Introduction
Pancreatic ductal adenocarcinoma (PDAC) is
associated with extremely high mortality rates due to rapid
progression and high incidences of metastases, peritoneal
dissemination and recurrence (1).
Therefore, there is an urgent need for new therapeutic strategies
that are focused on preventing the invasion and metastasis of
pancreatic cancer cells.
Nestin, a class VI intermediate filament, was first
described as a neuronal stem/progenitor cell marker that is
expressed in progenitor cells of various tissues, including
pancreas (2). Lineage-tracing
experiments have shown that exocrine cells in the pancreas are
derived from nestin-expressing progenitor cells (3–5). An
increased nestin expression has been reported in various tumors,
including pancreatic cancer (6).
Nestin immunoreactivity was found to be present in the cancer cells
in approximately 30% of PDAC cases. Moreover, nestin expression in
pancreatic cancer cells was found to correlate with nerve invasion
and the presence of cancer cells at the tumor resection margins
(7). Suppression of the nestin
expression was found to inhibit the invasion and migration of PDAC
cells in vitro, and inhibit liver metastasis in a xenograft
mouse model (8). These findings
suggest that nestin is important in the aggressiveness of
pancreatic cancer cells.
The epithelial-mesenchymal transition (EMT), in
which cells undergo a morphological switch from the epithelial
polarized phenotype to the mesenchymal fibroblastoid phenotype, is
considered to occur during cancer invasion and metastasis (9). Several distinct traits are conveyed
by EMT, including cell motility and invasiveness (10). As a result of EMT, epithelial cells
lose their defined cell-cell/cell-substratum contacts and their
structural/functional polarity, and become spindle-shaped and
morphologically similar to activated fibroblasts (11). At the molecular level, EMT is
defined by the loss of cell-cell adhesion molecules (e.g.,
E-cadherin and ZO-1), downregulation of epithelial differentiation
markers (e.g., cytokeratins and E-cadherin), transcriptional
induction of mesenchymal markers (e.g., vimentin, fibronectin, and
N-cadherin) and the nuclear localization of β-catenin (12). E-cadherin plays a major role in
EMT, and the Snail, Twist, and SIP-1/ZEB-2 proteins are all
repressors of the gene CDH1 that codes for E-cadherin.
We hypothesized that nestin regulates the migration
and invasion of PDAC cells via interactions with EMT factors. In
the present study, we modulated the nestin expression in PDAC cells
and investigated the corresponding changes in the mRNA and protein
levels of major EMT factors.
Materials and methods
Reagents and chemicals
The following reagents and chemicals were purchased:
FuGene HD transfection reagent from Roche Diagnostics (Mannheim,
Germany); geneticin from Gibco-BRL (Grand Island, NY, USA);
pBAsi-hU6 Neo DNA vector and FastPure RNA kit from Takara Bio, Inc.
(Tokyo, Japan); pAcGFP1-N1 vector from Clontech Laboratories
(Mountain View, CA, USA) and the High Capacity cDNA Reverse
Transcription kit. TaqMan Fast Universal PCR Master Mix, and TaqMan
Gene Expression Assays for nestin (Hs00707120_s1), E-cadherin
(Hs01013953_m1), Snail (Hs00195591_m1), Slug (Hs00950344_m1), Twist
(Hs00361186_m1), and 18S rRNA (Hs99999901_s1) were obtained from
Applied Biosystems, Inc. (Foster City, CA, USA). Rabbit polyclonal
anti-Snail antibody was purchased from Abcam (Cambridge, UK); Alexa
488-labeled goat anti-rabbit IgG antibody was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA); and Vectashield
H-1200 containing 4′,6-diamidino-2-phenylindole-2HCl (DAPI) was
obtained from Vector Laboratories (Burlingame, CA, USA). All other
chemicals and reagents were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Pancreatic cancer cell lines
PK-45H and KLM-1 human pancreatic cancer cells were
obtained from the Cell Resource Center for Biomedical Research,
Institute of Development, Aging, and Cancer, Tohoku University,
Japan. Cells were grown in RPMI-1640 medium containing 10% fetal
bovine serum (FBS) at 37°C under a humidified 5% CO2
atmosphere.
