Introduction
Burn wounds are classified as first-, second- or
third-degree according to the depth and severity of damage caused
to the inner layers of the skin (1,2).
Third-degree or full-thickness burns are particularly aggressive
wounds that present considerable challenges for healthcare
professionals since they extend below the epidermis and cause
damage to the dermis and subcutaneous adipose tissue (3-5).
This type of injury requires hospitalization and surgical
intervention due to high risk of infection, shock and death;
healing is slow and hypertrophic scarring is common (6). The worldwide incidence of burns is
close to 9 million injuries each year with >2.2% being fatal
(7,8). For non-fatal injury severe enough to
hospitalization, long-term physical and psychological consequences
include chronic pain, limited mobility, permanent scarring and
post-traumatic stress disorder (9,10). The
treatment of third-degree burns is individualized therapy depending
on medical condition and affected organs. Considering only the
wound healing aspect, the process is slower and more complex
compared with other types of burn. However, it still follows
several distinct stages: inflammation, proliferation and remodeling
(6). Various treatments are
employed to enhance skin healing and improve patient outcomes,
including skin grafts, polymeric membrane dressings, patches or gel
(G) containing growth factors, delivery of adult mesenchymal stem
cells to the wound and other skin substitutes (6,9,11-16).
Additionally, because burn wounds are particularly susceptible to
infection, topical antiseptics are crucial in wound care as they
help prevent infections (16).
Therefore, an antiseptic must be efficient and not hinder or
negatively intervene in the healing process. Silver sulfadiazine
(S; 1%) and nitrofurazone (NF; 0.2%) have been widely used due to
their effective antimicrobial properties and general safety in
improving healing (17-19).
However, despite their effectiveness, it is important to consider
factors such as availability, cost-effectiveness and the specific
requirements of each treatment modality must be evaluated to make
informed clinical decisions (20).
In recent years, there has been growing interest in alternative
antiseptics such as electrolyzed solutions (21-23).
Superoxidized electrolyzed solution (SES) is noted not only for
antimicrobial properties but also for its potential to efficiently
promote wound healing, offering a promising addition to
conventional treatment (22,23).
SES is produced from a saline solution of sodium
chloride activated through controlled electrolysis process and pH
control in a range of 6.5-7.5(23).
This process generates reactive species of chlorine and reactive
oxygen species (ROS) (24). SES key
reactive species include oxidant chlorine compounds, such as
hypochlorous acid (HOCl), and ROS species, such as hydrogen
peroxide (H2O2) (24-27).
Several studies reported that SES exhibits properties similar to
those of active compounds that are produced during the innate
immune response; these include antimicrobial activity, an
anti-inflammatory effects, decreased oxidative stress, promotion of
tissue regeneration and angiogenesis and immune modulation,
suggesting potential therapeutic benefits for wound healing and
infection control (24-27).
SES has been shown to modulate inflammatory
responses, promoting balanced healing and reducing excessive
inflammation that can impede tissue regeneration (28-31).
The regenerative properties of SES have also been explored in other
areas, such as chronic wound and tissue engineering (32). SES also has showed the ability to
stimulate angiogenesis, the formation of new blood vessels, which
is key for supplying nutrients and oxygen to healing tissues
(23,29,31,33).
Moreover, SES has potential in modulating the production of growth
factors and cytokines, facilitating tissue repair and regeneration
(28-31,33).
Furthermore, some studies have highlighted the
potential of SES in stimulating the proliferation and migration of
various types of cells involved in wound healing, including
keratinocytes and fibroblasts (26,28,32,34).
These cells serve essential roles in re-epithelialization and
production of extracellular matrix components necessary for tissue
regeneration. By promoting cell activities, SES may contribute to
accelerated wound closure and improved tissue regeneration
(28-31,33,34).
The present study aimed to assess the efficacy of
SES, at low (SES-low) and high (SES-high) concentrations of active
species and compare it with commonly used antimicrobials for wound
care in a preclinical murine burn wound model. Histological
evaluation, focusing on wound closure, collagen fiber formation and
orientation and the number of inflammatory cells, was performed to
determine healing score and status, enabling a comparison of the
efficacy of treatments and providing a comprehensive assessment of
the wound healing process. By evaluating the advantages and
disadvantages of treatments, the present results may contribute to
the strategies to improve burn wound care.
Materials and methods
Treatments
SES was administered at two concentrations as a
liquid solution and as gel formulations. The low-concentration SES
(SES-low) contained 20 parts per million active species of chlorine
and oxygen (0.002%), has a pH of 6.5-7.5 and oxidation-reduction
potential of ~850 mV (Estericide® Solución Antiséptica;
Esteripharma® S.A. de C.V.; cat. no. 0412C2016 SSA). Gel
formulation is commercially available as Estericide® Gel
Antiséptico (cat. no. 1594C2014 SSA). The high concentration SES
(SES-high) contained <80 parts per million (>0.008%) chlorine
(Microdacyn® Solución Antiséptica; Aerobal S.A. de C.V.,
México; cat. no. 1075C2003 SSA). The hydrogel formulation is
commercially available as Microdacyn® Hydrogel Gel
antiséptico and contains ≥40 parts per million of free chlorine
(cat. no. 0176C2014 SSA). NF ointment was used at a concentration
of 0.2% as the commercially available Furacin®
(Siegfried Rhein® S.A. de C.V; cat. no. 31258 SSA) and S
cream was used at a concentration of 1% as the commercially
available Bioargirol-C (Bioresearch de México S.A. de C.V; cat. no.
489M2000 SSA). These products are widely available and frequently
used in patients suffering burns covering <15 (adults) or 5%
(children) of their total body surface area (19). Physiological saline solution (0.9%
NaCl solution; PiSA Pharmaceuticals) was used as a placebo (Pl) in
the control group.
Animals
Male BALB/c mice (n=252; Inotiv; age, 10-14 weeks;
weight, 25-30 g) were used. The duration of the experimental
procedure was 32 days. Mice were randomly assigned to seven groups,
each comprising 36 animals as follows: Pl, SES-low, SES-low + G,
SES-high, SES-high + G, NF and S. All animals were kept at 21±2˚C
with 48% humidity in a 12/12-h light/dark cycle, with food and
water provided ad libitum. The mice were kept in cages, with
a maximum of 6 mice/cage.
The animal experiments were approved by the Research
Ethics Committee of the Colima State Institute of Cancerology,
Colima, Mexico (approval no. CIIECAN/06/19). Animals were handled
in accordance with institutional guidelines (35) and the official Mexican standard for
the care and use of laboratory animals (Official Mexican Standard
NOM-062-ZOO-1999: Technical specifications for the production,
care, and use of laboratory animals) (36-38),
in addition to the eighth edition of the Guide for the Care and Use
of Laboratory Animals prepared by the National Academy of Sciences
of the USA (2011) (38). Mice were
observed daily to assess for clinical signs of toxicity or
distress, and behavioral changes were evaluated by functional
observational battery parameters such ass salivation, lacrimation,
signs of distress, changes in eating and drinking, activity levels
and any signs of infection or discomfort at the wound site
(39-41).
Humane endpoints were weight loss >20% of body weight, severe
illness, infection or necrosis at the wound site or any signs of
severe distress, such as lack of grooming, abnormal posture or
reduced activity (37,42). No animals met the humane endpoints
for euthanasia before the end of the experiment and none were found
dead. Pain management included administering paracetamol (200
mg/kg) orally for the first 5 days (1,43-45)
and ketamine (120 mg/kg) and xylazine (15 mg/kg) were used during
burn induction and prior to euthanasia (45). Death was verified by cessation of
heartbeat and respiration, as well as the absence of reflexes
(46).
Full-thickness burn induction and
treatment
The scald burn model was established as described by
Abdullahi et al (47). At 1
day before the intervention, the dorsal area of the mice was shaved
and depilated with cream (Nair Sensible, Reckitt Benckiser) for 30
sec and residues were removed with warm water. On the day of the
intervention, mice were anesthetized using intraperitoneal ketamine
(120 mg/kg) and xylazine (15 mg/kg; PiSA
Pharmaceuticals®, Agropecuaria). Each mouse was placed
in a supine position on a template of flame-resistant plastic mold,
which included a window exposing the predetermined skin surface
area. A test tube with 95˚C water was brought into direct contact
with the exposed skin surface of the mouse for 7 sec, resulting in
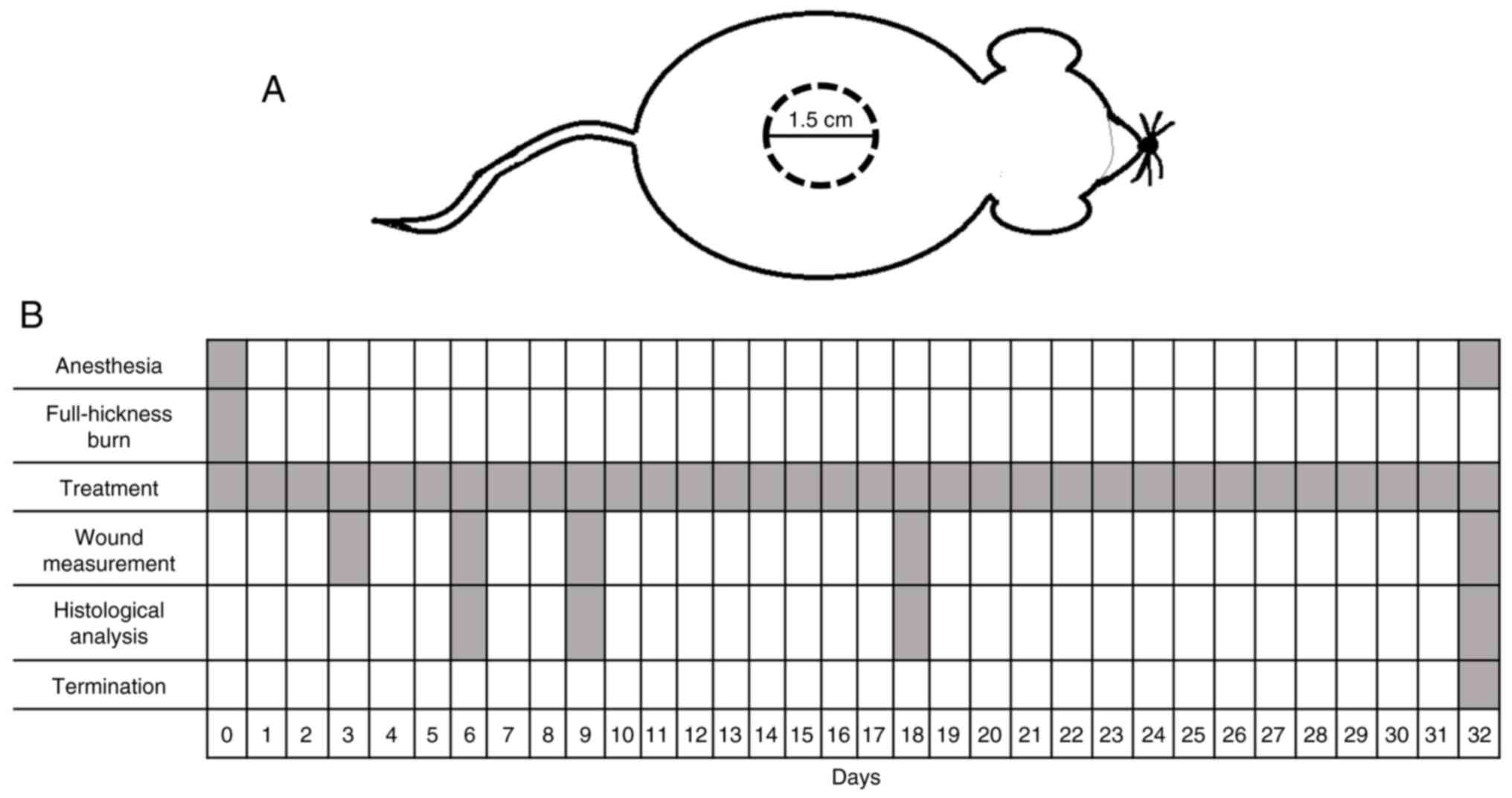
an oval burn with diameters of 1.5-2.0 cm (Fig. 1A).
