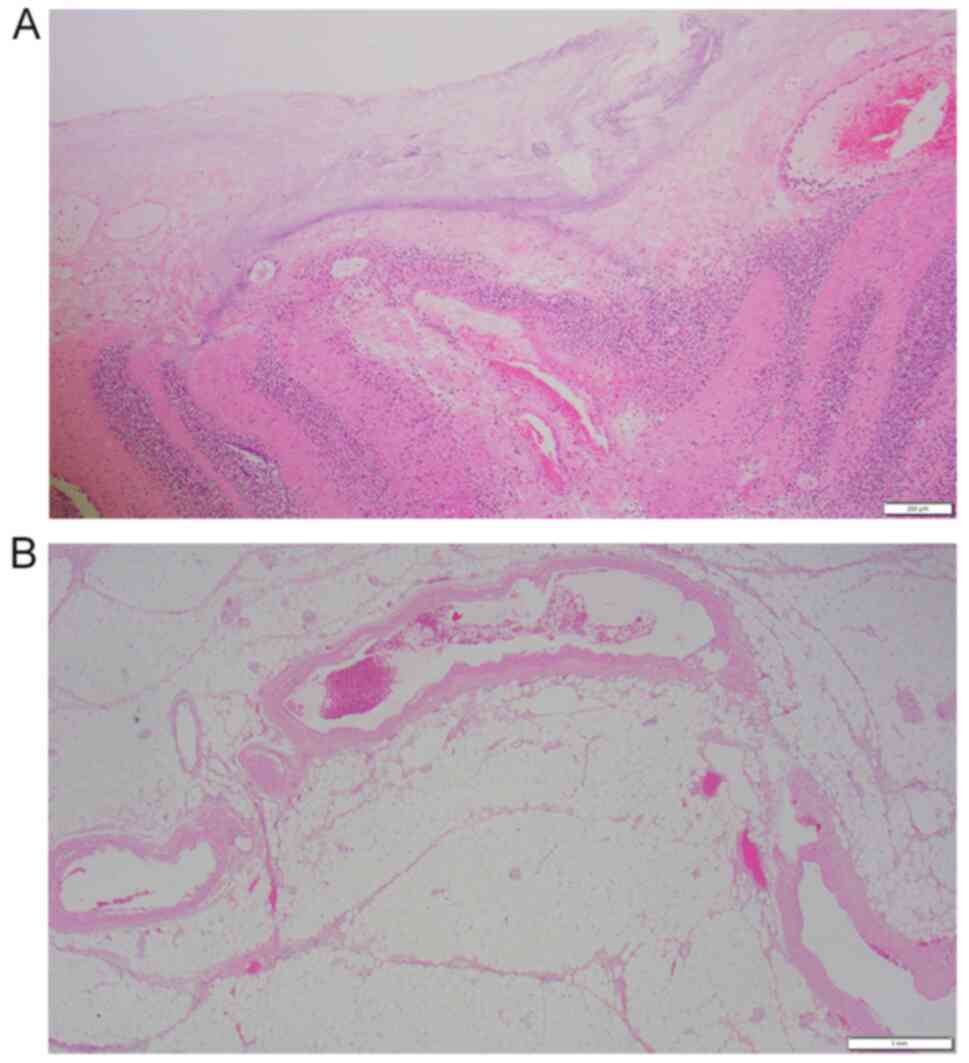
(Fig. 3). Histopathological
examination performed according to standard procedures showed
massive necrosis of the colon and proliferation of neutrophils. No
thrombi were observed in the blood vessels directly beneath the
necrotic mucosa, and the lumen remained patent (Fig. 4). Thereafter, the patient's general
condition improved and no further increase of the lesion in the
right maxillary region was observed. However, owing to a decline in
cognitive function and the presence of aspiration pneumonia, the
patient's performance status (8)
decreased from 2 to 3 and he was transferred to a recuperation
facility for palliative care. The patient's subsequent progress is
unknown.