Establishment of nestin short hairpin
RNA-transfected PK-45H cells
Human nestin short hairpin (sh) RNA was prepared as
previously reported (8). PK-45H
cells (1×105 cells/well) were plated in 6-well plates
and grown in 2 ml RPMI-1640 medium with 10% FBS. Transfections of
the nestin shRNA expression and sham vectors were performed using
FuGENE HD transfection reagent, according to the manufacturer’s
instructions. Independent colonies were isolated by ring cloning,
and expanded in 300 μg/ml geneticin. Cell lysates were
collected, and nestin mRNA was measured by quantitative RT-PCR
(qRT-PCR).
Establishment of nestin-expressed
vector-transfected KLM-1 cells
The full-length nestin cDNA fragment was ligated
into the pAcGFP1-N1 eukaryotic expression vector as reported in a
previous study (8). Transfections
with the nestin expression vector (Nes) and the empty vector (Mock)
were performed using FuGENE HD transfection reagent. The cells were
passaged and cultured with 600 μg/ml geneticin. Independent
colonies were isolated by ring cloning and expanded in 300
μg/ml geneticin.
qRT-PCR
Total RNA was extracted from cells and purified with
the FastPure RNA kit. One microgram of the total RNA sample was
used for reverse transcription (RT) with the High Capacity cDNA
Reverse Transcription kit following the manufacturer’s protocol.
qRT-PCR for nestin, E-cadherin, Snail, Slug, Twist and 18S rRNA was
performed with the StepOnePlus Real-Time PCR System (Applied
Biosystems, Inc.) using specific primers and a TaqMan probe.
Cycling conditions were as follows: denaturation for 20 sec at
95°C, followed by annealing for 40 cycles of 1 sec at 95°C and a
final extension for 20 sec at 60°C. qRT-PCR results were expressed
as the ratio of the target to 18S rRNA, the latter serving as an
internal standard. Gene expression levels were measured in
triplicate.
Immunocytochemistry
Cells were plated in 35-mm glass-bottomed dishes
(2×105 cells/dish) and incubated for 72 h at 37°C in a
humidified 5% CO2 atmosphere. The cells were fixed in 4%
paraformaldehyde solution for 15 min at room temperature, and
incubated overnight at 4°C with polyclonal rabbit anti-Snail
antibody. Cells were then incubated with Alexa 488-labeled
anti-rabbit IgG antibody (1:1000 dilution), and mounted in
Vectashield H-1200. Snail protein was visualized using a Digital
Eclipse C1 TE2000-E microscope (Nikon Instech Co., Ltd., Tokyo,
Japan). Fluorescent images were analyzed using control software
EZ-C1 (Nikon). Confocal settings, including the laser power and
detector sensitivity, were unchanged during the acquisition of all
images.
Statistical analysis
Quantitative data were shown as the mean ± SEM.
One-way ANOVA was used to compare data for each shRNA clone
separately for the corresponding values for each of two sham
clones. P<0.05 was considered to indicate a statistically
significant difference with respect to each of the two sham clones.
Data for transiently nestin gene-transfected cells and
corresponding Mock-transfected cells were assessed by the Student’s
t-test. Computations were performed using the Stat View J version
5.0 software package (SAS Institute, Inc., Cary, NC, USA).