A total of 12 mice/group was selected to investigate
wound size change. Treatment was applied once/day for 32
consecutive days, starting on day 0 of the study (the day of the
burn; Fig. 1B). Liquid was directly
applied to the burn site, resulting in a total volume of ~1 ml
product. For the gel, ~1 g was applied to the burn area using a
sterile plastic applicator. On days 6, 9, 18 and 32, 6 mice/group
were sacrificed by decapitation after being anesthetized as
previously described (48).
Wound size and macroscopic
evaluation
Periodic measurements of the wound area were taken
on days 3, 6, 9, 18 and 32 as described by Zhang et al
(49) with minor modification. The
mice were immobilized and the contour of the wound was traced using
a transparent graph sheet and marker. The resulting images were
analyzed to determine the burn wound area. Wound area reduction was
calculated using the following formula: Wound contraction
(%)=100-[(wound size x100)/mean value of day 0 wound size]. Changes
in morphology were documented by capturing images with a digital
camera (Nikon AF-S VR Micro Nikkor; Nikon Corporation) at a
constant focusing distance. The resulting images were analyzed
using Fiji2.0 software (National Institutes of Health) (50). All images were captured under the
same light and exposure.
Histopathological analysis
Samples of burn areas were surgically excised (1x1
cm) and rinsed with cold PBS following sacrifice and fixed in 10%
neutral buffered formalin at room temperature for 24 h, washed,
dehydrated with ethanol and embedded in paraffin. The obtained
blocks were cut into 5-mm-thick tissue sections, mounted on glass
slides, deparaffinized and rehydrated. Slides were stained with
hematoxylin-eosin (H&E) at room temperature for 30 min for
evaluation of inflammatory infiltration state and epithelial
regeneration, and with Masson's trichrome at room temperature for
60 min to analyze collagen fibers (51-55).
Each measurement was independently conducted by two qualified
scientists in a blinded manner, ensuring unbiased data collection
and analysis. Images were captured using a digital camera model
Axiocam MRC-5 connected to a t bright-field optical light
microscope model AxioPlan 2 M (Zeiss GmbH) with a motorized stage
(total magnification, x100, 200 and 400). MosaiX and Autofocus
modules were used to scan images of the entire sample surface and
the lesions were measured using a calibration line. All images were
captured under the same illumination and exposure times using the
AxioVs 40 V.4.7.0.0 image software (Carl Zeiss Imaging Solutions
GmbH). All histological data were obtained from 30 randomly
selected fields of view from 6 mice (5 data/mouse). Counts of total
inflammatory cells, polymorphonuclear neutrophils (PMNs) and
mononuclear leukocytes (MNC) were manually determined using five
randomly selected fields of view (magnification, x10 and 40). The
inflammatory infiltration state was determined according to degree
of inflammatory infiltrate, by assigning a semi-quantitative and
discontinuous score: 1-plenty; 2-moderate and 4-few (51,52,54).
Samples stained with Masson's trichrome were
analyzed using a Motic BA310E optical light microscope (Motic China
Group Co., Ltd.; magnification, x10). A total of three
microphotographs were captured for each tissue sample with a
Moticam 1080 digital camera (Motic China Group Co., Ltd.) under the
same lighting and exposure. The proportion, shape and type of
collagen fibers were analyzed using Fiji 2.0 software. Collagen
orientation was classified as follows: 1, vertical; 2 for mixed,
and 4 for horizontal. The collagen patterns were categorized as: 1
for reticular, 2, mixed, and 4 for fascicular. The amount of early
collagen was qualitatively evaluated as 1, profound; 2, moderate;
3, minimal and 4, absent. Mature collagen was classified as 1,
profound; 2, moderate and 4, minimal (52-55).
Epithelial regeneration was evaluated by assessing
the migration of cells to the wound edge, defined as the area where
epithelial cells meet the edge of the wound, divided by the
distance from the wound bed, the base of the wound where new tissue
is forming, multiplied by 100% and scored as follows: 0-0; 1- >0
and<50; 2, ≥50 <100; 3-100% and irregular thickness, and 4,
100% and normal thickness (56).
Additionally, quantitative (µm) and qualitative (yes/no)
measurements of epidermal detachment visualized as separation of
wound edges viewed at 2.5X magnification, as well as the thickness
of the epidermal lesion (40X magnification), were performed
(57). The number of blood vessels
and follicles/field was included for evaluation, along with the
presence or absence of scar tissue (58). Furthermore, a semi-quantitative
assessment of granulation tissue (1, deep; 2, moderate; 3, scant
and 4, absent) and a qualitative assessment of presence of the
stratum corneum at 2.5X magnification was performed (51,52,59).
Calculation of healing score and
status
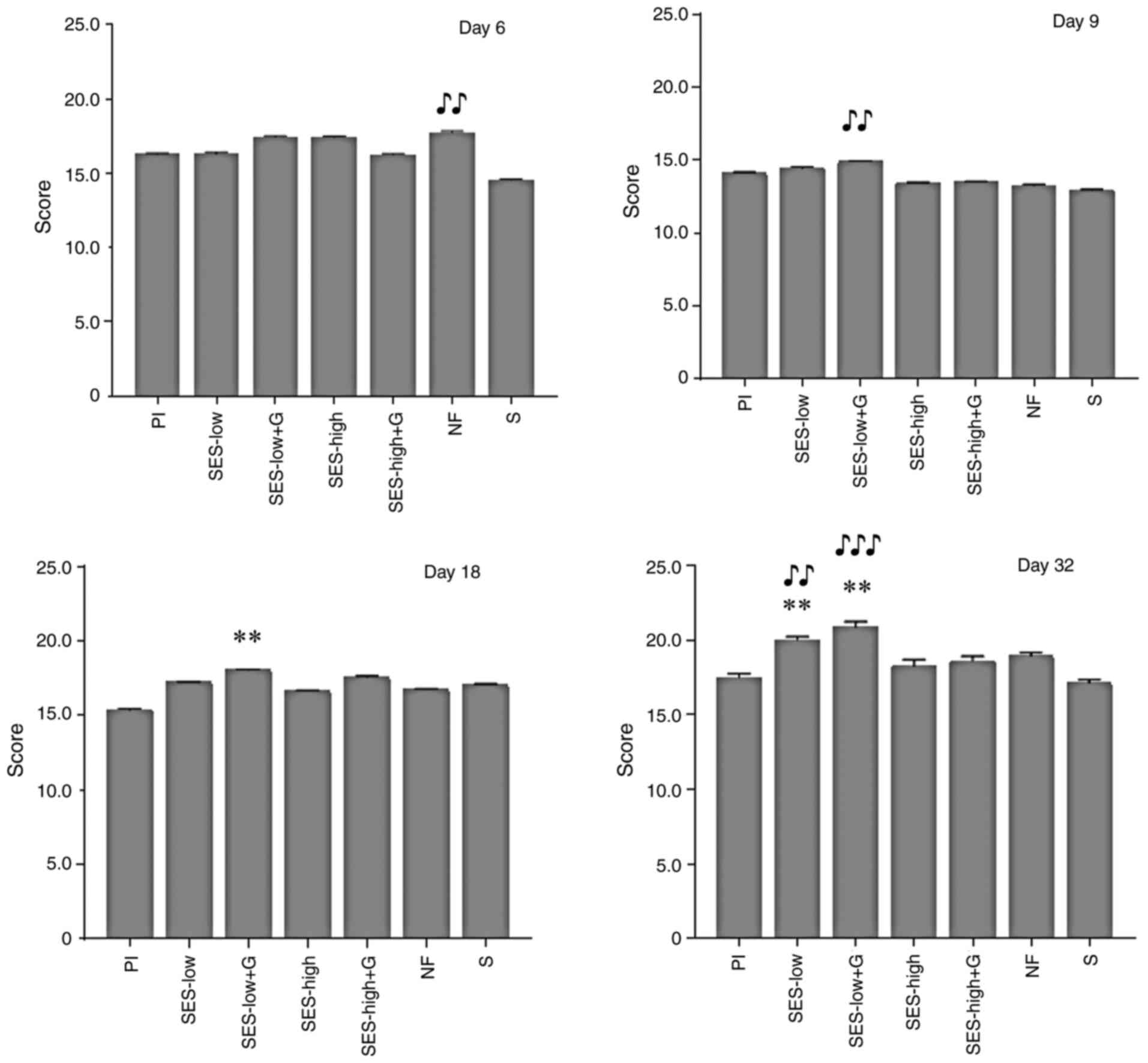
Wound healing score and status were determined as
described by Gupta and Kumar and Santos et al (51,52).
The parameters assessed included granulation tissue amount,
inflammatory infiltrate, collagen fiber orientation and pattern and
early and mature collagen amount. The total healing score was
calculated by adding the scores of individual criteria, with lower
scores indicating poorer wound healing. Healing status was graded
as follows: 8-11, poor; 12-15, acceptable and 16-19, good (51,52).
Statistical analysis
Data are presented as the mean and SEM (n=≥6. Normal
distribution of data was determined using the Shapiro-Wilk test.
Data were analyzed using one-way ANOVA for normally distributed
data (parametric) or Kruskal-Wallis test for non-normal or ordinal
data (non-parametric). Post hoc analysis was performed using
Bonferroni's comparisons or Mann-Whitney U test (non-parametric)
and Tukey's multiple comparison test (parametric). The statistical
analysis was performed using IBM SPSS version 20 software (IBM
Corp.) P<0.05 was considered to indicate a statistically
significant difference.
Results
SES-low + G and nitrofurazone
treatments induce the highest and early closure of the burn wound
area
Fig. 2 shows
representative pictures of the wound healing process and reduction
of burn areas, providing a visual and quantitative assessment of
the treatment outcomes.
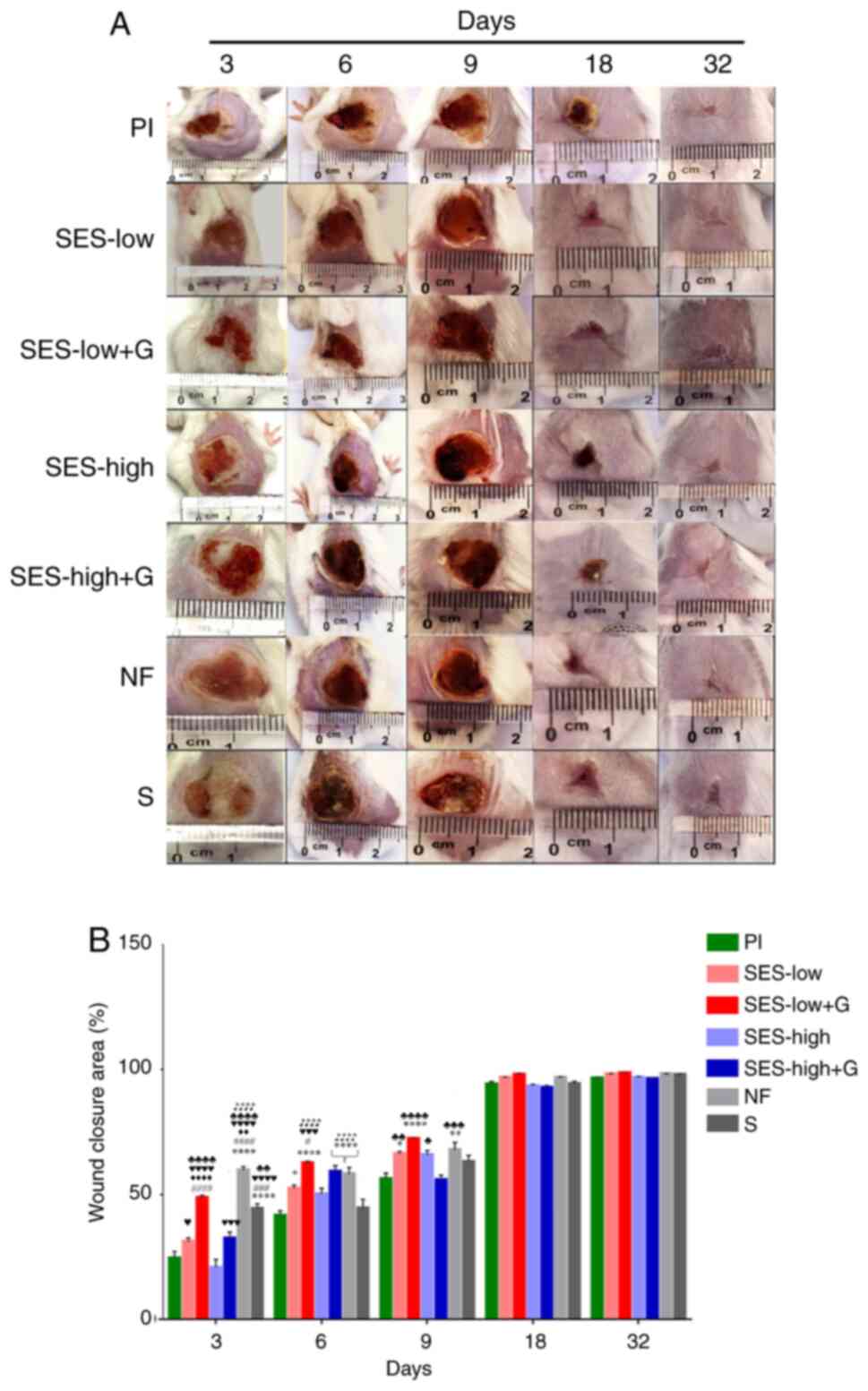 | Figure 2Macroscopic evaluation of burn wound
Healing. (A) Morphology and macroscopic appearance of burn wound
area. (B) Burn area reduction. *P<0.05,
**P<0.01 vs. Pl, ****P<0.0001 vs. Pl,
#P<0.05 vs. SES-low, ###P<0.001 vs.