Discussion
NOMI was first reported in 1958 by Ende (9) in a patient with intestinal necrosis
and heart failure. NOMI is known to cause ischaemia or necrosis of
the intestinal tract, despite the absence of organic obstruction of
the main mesenteric blood vessels. Heer et al (10) defined NOMI as follows: i) No
obstruction in the mesenteric arteries and veins in areas of
intestinal necrosis; ii) discontinuous necrosis and ischaemic
changes in the intestinal tract; and iii) histopathological
findings showing the presence of bleeding and necrosis. However, it
is defined by the lack of fibrin thrombus in the venules (10). The onset mechanism of NOMI involves
a reduction in intestinal blood flow due to factors such as
decreased cardiac output, reduced circulating blood volume,
mesenteric contraction and mesenteric vasospasm caused by sepsis or
the use of vasoconstrictive therapeutic agents. This pathological
condition leads to diminished intestinal blood flow, ultimately
resulting in intestinal ischaemia (3,4).
Furthermore, rapid acceleration of intestinal peristalsis and an
increase in intestinal pressure caused by faecal impaction or enema
has been reported to cause intestinal ischaemia (11). The risk factors for NOMI include
cardiovascular disease, myocardial infarction, sepsis, arrhythmia
and the use of catecholamines or digitalis (2). A search in PubMed (https://pubmed.ncbi.nlm.nih.gov) and J-stage
(https://www.jstage.jst.go.jp) for the
search terms (NOMI and chemotherapy) retrieved reports of only 15
cases of NOMI due to chemotherapy (Table I) (5,12-21).
The reason why most of the reported cases are Japanese is unclear
and further investigation is needed. Docetaxel was used in six
cases, paclitaxel in three cases and taxane-based anticancer drugs
were used in nine cases. The onset of NOMI after chemotherapy has
been linked to several factors: Mucosal damage due to the
inhibition of epithelial cell division and proliferation, a
pharmacological effect of taxane-based anticancer drugs (22); and vascular system damage resulting
from the inhibition of vascular smooth muscle cell proliferation
and migration and neointimal accumulation (23). The intestinal mucosa repeatedly
regenerates every 3rd to 4th day and intestinal mucosal damage
appears after the 3rd to 4th day of administration due to the
suppression of intestinal epithelial cell division and
proliferation by taxane-based anticancer drugs. The increased
intestinal pressure caused by chemotherapy-induced constipation
contributes to the onset of NOMI (5). Taxane-based anticancer drugs have been
approved for use against a wide range of malignant tumours,
including ovarian, breast and head and neck cancers. Intestinal
disorders such as gastrointestinal necrosis, perforation, bleeding,
ulcers and severe enteritis have been reported, although at a low
incidence (<0.1%) (24).
However, in the case of ischaemic colitis due to taxane-based
anticancer drugs, drug discontinuation has been found to ameliorate
the symptoms; in such cases, the causative drug should not be
re-administered (25). Seewaldt
et al (26) reported that
paclitaxel-induced intestinal necrosis is caused not only by
toxicity to the intestinal epithelium, but also by a history of
laparotomy, cancer progression, intraperitoneal radiotherapy and
chemotherapy. Intestinal necrosis occurs because of the effects of
paclitaxel on the intestinal epithelium in the intestinal tract,
where mucosal damage occurs. In the present case, the patient had a
history of surgery for colonic polyps and the necrotic segment of
the colon that was resected was the same segment previously
containing the polyps. Therefore, intestinal mucosal damage had
already occurred. In this case, the administration of anticancer
drugs was thought to be the cause of intestinal necrosis.