Results
Increase and decrease in expression
levels of nestin and E-cadherin in PK-45H and KLM-1 cells
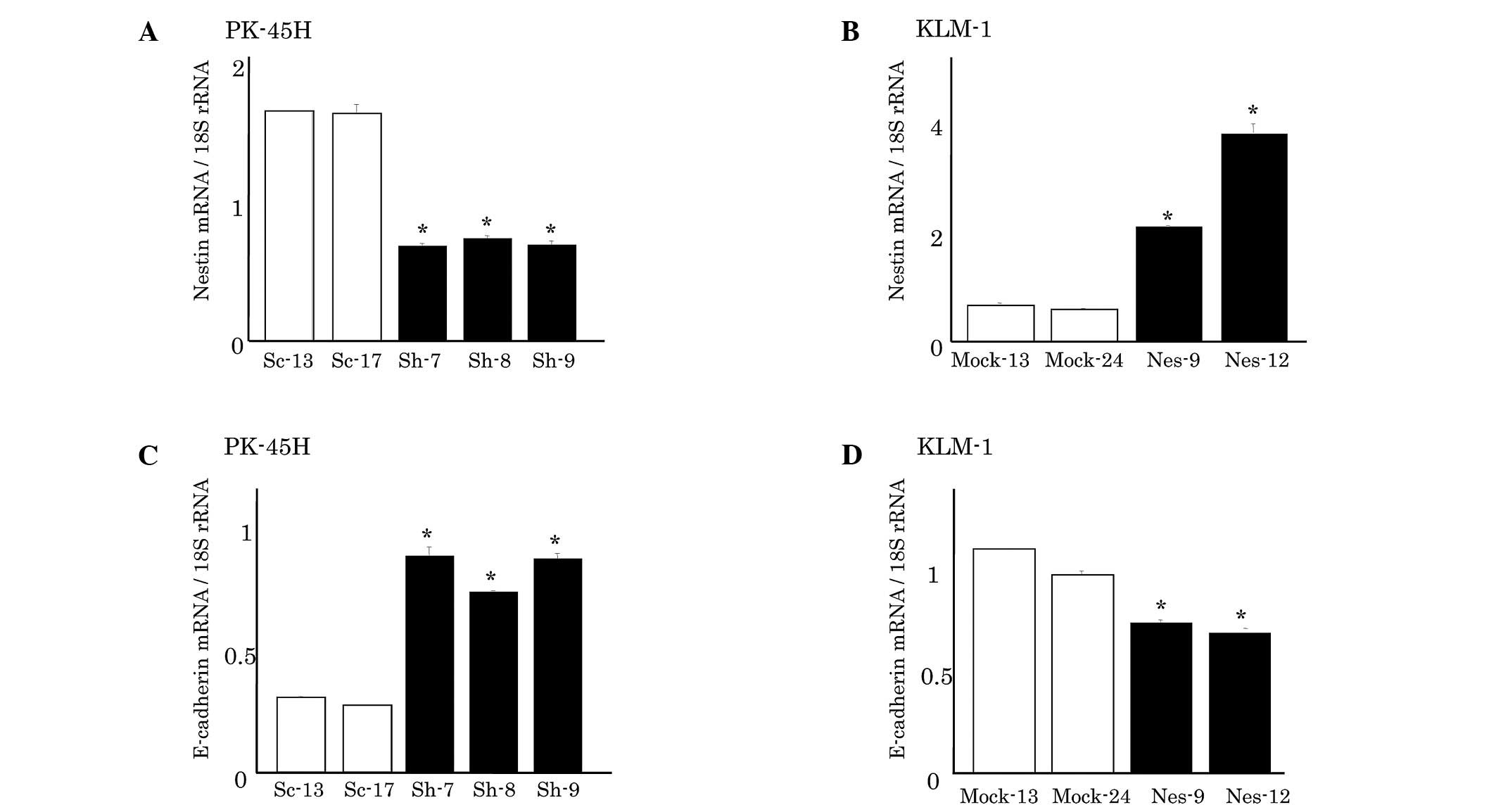
Nestin mRNA expression levels in PK-45H and KLM-1
cells were analyzed using qRT-PCR (Fig. 1). In PK-45H cells, nestin
shRNA-transfected clones (Sh-7, −8, and −9) exhibited a markedly
decreased nestin expression compared with the sham
vector-transfected cells (Sc-13 and −17) (Fig. 1A). Sh-7 cells showed the lowest
nestin expression, approximately 40% lower than that of Sc-13
cells. By contrast, KLM-1 cells originally expressed low levels of
nestin, and nestin-expressed vector-transfected KLM-1 cells (Nes-9
and −12) expressed higher nestin levels than the empty
vector-transfected clones (Mock-13 and −24) (Fig. 1B). Nes-12 cells showed the highest
nestin expression level, which was approximately eight times the
level of Mock-24 cells.