SES-low, ####P<0.0001 vs. SES-low,
♦♦P<0.01 vs. SES-low + G, ♦♦♦♦P<0.0001
vs. SES-low + G, ♥P<0.05 vs. SES-high,
♥♥♥P<0.001 vs. SES-high, ♥♥♥♥P<0.0001
vs. SES-high, ♣P<0.05 vs. SES-high + G,
♣♣P<0.01 vs. SES-high + G, ♣♣♣P<0.001
vs. SES-high + G, ♣♣♣♣P<0.0001 vs. SES-high + G,
♪♪♪♪P<0.0001 vs. S. SES, superoxidized electrolyzed
solution; NF, nitrofurazone; Pl, placebo; S, silver sulfadiazine;
and G, gel. |
On day 3, NF, SES-low + G, and S treatments
exhibited the smallest wound areas, with closure of 59.7±5.9,
48.7±4.0 and 44.3±7.1%, respectively (Table SI). The NF group exhibited
significantly greater wound closure compared with all other groups.
SES-low + G showed better wound healing compared to SES-high,
though not significantly different from the S group. Pl and
SES-high groups had the poorest closure (Table SII).
On day 6, wound closure was highest in the SES-low +
G (62.6±2.5%), NF (58.2±10.5%), and SES-high + G (59.2±9.2%) groups
(Tables SI and SII). SES-low +G treatment demonstrated
significantly better closure compared to the SES-low and SES-high
groups.
At day 9, the SES-low + G (72.4±1.4%) and NF
(67.8±12.0%) groups again exhibited the highest wound closure rates
(Table SII). These were
statistically different from the Pl and SES-high + G groups,
showing superior wound healing outcomes.
From day 18 to 32, all treatments groups
demonstrated similar wound healing progress, eventually reaching
full wound closure (Fig. 2A).
However, the SES-low + G and NF groups continued to exhibit the
smallest final wound areas (Fig.
2B, Table SI), with the
highest overall closure rates (Table
SII). While SES-low + G outperformed the other treatments on
days 6 and 9, the differences were not statistically significant
(Table SII).
SES-low + G and SES-low treatment
reduced inflammatory infiltrate in the burn wound area in the early
and late states of healing
To assess the inflammatory response number of
polymorphonuclear cells (PMNs) (Table
SIII) and monocytes (Table
SIV) at the days 6, 9, 18 and 32 of the wound healing process.
The total inflammatory infiltrate was also measured, and multiple
comparison tests were conducted to evaluate the significance of
these counts across treatments (Table
SV). As expected, abundant inflammatory infiltrate was observed
in the early stages of wound healing as part of the typical course
of the re-epithelization process (52,60-62)
and reached maximum values on day 9 (Fig. 3A). On day 6, the S, NF and SES-high
+ G groups exhibited the highest cell/field values (92.90±7.19,
72.80±5.35 and 54.30±5.87, respectively). S showed the most
abundant infiltrate, being significantly different from the rest of
the groups except with NF. On the other hand, SES-low (29.33±3.20),
SES-low + G (33.10±3.16) and SES-high (35.86±2.87) groups had
similar effects to Pl (26.06±4.43).
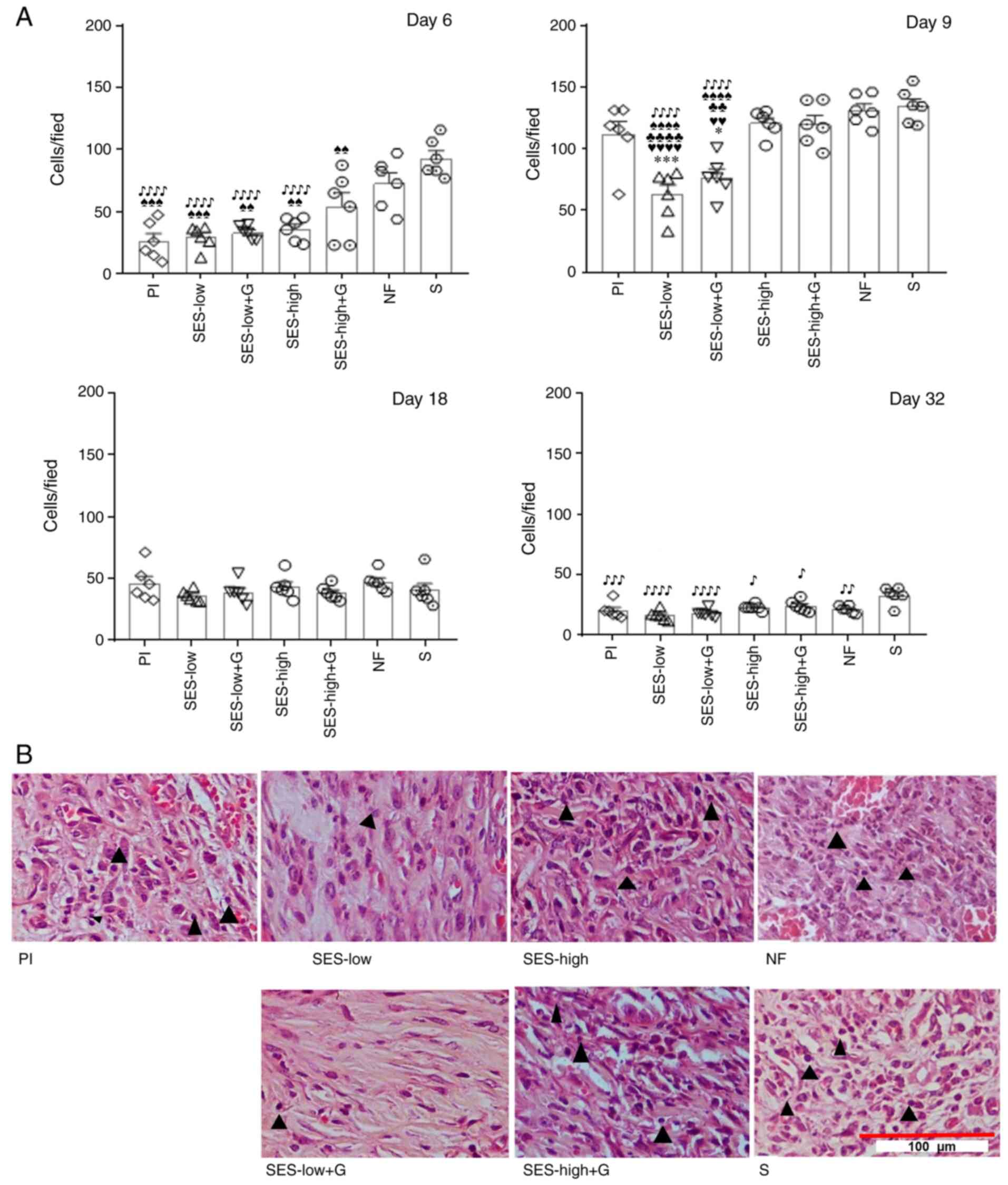 | Figure 3Inflammatory response and infiltrate
abundance on day 9. (A) Amount of total inflammatory cells was
significantly ifferent between the groups. *P<0.05,
***P<0.001 vs. Pl; ♥♥P<0.01,
♥♥♥♥P<0.0001 vs. SES-high; ♣♣P<0.01,
♣♣♣♣P<0.0001 vs. SES-high + G;
♠♠P<0.01, ♠♠♠P<0.001,
♠♠♠♠P<0.0001 vs. NF; ♪P<0.05,
♪♪P<0.01, ♪♪♪P<0.001,
♪♪♪♪P<0.0001 vs. S. (B) Inflammatory infiltrate
abundance at day 9. Histological evaluation with hematoxylin/eosin
staining, visualized with light microscopy (40X magnification). ▲
indicates macrophages. SES, superoxidized electrolyzed solution;
NF, nitrofurazone; Pl, placebo; S, silver sulfadiazine; and G,
gel. |
On day 9, a general and significant increase in
inflammatory infiltrate was observed (Table SV). However, SES-low (63.30±4.87)
and SES-low + G (77.10±5.04 cells/field) groups exhibited
significantly lower cell counts compared with all other groups. S
(134.75±5.58) and NF (131.50±5.19 cells/field) groups had the
highest levels of inflammatory infiltrate, followed by SES-high
(120.93±5.77 cells/field), SES-high + G (119.93±5.00 cells/field)
and Pl (110.90±6.47 cells/field) groups. No statistical differences
were observed between SES-high, SES-high + G, NF, S and Pl groups.
Fig. 3B shows histological images
on day 9, demonstrating the differences in inflammatory infiltrate
abundance, primarily macrophages (▲). SES-low and SES-low + G
produced less infiltration in the tissue, indicating an
anti-inflammatory.
On day 18, the inflammatory infiltrate all groups
became similar, without no significant differences observed-The
average cell count was 41.44±3.01 cells/field. By day 32, the
counts of total inflammatory cells decreased in all groups. The S
group exhibited the highest inflammatory infiltrate, with a mean
value of 33.04±2.76 cells/field, which was significantly higher
than the rest of the groups. SES-low and SES-low + G exhibited the
lowest cell counts, with mean values of 16.53±1.20 and 18.00±1.01
cells/field, respectively. The cell counts in the SES-low group
were significantly lower compared with those of the S and SES-high
+ G group (24.10±1.3). SES-low was better than SES-high at
modulating the inflammatory process. Additionally, S and NF groups
exhibited inflammatory infiltrate, suggesting an irritant effect.
In the specifics counts of PMNs, the SES-low group had consistently
lower cell counts throughout the study, maintaining modest values
compared to the S and NF groups, particularly on day 32 where
SES-low recorded 3.0±0.49 cells/field compared to S at 6.6±0.38
(Table SIII). Similarly, for
monocytes, the SES-low group showed reduced counts at all time
points, especially at day 32, with 13.5±1.54 cells/field compared
to S, with 26.4±2.26 cells/field (Table SIV). This suggests that the
treatment with SES-low and SES-low + G, may induce a regulated and
balanced inflammatory response at the early and late stages of the
healing process. S group induced a stronger pro-inflammatory
reaction, particularly evident at later stages of wound
healing.
