1. Introduction
Mango is a fruit-bearing tree cultivated in tropical
and sub-tropical regions of the world, with India being the largest
producer (1). The genus
Mangifera (family Anacardiaceae) comprises >70 species
and 1,000 varieties (2). Among the
various species, MI is the most widely found species across India,
China, Mexico, Brazil, Pakistan, Thailand and The Philippines. MI
is believed to have originated from the Indo-Burma region (2). The mango fruits are popular due to
sensorial properties, such as a bright colour, luscious flavour and
sweet taste (3). A large variety of
phytochemicals with distinct chemical structures and a range of
pharmacological properties have been reported in MI (1). Furthermore, various parts of MI have
been used in the traditional medicines of South Asian and African
countries. For example, extracts and decoctions prepared from
different parts of MI are used in the treatment of various ailments
and conditions, including anaemia, asthma, bronchitis, cough,
diarrhoea, dysentery, haemorrhage, hypertension, leucorrhoea, piles
and rheumatism, blisters and oral wounds, and animal bites
(1). Images of Mangifera
indica (MI) leaves, bark and fruits are presented in Fig. 1A (left panel, middle panel and right
panel, respectively).
Mangifera zeylanica (MZ), a species in the
mango family, is a plant endemic to Sri Lanka. Similar to MI, MZ
also bears edible fruits (4). The
tree grows up to a height of 10-30 m and branches from a stout
trunk. Compared with MI, MZ possesses smaller leaves (7-13 cm in
length, dark green and shiny) and fruits (3-5 cm long and ripen
fruits are yellow in colour) (Fig.
1B). The seed occupies a larger volume of the fruit than the
fruit pulp (4). In Sri Lankan
traditional medicine, the bark of MZ has been used in the treatment
of various ailments and diseases, including cancer (1). According to the International Union
for Conservation of Nature (IUCN), MZ has been classified as a
vulnerable species (5). MZ leaves,
bark and fruits are presented in Fig.
1B (left panel, middle panel and right panel,
respectively).
Epigenetics modifications arise due to alterations
in gene function that cannot be endorsed to DNA sequence
modifications. Two key biological processes, DNA methylation and
histone modifications (histone acetylation, deacetylation
sumoylation and ubiquitylation) have been reported to largely
contribute to such epigenetics modifications (6). In normal cells, it has been reported
that the functions of a number of genes are highly regulated
through DNA methylation and histone modifications (6). Given that epigenetic modifications
tightly control gene expression, it is not surprising that any
irregularity in the epigenetic modification is associated with
aberrant gene functions (7).
Notably, the dysregulation of epigenetics modifications have been
reported in a range of human cancers (8). Several phytochemicals have been
identified as promising drug candidates which can re-establish
aberrant epigenetic profiles (9).
The present review article discusses the biological and
pharmacological effects of various extracts and phytochemicals of
MI and MZ, as well as the effects of major mango bio-active
compounds on epigenetic modifications.
2. Medicinal properties of MI
MI is one of the widely used medicinal plants. A
number of pre-clinical investigations have reported a range of
pharmacological effects of different MI extracts (1). MI is well known to possess
polyphenolic compounds, which have been found to have diverse
biological activities (1).
Different parts of MI, including the leaves, stem, flowers, fruits
and seeds possess essential nutrients, such as vitamins and
minerals (10). The following
sections describe some experimentally validated pharmacological
effects (antioxidant, anti-inflammatory, anti-diabetic,
anti-obesity and anti-microbial properties) of different parts
(leaves, bark, seed and fruit peel and flesh) of MI.
Antioxidant properties
A number of studies have demonstrated the
antioxidant properties of extracts prepared from different parts of
MI. Dhital (11) demonstrated that
the leaf methanol extract of MI exerts moderate free radical
scavenging effects (12). In
another study, among the aqueous bark extracts prepared from the
Keitt, Kent, Honey (originally Ataulfo) and Tommy Atkins MI
varieties, the aqueous bark extract of Kent was reported to possess
the most pronounced antioxidant properties. Moreover, the Ataulfo
extract was found to exert the most prominent cellular anti-oxidant
effects (12). The leaf methanol
extract of Mahajanaka, a mango variety found in Thailand, has been
shown to exert potent free radical scavenging effects. The same
leaf extract has also been shown to inhibit
lipopolysaccharide-induced nitric oxide production in RAW264.7
cells (13). In another study by
John et al 2012, exposure to an aqueous ethanol extract of
MI stem bark increased the red blood cell count in Wistar strain
albino rats, indicating its ability to enhance erythropoiesis and
exert protective effects against oxidative damage. Furthermore, the
increment of monocytes and neutrophils following exposure to the
aqueous ethanol extract indicated its ability to enhance and
modulate immunological activities (14). The leaf ethanolic extract of MI has
been found to have antioxidant properties in vivo. In a
previous study, the attenuation of cerebral oxidative status in
rats was observed when the rats were fed various different doses of
MI leaf ethanol extract. Moreover, increased levels of
malondialdehyde, and reduced superoxide dismutase and glutathione
peroxidase activities were observed in the hippocampus of the
tested animals (15). The ethanolic
extract of MI fruits has also been found to exert protective
effects against cognitive impairment and oxidative stress (16). These observations indicate the
ability of the MI plant extract to function as an antioxidant in
vitro and in vivo.
Anti-inflammatory properties
The anti-inflammatory properties of mango peel, seed
and pulp of Sri Lankan mango varieties have been investigated
(17). Experiments conducted using
the human red blood cell membrane stabilization assay demonstrated
that the peel, pulp and seed ethyl acetate extracts of three
different mango varieties (Willard, Vellaicolomban and
Karthacolomban) exerted anti-inflammatory activities. Among these,
the ethyl acetate extract of Karthacolomban seeds exhibited the
highest anti-inflammatory activity (17). The ethanol leaf extract of MI was
found to possess analgesic and anti-inflammatory effects
in-vivo (18). Wistar
Hannover rats treated with MI leaf ethanol extract have also been
shown to exhibit diminished inflammatory activities induced by 4%
formalin (19). In the study by Kim
et al (20), polyphenolic
derivatives of MI were shown to modulate dextran sulfate sodium
(DSS)-induced colitis in rats, suggesting that MI polyphenolic
derivatives can attenuate inflammatory responses. In another study,
the methanol extract of MI stem bark was found to exert
anti-inflammatory effects against DSS-induced colitis (21). Moreover, the administration of
aqueous stem bark extracts of MI resulted in a reduction in the
levels of thiobarbituric acid reactive substances, the expression
of tumour necrosis factor-α (TNF-α), cyclooxigenase-2, inducible
nitric oxide synthase and TNF receptor-2 in colonic tissue and, and
in serum TNF-α and interleukin (IL)-6 levels (21). Collectively, these findings suggest
that mango peel, seed and leaf extracts possess anti-inflammatory
properties.
Anti-diabetic and anti-obesity
effects
The study by Perpétuo and Salgado (22) demonstrated the effects of diets
containing mango flour on blood glucose levels in diabetic rats.
Diabetic rats fed mango flour exhibited reduced blood glucose
levels from the 10th day of feeding. Moreover, a 64% reduction in
glycogen levels was observed compared to the control group
(22). MI flavonoids have been
reported to reduce blood glucose levels in Swiss albino mice with
induced diabetes (23).
Furthermore, MI leaf aqueous extract was found to increase
high-density lipoprotein levels (23). The ethanol (95%) extract of MI
leaves and mangiferin modulated the endocannabinoid (CB) system and
peroxisome proliferator-activated receptor-γ (PPARγ) mRNA
expression in cafeteria diet-fed rats, suggesting a possible role
for MI in controlling obesity and metabolic syndrome (24). The endocannabinoid system has been
reported to contribute to weight gain and glucose intolerance,
while PPARγ is one the key regulators of adipose cell development
and differentiation (24).
The peel acetone extract of MI possesses
hypoglycaemic effects. The study by Gondi and Rao illustrated that
the leaf extract of MI can be used against streptozotocin-induced
diabetes in rats (25). Rats fed
various doses of MI peel extracts were found to have lower levels
of glycated haemoglobin (25).
Narasimhan et al (26) also
demonstrated that albino rats of the Wistar strain fed various
doses of ferulic acid, a major compound found in mango, exhibited
an enhanced glycogen synthesis, improved blood glucose tolerance
and reduced gluconeogenic enzyme activities. Moreno et al
(27) demonstrated that MI extracts
inhibited the action of lipoprotein lipase and hormone sensitive
lipase in male Wistar rats. Animals receiving stem bark and leaf
extracts exhibited an inhibition of isoproterenol-stimulated
glycerol release and an increment in faecal fat. Moreover, animals
receiving MI leaf extract also exhibited an inhibition of
pancreatic lipase (27). A study on
lean and obese individuals identified that a mango supplementation
decreased plasminogen activator inhibitor-1, glycated haemoglobin
and inflammatory cytokine (IL-8 and monocyte chemoattractant
protein-1) levels in obese individuals and controlled blood
pressure, indicating a positive role of mango consumption against
metabolic and obesity-related chronic diseases (28).