Furthermore, we examined the expression of EMT
markers, including E-cadherin, a major marker for epithelial cells
(Fig. 1). The nestin-suppressed
PK-45H clones exhibited a markedly increased E-cadherin expression
(Fig. 1C). By contrast, the
nestin-overexpressed KLM-1 clones showed significantly decreased
E-cadherin mRNA levels (Fig.
1D).
mRNA expression and immunostaining of
Snail in PK-45H and KLM-1 cells
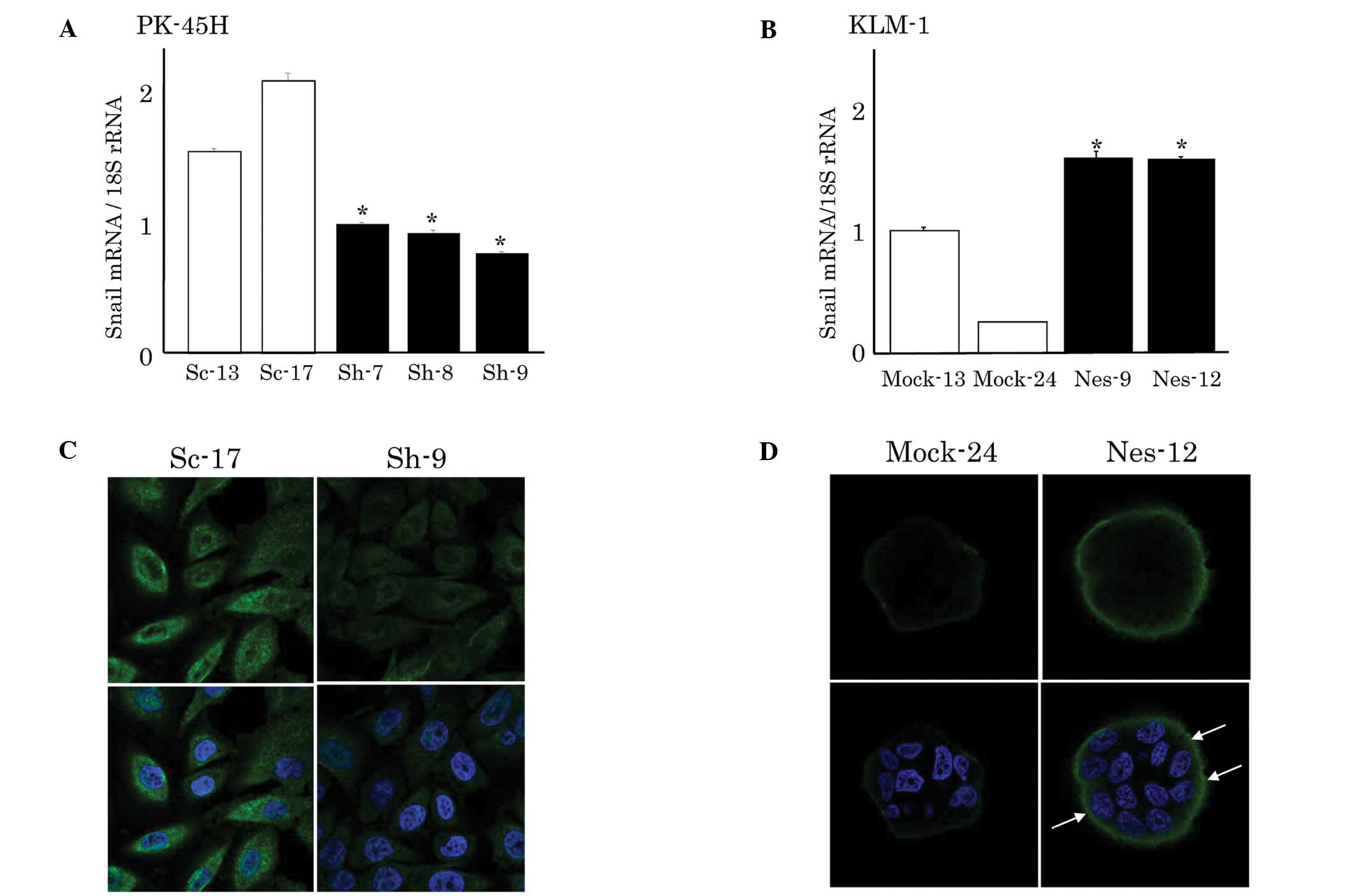
To investigate whether altered nestin expression
levels affected E-cadherin expression through transcriptional
factors, we analyzed the expression of Snail, Slug and Twist.