SES-low + G, SES-low and Nitrofurazone
treatments induced a more advanced progression in re-epithelization
of burn wound area
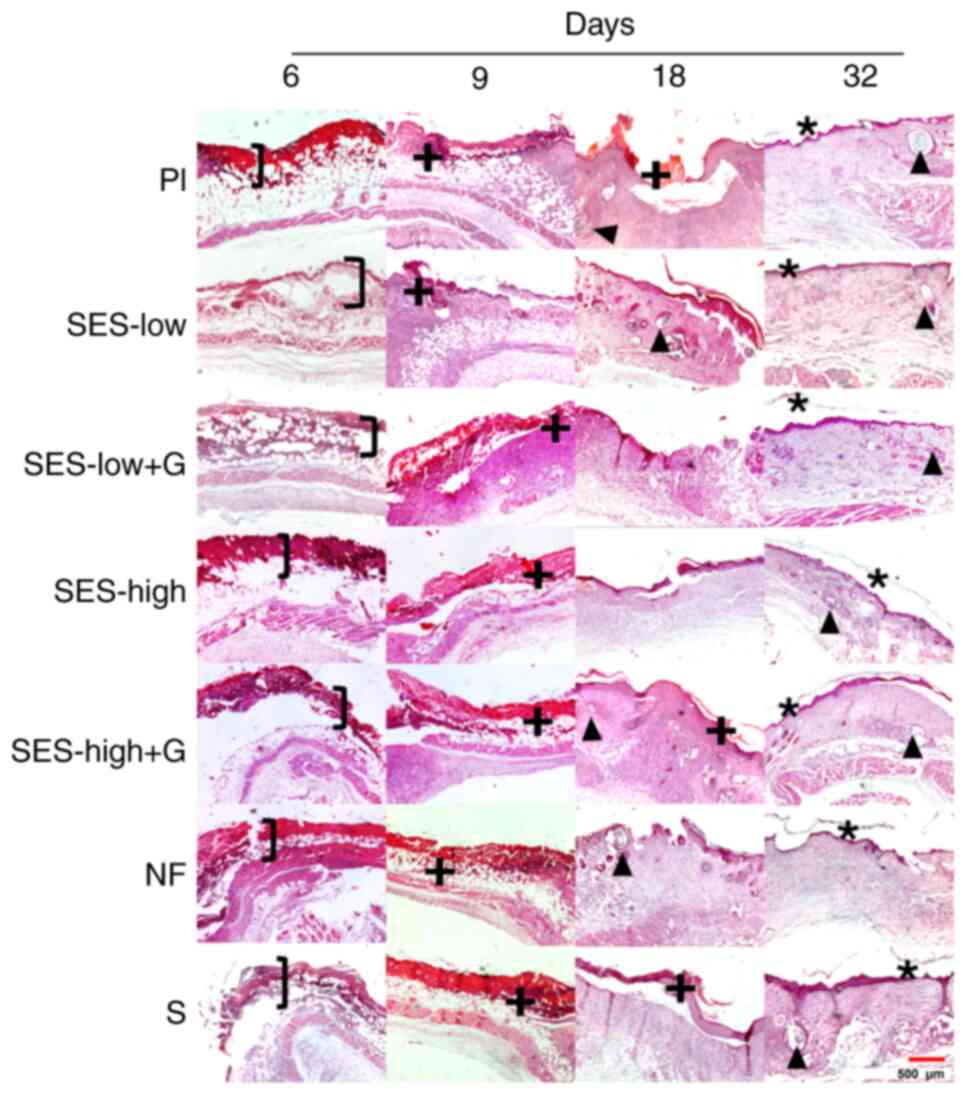
On day 6, all groups exhibited epidermal detachment
(Fig. 4). However, SES-low and
SES-low + G groups showed a more defined lesion with indications of
dermal recovery and greater differentiation of cutaneous layers,
while Pl showed deeper burn damage. Subsequently, on day 9, a
serohemorrhagic crust was present in all groups, indicating an
ongoing repair process (+). By day 18, the repair and
re-epithelization was indicated by epithelial edge junctions and
the hair follicle presence (▲). The Pl, SES-high and NF groups did
not show epithelial edge union, the process where wound edges come
together as new skin forms, while SES-low, SES-low + G, S and
SES-high + G-treated groups exhibited complete junction of
epithelial edges. Additionally, traces of serohemorrhagic crust
were observed in the Pl, SES-high + G and S groups. The presence of
hair follicles in SES-low, SES-low + G and NF groups indicated a
more advanced repair process (63-65).
Furthermore, on day 32, complete healing and re-epithelization of
the burn was observed in all groups, as evidenced by the union of
edges, indicating full closure of the wound, and presence of a
stratum corneum and hair follicles. The SES-low, SES-low + G,
SES-high + G and NF groups showed a thicker stratum corneum (*),
compared with Pl, SES-high, and S groups. Therefore, NF, SES-low +
G and SES-low groups demonstrated a more advanced progression
towards re-epithelization, characterized by a compact and
well-defined serohemorrhagic crust.
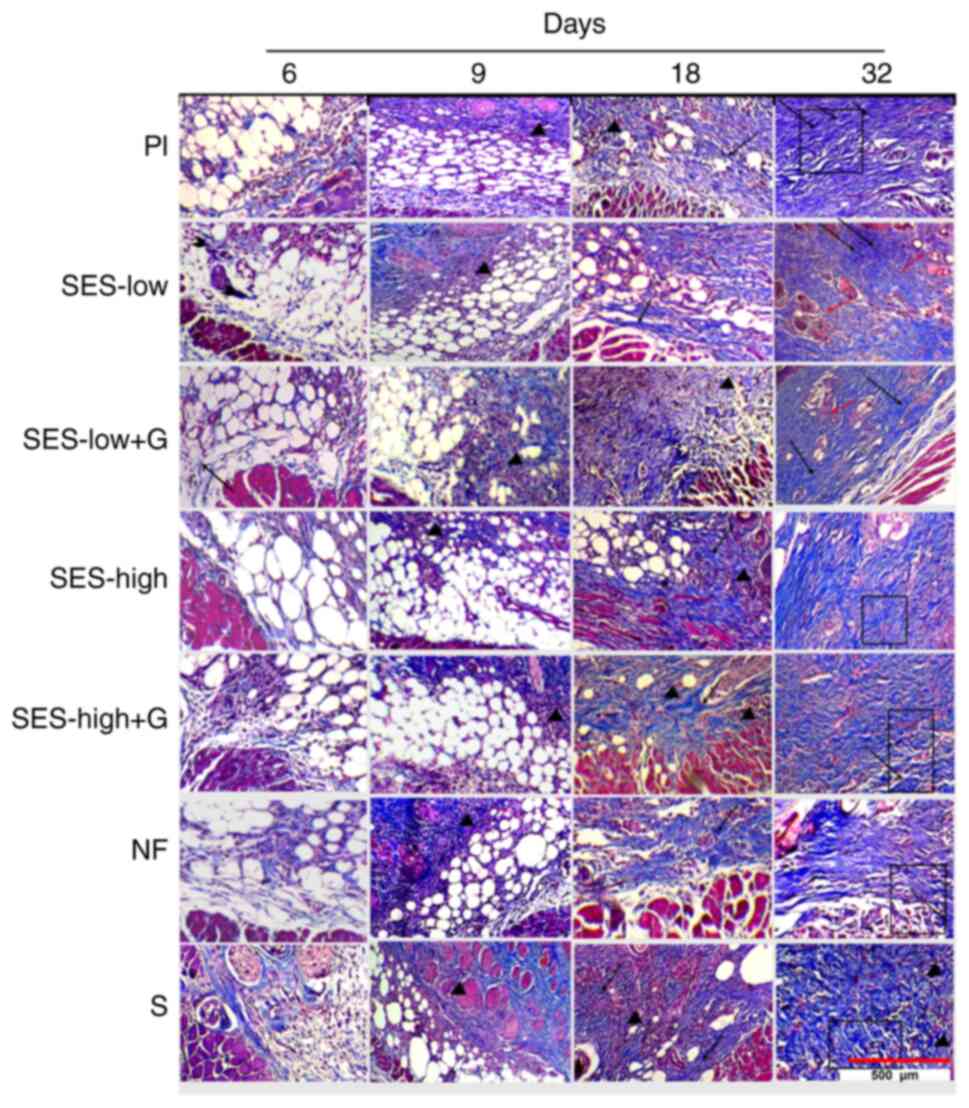
SES-low + G, SES-low and Nitrofurazone generate
better collagen matrix reorganization. The analysis of collagen
parameters at days 6, 9, 18, and 32, was performed using Mason's
staining (Fig. 5). The scores for
orientation and amount of early or mature collagen at day 32 are
presented in Fig. 6. Scores for
days 6, 9 and 18 are shown in Fig.
S1, Fig. S2 and Fig. S3, respectively while Table SVI, Table SVII and Table SVIII provide the statistical
analysis of these parameters. The collagen pattern showed no
significant differences between groups on any of the days.