Furthermore, lower gastrointestinal endoscopy revealed a large
amount of stool, and increased intestinal pressure owing to
constipation was considered a contributing factor to the onset of
NOMI. In cases of delayed diagnosis, the prognosis is poor, with a
mortality rate of 58-70%, being the highest for those with AMI
(1). The underlying reason for this
is the lack of distinctive clinical symptoms, leading to a delayed
diagnosis (27). In the early
stages of onset, 20-30% of the patients do not complain of
abdominal pain. When abdominal pain does occur, its location and
severity can vary widely. However, as ischaemia progresses,
patients typically develop persistent abdominal pain, melena and
flatulence. With further disease progression, symptoms of
peritoneal irritation, such as muscular guarding and Blumberg's
sign, become evident (2,5). Blood tests show elevated levels of
C-reactive protein, aspartate aminotransferase, alanine
aminotransferase, creatine phosphokinase, lactate dehydrogenase and
lactate, as well as metabolic acidosis, but these are not specific
to the diagnosis of NOMI (2). The
usefulness of CT in the diagnosis of NOMI has been reported
(29). In the past, diagnosis with
CT was considered difficult, but in recent years, advances in
multidetector-CT have improved the ability to visualise blood flow
in the blood vessels, intestines and mesentery, thereby improving
the diagnostic performance. Contrast-enhanced CT is essential for
diagnosis, and findings, such as decreased or absent contrast
effects on the intestinal wall, which are signs of intestinal
ischaemia, can be observed. In the present case, NOMI was suspected
and additional contrast-enhanced CT revealed ischaemia from the
transverse colon to the descending colon. Treatment with
vasodilators is considered useful if there is no intestinal
necrosis; however, surgery is required if the intestinal tract has
reached an irreversible ischaemic state (2,5,6).
Therefore, early diagnosis of intestinal necrosis, which indicates
the need for surgery, is crucial. NOMI can manifest as a shock and
can be fatal, requiring immediate surgical treatment (2). If abdominal symptoms such as abdominal
pain and vomiting occur during drug therapy, intestinal ischaemia
should be suspected and discontinuation of anticancer drugs should
be considered.
 | Table IPreviously reported cases of
non-occlusive mesenteric ischaemia during drug therapy. |
Table I
Previously reported cases of
non-occlusive mesenteric ischaemia during drug therapy.
| Author(s), year | Age, years/sex | Cancer type | Drug therapy
regimen | Outcome | (Refs.) |
|---|
| Pearson et al,
2008 | 53/female | Metastatic liver
cancer | CDDP, ADM MMC | Survival | (12) |
| Yoshida et al,
2020 | 63/female | Oropharyngeal
cancer | DOC, CDDP, 5-FU | Death | (5) |
| Yoshida et al,
2020 | 71/male | Oropharyngeal
cancer | DOC, CDDP, 5-FU | Death | (5) |
| Tanaka et al,
2018 | 74/male | Oropharyngeal
cancer | DOC, CDDP, 5-FU | Survival | (13) |
| Wada et al,
2017 | 79/male | Prostate cancer | DOC | Survival | (14) |
| Wada et al,
2017 | 74/male | Oropharynx
cancer | DOC, CDDP, 5-FU | Survival | (14) |
| Ikeda et al,
2007 | 84/female | Breast cancer | PTX | Survival | (15) |
| Awano et al,
2013 | 80/female | Lung
adenocarcinoma | Gefitinib | Death | (16) |
| Matsuzawa et
al, 2015 | 74/female | Melanoma | PTX, CBDCA | Survival | (17) |
| Yamane et al,
2015 | 68/male | Small cell lung
cancer | CDDP, ETP | Survival | (18) |
| Nagano et al,
2021 | 74/male | Oropharyngeal
cancer | S-1 (+
radiation) | Death | (19) |
| Nagano et al,
2021 | 69/male | Oropharyngeal
cancer | DOC, CDDP, 5-FU | Death | (19) |
| Kuwayama et
al, 2022 | 58/male | Hodgkin lymphoma | Nivolumab | Survival | (20) |
| Oikawa et al,
2022 | 76/male | Glioblastoma | Bevacizumab | Death | (21) |
| Present case,
2024 | 85/male | Maxillary cancer | PTX, CBDCA, Cmab | Survival | - |
In conclusion, if a patient has a history of
abdominal surgery or evidence of intestinal mucosal disorders (such
as constipation), early imaging examinations (including
contrast-enhanced CT) and consultation with a gastroenterologist
should be considered to rule out NOMI.