However, Twist expression was not high enough to detect, which is
consistent with previous findings (13). Suppression of nestin expression
significantly decreased the Snail mRNA expression in PK-45H cells
compared with that in sham-transfected Sc clones (Fig. 2A). In addition, the Snail protein
expression was apparently decreased in the cytoplasm and nucleus of
Sh-9 cells (Fig. 2C). By contrast,
Snail mRNA expression was significantly higher in the
nestin-expressed vector-transfected KLM-1 cells compared to the
Mock-transfected cells (Fig. 2B).
Similarly, an increased Snail protein expression was detected in
these cells (Nes-9 and −12) (Fig.
2D).
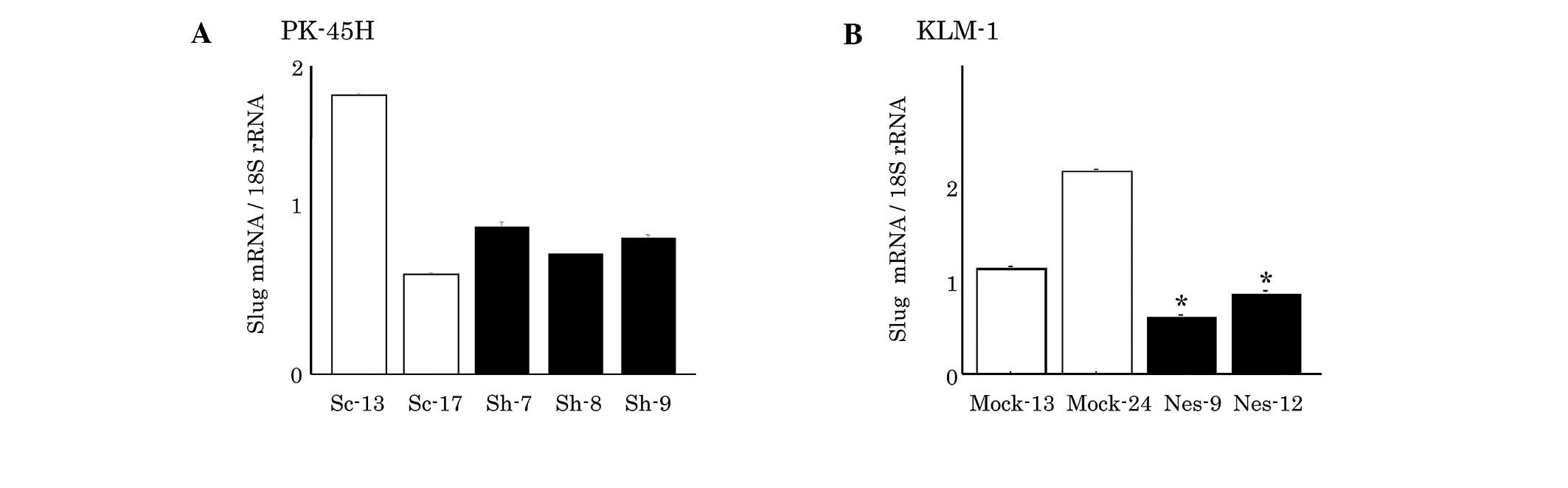
mRNA expression of Slug in PK-45H and
KLM-1 cells
Slug expression was not significantly different
between the Sc and nestin-suppressed Sh PK-45H clones (Fig. 3A). However, the
nestin-overexpressed KLM-1 cells showed a significantly decreased
Slug mRNA expression (Fig.