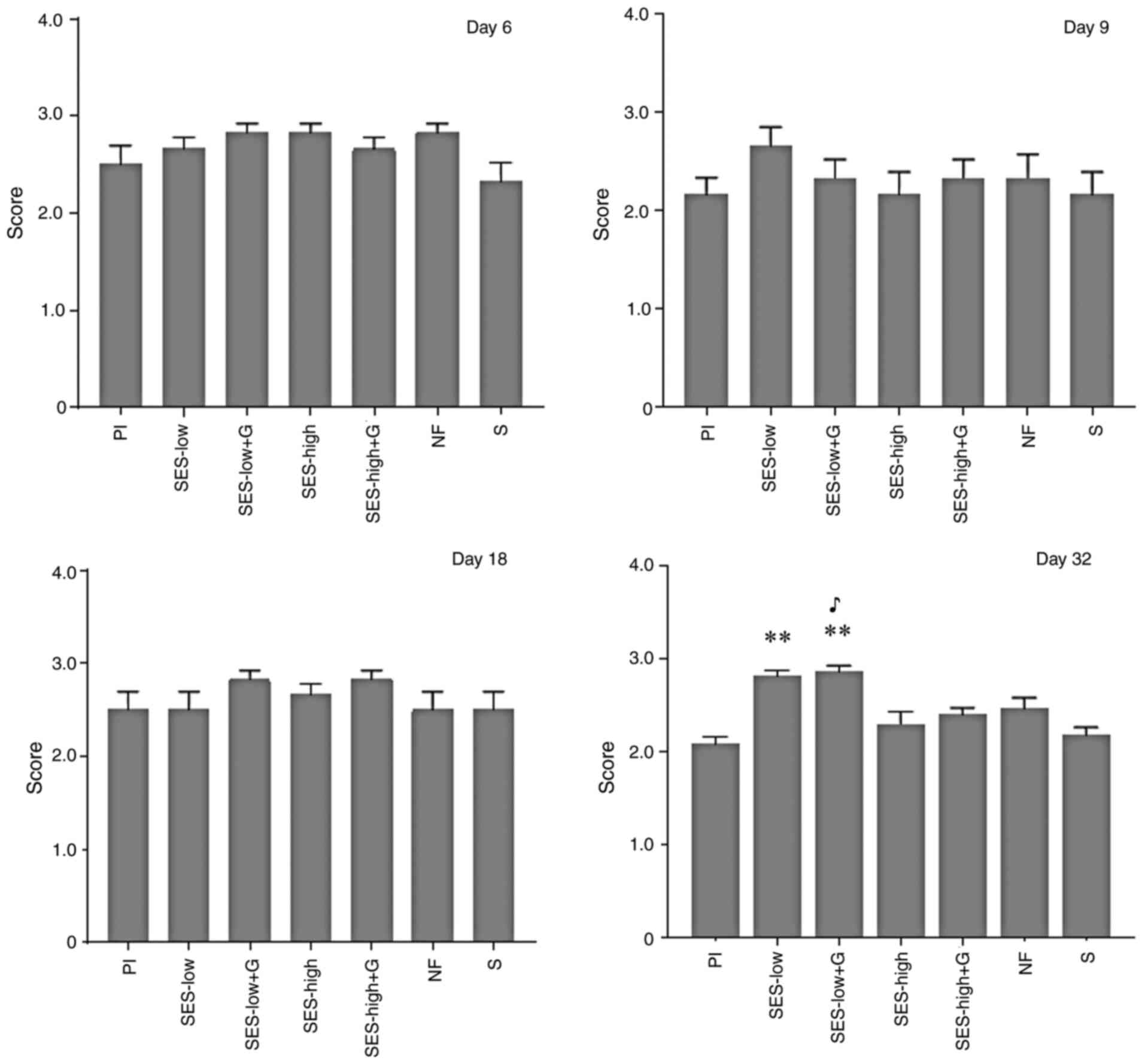
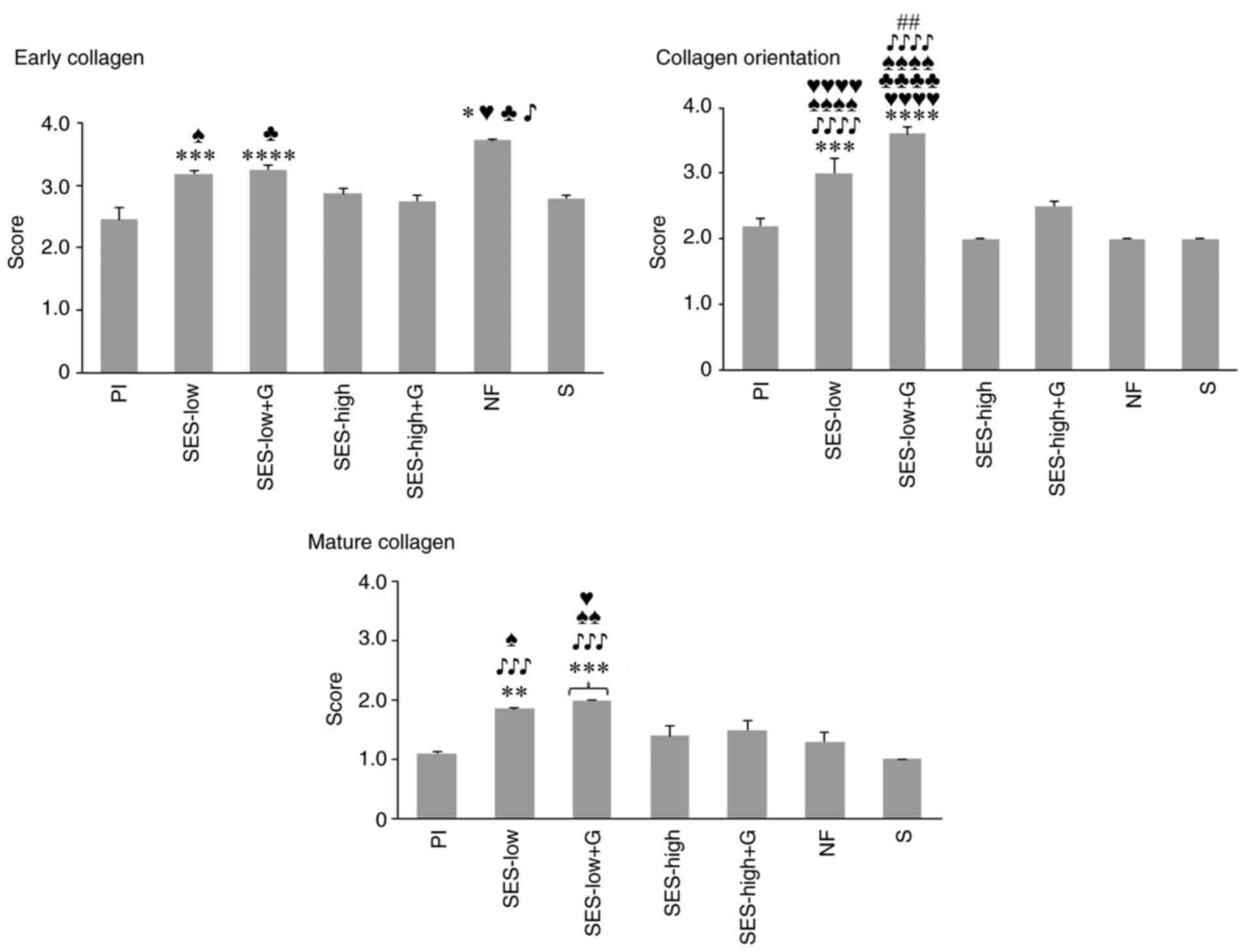 | Figure 6Semi-quantitative scores for early
and mature collagen and collagen orientation at day 32.
*P<0.05 vs. Pl, **P<0.01 vs. Pl,
***P<0.001 vs. Pl, ****P<0.0001 vs. Pl,
##P<0.01 vs. SES-low, ♥P<0.05 vs.
SES-high, ♥♥♥♥P<0.0001 vs. SES-high,
♣P<0.05 vs. SES-high + G, ♣♣♣♣P<0.0001
vs. SES-high + G, ♠P<0.05 vs. NF,
♠♠P<0.01 vs. NF, ♠♠♠♠P<0.0001 vs. NF,
♪P<0.05 vs. S, ♪♪♪P<0.001 vs. S,
♪♪♪♪P<0.0001 vs. S. SES, superoxidized electrolyzed
solution; NF, nitrofurazone; Pl, placebo; S, silver sulfadiazine;
and G, gel. |
Granulation tissue and inflammatory infiltrate were
predominant at the early stages of wound healing with collagen
deposition mainly early collagen (light blue) observed (Fig. 5). As wound healing progressed,
increased collagen deposition was noted, with agglomeration of
mature collagen, stained as deep blue, in SES-low, SES-low + G,
SES-high, SES-high + G and NF groups, alongside a gradual decrease
in inflammatory infiltrate and granulation tissue. By day 32 of
follow-up, the collagen matrix reached its maximum in all groups,
with statistical differences noted for minimal or absent deposition
of young collagen in NF, SES-low and SES-low + G groups (Fig. 6) (66,67).
Clear differences in the aggregation and organization of mature
collagen were observed among treatments. In comparison with
SES-low, S, NF and Pl showed thicker and more irregular deposition
of mature collagen, with greater collagen matrix deposition.
Vascularization and newly formed hair follicles were also noted in
the SES-low group (Fig. 5)
(66). The semi-quantitative
analysis of collagen matrix deposition is shown in Fig. S1, Fig.
S2 and Fig. S3.
At day 32, SES-low + G and SES-low exhibited the
most uniform and horizontal orientation of collagen fibers,
followed by SES-high (Fig. 5). This
indicated better collagen matrix reorganization and improved final
healing process (Fig. S4).
Orientation analysis of collagen fibers at day 32 revealed
significant differences between SES-low, SES-low + G and S, NF, Pl,
and SES-high (Fig. 6). According to
semiquantitative scores, NF was the third best treatment for
inducing organized collagen matrix deposition, though no
significant difference was observed compared to SES-high (Fig. S1, Fig.
S2 and Fig. S3).
SES-low + G and SES-low treatments had
the best Healing scores
At day 32, granulation tissue was absent and an
uniform collagen pattern across the groups. This indicates that the
healing process was complete and comparable in terms of collagen
deposition, so all groups were rated with the highest score for
this parameter. Additionally, scores for the amount of inflammatory
infiltrate and type/abundance of deposited collagen were assigned.
The highest scores were assigned to treatments that induced the
lowest inflammatory infiltrate (Fig.
3; Table SIII, Table SIV and Table SV). For quality of collagen
deposition, the highest values were registered for treatments that
produced an organized matrix, composed by horizontal deposition
(Fig. 6; Table SVI, Table SVII and Table SVIII). The higher the healing
score, the more favorable outcome in terms of tissue repair. A high
healing score suggested that the healing process progressed well
and achieved the desired results. At day 32, SES-low + G and
SES-low demonstrated the highest healing scores (20.85±0.36 and
20.03±0.19, respectively) compared with Pl with 17.50±0.22 and S
with (17.11±0.20 (Fig. 7; Table SIX). SES-high, SES-high + G and NF
had similar performance (~18 points) and without significant
differences between them or the rest of the groups. It is
interesting to notice that on day 6, treatments with the highest
healing scores were NF, SES-low and SES-low + G; NF was
significantly different compared with S. At day 9, SES-low + G,
SES-low and Pl groups had the highest values; only SES-low + G was
significantly different compared with S. These results partially
coincide with the speed of wound closure observed in Fig. 2, which demonstrates that proper
wound healing is not only matter of fast wound closure.
SES-low + G had the best Healing
status
There was a significant difference in healing status
at day 32 between Pl and SES-low and SES-low + G (P<0.01), as
well as between S and SES-Low + Gel. At this day, SES-low + G
yielded the best healing status (Fig.
8; Table SX). This effect can
be attributable to the low inflammatory infiltrate observed in such
groups, during wound healing evolution and particularly at day 9.
On the contrary, treatments with Pl and S had the worst healing
status, with no significant difference between them. No significant
differences in healing status were observed between groups at days
6, 9, or 18 (Fig. 8; Table SX).
Discussion
Successful treatment of full-thickness burns is a
global challenge due to severity of the wounds and a health issue
due to the impact in life quality of affected individuals (6,51,66).
The healing of third-degree burns is a dynamic and complex process
characterized by inflammatory, proliferative and remodeling phases,
which result in regeneration and re-epithelization of affected
tissues (11,60).
The inflammatory phase is characterized by
chemotaxis of different cells to the injured site, release of
histamine, pro-inflammatory factors, vasodilation, diapedesis and
activation of white blood cells and fibroblasts (11,60).
Pro-oxidant mechanisms, hemostasis and removal of dead tissue and
foreign and microbial material also occur (11,60).
In the proliferative phase, migration of keratinocytes, fibroblasts
and endothelial cells occurs, resulting in new epithelization, with
formation of fibronectin, collagen fibers, granulation tissue and
neovascularization (53,60). A key driving force of this phase is
growth factors produced by activated neutrophils and macrophages
during the inflammatory phase (60,67).
In the remodeling stage, the concentration of fibroblasts
decreases, excess collagen is degraded and various enzymes and
growth factors in the extracellular fluids that accumulate in and
around a wound promote tissue repair (60,68,69).
In severe burns, most affected tissue may be non-viable or
necrotic, with poor or compromised vascularity; this inhibits the
influx of white blood cells, such as neutrophils, into the injury
site, complicating the healing process, while the risk of infection
increases (68). Use of antiseptics
and healing agents is key to prevent complications. Ideally,
antiseptics must be effective to avoid infection and non-toxic to
prevent wound healing inhibition.
The present study investigated the effect of
different antiseptics on the quality of wound repair. SES-low and
SES-low + G yielded the best wound healing parameters. NF showed a
good performance but was significantly worse than SES-low + G in
terms of orientation of the collagen and presence of mature
collagen at day 32. All treatments produced wound closure, however
SES-low significantly improved wound healing compared with Pl and S
due to the anti-inflammatory effect. S exhibited significantly
higher levels of inflammation infiltrate than the rest of the
groups, while NF and SES-high produced less favorable healing
process than SES-low.
As aforementioned, the active species of SES mimics
the active species of chlorine and ROS produced by some white blood
cells; this may explain the non-irritant effect of SES-low.
Additionally, SES promotes wound healing by increasing oxygenation
of the lesion, regulating inflammatory activity and modulating
several immune-redox processes (23,26,28,70,71).
ROS participate in the synthesis and deposition of
collagen and other proteins such as elastin in a
concentration-dependent manner (72-76).
Exposure of fibroblasts to low concentrations of ROS increases
elastin mRNA synthesis platelet activation is detected when dermal
glycoprotein-VI is exposed to ROS (74-75).