Acknowledgements
The authors express their great appreciation to
Professor Kenichi Hirabayashi (Department of Diagnostic Pathology,
Faculty of Medicine, University of Toyama, Toyoma, Japan) and Dr
Takashi Minamisaka (Department of Diagnostic Pathology, Toyama
University Hospital, Toyoma, Japan) for histopathological
analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AI, RI, KS, DT, MT and SY treated the patient with
PCE. AI, KS and KF performed the follow-up. AI, SY, RI and MN
drafted the manuscript. AI, RI, SY and MN prepared the figures and
table. AI, SY and MN confirm the authenticity of all the raw data.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mazzei MA, Guerrini S, Cioffi Squitieri N,
Vindigni C, Imbriaco G, Gentli F, Berritto D, Mazzei FG, Grassi R
and Volterrani L: Reperfusion in non-occlusive mesenteric ischaemia
(NOMI): Effectiveness of CT in an emergency setting. Br J Radiol.
89(20150956)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Suzuki S, Kondo H, Furukawa A, Kawai K,
Yamamoto M and Hirata K: The treatment and diagnosis of
non-occlusive mesenteric ischemia (NOMI). J Abdom Emerg Med.
35:177–185. 2015.
|
|
3
|
Wiesner W, Khurana B, Ji H and Ros PR: CT
of acute bowel ischemia. Radiology. 226:635–650. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Furukawa A, Kanasaki S, Kono N, Wakamiya
M, Tanaka T, Takahashi M and Murata K: CT diagnosis of acute
mesenteric ischemia from various causes. AJR Am J Roentgenol.
192:408–416. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yoshida M, Kobayashi T and Hyodo M: Two
cases of non-occlusive mesenteric ischemia after chemotherapy for
head and neck cancer. Pract Oto-Rinno-Laryngol. 113:175–181.
2020.
|
|
6
|
Yukaya T, Kajiyama K, Koga C, Abe T,
Harimoto N, Miyazaki M, Yamashita T, Kai M, Shirabe K and Nagaie T:
Study of predictive factors for the prognosis in patients with non
occlusive mesenteric ischemia (NOMI). J Abdom Emerg Med.
31:1009–1014. 2011.
|
|
7
|
Teasdale G and Jennett B: Assessment of
coma and impaired consciousness. A practical scale. Lancet.
2:81–84. 1974.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
9
|
Ende N: Infarction of the bowel in cardiac
failure. N Engl J Med. 258:879–881. 1958.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heer FE, Silen W and French SW: Intestinal
gangrene without apparent vascular occlusion. Am J Surg.
110:231–238. 1965.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamakoshi H, Kanai T, Kurihara H, Ishikawa
T, Ozawa K and Ueno H: Clinical study on forty-six cases of
ischemic colitis. J Jpn Soc Coloproctol. 55:307–312. 2002.
|
|
12
|
Pearson AC, Steinberg S, Shah MH and
Bloomston M: The complicated management of a patient following
transarterial chemoembolization for metastatic carcinoid. World J
Surg Oncol. 6(125)2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tanaka H, Tsukahara K, Okamoto I, Kojima
R, Hirasawa K and Sato H: A case of septicemia due to nonocclusive
mesenteric ischemia occurring in induction chemotherapy. Case Rep
Otolaryngol. 2018(7426819)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wada T, Katsumata K, Kuwahara H, Matsudo
T, Murakoshi Y, Shigoka M, Enomoto M, Ishizaki T, Kasuya K and
Tsuchida A: Two cases of non-occlusive mesenteric ischemia that
developed after chemotherapy. Gan To Kagaku Ryoho. 44:1396–1398.