3B).
Discussion
The correlation between the cytoskeletal proteins
nestin and E-cadherin in cancer cells remains to be elucidated.
Thyroid carcinomas exhibit a high nestin expression, while
E-cadherin expression is not detected in anaplastic
(undifferentiated) carcinoma. By contrast, E-cadherin, but not
nestin, is expressed in papillary and follicular carcinomas
(14). The association between
these proteins in pancreatic cancer has yet to be adequately
clarified. Results of our previous study have shown an increased
E-cadherin expression in nestin-suppressed PDAC cells (8). Findings of the present study have
shown that nestin overexpression induced the E-cadherin expression,
strengthening the association between nestin and E-cadherin.
EMT occurs during various developmental processes,
such as gastrulation, neural crest migration and heart formation
(15–18). EMT is also involved in pathological
processes, such as fibrosis and metastasis (19). The molecular mechanisms controlling
EMT have only recently begun to emerge, and key roles have been
identified for the zinc finger transcription factors Snail (Snail1)
and Slug (Snail2) (20,21). In the present study, E-cadherin was
negatively correlated with Snail expression, which is consistent
with previous reports that indicate Snail is a repressor of
E-cadherin and directly regulates E-cadherin expression via the
integrin-linked kinase (ILK) pathway (22). We observed that the Snail
expression responded to the increase and decrease of nestin,
indicating that nestin is closely correlated with EMT and may thus
modulate EMT.
Slug was found to behave differently to Snail. In
chicks, Slug has been identified in two developmental processes
involving EMT, as a key regulator of mesoderm formation and neural
crest migration (23). Another
study reported differential effects of Snail and Slug in pancreatic
cancer, relating to the interplay between Rho signaling and
β1-integrin, and leading to differences in cell migration and
scattering (24). Our findings
suggest that Slug expression is less involved in EMT via the
nestin-induced change of E-cadherin. Twist was not detected in this
study; however, it may be involved. Twist reportedly may act
through a different mechanism compared to Snail and Zeb proteins
(25), and immunohistochemical
analysis has detected a decreased expression of nuclear Twist in
malignant pancreatic epithelium (26).
In conclusion, the results of this study suggest
that nestin may modulate the expression of E-cadherin and Snail,
thereby affecting the migration and metastasis of pancreatic
cancer.
Acknowledgements
The authors thank Dr Z. Naito for his
helpful discussion and Ms. K. Kawahara, Mr. Y. Yanagisawa and Ms.
Y. Kawamoto (Department of Pathology, Integrative Oncological
Pathology) for their excellent technical assistance. This study was
supported by a Grant-in-Aid for Young Scientists (A, no. 22689038
to Y. Matsuda and B, no. 24791451 to M. Hagio), a Grant-in-Aid for
Challenging Exploratory Research (no. 23650604 to Y. Matsuda) and a
Grant-in-Aid for Scientific Research (C, no. 22591531 to T.
Ishiwata) from the Japan Society for the Promotion of Sciences.
This study was also supported by a grant from the Pancreas Research
Foundation of Japan to M. Hagio.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008.
|
|
2.
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990.
|
|
3.
|
Esni F, Stoffers DA, Takeuchi T and Leach
SD: Origin of exocrine pancreatic cells from nestin-positive
precursors in developing mouse pancreas. Mech Dev. 121:15–25.