On the other hand, high concentrations of reactive oxygen species
(ROS) are associated with oxidative damage, which can significantly
impact wound healing (74,75,77).
High ROS levels are known to cause oxidative damage to various
cellular components, including lipids, proteins, and nucleic acids
(74,75,77).
This oxidative damage can inhibit collagen synthesis, leading to
reduced fibroblast function and a weakened extracellular matrix
(78). The fragmentation of
collagen fibers due to high ROS levels further compromises the
structural integrity of the tissue, making it more susceptible to
complications and delayed healing (77). Excessive ROS can also lead to
abnormal cross-linking of collagen fibers, resulting in stiffer and
less elastic tissue, which negatively affects the remodeling
process and can lead to the formation of fibrotic scars (77). Additionally, elevated ROS levels
contribute to skin aging, wrinkles, and a loss of elasticity, which
can impair the tissue's ability to heal effectively in epithelial
tissue (73,74,76).
Active species of chlorine and oxygen in SES
regulate the secretion/inhibition of specific cytokines, such as
TGF-β and EGF, which attract and stimulate the proliferation of
fibroblasts and keratinocytes, key cells in new tissue formation
and re-epithelization (71). Also,
SES serves as an immunomodulatory factor, inhibiting the secretion
of TNF-α and IL-6 (26,28,70,71).
SES can upregulate the production and utilization of intracellular
calcium, as well as matrix metalloproteinases 1 and 9, which play
an essential role in all stages of wound healing by modifying the
wound matrix, allowing cell migration and promoting faster tissue
healing and remodeling (71,78,79).
However, the exact mechanisms by which these active species
interact with cells, potentially causing damage or facilitating
repair, have not been fully elucidated. Active species of chlorine
and oxygen in SES can impact cellular integrity via oxidative
stress and inflammation (25,70),
however, both previous evidence and the present study demonstrate a
beneficial effect of topical SES-low application on wound healing
without notable adverse effects (25,30,80,81).
Future research should focus on elucidating the underlying
mechanisms, examining how these active species influence cytokine
regulation, and understanding their impact on cellular function and
integrity. This deeper understanding will provide a clearer picture
of the dual role of chlorine and oxygen species in both potential
cell damage and healing, contributing to more comprehensive
understanding of how SES supports wound repair at the molecular and
cellular level.
SES-low and SES-low + G exhibited better collagen
scores and healing status than SES-high and SES-high + G. NF,
SES-high and SES-high + G treatments did not significantly impede
wound healing, but produced an irritant effect as evidenced by the
increased inflammatory infiltrate and less organized collagen
deposition observed in these groups resulting in a lower healing
status. This was also observed for S treatment, due to the
increased inflammatory cell infiltration and less efficient
collagen matrix deposition. None of the treatments were worse than
Pl. SES-low and SES-low + G treatments were significantly better
than Pl and S groups. The controlled inflammatory cell infiltration
response and increased wound healing quality in the SES-low groups
were consistent with previous reports on wound healing and
anti-inflammatory activity of SES (24,25,27).
In a wound healing the transition from early to mature collagen is
a positive indicator of proper healing because it promotes the
generation of complex structures oriented for tensile strength
restoration (66). However,
excessive deposition and/or agglomeration is indicative of abnormal
or hypertrophic wound healing (82,83).
Then the organization of the collagen matrix is crucial for
high-quality wound healing. horizontal orientation of collagen
fibers facilitates recovery of the damaged area and is associated
with a better organization and function of scar tissue (66).
The moisture of injury is also another factor for
proper wound evolution. In comparison with dry environments,
moisturized wounds show reduced necrosis and inflammatory
infiltrate, as well as increased angiogenesis and faster and better
quality of healing (84-87).
SES-low + G showed the best healing status, which may be explained
by increased wound moisture and prevalence of the SES in the
lesion; pattern of collagen formation was more structured
(horizontal), with less accumulation of mature collagen and
inflammatory infiltrate. SES-high resulted in similar performance
to NF in terms of collagen formation/remodeling and fiber
orientation but healing quality was lower compared with SES-low.
Sulfadiazine, one of the most commonly antimicrobial topical
dressings used by physicians for treatment of second and
third-degree burns had the poorest performance in wound healing due
to a prolonged inflammatory effect (88,89).
Previous studies have reported that sulfadiazine slows wound
healing (90,91). Additionally, silver particles may
activate inflammatory responses when recognized as foreign material
or as an antigen (92,93). Clinically, other adverse effects
have been observed following the use of S, such as black scarring,
restricted wound penetration, which refers to the reduced ability
of the treatment to effectively reach deeper layers of the wound.
Additionally, its use has been associated with hypersensitivity
reactions, renal toxicity, and leukopenia, (89,91).
Consequently, long-term use of is not recommended due to these
potential risks and side effects (89).
The present pre-clinical model demonstrated that
SES-low was the best antimicrobial substance for wound care since
it did not compromise tissue repair and promoted high-quality wound
healing, especially in combination with G. Nevertheless, the
present study had limitations, such as use of the mouse model,
which does not fully replicate complexities of wound healing in
humans. For example, human skin is thicker and more complex than
mouse skin with a wound healing processes that involves
re-epithelialization and scar formation, while mice heal rapidly
and primarily to wound contraction. Besides, human-wounds involve
more prolonged and meticulous immune response that those in mice
(47,55,94).
However, it is an accepted model as a first approach to these skin
repair processes and has been used to analyses the effect of SES on
cutaneous wounds (29,71).
Additionally, more detailed studies such as
immunohistochemistry and quantification of pro-/anti-inflammatory
biomarkers are required to understand the wound healing mechanisms
at molecular and cellular levels. Nonetheless, the present study
demonstrated the potential of SES-low as an alternative, to promote
high quality wound healing. Clinical trials or case reports are
necessary to validate these findings.
While the present findings demonstrated the
potential of SES-low as an effective treatment for enhancing wound
healing in burn injury, translating these results into clinical
practice requires consideration. Determining the optimal dosing and
treatment frequency is key to achieve consistent therapeutic
outcomes in human patients, as the frequency and concentration of
SES application in the present study were tailored to the animal
model and human skin may respond differently, necessitating dose
adjustments. Although products based on SES are already available
on the market as adjuvants for the treatment of acute and chronic
wounds, it remains important to explore their precise formulation,
dosing and application in clinical scenarios. For example, many of
these products recommend cleaning the wound with water and soap,
drying it and performing debridement with sterile gauze before
applying SES directly to the wound three times/day or as directed
by the physician (95,96), whereas the present laboratory study
used a single daily dose. Therefore, it is necessary to establish
new paradigms to confirm safety and effectiveness of SES in
treating deep partial-thickness wounds and refine these guidelines
for optimal use.
Moreover, it is key to consider and study potential
interactions between SES and other topical products, commonly used
in the integral treatment of these kind of injuries (6,9). As
aforementioned before, third-degree burns require individualized
therapy depending on medical condition and severity of the injurie,
for example, moisturizers, polymeric membrane dressing with or
without growing factors, and skin grafts are typically included in
full-thickness burn management (11-16).
These interactions may influence the efficacy of SES as
antimicrobial, or the performance of the other adjuvants and
dressing agents used to promote wound healing, or the tolerance of
the patient to the integral treatment. For example, the present
study demonstrated irritation and prolonged inflammation following
treatment with SES-high and S but the specific impact of these side
effects on the animal model was not elaborated. These adverse
effects may lead to delayed wound closure or compromised tissue
integrity, posing challenges in patient care. Strategies to
mitigate these side effects, such as investigating the
compatibility of antimicrobial substances with additional adjuvants
and dressings, and adjusting their posology and frequency of
administration should be explored to enhance patient outcomes.
More studies are needed to understand the dynamics
of antimicrobials in these kind of integral burn treatments,
especially the SES-based therapies, ensuring practicality and
benefits in real-world scenarios while balancing therapeutic
efficacy with safety in both preclinical and clinical settings.
Patients with burns typically receive comprehensive treatment
regimens, including hyperbaric oxygen therapy and pharmaceutical
interventions, which were not considered in the present animal
model. The present study demonstrates SES as a potential effective
adjunctive therapy for wound healing, but its integration with
established treatments such as hyperbaric oxygen therapy and
pharmaceuticals requires further exploration. Future research
should investigate the combined effects of SES with these standard
therapies to determine how SES can be optimized alongside
conventional burn care practices. This approach will provide a more
comprehensive understanding of SES and its potential benefits when
used in conjunction with existing treatments in clinical
settings.
Supplementary Material
Collagen characteristics evaluated on
day 6. *P<0.05 vs. Pl, **P<0.01 vs. Pl,
***P<0.001 vs. Pl, #P<0.05 vs. SES-low,
##P<0.01 vs. SES-low, ###P<0.001 vs.
SES-low, ♦P<0.05 vs. SES-low + G,
♦♦P<0.01 vs. SES-low + G, ♦♦♦P<0.001
vs. SES-low + G, ♥P<0.05 vs. SES-high,
♥♥♥P<0.001 vs. SES-high, ♣♣P<0.01 vs.
SES-high + G, ♣♣♣P<0.001 vs. SES-high + G,
♪P<0.05 vs. S, ♪♪P<0.01 vs. S. SES,
superoxidized electrolyzed solution; NF, nitrofurazone; Pl,
placebo; S, silver sulfadiazine; and G, gel.
Collagen characteristics evaluated on
day 9. #P<0.05 vs. SES-low, ♦P<0.05 vs.
SES-low + G, ♦♦P<0.01 vs. SES-low + G,
♥♥P<0.01 vs. SES-high, ♠♠P<0.01 vs. NF,
♠♠♠P<0.001 vs. NF, ♪P<0.05 vs. S,
♪♪♪P<0.001 vs. S. SES, superoxidized electrolyzed
solution; NF, nitrofurazone; Pl, placebo; S, silver sulfadiazine;
and G, gel.
Collagen characteristics evaluated on
day 18. **P<0.01 vs. Pl, ***P<0.001 vs.
Pl, #P<0.05 vs. SES-low, ##P<0.01 vs.
SES-low, ♥P<0.05 vs. SES-high, ♠♠P<0.01
vs. NF, ♪P<0.05 vs. S, ♪♪P<0.01 vs. S.
SES, superoxidized electrolyzed solution; NF, nitrofurazone; Pl,
placebo; S, silver sulfadiazine; and G, gel.
Representative Masson's staining. Day
(A) 9 and (B) 18. Magnification, x40. SES, superoxidized
electrolyzed solution; NF, nitrofurazone; Pl, placebo; S, silver
sulfadiazine; and G, gel.
Mean wound area(mm2).
Mean wound closure (%).
Polymorphonuclear cell count.
Monocyte count.
Mean inflammatory infiltrate.
Histology analysis of early collagen
with Masson staining.
Histology analysis of the collagen
orientation with Masson staining.
Histology analysis of mature collagen
with Masson staining.
Healing score.
Healing status.
Acknowledgements
The authors would like to Professor Julio V.
Barrios Nuñez (University of Colima, Colima, Mexico) for assistance
with English language editing.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
IDE and BAPM conceived the study and revised the
manuscript. NSAC, MLMF, CMR, DCC and MWG designed the methodology.
AEHR, GAHF and MLMF analyzed and interpretation of data. MWG, ACL,
and MDM, IPRS and GAHF analyzed the data. ARH, OBG, and CBCA
performed the experiments. IDE, GAHF, BAPM and ACL wrote the
manuscript. GAHF and ACL revised the manuscript. IDE and MLMF
supervised the study. IDE and BAPM confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the Colima State Institute of Cancerology
(Colima, Mexico; approval no. CIIECAN/06/19).
Patient consent for publication
Not applicable.
Competing interests
BPM and ACL are employees at Esteripharma but did
not participate in the decision to publish the results of the
study, nor in its development or data collection. The rest of the
authors declare that they have no competing interests.
References
|
1
|
Summer GJ, Puntillo KA, Miaskowski C,
Green PG and Levine JD: Burn injury pain: The continuing challenge.