Anti-microbial properties
Acetone extracts of MI leaves have been shown to
exhibit antibiotic activity against antibiotic-sensitive and
multidrug-resistant bacteria, such as Salmonella typhi,
Staphylococcus aureus and Salmonella typhimurium
(29). Furthermore, a methanol
extract of the seed kernal of MI has been found to exert inhibitory
effects against Staphylococcus aureus, Escherichia
coli and Bacillus subtilis (29). In the study by Manzur et al
(30), aqueous and ethanolic
extracts of MI leaves were found to exert inhibitory effects
against Stapylococcus species isolated from cows. In another
study, Mushore and Matuvhunye (31)
demonstrated that an aqueous extract of stem bark of MI can reduce
the growth of S. aureus. A recent study demonstrated that
the hexane peel extract prepared from the Sindhura and Banisha
mango varieties found in India exerted growth inhibitory effects
against Escherichia coli (32). In addition, methanolic and ethyl
acetate peel extracts was found to exert inhibitory effects against
Bacillus subtilis and Pseudomonas aeruginosa, respectively
(32). These findings suggest the
potential use of MI extracts and by-products as anti-microbial
agents. Further studies are required however, to elucidate
anti-microbial mechanisms and to isolate active compounds.
Anticancer properties
Various extracts and compounds isolated from MI have
been reported to exert anticancer effects in vitro and in
vivo. Noratto et al (33) evaluated the anticancer effects of
polyphenolic extracts prepared from five mango varieties (Haden,
Francis, Honey, Kent and Tommy Atkins) in breast (MDA-MB-231), lung
(A549), prostate (LNCaP), colon (SW-480) and leukaemia (Molt-4)
cancer cell lines. The Ataulfo and Haden polyphenolic extracts
exhibited cell growth inhibitory potential in all tested cancer
cell lines in a dose-dependent manner. The Ataulfo extract
exhibited the most potent inhibitory effects in the MDA-MB-231
cells, while the Haden extract displayed the most prominent
inhibitory effects in the Molt-4 cells. Fitriasih et al
(34) also demonstrated that the
treatment of MCF-7 breast cancer cells with a methanol extract of
MI leaves induced apoptosis by decreasing Bcl-2 expression
and increasing Bax expression. In another study, when
MDA-MB-231 cells were exposed to polyphenols extracted from MI
pulp, cell growth was inhibited and the expression of PI3K
signalling-associated miRNAs was modulated (35). Furthermore, Abdullah et al
(36) reported that MI seed
ethanolic extract induced the apoptosis of MCF-7 breast cancer
cells.
Notably, major polyphenols, such as mangiferin,
norathyriol and quercetin have been reported to exert inhibitory
effects against P-glycoprotein, a member of the adenosine
triphosphate binding cassette transporter superfamily responsible
for multidrug resistance in human cancers (37). The PI3K/AKT/mTOR signalling pathway
is a frequently activated signalling pathway in a range of human
cancers (38). Banerjee et
al (39) demonstrated that when
BT474 breast cancer cells were exposed to MI pulp extracts, the
expression of PI3K signalling-associated proteins, such as p-PI3K,
p-AKT and AKT was reduced. Furthermore, female athymic BALB/c nude
mice bearing BT474 tumours, exhibited a reduction in tumour size
and PI3K signalling-associated proteins when fed the pulp extract
(39). These findings indicate that
MI extracts and phytochemicals may have potential for use in the
development of drugs for cancer treatment. However, the possible
toxic effects of mango extracts and compounds need to be determined
and managed before using these as therapeutics for cancer in
clinical practice.
3. Medicinal properties of MZ
Although MZ has been used in Sri Lankan traditional
medicine against several diseases and conditions. including cancer,
the scientific validation of its use for cancer treatments was only
recently initiated (40). Hexane
and chloroform extracts of MZ bark have been reported to exert
anticancer effects in breast and ovarian cancer cells in
vitro (40,41). Moreover, two new halogenated
compounds and a new resorcinolic lipid were isolated from the bark
of MZ (41,42). The scientific evidence related to
the anticancer effects of MZ extracts and compounds is briefly
discussed below.
Anticancer properties exerted by
various MZ extracts and isolated compounds
Two new halogenated compounds (chloromangiferamide
and bromomangiferic acid), quercetin and catechin (41), as well as a new resorcinolic lipid
(42) were isolated from the bark
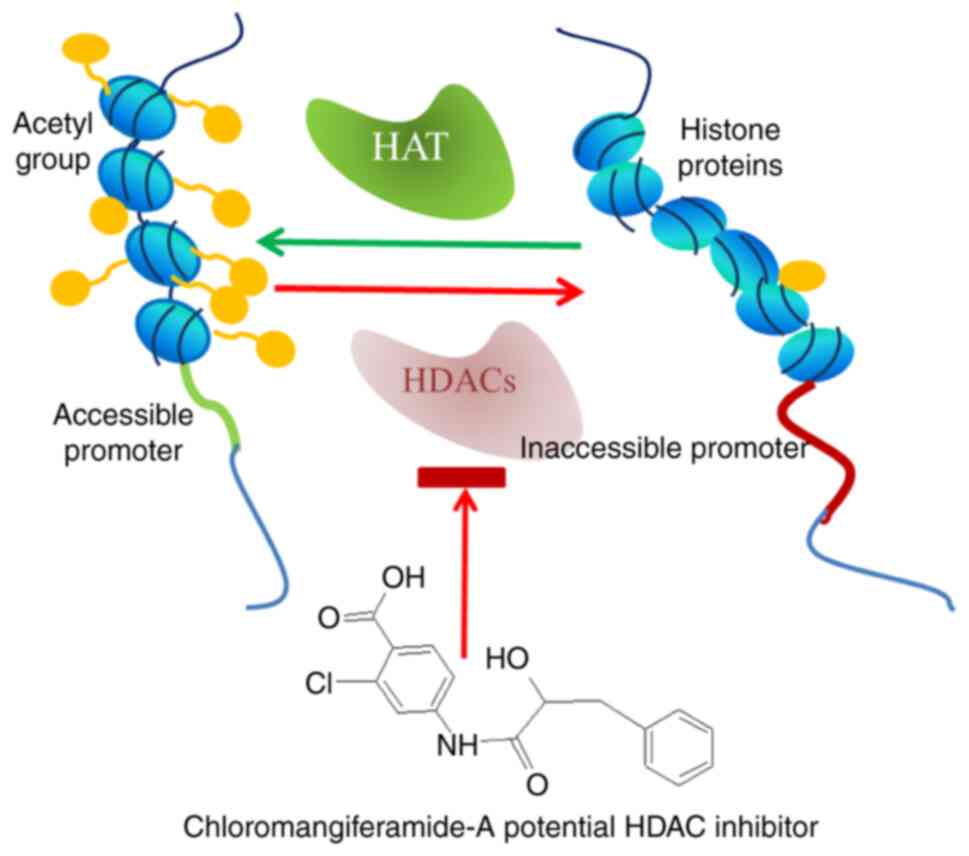
of MZ. Of these compounds, chloromangiferamide have been shown to
exert selective anticancer effects in MDA-MB-231 triple-negative
breast cancer cells with less cytotoxicity to MCF-10A normal
mammary epithelial cells. Furthermore, experiments performed using
qPCR assays revealed that chloromangiferamide regulated the
expression of genes associated with the cell cycle, apoptosis,
topoisomerases, drug metabolism, receptor tyrosine kinase
signalling, histone deacetylases (HDAC1-4, 6-8 and 11), protein
kinases, phosphatases, growth factors and PI3K signalling in
MDA-MB-231 cells (41). The
isolated new resorcinolic lipid has been shown to exert potent
cytotoxic effects through a mechanism related to oxidative stress
in MCF-7 oestrogen receptor positive breast cancer cells (42).
Studies conducted with MZ bark extracts have also
demonstrated notable scientific findings, which support its
traditional use in cancer treatment. The hexane extract of MZ bark
was previously reported to exert cytotoxic effects in breast (MCF-7
and MDA-MB-231) and ovarian (SKOV-3) cancer cells through the
induction of apoptosis. The gas chromatography-mass spectrometry
(GC-MS) analysis of the hexane extract of MZ bark identified
certain unknown compounds, which indicated the presence of new
phytochemicals in the bark of MZ (40). Furthermore, the chloroform extract
of MZ fruit peel induced the apoptosis of MCF-7 breast cancer cells
through a mechanism related to oxidative stress, suggesting the
potential use of MZ fruit peel as a cost-effective source for
anticancer compounds (43).
However, in order to elucidate the complete anticancer mechanisms
exerted by MZ extracts and isolated compounds, in vivo
investigations are necessary to determine the in vivo
anticancer efficacy and toxic effects of the MZ extracts.