2017.PubMed/NCBI(In Japanese).
|
|
15
|
Ikeda K, Hanba H, Nagano k, Shimizu S,
Nakazawa K, Nishiguchi Y, Ogawa Y and Inoue K: Paclitaxel (Taxol)
associated bowels necrosis in the patient with local progressive
breast cancer. J Jpn Coll Surg. 32:638–642. 2007.
|
|
16
|
Awano N, Kondoh K, Andoh T, Ikushima S,
Kumasaka T and Takemura T: Three cases of lung carcinoma
complicated by gastrointestinal necrosis and perforation. Jpn J
Lung Cancer. 53:809–814. 2013.
|
|
17
|
Matsuzawa M, Harada K, Hosomura N, Amemiya
H, Ando N, Inozume T, Kawamura T, Shibasaki N and Shimada S:
Non-occlusive mesenteric ischemia after chemotherapy for metastatic
melanoma. J Dermatol. 42:105–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamane H, Fukuda N, Nishino K, Yoshida K,
Ochi N, Yamagishi T, Honda Y, Kawamoto H, Monobe Y, Mimura H, et
al: Non-occlusive mesenteric ischemia after splenic metastasectomy
for small-cell lung cancer. Intern Med. 54:743–747. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nagano H, Fujiwara Y, Matsuzaki H,
Umakoshi M, Ohori J and Kurono Y: Three cases of non-occlusive
mesenteric ischemia that developed after head and neck cancer
therapy. Auris Nasus Larynx. 48:1193–1198. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kuwayama N, Hoshino I, Gunji H, Kurosaki
T, Nabeya Y and Takayama W: A case of NOMI during treatment with
nivolumab for recurrent Hodgkin lymphoma. J Jpn Surg Assoc.
83:92–97. 2022.
|
|
21
|
Oikawa N, Kinoshita M, Yamamura M, Uno T,
Ichinose T, Sabit H, Hayashi T, Inoue D, Harada K and Nakada M:
Non-occlusive mesenteric ischemia during bevacizumab treatment for
glioblastoma: A case report. Acta Neurochir (Wien). 164:2767–2771.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Klauber N, Parangi S, Flynn E, Hamel E and
D'Amato RJ: Inhibition of angiogenesis and breast cancer in mice by
the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer
Res. 57:81–86. 1997.PubMed/NCBI
|
|
23
|
Britt LG and Cheek RC: Nonocclusive
mesenteric vascular disease: Clinical and experimental
observations. Ann Surg. 169:704–711. 1969.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kikuchi O, Saito D, Ikezaki O, Mitsui T,
Miura M, Sakuraba A, Yamada Y, Hayashida M, koyama G, et al: A case
of Ischemic colitis occurring in chemotherapy for ovarian cancer.
Prog Dig Endosc. 88:69–72, 61. 2016.
|
|
25
|
Nakamura S, Nogami K, Hida N, Iimuro M,
Yokoyama Y, Kamikozuru K, Nakamura M, Kawai M, Fukunaga K, et al:
Two cases of ischemic colitis associated with paclitaxel-based
chemotherapy in patients with ovarian cancer. 48:1785–1790.
2013.
|
|
26
|
Seewaldt VL, Cain JM, Goff BA, Tamimi H,
Greer B and Figge D: A retrospective review of
paclitaxel-associated gastrointestinal necrosis in patients with
epithelial ovarian cancer. Gynecol Oncol. 67:137–140.
1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Al-Diery H, Phillips A, Evennett N,
Pandanaboyana S, Gilham M and Windsor JA: The pathogenesis of
nonocclusive mesenteric ischemia: Implications for research and
clinical practice. J Intensive Care Med. 34:771–781.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mitsuyoshi A, Obama K, Shinkura N, Ito T
and Zaima M: Survival in nonocclusive mesenteric ischemia: Early
diagnosis by multidetector row computed tomography and early
treatment with continuous intravenous high-dose prostaglandin E(1).
Ann Sur. 246:229–235. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Woodhams R, Nishimaki H, Fujii K, Kakita S
and Hayakawa K: Usefulness of multidetector-row CT (MDCT) for the
diagnosis of non-occlusive mesenteric ischemia (NOMI): Assessment
of morphology and diameter of the superior mesenteric artery (SMA)
on multi-planar reconstructed (MPR) images. Eur J of Radio.
76:96–102. 2010.PubMed/NCBI View Article : Google Scholar
|