J Pain. 8:533–548. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Warby R and Maani CV: Burn classification.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing,
2024.
|
|
3
|
Stiles K: Emergency management of burns:
Part 2. Emerg Nurse. 26:36–41. 2018.PubMed/NCBI
|
|
4
|
Eyvaz K, Kement M, Balin S, Acar H, Kündeş
F, Karaoz A, Civil O, Eser M, Kaptanoglu L, Vural S and Bildik N:
Clinical evaluation of negative-pressure wound therapy in the
management of electrical burns. Turk J Trauma Emerg Surg.
24:456–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu YT, Chen KH, Ban SL, Tung KY and Chen
LR: Evaluation of leap motion control for hand rehabilitation in
burn patients: An experience in the dust explosion disaster in
Formosa Fun Coast. Burns. 45:157–164. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Markiewicz-Gospodarek A, Kozioł M, Tobiasz
M, Baj J, Radzikowska-Büchner E and Przekora A: Burn wound healing:
Clinical complications, medical care, treatment, and dressing
types: The current state of knowledge for clinical practice. Int J
Environ Res Public Health. 19(1338)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
James SL, Lucchesi LR, Bisignano C, Castle
CD, Dingels ZV, Fox JT, Hamilton EB, Henry NJ, McCracken D, Roberts
NLS, et al: Epidemiology of injuries from fire, heat and hot
substances: Global, regional and national morbidity and mortality
estimates from the global burden of disease 2017 study. Inj Prev.
26 (Supp 1):i36–i45. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abarca L, Guilabert P, Martin N, Usúa G,
Barret JP and Colomina MJ: Epidemiology and mortality in patients
hospitalized for burns in Catalonia, Spain. Sci Rep.
13(14364)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yakupu A, Zhang J, Dong W, Song F, Dong J
and Lu S: The epidemiological characteristic and trends of burns
globally. BMC Public Health. 22(1596)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Giummarra MJ, Casey SL, Devlin A, Ioannou
LJ, Gibson SJ, Georgiou-Karistianis N, Jennings PA, Cameron PA and
Ponsford J: Co-occurrence of posttraumatic stress symptoms, pain,
and disability 12 months after traumatic injury. Pain Rep.
2(e622)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tiwari VK: Burn wound: How it differs from
other wounds? Indian J Plast Surg. 45:364–373. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hoang DM, Pham PT, Bach TQ, Ngo ATL,
Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, et
al: Stem cell-based therapy for human diseases. Signal Transduct
Target Ther. 7(272)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shu DY, Hutcheon AEK, Zieske JD and Guo X:
Epidermal growth factor stimulates transforming growth factor-beta
receptor type II expression in corneal epithelial cells. Sci Rep.
9(8079)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rothe MJ and Falanga V: Growth factors and
wound healing. Clin Dermatol. 9:553–559. 1991.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Negut I, Grumezescu V and Grumezescu A:
Treatment strategies for infected wounds. Molecules.
23(2392)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Norman G, Christie J, Liu Z, Westby MJ,
Jefferies JM, Hudson T, Edwards J, Mohapatra DP, Hassan IA and
Dumville JC: Antiseptics for burns. Cochrane Database Syst Rev.
7(CD011821)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lagziel T, Asif M, Born L, Quiroga LH,
Duraes E, Slavin B, Shetty P, Caffrey J and Hultman CS: Evaluating
the efficacy, safety, and tolerance of silver sulfadiazine
dressings once daily versus twice daily in the treatment of burn
wounds. J Burn Care Res. 42:1136–1139. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kazemzadeh J, Yousefiazar A and Zahedi A:
Amniotic membrane dressing versus nitrofurazone-impregnated
dressing in the treatment of second-degree burn wounds: A
randomized clinical trial. Wounds. 34:11–16. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Webber CE, Glanges E and Crenshaw CA:
Treatment of second degree burns: Nitrofurazone, povidone-iodine,
and silver sulfadiazine. JACEP. 6:486–490. 1977.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yamakawa S and Hayashida K: Advances in
surgical applications of growth factors for wound healing. Burns
Trauma. 7(10)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Toral-Freyre SDC, Castillejos-López M,
Hernández A, Becerril-Vargas E, Mújica-Sánchez MA, Mendoza-Romero
VM, Casino-Ríos A, González-Mancera G, Cabrera-Licona A and
Mervitch-Sigal N: Uso de una solución electrolizada de
superoxidación para desinfectar mascarillas de ventilación mecánica
no invasiva. Neumol Cir Torax. 81:224–231. 2022.(In Spanish).
|
|
22
|
Cárdenas AM, Campos-Bijit V, Di Francesco
F, Schwarz F, Cafferata EA and Vernal R: Electrolyzed water for the
microbiologic control in the pandemic dental setting: A systematic
review. BMC Oral Health. 22(579)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan P, Daliri EBM and Oh DH: New clinical
applications of electrolyzed water: A review. Microorganisms.
9(136)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Delgado-Enciso I, Paz-Garcia J,
Barajas-Saucedo C, Mokay-Ramírez KA, Meza-Robles C, Lopez-Flores R,
Delgado-Machuca M, Murillo-Zamora E, Toscano-Velazquez JA,
Delgado-Enciso J, et al: Safety and efficacy of a COVID-19
treatment with nebulized and/or intravenous neutral electrolyzed
saline combined with usual medical care vs usual medical care
alone: A randomized, open-label, controlled trial. Exp Ther Med.
22(915)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aurelien-Cabezas NS, Paz-Michel BA,
Jacinto-Cortes I, Delgado-Enciso OG, Montes-Galindo DA,
Cabrera-Licona A, Zaizar-Fregoso SA, Paz-Garcia J, Ceja-Espiritu G,
Melnikov V, et al: Protective effect of neutral electrolyzed saline
on gentamicin-induced nephrotoxicity: Evaluation of histopathologic
parameters in a murine model. Medicina (Kaunas).
59(397)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
González-Cantú CC, Torres-Muñoz Á,
Urrutia-Baca VH, Sánchez-García GA and De La Garza-Ramos MA:
Antibacterial efficacy of a pH-neutral electrolyzed super-oxidized
solution for nonsurgical periodontal treatment. Heliyon.
8(e12291)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gutiérrez-García R, De La Cerda-Ángeles
JC, Cabrera-Licona A, Delgado-Enciso I, Mervitch-Sigal N and
Paz-Michel BA: Nasopharyngeal and oropharyngeal rinses with neutral
electrolyzed water prevents COVID-19 in front-line health
professionals: A randomized, open-label, controlled trial in a
general hospital in Mexico City. Biomed Rep. 16(11)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Medina-Tamayo J, Sánchez-Miranda E,
Balleza-Tapia H, Ambriz X, Cid ME, González-Espinosa D, Gutiérrez
AA and González-Espinosa C: Super-oxidized solution inhibits
IgE-antigen-induced degranulation and cytokine release in mast
cells. Int Immunopharmacol. 7:1013–1024. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fadriquela A, Sajo MEJ, Bajgai J, Kim DH,
Kim CS, Kim SK and Lee KJ: Effects of strong acidic electrolyzed
water in wound healing via inflammatory and oxidative stress
response. Oxid Med Cell Longev. 2020(2459826)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zaizar-Fregoso SA, Paz-Michel BA,
Rodriguez-Hernandez A, Paz-Garcia J, Aurelien-Cabezas NS,
Tiburcio-Jimenez D, Melnikov V, Murillo-Zamora E, Delgado-Enciso
OG, Cabrera-Licona A, et al: Systemic administration of neutral
electrolyzed saline as a novel treatment for rheumatoid arthritis
reduces mechanical and inflammatory damage to the joints:
Preclinical evaluation in mice. Evid Based Complement Alternat Med.
2022(1717614)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Opneja A, Kapoor S and Stavrou EX:
Contribution of platelets, the coagulation and fibrinolytic systems
to cutaneous wound healing. Thromb Res. 179:56–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mishra B, Sharma DJ and Arora C:
Comparative study of the efficacy of ionic silver solution and
super oxidized solution in the management of chronic wounds. Med J
Armed Forces India. 79:40–45. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
García JP, Maldonado RA, Díaz RI, Muñiz J
and Rodríguez HA: Sustitución del uso de solución salina
fisiológica como irrigante en el manejo de pacientes sépticos y
quirúrgicos por solución electrolizada. Rev Mex Cir Bucal
Maxilofac. 7:46–52. 2011.(In Spanish).
|
|
34
|
Yahagi N, Kono M, Kitahara M, Ohmura A,
Sumita O, Hashimoto T, Hori K, Ning-Juan C, Woodson P, Kubota S, et
al: Effect of electrolyzed water on wound healing. Artif Organs.
24:984–987. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
United Mexican States-Ministry of Health:
Regulation of the General Health Law on Health Research. Official
Journal of the Federation: 1-31, 1987 (In Spanish). https://www.diputados.gob.mx/LeyesBiblio/regley/Reg_LGS_MIS.pdf.
|
|
36
|
Ministry of Agriculture GDRP and A:
Mexican norm NOM-0062-ZOO-1999 entitled Technical specifications
for the production, care and use of laboratory animals. Government
of Mexico, Mexico, 1999 (In Spanish). https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF.
|
|
37
|
de Aluja AS: Laboratory animals and
official Mexican norms (NOM-062-ZOO-1999). Gac Med Mex.
138:295–298. 2002.PubMed/NCBI(In Spanish).
|
|
38
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition. Washington (DC): National Academies Press (US), 2011.
|
|
39
|
Mels C, Niebuhr K, Futschik A, Rault JL
and Waiblinger S: Development and evaluation of an animal health
and welfare monitoring system for veterinary supervision of pullet
farms. Prev Vet Med. 217(105929)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mattsson JL, Spencer PJ and Albee RR: A
performance standard for clinical and functional observational
battery examinations of rats. J Am Coll Toxicol. 15:239–254.
1996.
|
|
41
|
Redfern WS, Dymond A, Strang I, Storey S,
Grant C, Marks L, Barnard C, Heys C, Moyser K, Greenwood K, et al:
The functional observational battery and modified Irwin test as
global neurobehavioral assessments in the rat: Pharmacological
validation data and a comparison of methods. J Pharmacol Toxicol
Methods. 98(106591)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Talbot SR, Biernot S, Bleich A, van Dijk
RM, Ernst L, Häger C, Helgers SOA, Koegel B, Koska I, Kuhla A, et
al: Defining body-weight reduction as a humane endpoint: A critical
appraisal. Lab Anim. 54:99–110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
McIntyre MK, Clifford JL, Maani CV and
Burmeister DM: Progress of clinical practice on the management of
burn-associated pain: Lessons from animal models. Burns.
42:1161–1172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Girtler R and Gustorff B: Schmerztherapie
bei verbrennungen. Anaesthesist. 60:243–250. 2011.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
45
|
Animal Experimentation Center Ethics
Committee (CEUMA): Surgical Analgesia Refinement in Surgical
Protocols in Experimental Animals. Málaga, 2019 (In Spanish).
https://www.fmvz.unam.mx/fmvz/principal/archivos/cicuae/Protocolos_analgesia_roedores.pdf.
|
|
46
|
American Veterinary Medical Association:
AVMA Guidelines for the euthanasia of animals: 2013 Edition. 5-95,
2013. https://www.in.gov/boah/files/AVMA_Euthanasia_Guidelines.pdf.
|
|
47
|
Abdullahi A, Amini-Nik S and Jeschke MG:
Animal models in burn research. Cell Mol Life Sci. 71:3241–3255.
2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Cortes-Alvarez SI, Delgado-Enciso I,
Rodriguez-Hernandez A, Hernandez-Fuentes GA, Aurelien-Cabezas NS,
Moy-Lopez NA, Cortes-Alvarez NY, Guzman-Muñiz J, Guzman-Esquivel J,
Rodriguez-Sanchez IP, et al: Efficacy of hot tea infusion vs
ethanolic extract of moringa oleifera for the simultaneous
treatment of nonalcoholic fatty liver, hyperlipidemia, and
hyperglycemia in a murine model fed with a high-fat diet. J Nutr
Metab. 2024(2209581)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang L, Hu Q, Jin H, Yang Y, Yang Y, Yang
R, Shen Z and Chen P: Effects of ginsenoside Rb1 on second-degree
burn wound healing and FGF-2/PDGF-BB/PDGFR-β pathway modulation.