Preliminary investigations conducted in the authors'
laboratories identified that extracts prepared from MZ leaves
exerted anticancer effects in breast and lung cancer cells in
vitro (unpublished data). In vitro investigations
performed using MI and MZ extracts are summarized in Table I, while in vivo
investigations performed using various MI extracts are listed in
Table II. The chemical structures
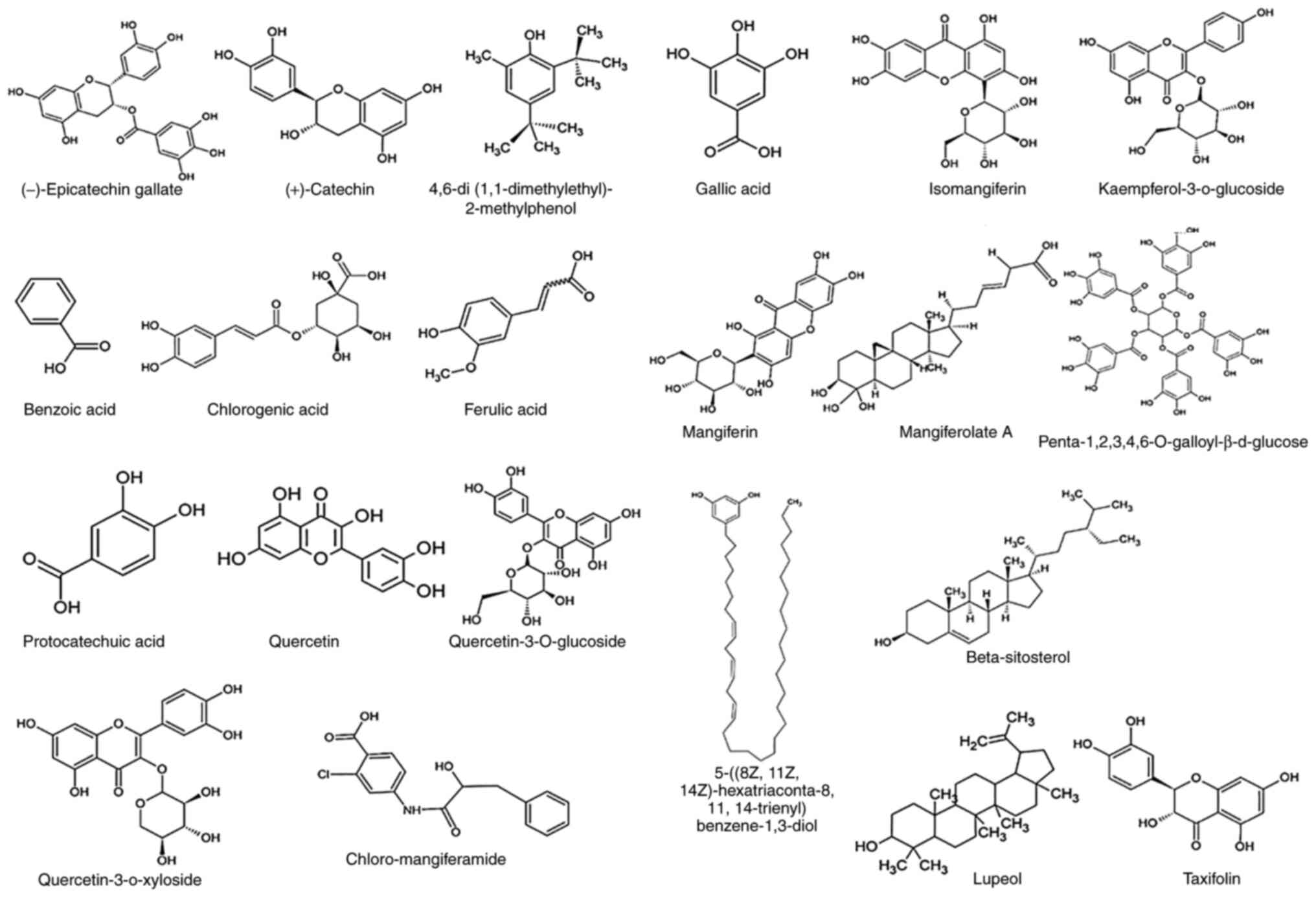
of the compounds present in MI and MZ are illustrated in Fig. 2. In addition, the pharmacological
activities of various compounds isolated from MI and MZ are
summarised in Table III. A
comparison of the anticancer activities of various MI and MZ
extracts is presented in Table IV.
Furthermore, the potential efficacy of chloromangiferamide to
target HDAC genes in MDA-MB-231 triple-negative breast cancer cells
is illustrated by the schematic diagram in Fig. 3.
 | Table IIn vitro and in vivo
studies conducted with different extracts/parts of Mangifera
indica and Mangifera zeylanica. |
Table I
In vitro and in vivo
studies conducted with different extracts/parts of Mangifera
indica and Mangifera zeylanica.
| Plant | Plant part and
extract |
Disease/condition | Cell line/s
used | Pathway/s | (Refs.) |
|---|
| Mangifera
indica | Ethanol and PBS
extracts of peel | Colorectal
cancer | HT29, Caco-2 and
HCT116 | γH2AX-mediated
genotoxicity and apoptosis | (44) |
| | Ethanol extract of
seed kernel | Breast cancer | MDA-MB-231 and
MCF-7 | Increments in
neutral red uptake and lactate dehydrogenase release and enhanced
cytotoxicity | (45,46) |
| | Aqueous extract of
fruits | Leukaemia | B-lymphocytes from
patients | Intrinsic pathway
of apoptosis and induction of oxidative stress | (47) |
| | Ethanol extract of
seed | Breast cancer | MCF-7 | Upregulation of
Bax, cytochrome c, p53 and caspases, | (36) |
| | | | | Reduction of GSH
and Bcl-2 levels and induction of oxidative stress | (36) |
| | Ethanol (80%)
extract of pomace | Malignant
tumours | HepG2, MCF-7, A549,
HeLa, A2780, HCT-116 and BGC-823 | Induction of
apoptosis | (48) |
| | Ethanol (80%)
extract of peel | Breast cancer | MCF-7 | Downregulation of
CYP19A1 leading to decreased aromatase activity | (49) |
| | Methanol extract of
peel | Breast cancer | MCF-7 | Inhibitory effects
on Ca2+ channels | (50) |
| | | | BT474 | Suppression of the
PI3K/AKT pathway | (39) |
| | | | | Modulation of the
miR-126 expression | (39) |
| | Mango beverage | Inflammation in
intestinal colitis | CCD-18Co | Modulation of the
miR-126/PI3K/AKT/mTOR axis | (51) |
| Mangifera
zeylanica | Peel chloroform
extract | Breast cancer | MCF-7 | Induction of
oxidative stress | (43) |
| | Hexane extract of
bark | Breast and ovarian
cancer | MCF-7, MDA-MB-231
and SKOV-3 | Induction of
apoptosis | (42) |
 | Table IIIn vivo pharmacological
activities of various extracts/major compounds of Mangifera
indica. |
Table II
In vivo pharmacological
activities of various extracts/major compounds of Mangifera
indica.
| Extract |
Disease/condition | Test animals | Effects | (Refs.) |
|---|
| Aqueous extract of
leaves | Diabetics | Wistar rat | Increment of
insulin sensitivity and levels in diabetic animals | (52) |
| Ethanol extract of
leaves | Obesity | Wistar rat | Modulation of
endocannabinoid system and PPARγ expression leading to the control
of metabolic syndrome and obesity | (24) |
| Mango
polyphenols | Anti-tumorigenic
properties | Female athymic
BALB/c nude mice | Suppression of the
PI3K/AKT pathway; modulation of miR-126 expression | (39) |
| Stem bark extract
and leaf extract (ethanol) | Obesity | Male Wistar
rats | Inhibition of
pancreatic lipase and lipoprotein lipase led to reduce the lipid
uptake in intestine and free fatty acids in adipose tissue | (27) |
| Mangiferin | Iron overload in
animal models | Sprague-Dawley
rats | Reduction of iron
accumulation in liver, spleen and heart | (53) |
| | Cisplatin-induced
nephrotoxicity | Male Wistar albino
rats | Protection of
kidney cells from apoptosis through modulation of the MAP kinase
pathway | (54) |
 | Table IIICompounds isolated from different
Mangifera species and their effects on cancer signalling
pathways. |
Table III
Compounds isolated from different
Mangifera species and their effects on cancer signalling
pathways.
| Mangifera
species | Identified
compounds | Cell lines
used | Pathway/s
affected | (Refs.) |
|---|
| Mangifera
indica | (-)-Epicatechin
gallate | SCC7, Tu177 and
Tu212 | Inhibition of the
canonical Wnt pathway | (55,56) |
| | | | Downregulation of
cyclin D1 expression | (55,56) |
| | 4,6-di
(1,1-Dimethylethyl)-2-methylphenol | MCF-7 and
MDA-MB-231 | Apoptosis and
oxidative stress | (12,45) |
| | Chlorogenic
acid | HCT116 | Generation of
reactive oxygen species | (57,58) |
| | | HT29 | Cell cycle arrest
at S phase | (57,58) |
| | Ferulic acid | HeLa and Caski | Downregulation of
MMP-9 expression | (59) |
| | Gallic acid | SMMC-7721 | Induction of
apoptosis | (60) |
| | Isomangiferin | MCF-7 | Induction of
apoptosis by inhibition of VEGFR 2 kinase | (61) |
| | Kaempferol
3-O-β-D-glucoside | HL-60 | Intrinsic pathway
of apoptosis Generation of reactive oxygen species Induction of
JNK/SAPK and ERK1/2 signalling | (56,62) |
| | Mangiferin | HL-60 | Inhibition of ATR,
Chk1, Wee1, Akt, and Erk1/2 phosphorylation | (63) |
| | | K562 | Overexpression of
BCR and ABL | (64) |
| | | OVCAR3 | Induction of
apoptosis through the Notch3 pathway | (65) |
| |
Penta-1,2,3,4,6-O-galloyl-β-d-glucose | LNCaP | Apoptosis | (33,66) |
| | Protocatechuic
acid | HL-60 | Suppression of
Bcl-2 and upregulation of Bax | (33) |
| | Quercetin | MGC803 | Modulation of the
PI3K/Akt/mTOR pathway | (67) |
| | | Nalm6 | Cell cycle arrest
at S phase | (68) |
| | | HCT116 and
HT29 | Suppression of
HSP27 | (57,68) |
| Mangifera
zeylanica |
Chloro-mangiferamide | MDA-MB-231 | Apoptosis,
downregulation of protein kinases, histone deacetylases and
heat-shock proteins | (41) |
| Mangifera
casturi | Lupeol | HCT116 | Canonical Wnt
pathway | (69,70) |
| Mangifera
pajang | Taxifolin | HCT119 and
HT29 | Canonical Wnt
pathway and cell cycle | (71,72) |
 | Table IVComparison of anticancer activities
of different parts of MI and MZ and grapes (Vitis
vinifera). |
Table IV
Comparison of anticancer activities
of different parts of MI and MZ and grapes (Vitis
vinifera).
| Plant part | Mangifera
indica | Mangifera
zeylanica | Vitis
vinifera | (Refs.) |
|---|
| Bark | Induces apoptosis
of MCF-7 breast cancer cells | Induces apoptosis
of MCF-7 cells | Not reported | (42,73) |
| Leaves | Induces apoptosis
of MCF-7 breast cancer cells by upregulating Bax and
downregulating Bcl | Induces apoptosis
of NCI-H292 lung cancer cells by upregulating p53, Bax and
downregulating Survivin (unpublished data) | Water and ethanol
extracts increase the expression of Bax in HUVECs and HepG2
liver cancer cells | (34,74) |
| Fruit pulp | Mango beverage
inhibits growth in CCD-18Co by modulating miR-126/PI3K/AKT/mTOR
axis | Not reported | Induces autophagy
in MCF-7 breast cancer cells | (39,75) |
| Seed | Ethanolic extract
enhances cytotoxicity to MCF-7 and MDA-MB-231 breast cancer
cells | Hexane extract
shows cytotoxicity to NCI-H292 lung cancer cells (unpublished
data) | Increases apoptosis
in DU145 and LNCaP prostate cancer cells | (36,76) |
| Fruit
peel/skin | Ethanol extract
inhibits MCF-7 breast cancer cell growth by downregulating the
CYP19A1 gene. Methanol extract inhibits Ca2+
channels in MCF-7 cells | Chloroform extract
induces oxidative stress in MCF-7 breast cancer cells | Induces apoptosis
of prostate cancer cells by targeting the phosphatidylinositol
3-kinase-Akt and mitogen-activated protein kinase survival
pathways | (43,49,77) |
4. Epigenetic modifications in human
cancer
Epigenetic modifications can be defined as heritable
alterations of gene expression levels which are independent of DNA
sequences (6). Apart from the DNA
methylation and histone modifications, microRNAs (miRNAs/miRs) also
contribute to the epigenetic regulation of gene expression
(78). It has been reported that
the regulation of epigenetic processes is largely controlled by the
environmental conditions, lifestyle, developmental stages,
pathological conditions and diet (79,80).
During the development and cellular differentiation of an organism,
cell type-specific epigenetic patterns developed define normal
pattern of gene functions in each cell type (80). Furthermore, it has been reported
that epigenetic modifications play a key role in the development of
a number of diseases, including cancer, autoimmune diseases,
neurodegenerative diseases and psychiatric disorders (81,82).
Therefore, understanding the role of epigenetics in human diseases
and identification potential drug targets which can target aberrant
epigenetic alterations will be extremely beneficial.
Chromatin structure
Nucleic acid material of eukaryotes consists of DNA
and histone proteins (83). Histone
proteins are found as octamers and are wrapped by 1.65 turns of
DNA. These octamers consist of two copies of histone subunits known
as H2A, H2B, H3 and H4. The N-terminal tails of these subunits
contain higher amounts of lysine resulting in an overall positive
charge. The chromatin structure functions as a protective mechanism
for genetic information and regulatory mechanism to control the
access to enzymes and proteins necessary for DNA replication and
gene expression (84).
DNA methylation and its role in human
cancers
DNA methylation exclusively takes place in the
cytosine bases of CpG dinucleotides in the human genome (85). Short CpG contents (0.5-4 kb),
referred to as CpG islands, are commonly found in the human gene
promoter regions (86). The
epigenetic silencing of tumour suppressor genes (for example
CDKN1C, CDKN2A, RUNX3, WT1, FOXA2, DAPK, TMS1, BCL2, HOXD11,
GPC3, LAMA3 and LKB1) due to the hypermethylation of CpG
islands is frequently observed in human cancers (86). Several oncogenes are upregulated
through promoter hypermethylation (6). For example, the promoter of the gene
SOSTDC1, which encodes for a bone morphogenetic protein, is
frequently upregulated through hypermethylation (85,86).
The FLT4 gene, that encodes a tyrosine kinase receptor for
vascular endothelial growth factors C and D, is frequently
upregulated through promoter hypermethylation. CYBA, a gene
that encodes for cytochrome B light chain is also upregulated
through DNA hypermethylation (86).
APC is a tumour suppressor gene known to regulate the Wnt
signalling pathway. In colorectal cancer the hypermethylation of
the APC gene promoter induces the downregulation of
APC (87). The RASAL2
gene that encodes for Ras-GTPase-activating protein 2 is also
upregulated through promoter hypomethylation in distinct human
cancers (88). DNA methyl
transferase inhibitors are useful drugs in cancer treatments.
Deoxycytidine is one of the very first drugs which undergoes a
series of phosphorylation steps and incorporates into CpG sites of
DNA, leading to the formation of covalent bonds between DNA methyl
transferase 1 (DNMT1) catalytic sites (89,90).
5-Aza-2'-deoxycytidine is a Food and Drug Administration
(FDA)-approved DNMT inhibitor used in the treatment of
myelodysplastic syndromes (91).
Histone modifications in cancer
Histone acetylation is another major epigenetic
modification event, which involves the acetylation of lysine
residues at the ε-amino groups of histones (92). This epigenetic modification is
controlled by two different groups of enzymes known as histone
acetyl transferases (HATs) and histone deacetylases (HDACs). HATs
transfer the acetyl group from Acetyl-Co A to amino acid group of
the targeted lysine residues found in the histone tails, while
HDACs counteract the actions of HATs (92).
Based on the structural homology, HATs are
classified into three different families known as Gcn5-related
N-acetyltransferases (GNATs), MOZ, Ybf2, Sas2, TIP60 (MYST) and
orphan [p300/CREB binding protein (CBP) and nuclear receptors]
(93). These enzymes neutralize
positive charges of the histone proteins, resulting in weakened
interactions between histones and DNA, rendering chromatin less
condensed and DNA more accessible to transcription factors
(94). Histone acetylation is
however targeted to specific regions of DNA through sequences
specific co-factors of HATs, including CBP, p300, MYST, and GNAT.
The deacetylation of histones leads to condensed chromatin,
resulting in the transcriptional silencing of genes (95).
Apart from the acetylation of histones, several
other modifications, such as the phosphorylation, methylation and
ubiquitination of histone subunits have also been identified.
Research performed using normal tissues, tumours and mouse models
has reported that the loss of acetylated Lys16 (K16-H4) of histone
H4 is a frequent event in human cancers (95). Moreover, a reduction in histone
acetylation has been shown to be associated with invasion and
metastasis in gastrointestinal tumours (95). Mutations, such as missense
mutations, truncations, translocations and frame shift mutations of
different families of HATs have been identified in several human
cancers, including colorectal, head, neck, breast and lung cancers
(96).
Investigations carried out with histone deacetylase
inhibitors (HDACis) have proven the role of HDACs in the regulation
of p21 protein (97). p21 Protein
is a cyclin-dependant kinase inhibitor which inhibits cyclin D1, A
and E. The inhibition of p21 leads to cell cycle arrest at the G1
or G2/M phases (98). In prostate
cancer cells, exposure to trichostatin A, an HDAC inhibitor, has
been found to induce the activation of p-glycoprotein expression
through the modulation of the histone markers, H3K4me2, H3K4me3,
H3K9Ac, H4Ac and H3Ac, indicating an involvement of an epigenetic
mechanism in the regulation of p-glycoprotein expression (99).
HDACis, also known as chromatin-modifying agents,
have been identified as a promising class of anticancer agents.
HDACis have the ability to re-establish dysregulated acetylation
profiles of cancer cells and re-activate the expression of
epigenetically silenced tumour suppressor genes, allowing cancer
cells to undergo programmed cell death (apoptosis) (6). To date, several pan or isoform
specific HDACis (natural and synthetic) have been developed and,
these HDACis allow for the identification of the role of various
HDACs in tumorigenesis (100).
Vorinostat (SAHA) for the treatment of cutaneous T-cell lymphomas
(CTCL), panobinostat (LBH-589) for the treatment of multiple
myeloma, belinostat (PXD101) for the treatment of peripheral T-cell
lymphomas (PTCL) and romidepsin for the treatment of CTCL and PTCL
are the only four FDA-approved HDACis in clinical use to date
(100). Natural compounds, such as
curcumin, epigallocatechin gallate, sulforaphanes, kaempferol,
resveratrol and butein are some examples for natural HDACis
(7).
miRNAs
A group of short RNA molecules (18-22 nucleotides),
known as miRNAs, also contribute to the epigenetic regulation of
gene expression (101). miRNAs are
transcribed by RNA polymerase II to yield primary-miRNAs
(pri-miRNAs), which are then cleaved into 65-70 nucleotide-long
hairpin RNA duplexes by DROSHA and PASHA (microprocessors)
(101,102). These are then exported to the
cytoplasm and further cleaved by a protein complex known as Dicer
to generate functional miRNAs. A complex comprised of miRNAs and a
protein known as RISC (RNA induced silencing complex) bind to the
target mRNA and degrade it, causing gene silencing (103). miRNAs play crucial roles in the
development, apoptosis, cell differentiation and proliferation
under normal conditions (104).