Chin Med. 16(45)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 Years of image analysis. Nat Methods.
9:671–675. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gupta A and Kumar P: Assessment of the
histological state of the healing wound. Plast Aesthet Res.
2:239–242. 2015.
|
|
52
|
Santos TS, Santos IDDD, Pereira-Filho RN,
Gomes SVF, Lima-Verde IB, Marques MN, Cardoso JC, Severino P, Souto
EB and Albuquerque-Júnior RLC: Histological evidence of wound
healing improvement in rats treated with oral administration of
hydroalcoholic extract of vitis labrusca. Curr Issues Mol Biol.
43:335–352. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Holzer-Geissler JCJ, Schwingenschuh S,
Zacharias M, Einsiedler J, Kainz S, Reisenegger P, Holecek C,
Hofmann E, Wolff-Winiski B, Fahrngruber H, et al: The impact of
prolonged inflammation on wound healing. Biomedicines.
10(856)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sultana J, Molla MR, Kamal M, Shahidullah
M, Begum F and Bashar MA: Histological differences in wound healing
in Maxillofacial region in patients with or without risk factors.
Bangladesh J Pathol. 24:3–8. 1970.
|
|
55
|
Masson-Meyers DS, Andrade TAM, Caetano GF,
Guimaraes FR, Leite MN, Leite SN and Frade MAC: Experimental models
and methods for cutaneous wound healing assessment. Int J Exp
Pathol. 101:21–37. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Onesti MG, Fioramonti P, Carella S and
Maruccia M: The importance of periwound skin in the treatment of
‘difficult wound’. G Chir. 32:83–88. 2011.PubMed/NCBI(In Italian).
|
|
57
|
Maurizi E, Adamo D, Magrelli FM, Galaverni
G, Attico E, Merra A, Maffezzoni MBR, Losi L, Genna VG, Sceberras V
and Pellegrini G: Regenerative medicine of epithelia: Lessons from
the past and future goals. Front Bioeng Biotechnol.
9(652214)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liew YM, McLaughlin RA, Gong P, Wood FM
and Sampson DD: In vivo assessment of human burn scars through
automated quantification of vascularity using optical coherence
tomography. J Biomed Opt. 18(061213)2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cardoso JC, Veraitch O, Gianotti R,
Ferrara G, Tomasini CF, Singh M, Zalaudek I and Stefanato CM:
‘Hints’ in the horn: Diagnostic clues in the stratum corneum. J
Cutan Pathol. 44:256–278. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lateef Z, Stuart G, Jones N, Mercer A,
Fleming S and Wise L: The cutaneous inflammatory response to
thermal burn injury in a murine model. Int J Mol Sci.
20(538)2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Andritoiu CV, Andriescu CE, Danu M, Lungu
C, Ivanescu B, Havarneanu C and Popa M: Evaluation of the wound
healing potential of some natural polymers on three experimental
models. Pharmaceuticals (Basel). 14(465)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tang X, Wang X, Sun Y, Zhao L, Li D, Zhang
J, Sun H and Yang B: Magnesium oxide-assisted dual-cross-linking
bio-multifunctional hydrogels for wound repair during
full-thickness skin injuries. Adv Funct Mater. 31(2105718)2021.
|
|
63
|
Itoi E, Minagawa H, Yamamoto N, Seki N and
Abe H: Are pain location and physical examinations useful in
locating a tear site of the rotator cuff? Am J Sports Med.
34:256–264. 2006.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ito M, Yang Z, Andl T, Cui C, Kim N,
Millar SE and Cotsarelis G: Wnt-dependent de novo hair follicle
regeneration in adult mouse skin after wounding. Nature.
447:316–320. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Oshimori N and Fuchs E: Paracrine TGF-β
signaling counterbalances BMP-mediated repression in hair follicle
stem cell activation. Cell Stem Cell. 10:63–75. 2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Schultz GS, Chin GA, Moldawer L and
Diegelmann RF: Principles of wound healing. Fitridge R and Thompson
M (eds). In: Mechanisms of Vascular Disease: A Reference Book for
Vascular Specialists [Internet]. Adelaide (AU): University of
Adelaide Press, 2011.
|
|
67
|
Englbrecht M, Tarner IH, van der Heijde
DM, Manger B, Bombardier C and Müller-Ladner U: Measuring pain and
efficacy of pain treatment in inflammatory arthritis: A systematic
literature review. J Rheumatol Suppl. 90:3–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Rose LF and Chan RK: The burn wound
microenvironment. Adv Wound Care (New Rochelle). 5:106–118.
2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Evers LH, Bhavsar D and Mailänder P: The
biology of burn injury. Exp Dermatol. 19:777–783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Montesinos-Peña NE, Hernández-Valencia M,
Delgado-Enciso I, Herrera-Leal A and Paz-Michel BA: Evaluación de
un gel antiséptico de aplicación intravaginal para pacientes con
infecciones cervicovaginales multitratadas. Ginecol Obstet Méx.
87:454–466. 2019.
|
|
71
|
You HS, Fadriquela A, Sajo MEJ, Bajgai J,
Ara J, Kim CS, Kim SK, Oh JR, Shim KY, Lim HK and Lee KJ: Wound
healing effect of slightly acidic electrolyzed water on cutaneous
wounds in hairless mice via immune-redox modulation. Biol Pharm
Bull. 40:1423–1431. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Papaccio F, D'Arino A, Caputo S and Bellei
B: Focus on the contribution of oxidative stress in skin aging.
Antioxidants (Basel). 11(1121)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Siwik DA, Pagano PJ and Colucci WS:
Oxidative stress regulates collagen synthesis and matrix
metalloproteinase activity in cardiac fibroblasts. Am J Physiol
Cell Physiol. 280:C53–C60. 2001.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kawaguchi Y, Tanaka H, Okada T, Konishi H,
Takahashi M, Ito M and Asai J: Effect of reactive oxygen species on
the elastin mRNA expression in cultured human dermal fibroblasts.
Free Radic Biol Med. 23:162–165. 1997.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Carrim N, Walsh TG, Consonni A, Torti M,
Berndt MC and Metharom P: Role of focal adhesion tyrosine kinases
in GPVI-dependent platelet activation and reactive oxygen species
formation. PLoS One. 9(e113679)2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Jang JY, Min JH, Chae YH, Baek JY, Wang
SB, Park SJ, Oh GT, Lee SH, Ho YS and Chang TS: Reactive oxygen
species play a critical role in collagen-induced platelet
activation via SHP-2 oxidation. Antioxid Redox Signal.
20:2528–2540. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Pérez-Sánchez A, Barrajón-Catalán E,
Ruiz-Torres V, Agulló-Chazarra L, Herranz-López M, Valdés A,
Cifuentes A and Micol V: Rosemary (Rosmarinus officinalis) extract
causes ROS-induced necrotic cell death and inhibits tumor growth in
vivo. Sci Rep. 9(808)2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Caley MP, Martins VLC and O'Toole EA:
Metalloproteinases and wound healing. Adv Wound Care (New
Rochelle). 4:225–234. 2015.
|
|
79
|
Belkhiri A, Richards C, Whaley M, McQueen
SA and Orr FW: Increased expression of activated matrix
metalloproteinase-2 by human endothelial cells after sublethal H2O2
exposure. Lab Invest. 77:533–539. 1997.PubMed/NCBI
|
|
80
|
Delgado-Enciso I, Paz-Garcia J,
Valtierra-Alvarez J, Preciado-Ramirez J, Olmedo-Buenrostro BA,
Delgado-Enciso J, Guzman-Esquivel J, Barajas-Saucedo CE,
Ceja-Espiritu G, Rodriguez-Sanchez IP, et al: A novel cell-free
formulation for the treatment of knee osteoarthritis generates
better patient-reported health outcomes in more severe cases. J
Orthop Surg (Hong Kong). 28(2309499020938121)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Delgado-Enciso I, Paz-Garcia J,
Rodriguez-Hernandez A, Madrigal-Perez VM, Cabrera-Licona A,
Garcia-Rivera A, Soriano-Hernandez AD, Cortes-Bazan JL,
Galvan-Salazar HR, Valtierra-Alvarez J, et al: A promising novel
formulation for articular cartilage regeneration: Preclinical
evaluation of a treatment that produces SOX9 overexpression in
human synovial fluid cells. Mol Med Rep. 17:3503–3510.
2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhang XR, Ryu U, Najmiddinov B, Trinh TTT,
Choi KM, Nam SY and Heo CY: Effect of silicone patch containing
metal-organic framework on hypertrophic scar suppression. In Vivo.
38:235–245. 2024.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Cheng W, Rong Y, Ning F and Zhang GA: The
content and ratio of type I and III collagen in skin differ with
age and injury. Afr J Biotechnol. 10:2524–2529. 2011.
|
|
84
|
Dyson M, Young SR, Hart J, Lynch JA and
Lang S: Comparison of the effects of moist and dry conditions on
the process of angiogenesis during dermal repair. J Invest
Dermatol. 99:729–733. 1992.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Vogt PM, Andree C, Breuing K, Liu PY,
Slama J, Helo G and Eriksson E: Dry, moist, and wet skin wound
repair. Ann Plast Surg. 34:493–500. 1995.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Barnett A, Berkowitz RL, Mills R and
Vistnes LM: Comparison of synthetic adhesive moisture vapor
permeable and fine mesh gauze dressings for split-thickness skin
graft donor sites. Am J Surg. 145:379–381. 1983.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Carter K: Hydropolymer dressings in the
management of wound exudate. Br J Community Nurs. 8 (9
Suppl):S10–S16. 2003.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Dai T, Huang YY, Sharma SK, Hashmi JT,
Kurup DB and Hamblin MR: Topical antimicrobials for burn wound
infections. Recent Pat Antiinfect Drug Discov. 5:124–151.
2010.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Levin NJ, Erben Y, Li Y, Brigham TJ and
Bruce AJ: A systematic review and meta-analysis comparing burn
healing outcomes between silver sulfadiazine and Aloe vera. Cureus.
14(e30815)2022.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Muller MJ, Hollyoak MA, Moaveni Z, Brown
TLH, Herndon DN and Heggers JP: Retardation of wound healing by
silver sulfadiazine is reversed by Aloe vera and nystatin. Burns.
29:834–836. 2003.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Hosseinimehr SJ, Khorasani G, Azadbakht M,
Zamani P, Ghasemi M and Ahmadi A: Effect of aloe cream versus
silver sulfadiazine for healing burn wounds in rats. Acta
Dermatovenerol Croat. 18:2–7. 2010.PubMed/NCBI
|
|
92
|
Cascione M, Rizzello L, Manno D, Serra A
and De Matteis V: Green silver nanoparticles promote inflammation
shutdown in human leukemic monocytes. Materials (Basel).
15(775)2022.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Bartucci R, van der Meer AZ, Boersma YL,
Olinga P and Salvati A: Nanoparticle-induced inflammation and
fibrosis in ex vivo murine precision-cut liver slices and effects
of nanoparticle exposure conditions. Arch Toxicol. 95:1267–1285.
2021.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Zomer HD and Trentin AG: Skin wound
healing in humans and mice: Challenges in translational research. J
Dermatol Sci. 90:3–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
PLM Latin America: Estericide antiseptic
solution-PLM. PLM: 1, 2023 (In Spanish). https://www.medicamentosplm.com/Home/productos/estericide.solucion.antiseptica.solucion.topica/1365/101/33563/186.
|
|
96
|
Vademecum V: Estericide antiseptic
solution. Vademecum: 1, 2023 (In Spanish). https://www.vademecum.es/mexico/medicamento/1277798/estericide-solucion-antiseptica.
|