There is experimental evidence to indicate that a
considerable number of miRNAs can play oncogenic or
tumour-suppressive roles (105).
The upregulation of oncogenic miRNAs, also referred as oncomirs, is
frequently observed in a range of human cancers (105). It has been reported that any
functional irregularity in the expression and function of tumour
suppressor miRNAs can cause tumorigenesis (105). The Let-7 family miRNAs (let-7a,
let-7b and let-7g), miR-205, members of the miR-200 family
(miR-200a, b and c, miR141 and miR-429), miR-200c, miR-145 and
miR-142-3p have been reported to function as tumour suppressor
miRNAs, while oncogenic roles of miRNAs, such as miR-21, miR-10a,
miR-10b, miR-155, miR-17-92, miR-17-5p, miR-27a, miR-96 and miR-182
have also been identified (98,106,107). It has been found that epigenetic
modifications, such as histone deacetylation in promoter sites and
DNA methylation in CpG islands cause alternations in mRNA
expression. Therefore, epigenetic mechanisms provide promising drug
targets since DNA-demethylating agents and HDACis can be used to
re-establish mRNA expression implicated due to epigenetic
alternations (99). Several miRNAs
have also been identified to play a regulatory role in cancer stem
cells (CSCs). For example, the Let-7 family miRNAs have been
reported to play a role in CSC differentiation (99).
Mango compounds as epigenetic
modifiers
Several mango compounds have been reported to
function as epigenetic modulators. Epicatechin gallate has been
found to upregulate HDAC5 and HDAC7, while downregulating HDAC 1
and HDAC3. Moreover, the DNMT inhibitory activities of catechin
have also been reported (108).
Experiments performed using A/J mice have revealed
that epicatechin gallate leads to a significant elevation in the
levels of miRNAs related to lung tumours induced by
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. These miRNAs
include mmu-miR-2137, mmu-miR-449a, mmu-miR-144, mmu-miR-193,
mmu-miR-5030 and mmu-miR-2861. Moreover, the downregulation of
mmu-miR-969, mmu-miR-449c, mmu-miR-7a, mmu-miR-205 and mmu-miR-218
has also been observed due to the exposure to epicatechin gallate
(109).
A previous study performed using ATDC5 cells
revealed that mangiferin upregulated miR-181a expression. The
upregulation of miR-181a resulted in protection from
lipopolysaccharide-induced cell damage, by suppressing the levels
of PTEN and the NF-κB pathway (110). Another study performed using
glioma cells demonstrated that mangiferin upregulated miR-15b
expression, resulting in the suppression of MMP-9 in U87 cells,
indicating the ability of mangiferin to reduce metastasis (111).
5. Phytochemicals common to both MI and
MZ
Mangiferin is one of the well-known phytochemicals
found in MI. Mangiferin has also been isolated from the bark of MZ
(112). The therapeutic potential
of mangiferin has also been investigated. Mangiferin has been found
to exert preventive roles against mitochondrial depolarization,
oxidative stress and neuronal death (112). Lemus-Molina et al (113) demonstrated that the consumption of
MI extracts rich in mangiferin attenuated neuronal death during
normal aging and in neurodegenerative disorders. Another study
reported that mangiferin exerted potent antioxidant effects
(114). Mangiferin has also been
reported to exert cytotoxic effects in cancer cells (63,64).
Another study states that mangiferin can protect rats from cardiac
and renal damage (115). Rajendran
et al (116) demonstrated
cytoprotective and antioxidant effects of mangiferin. Apart from
these effects, mangiferin is capable of antagonizing the cytopathic
effect of HIV in vitro (117). Furthermore, a previous study
demonstrated that mangiferin induced apoptosis through the
PKC/NF-κB pathway and the cell cycle arrest of multiple myeloma
cells (118). Biersack (119) reported that mangiferin can mediate
the regulation of tumor suppressor miRNAs including miR-15b and
miR-182.
Apart from mangiferin, gallic acid and quercetin are
commonly found in the genus Mangifera. In a previous study,
Ediriweera et al (41)
isolated quercetin from the bark of MZ. The anti-proliferative
effects of gallic acid mediated by the epigenetic regulation of
miRNAs have been reported in glioma T98G cells (120). Sundaram et al (121) demonstrated that quercetin exerted
anti-proliferative effects in human cervical cancer cells.
Quercetin treatment has been shown to decrease the activity of
DNMTs, increasing the global acetylation of H3 and H4, and induce
the enrichment of acetylated histone H3 and H4 to the promoters of
genes related to apoptosis (121).
Previously, PLGA [poly(lactic-co-glycolic acid)]-loaded gold
nanoparticles prepared with quercetin were found to exert
anti-proliferative effects in hepatocellular carcinoma cells
through down-regulation of HDAC-Akt activities (122). On the whole, these studies
indicate that phytochemicals from MI and MZ are useful epigenetic
regulators.
6. Conclusion
MI is a pharmacologically and phytochemically
diverse plant. Extracts prepared from different parts (skin and
pulp of the fruit, leaves, bark, roots and seeds) of MI are used in
traditional medicine for the treatment of numerous diseases and
ailments. The fruits of MI are rich in vitamins, essential amino
acids and a range of polyphenols. In vitro and in
vivo experimental evidence indicates that different extracts
and major phytochemicals of MI can lead to beneficial pre-clinical
outcomes against several health conditions, including cancer
(1). Mango phytochemicals and mango
extracts have been reported to target aberrantly expressed cancer
signalling pathways (e.g., the PI3K/AKT/mTOR signalling pathway)
(1,39). Some major mango polyphenols have the
ability to re-store dysregulated epigenetic profiles in human
cancers. Of note, in vivo experimental evidence demonstrates
that MI extracts and some major compounds exert less toxic effects,
justifying their use in human systems without causing adverse
side-effects (14-19,21,22,25,26,29).
MZ is an endemic mango species found in Sri Lanka.
The bark of MZ has been used in Sri Lankan traditional medicine for
the treatment of a number of diseases, including cancer. Efforts
have been made to validate its traditional use scientifically. For
example, hexane and chloroform extracts of its bark and certain
novel compounds isolated from the bark of MZ have been reported to
exert anticancer effects in breast and ovarian cancer cells
(41,42). Moreover, peel chloroform extract
prepared from MZ fruits also exerts anticancer effects, indicating
that the anticancer potential of MZ is not limited to its bark.
However, to obtain a clear anticancer profile for MZ, in
vivo studies with distinct mouse models for cancer and the
evaluation of the toxic effects of MZ extracts are necessary.
Dysregulated epigenetic events have been reported to drive
tumorigenesis. A number of phytochemicals have exhibited the
ability to re-establish aberrant epigenetic profiles in a range of
human cancers. The present review article highlighted the ability
of certain mango compounds to function as epigenetic modifiers.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Department of
Science and Technology, Government of India (grant no.
DST/INT/SL/P-12/2016) and the Ministry of Science, Technology and
Research, Sri Lanka (grant no. MSTR/TRD/AGR/3/02/08).
Availability of data and materials
Not applicable.
Authors' contributions
All authors (IS, AV, NP, MKE, DN, SRS, KHT and VV)
contributed to the conceptualization, writing, drafting, revising,
editing and reviewing of the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: A review on ethnopharmacological applications, pharmacological
activities, and bioactive compounds of Mangifera indica
(mango). Evid Based Complement Alternat Med.
2017(6949835)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yadav D and Singh S: Mango: History origin
and distribution. J Pharmacogn Phytochem. 6:1257–1262. 2017.
|
|
3
|
Maldonado-Celis ME, Yahia EM, Bedoya R,
Landázuri P, Loango N, Aguillón J, Restrepo B and Guerrero Ospina
JC: Chemical composition of mango (Mangifera indica L.)
fruit: Nutritional and phytochemical compounds. Front Plant Sci.
10(1073)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Weerarathne WAPG, Samarajeewa PK and
Nilanthi RMR: Genetic diversity of etamba in Sri Lanka. J Trop Agri
Res Ext. 8:107–112. 2005.
|
|
5
|
IUCN. The IUCN Red List of Threatened
Species. Version 2021-3. 2021. https://www.iucnredlist.org/species/31400/9630295.
Accessed January 21, 2022.
|
|
6
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Emerging role of histone deacetylase inhibitors as
anti-breast-cancer agents. Drug Discov Today. 24:685–702.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Thakur VS, Deb G, Babcook MA and Gupta S:
Plant phytochemicals as epigenetic modulators: Role in cancer
chemoprevention. AAPS J. 16:151–163. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ghasemi S: Cancer's epigenetic drugs:
Where are they in the cancer medicines? Pharmacogenomics J.
20:367–379. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shankar E, Kanwal R, Candamo M and Gupta
S: Dietary phytochemicals as epigenetic modifiers in cancer:
Promise and challenges. Semin Cancer Biol. 40-41:82–99.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Princwill-Ogbonna IL, Ogbonna PC and
Ogujiofor IB: Proximate composition, vitamin, mineral and
biologically active compounds levels in leaves of Mangifera
indica (Mango), Persea Americana (Avocado pea), and
Annona muricata (Sour Sop). J Appl Sci Environ Manage.
23(65)2019.
|
|
11
|
Dhital KS: Phytochemical screening and
antioxidant activities of Mangifera indica leaves grown in
temperate region of the Nepal. J Pharmacogn Phytochem. 6:205–209.
2017.
|
|
12
|
Vazquez-Olivo G, Antunes-Ricardo M,
Gutiérrez-Uribe JA, Osuna-Enciso T, León-Félix J and Heredia JB:
Cellular antioxidant activity and in vitro intestinal permeability
of phenolic compounds from four varieties of mango bark
(Mangifera indica L.). J Sci Food Agric. 99:3481–3489.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khumpook T, Saenphet S, Tragoolpua Y and
Saenphet K: Anti-inflammatory and antioxidant activity of Thai
mango (Mangifera indica Linn.) leaf extracts. Comp Clin
Path. 28:157–164. 2019.
|
|
14
|
John OR, Yahaya AA and Emmanuel A: Aqueous
ethanolic extract of Mangifera indica stem bark effect on
the biochemical and haematological parameters of albino rats. Arch
Appl Sci Res. 4:1618–1622. 2012.
|
|
15
|
Al Omairi NE, Radwan OK, Alzahrani YA and
Kassab RB: Neuroprotective efficiency of Mangifera indica
leaves extract on cadmium-induced cortical damage in rats. Metab
Brain Dis. 33:1121–1130. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wattanathorn J, Muchimapura S, Thukham-Mee
W, Ingkaninan K and Wittaya-Areekul S: Mangifera indica
Fruit extract improves memory impairment, cholinergic dysfunction,
and oxidative stress damage in animal model of mild cognitive
impairment. Oxid Med Cell Longev. 2014(132097)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuganesan A, Thiripuranathar G, Navaratne
A and Paranagama P: Antioxidant and anti-inflammatory activities of
peels, pulps and seed kernels of three common mango (Mangifera
indical L.) varieties in Sri Lanka. Int. J Pharm Sci Res.
8:70–78. 2017.
|
|
18
|
Hassan M, Khan S, Shaikat A, Hossain M,
Hoque M, Ullah M and Islam S: Analgesic and anti-inflammatory
effects of ethanol extracted leaves of selected medicinal plants in
animal model. Vet World. 6:68–71. 2013.
|
|
19
|
Márquez L: Anti-inflammatory effects of
Mangifera indica L. extract in a model of colitis. World J
Gastroenterol. 16:4922–4931. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim H, Krenek KA, Fang C, Minamoto Y,
Markel ME, Suchodolski JS, Talcott ST and Mertens-Talcott SU:
Polyphenolic derivatives from mango (Mangifera indica L.)
modulate fecal microbiome, short-chain fatty acids production and
the HDAC1/AMPK/LC3 axis in rats with DSS-induced colitis. J Funct
Foods. 48:243–251. 2018.
|
|
21
|
Márquez L, Pérez-Nievas BG, Gárate I,
García-Bueno B, Madrigal JL, Menchén L, Garrido G and Leza JC:
Anti-inflammatory effects of Mangifera indica L. extract in
a model of colitis. World J Gastroenterol. 16:4922–4931.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Perpétuo GF and Salgado JM: Effect of
mango (Mangifera indica, L.) ingestion on blood glucose
levels of normal and diabetic rats. Plant Foods Hum Nutr. 58:1–12.
2003.
|
|
23
|
Saleem M, Tanvir M, Akhtar MF, Iqbal M and
Saleem A: Antidiabetic potential of Mangifera indica L. cv.
Anwar Ratol leaves: Medicinal application of food wastes. Medicina
(Kaunas). 55(353)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brito LF, Gontijo DC, Toledo RCL, Barcelos
RM, de Oliveira AB, Brandão GC, de Sousa LP, Ribeiro SMR, Leite
JPV, Fietto LG and de Queiroz JH: Mangifera indica leaves
extract and mangiferin modulate CB1 and PPARγ receptors and others
markers associated with obesity. J Funct Foods. 56:74–83. 2019.
|
|
25
|
Gondi M and Prasada Rao UJS: Ethanol
extract of mango (Mangifera indica L.) peel inhibits
α-amylase and α-glucosidase activities, and ameliorates diabetes
related biochemical parameters in streptozotocin (STZ)-induced
diabetic rats. J Food Sci Technol. 52:7883–7893. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Narasimhan A, Chinnaiyan M and Karundevi
B: Ferulic acid exerts its antidiabetic effect by modulating
insulin-signalling molecules in the liver of high-fat diet and
fructose-induced type-2 diabetic adult male rat. Appl Physiol Nutr
Metab. 40:769–781. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moreno DA, Ripoll C, Ilic N, Poulev A,
Aubin C and Raskin I: Inhibition of lipid metabolic enzymes using
Mangifera indica extracts. J Food Agric Environ. 4:21–26.
2006.
|
|
28
|
Barnes RC, Kim H, Fang C, Bennett W, Nemec
M, Sirven MA, Suchodolski JS, Deutz N, Britton RA, Mertens-Talcott
SU and Talcott ST: Body mass index as a determinant of systemic
exposure to gallotannin metabolites during 6-week consumption of
mango (Mangifera indica L.) and modulation of intestinal
Microbiota in lean and obese individuals. Mol Nutr Food Res.
63(1800512)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hannan A, Asghar S, Naeem T, Ikram Ullah
M, Ahmed I, Aneela S and Hussain S: Antibacterial effect of mango
(Mangifera indica Linn.) leaf extract against antibiotic
sensitive and multi-drug resistant Salmonella typhi. Pak J Pharm
Sci. 26:715–719. 2013.PubMed/NCBI
|
|
30
|
Manzur AG, Sm Junior V, Morais-Costa F,
Mariano EG, Careli RT, da Silva LM, Coelho SG, de Almeida AC and
Duarte ER: Extract of Mangifera indica L. leaves may reduce
biofilms of Staphylococcus spp. in stainless steel and
teatcup rubbers. Food Sci Technol Int. 26:11–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mushore J and Matuvhunye M: Antibacterial
properties of Mangifera indica on Staphylococcus
aureus. Afr J Clin Exp Microbiol. 14:62–74. 2013.
|
|
32
|
Umamahesh K, Ramesh B, Kumar BV and Reddy
OV: In vitro anti-oxidant, anti-microbial and anti-inflammatory
activities of five Indian cultivars of mango (Mangifera
indica L.) fruit peel extracts. J Herb Med Pharmacol.
8:238–247. 2019.
|
|
33
|
Noratto GD, Bertoldi MC, Krenek K, Talcott
ST, Stringheta PC and Mertens-Talcott SU: Anticarcinogenic effects
of polyphenolics from mango (Mangifera indica) Varieties. J
Agric Food Chem. 58:4104–4112. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fitriasih F, Komariyah SM, Sandra F,
Pratiwi N and Hidayati DN: Mangifera indica L. leaves
extract induced intrinsic apoptotic pathway in MCF-7 cells by
decreasing Bcl-2 expression and inducing Bax expression. Indones J
Cancer Chemoprevention. 10(1)2019.
|
|
35
|
Arbizu-Berrocal SH, Kim H, Fang C, Krenek
KA, Talcott ST and Mertens-Talcott SU: Polyphenols from mango
(Mangifera indica L.) modulate PI3K/AKT/mTOR-associated
micro-RNAs and reduce inflammation in non-cancer and induce cell
death in breast cancer cells. J Funct Foods. 55:9–16. 2019.
|
|
36
|
Abdullah AS, Mohammed A, Rasedee A and
Mirghani M: Oxidative stress-mediated apoptosis induced by
ethanolic mango seed extract in cultured estrogen receptor positive
breast cancer MCF-7 cells. Int J Mol Sci. 16:3528–3536.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chieli E, Romiti N, Rodeiro I and Garrido
G: In vitro effects of Mangifera indica and polyphenols
derived on ABCB1/P-glycoprotein activity. Food Chem Toxicol.
47:2703–2710. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Banerjee N, Kim H, Krenek K, Talcott ST
and Mertens-Talcott SU: Mango polyphenolics suppressed tumor growth
in breast cancer xenografts in mice: Role of the PI3K/AKT pathway
and associated microRNAs. Nutr Res. 35:744–751. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ediriweera MK, Tennekoon KH, Samarakoon
SR, Thabrew I and Dilip de Silva E: A study of the potential
anticancer activity of Mangifera zeylanica bark: Evaluation
of cytotoxic and apoptotic effects of the hexane extract and
bioassay-guided fractionation to identify phytochemical
constituents. Oncol Lett. 11:1335–1344. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ediriweera MK, Tennekoon KH, Adhikari A,
Samarakoon SR, Thabrew I and de Silva ED: New halogenated
constituents from Mangifera zeylanica Hook.f. and their potential
anti-cancer effects in breast and ovarian cancer cells. J
Ethnopharmacol. 189:165–174. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ediriweera MK, Tennekoon KH, Samarakoon
SR, Adhikari A, Thabrew I and Dilip de Silva E: Isolation of a new
resorcinolic lipid from Mangifera zeylanica Hook.f. bark and its
cytotoxic and apoptotic potential. Biomed Pharmacother. 89:194–200.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ediriweera MK, Tennekoon KH, Samarakoon
SR, Thabrew I and De Silva ED: Induction of apoptosis in MCF-7
breast cancer cells by Sri Lankan endemic mango (Mangifera
zeylanica) fruit peel through oxidative stress and analysis of its
phytochemical constituents: Anticancer effects of Mangifera
zeylanica Peel. J Food Biochem. 41(e12294)2017.
|
|
44
|
Lauricella M, Lo Galbo V, Cernigliaro C,
Maggio A, Palumbo Piccionello A, Calvaruso G, Carlisi D, Emanuele
S, Giuliano M and D'Anneo A: The Anti-cancer effect of Mangifera
indica L. Peel extract is associated to γH2AX-mediated
apoptosis in colon cancer cells. Antioxidants (Basel).
8(422)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Abdullah AS, Mohammed AS, Abdullah R,
Mirghani ME and Al-Qubaisi M: Cytotoxic effects of Mangifera
indica L. kernel extract on human breast cancer (MCF-7 and
MDA-MB-231 cell lines) and bioactive constituents in the crude
extract. BMC Complement Altern Med. 14(119)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yap KM, Sekar M, Seow JL, Gan SH, Bonam
SR, Rani NNIM, Lum PT, Subramaniyan V, Wu YS, Fuloria NK and
Fuloria S: Mangifera indica (Mango): A promising medicinal
plant for breast cancer therapy and understanding its potential
mechanisms of action. Breast Cancer (Dove Med Press). 13:471–503.
2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ayatollahi A, Rahmati J, Salimi A and
Pourahmad J: A comparison of cytotoxic effects of Mangifera
indica L. and Juglans RegiaAqueous extract on human
chronic Lymphocytic leukemia. Iran J Pharm Res. 18:1843–1853.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hu H, Zhao Q, Pang Z, Xie J, Lin L and Yao
Q: Optimization extraction, characterization and anticancer
activities of polysaccharides from mango pomace. Int J Biol
Macromol. 117:1314–1325. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shaban NZ, Hegazy WA, Abdel-Rahman SM,
Awed OM and Khalil SA: Potential effect of Olea europea
leaves, Sonchus oleraceus leaves and Mangifera indica
peel extracts on aromatase activity in human placental microsomes
and CYP19A1 expression in MCF-7 cell line: Comparative study. Cell
Mol Biol (Noisy-le-grand). 62:11–19. 2016.PubMed/NCBI
|
|
50
|
Taing MW, Pierson JT, Shaw PN, Dietzgen
RG, Roberts-Thomson SJ, Gidley MJ and Monteith GR: Mango fruit
extracts differentially affect proliferation and intracellular
calcium signalling in MCF-7 human breast cancer cells. J Chem.
2015(613268)2015.
|
|
51
|
Kim H, Banerjee N, Barnes RC, Pfent CM,
Talcott ST, Dashwood RH and Mertens-Talcott SU: Mango polyphenolics
reduce inflammation in intestinal colitis-involvement of the
miR-126/PI3K/AKT/mTOR axis in vitro and in vivo: Mango
polyphenolics suppress colitis. Mol Carcinog. 56:197–207.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Villas Boas GR, Rodrigues Lemos JM, de
Oliveira MW, dos Santos RC, Stefanello da Silveira AP, Barbieri
Bacha F, Ito CNA, Bortolotte Cornelius E, Brioli Lima F, Sachilarid
Rodrigues AM, et al: Aqueous extract from Mangifera indica
Linn. (Anacardiaceae) leaves exerts long-term hypoglycemic effect,
increases insulin sensitivity and plasma insulin levels on diabetic
Wistar rats. PLoS One. 15(e0227105)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Estuningtyas A, Setiabudy R, Wahidiyat P
and Freisleben HJ: The role of mangiferin in the prevention of
experimentally induced iron overload in an animal model. Drug Res
(Stuttg). 69:234–240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sahu AK, Verma VK, Mutneja E, Malik S, Nag
TC, Dinda AK, Arya DS and Bhatia J: Mangiferin attenuates
cisplatin-induced acute kidney injury in rats mediating modulation
of MAPK pathway. Mol Cell Biochem. 452:141–152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lim YC, Lee SH, Song MH, Yamaguchi K, Yoon
JH, Choi EC and Baek SJ: Growth inhibition and apoptosis by
(-)-epicatechin gallate are mediated by cyclin D1 suppression in
head and neck squamous carcinoma cells. Eur J Cancer. 42:3260–3266.
2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Coelho EM, de Souza MEAO, Corrêa LC, Viana
AC, de Azevêdo LC and dos Santos Lima M: Bioactive compounds and
antioxidant activity of mango peel liqueurs (Mangifera
indica L.) produced by different methods of maceration.
Antioxidants (Basel). 8(102)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Abbasi AM, Guo X, Fu X, Zhou L, Chen Y,
Zhu Y, Yan H and Liu RH: Comparative assessment of phenolic content
and in vitro antioxidant capacity in the pulp and peel of mango
cultivars. Int J Mol Sci. 16:13507–13527. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hou N, Liu N, Han J, Yan Y and Li J:
Chlorogenic acid induces reactive oxygen species generation and
inhibits the viability of human colon cancer cells. Anticancer
Drugs. 28:59–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gao J, Yu H, Guo W, Kong Y, Gu L, Li Q,
Yang S, Zhang Y and Wang Y: The anticancer effects of ferulic acid
is associated with induction of cell cycle arrest and autophagy in
cervical cancer cells. Cancer Cell Int. 18(102)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sun G, Zhang S, Xie Y, Zhang Z and Zhao W:
Gallic acid as a selective anticancer agent that induces apoptosis
in SMMC-7721 human hepatocellular carcinoma cells. Oncol Lett.
11:150–158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang B, Shen J, Wang Z, Liu J, Ning Z and
Hu M: Isomangiferin, a novel potent vascular endothelial growth
factor receptor 2 kinase inhibitor, suppresses breast cancer
growth, metastasis and angiogenesis. J Breast Cancer. 21:11–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Burmistrova O, Quintana J, Díaz JG and
Estévez F: Astragalin heptaacetate-induced cell death in human
leukemia cells is dependent on caspases and activates the MAPK
pathway. Cancer Lett. 309:71–77. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Peng ZG, Yao YB, Yang J, Tang YL and Huang
X: Mangiferin induces cell cycle arrest at G2/M phase through
ATR-Chk1 pathway in HL-60 leukemia cells. Genet Mol Res.
14:4989–5002. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Peng ZG, Luo J, Xia LH, Chen Y and Song
SJ: CML cell line K562 cell apoptosis induced by mangiferin.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 12:590–594. 2004.PubMed/NCBI(In Chinese).
|
|
65
|
Zou B, Wang H, Liu Y, Qi P, Lei T, Sun M
and Wang Y: Mangiferin induces apoptosis in human ovarian
adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol
Rep. 38:1431–1441. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mohan CG, Deepak M, Viswanatha GL, Savinay
G, Hanumantharaju V, Rajendra CE and Halemani PD: Anti-oxidant and
anti-inflammatory activity of leaf extracts and fractions of
Mangifera indica. Asian Pac J Trop Med. 6:311–314.
2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Shen X, Si Y, Wang Z, Wang J, Guo Y and
Zhang X: Quercetin inhibits the growth of human gastric cancer stem
cells by inducing mitochondrial-dependent apoptosis through the
inhibition of PI3K/Akt signaling. Int J Mol Med. 38:619–626.
2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Srivastava S, Somasagara RR, Hegde M,
Nishana M, Tadi SK, Srivastava M, Choudhary B and Raghavan SC:
Quercetin, a natural flavonoid interacts with DNA, arrests cell
cycle and causes tumor regression by activating mitochondrial
pathway of apoptosis. Sci Rep. 6(24049)2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Pardede A and Koketsu M: Antioxidant and
antileukemic activity of chemical components from bark of Mangifera
casturi. Comp Clin Path. 26:499–504. 2017.
|
|
70
|
Wang Y, Hong D, Qian Y, Tu X, Wang K, Yang
X, Shao S, Kong X, Lou Z and Jin L: Lupeol inhibits growth and
migration in two human colorectal cancer cell lines by suppression
of Wnt-β-catenin pathway. Onco Targets Ther. 11:7987–799.
2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ahmad S, Sukari MA, Ismail N, Ismail IS,
Abdul AB, Abu Bakar MF, Kifli N and Ee GC: Phytochemicals from
Mangifera pajang Kosterm and their biological activities. BMC
Complement Altern Med. 15(83)2015.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Razak S, Afsar T, Ullah A, Almajwal A,
Alkholief M, Alshamsan A and Jahan S: Taxifolin, a natural
flavonoid interacts with cell cycle regulators causes cell cycle
arrest and causes tumor regression by activating Wnt/β-catenin
signaling pathway. BMC Cancer. 18(1043)2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ediriweera MK, Tennekoon KH, Samarakoon
SR, Thabrew I and De Silva ED: Cytotoxic and apoptotic effects of
the bark of two common mango (Mangifera indica) varieties
from Sri Lanka on breast and ovarian cancer cells. Br J Pharm Res.
10:1–7. 2016.
|
|
74
|
Ferhi S, Santaniello S, Zerizer S,
Cruciani S, Fadda A, Sanna D, Dore A, Maioli M and D'hallewin G:
Total phenols from grape leaves counteract cell proliferation and
modulate apoptosis-related gene expression in MCF-7 and HepG2 human
cancer cell lines. Molecules. 24(612)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Scarlatti F, Maffei R, Beau I, Codogno P
and Ghidoni R: Role of non-canonical Beclin 1-independent autophagy
in cell death induced by resveratrol in human breast cancer cells.
Cell Death Differ. 15:1318–1329. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Jo EH, Lee SJ, Ahn NS, Park JS, Hwang JW,
Kim SH, Aruoma OI, Lee YS and Kang KS: Induction of apoptosis in
MCF-7 and MDA-MB-231 breast cancer cells by Oligonol is mediated by
Bcl-2 family regulation and MEK/ERK signaling. Eur J Cancer Prev.
16:342–347. 2007.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Morré DM and Morré DJ: Anticancer activity
of grape and grape skin extracts alone and combined with green tea
infusions. Cancer Lett. 238:202–209. 2006.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Schuebel K, Gitik M, Domschke K and
Goldman D: Making sense of epigenetics. Int J Neuropsychopharmacol.
19(pyw058)2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Szyf M and Meaney MJ: Epigenetics,
behaviour, and health. Allergy Asthma Clin Immunol. 4:37–49.
2008.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hamilton JP: Epigenetics: Principles and
practice. Dig Dis. 29:130–135. 2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Rozek LS, Dolinoy DC, Sartor MA and Omenn
GS: Epigenetics: Relevance and implications for public health. Annu
Rev Public Health. 35:105–122. 2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Moosavi A and Motevalizadeh Ardekani A:
Role of epigenetics in biology and human diseases. Iran Biomed J.
20:246–258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Margueron R and Reinberg D: Chromatin
structure and the inheritance of epigenetic information. Nat Rev
Genet. 11:285–296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Jones PA and Laird PW: Cancer epigenetics
comes of age. Nat Genet. 21:163–167. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
85
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
86
|
Rauluseviciute I, Drabløs F and Rye MB:
DNA hypermethylation associated with upregulated gene expression in
prostate cancer demonstrates the diversity of epigenetic
regulation. BMC Med Genomics. 13(6)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Liang TJ, Wang HX, Zheng YY, Cao YQ, Wu X,
Zhou X and Dong SX: APC hypermethylation for early diagnosis of
colorectal cancer: A meta-analysis and literature review.
Oncotarget. 8:46468–46479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
McLaughlin SK, Olsen SN, Dake B, De Raedt
T, Lim E, Bronson RT, Beroukhim R, Polyak K, Brown M, Kuperwasser C
and Cichowski K: The RasGAP gene, RASAL2, is a tumor and metastasis
suppressor. Cancer Cell. 24:365–378. 2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Williams BP, Pignatta D, Henikoff S and
Gehring M: Methylation-sensitive expression of a DNA demethylase
gene serves as an epigenetic rheostat. PLoS Genet.
11(e1005142)2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Da Costa EM, McInnes G, Beaudry A and
Raynal NJ: DNA methylation-targeted drugs. Cancer J. 23:270–276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Christman JK: 5-Azacytidine and
5-aza-2'-deoxycytidine as inhibitors of DNA methylation:
Mechanistic studies and their implications for cancer therapy.
Oncogene. 21:5483–5495. 2002.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Javaid N and Choi S: Acetylation- and
methylation-related epigenetic proteins in the context of their
targets. Genes (Basel). 8(196)2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Handy DE, Castro R and Loscalzo J:
Epigenetic modifications: Basic mechanisms and role in
cardiovascular disease. Circulation. 123:2145–2156. 2011.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Eberharter A and Becker PB: Histone
acetylation: A switch between repressive and permissive chromatin.
Second in review series on chromatin dynamics. EMBO Rep. 3:224–229.
2002.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Ropero S and Esteller M: The role of
histone deacetylases (HDACs) in human cancer. Mol Oncol. 1:19–25.
2007.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Shanmugam MK, Arfuso F, Arumugam S,
Chinnathambi A, Jinsong B, Warrier S, Wang LZ, Kumar AP, Ahn KS,
Sethi G and Lakshmanan M: Role of novel histone modifications in
cancer. Oncotarget. 9:11414–11426. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Ju R and Muller MT: Histone deacetylase
inhibitors activate p21(WAF1) expression via ATM. Cancer Res.
63:2891–2897. 2003.PubMed/NCBI
|
|
98
|
Shamloo U and Usluer S: p21 in cancer
research. Cancers (Basel). 11(1178)2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Ediriweera MK and Cho SK: Targeting miRNAs
by histone deacetylase inhibitors (HDACi): Rationalizing
epigenetics-based therapies for breast cancer. Pharmacol Ther.
206(107437)2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Suraweera A, O'Byrne KJ and Richard DJ:
Combination therapy with histone deacetylase inhibitors (HDACi) for
the treatment of cancer: Achieving the full therapeutic potential
of HDACi. Front Oncol. 8(92)2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Lau PW and MacRae IJ: The molecular
machines that mediate microRNA maturation. J Cell Mol Med.
13:54–60. 2008.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wei JW, Huang K, Yang C and Kang CS:
Non-coding RNAs as regulators in epigenetics. Oncol Rep. 37:3–9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Moutinho C and Esteller M: MicroRNAs and
epigenetics. Adv Cancer Res. 135:189–220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Mazzio EA and Soliman KFA: HTP
Nutraceutical screening for histone deacetylase inhibitors and
effects of HDACis on Tumor-suppressing miRNAs by Trichostatin A and
Grapeseed (Vitis vinifera) in HeLa cells. Cancer Genomics
Proteomics. 14:17–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Nguyen DD and Chang S: Development of
novel therapeutic agents by inhibition of oncogenic MicroRNAs. Int
J Mol Sci. 19(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther.
1(15004)2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Krutovskikh VA and Herceg Z: Oncogenic
microRNAs (OncomiRs) as a new class of cancer biomarkers.
Bioessays. 32:894–904. 2010.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Ciesielski O, Biesiekierska M and
Balcerczyk A: Epigallocatechin-3-gallate (EGCG) alters histone
acetylation and methylation and impacts chromatin architecture
profile in human endothelial cells. Molecules.
25(2326)2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Zhou H, Chen JX, Yang CS, Yang MQ, Deng Y
and Wang H: Gene regulation mediated by microRNAs in response to
green tea polyphenol EGCG in mouse lung cancer. BMC Genomics. 15
(Suppl 11)(S3)2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Ma Y, Liu Y, Ma Y, Jiang N, Wang L, Wang
B, Niu W, Hu Y, Lin Q and Yu B: Mangiferin relieves
lipopolysaccharide-induced injury by up-regulating miR-181a via
targeting PTEN in ATDC5 cells. Front Pharmacol.
11(137)2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Xiao J, Liu LI, Zhong Z, Xiao C and Zhang
J: Mangiferin regulates proliferation and apoptosis in glioma cells
by induction of microRNA-15b and inhibition of MMP-9 expression.
Oncol Rep. 33:2815–2820. 2015.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Alberdi E, Sánchez-Gómez MV, Ruiz A,
Cavaliere F, Ortiz-Sanz C, Quintela-López T, Capetillo-Zarate E,
Solé-Domènech S and Matute C: Mangiferin and Morin attenuate
oxidative stress, mitochondrial dysfunction, and neurocytotoxicity,
induced by amyloid beta oligomers. Oxid Med Cell Longev.
2018(2856063)2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Lemus-Molina Y, Sánchez-Gómez MV,
Delgado-Hernández R and Matute C: Mangifera indica L.
extract attenuates glutamate-induced neurotoxicity on rat cortical
neurons. Neurotoxicology. 30:1053–1058. 2009.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Ajila CM, Rao LJ and Rao UJ:
Characterization of bioactive compounds from raw and ripe
Mangifera indica L. peel extracts. Food Chem Toxicol.
48:3406–3411. 2010.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Muruganandan S, Gupta S, Kataria M, Lal J
and Gupta PK: Mangiferin protects the streptozotocin-induced
oxidative damage to cardiac and renal tissues in rats. Toxicology.
176:165–173. 2002.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Rajendran P, Ekambaram G and Sakthisekaran
D: Effect of mangiferin on benzo(a)pyrene induced lung
carcinogenesis in experimental Swiss albino mice. Nat Prod Res.
22:672–680. 2008.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Wang RR, Gao YD, Ma CH, Zhang XJ, Huang
CG, Huang JF and Zheng YT: Mangiferin, an anti-HIV-1 agent
targeting protease and effective against resistant strains.
Molecules. 16:4264–4277. 2011.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Takeda T, Tsubaki M, Kino T, Yamagishi M,
Iida M, Itoh T, Imano M, Tanabe G, Muraoka O, Satou T and Nishida
S: Mangiferin induces apoptosis in multiple myeloma cell lines by
suppressing the activation of nuclear factor kappa B-inducing
kinase. Chem Biol Interact. 251:26–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Biersack B: Current state of phenolic and
terpenoidal dietary factors and natural products as non-coding
RNA/microRNA modulators for improved cancer therapy and prevention.
Noncoding RNA Res. 1:12–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Paolini A, Curti V, Pasi F, Mazzini G,
Nano R and Capelli E: Gallic acid exerts a protective or an
anti-proliferative effect on glioma T98G cells via dose-dependent
epigenetic regulation mediated by miRNAs. Int J Oncol.
46:1491–1497. 2015.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Sundaram KM, Hussain A, Haque S, Raina R
and Afroze N: Quercetin modifies 5'CpG promoter methylation and
reactivates various tumor suppressor genes by modulating epigenetic
marks in human cervical cancer cells. J Cell Biochem.
120:18357–18369. 2019.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Bishayee K, Khuda-Bukhsh AR and Huh SO:
PLGA-loaded Gold-nanoparticles precipitated with quercetin
downregulate HDAC-Akt activities controlling proliferation and
activate p53-ROS crosstalk to induce apoptosis in hepatocarcinoma
cells. Mol Cells. 38:518–527. 2015.PubMed/NCBI View Article : Google Scholar
|