2004.
|
|
4.
|
Delacour A, Nepote V, Trumpp A and Herrera
PL: Nestin expression in pancreatic exocrine cell lineages. Mech
Dev. 121:3–14. 2004.
|
|
5.
|
Ishiwata T, Kudo M, Onda M, Fujii T,
Teduka K, Suzuki T, Korc M and Naito Z: Defined localization of
nestin-expressing cells in L-arginine-induced acute pancreatitis.
Pancreas. 32:360–368. 2006.
|
|
6.
|
Parry S, Savage K, Marchio C and
Reis-Filho JS: Nestin is expressed in basal-like and triple
negative breast cancers. J Clin Pathol. 61:1045–1050. 2008.
|
|
7.
|
Carrière C, Seeley ES, Goetze T,
Longnecker DS and Korc M: The Nestin progenitor lineage is the
compartment of origin for pancreatic intraepithelial neoplasia.
Proc Natl Acad Sci USA. 104:4437–4442. 2007.
|
|
8.
|
Matsuda Y, Naito Z, Kawahara K, Nakazawa
N, Korc M and Ishiwata T: Nestin is a novel target for suppressing
pancreatic cancer cell migration, invasion and metastasis. Cancer
Biol Ther. 11:512–523. 2011.
|
|
9.
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelialmesenchymal transition in cancer: parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010.
|
|
10.
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: role
of phosphatidylinositol 39 kinase/AKT pathways. Oncogene.
24:7443–7454. 2005.
|
|
11.
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005.
|
|
12.
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011.
|
|
13.
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007.
|
|
14.
|
Liu J and Brown RE: Immunohistochemical
detection of epithelialmesenchymal transition associated with
stemness phenotype in anaplastic thyroid carcinoma. Int J Clin Exp
Pathol. 3:755–762. 2010.
|
|
15.
|
Hay ED: An overview of
epithelio-mesenchymal transformation. Acta Anat (Basel). 154:8–20.
1995.
|
|
16.
|
Birchmeier C, Birchmeier W and
Brand-Saberi B: Epithelialmesenchymal transitions in cancer
progression. Acta Anat (Basel). 156:217–226. 1996.
|
|
17.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454.
2002.
|
|
18.
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003.
|
|
19.
|
Taylor MA, Parvani JG and Schiemann WP:
The pathophysiology of epithelial-mesenchymal transition induced by
transforming growth factor-beta in normal and malignant mammary
epithelial cells. J Mammary Gland Biol Neoplasia. 15:169–190.
2010.
|
|
20.
|
Savagner P: Leaving the neighborhood:
molecular mechanisms involved during epithelial-mesenchymal
transition. Bioessays. 23:912–923. 2001.
|
|
21.
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005.
|
|
22.
|
Schaeffer DF, Assi K, Chan K, Buczkowski
AK, Chung SW, Scudamore CH, Weiss A, Salh B and Owen DA: Tumor
expression of integrin-linked kinase (ILK) correlates with the
expression of the E-cadherin repressor snail: an
immunohistochemical study in ductal pancreatic adenocarcinoma.
Virchows Arch. 456:261–268. 2010.
|
|
23.
|
Nieto MA, Sargent MG, Wilkinson DG and
Cooke J: Control of cell behavior during vertebrate development by
Slug, a zinc finger gene. Science. 264:835–839. 1994.
|
|
24.
|
Shields MA, Krantz SB, Bentrem DJ,
Dangi-Garimella S and Munshi HG: Interplay between β1-integrin and
Rho signaling regulates differential scattering and motility of
pancreatic cancer cells by snail and Slug proteins. J Biol Chem.
287:6218–6229. 2012.
|
|
25.
|
Montserrat N, Gallardo A, Escuin D,
Catasus L, Prat J, Gutiérrez-Avignó FJ, Peiró G, Barnadas A and
Lerma E: Repression of E-cadherin by SNAIL, ZEB1, and TWIST in
invasive ductal carcinomas of the breast: a cooperative effort? Hum
Pathol. 42:103–110. 2011.
|
|
26.
|
Cates JM, Byrd RH, Fohn LE, Tatsas AD,
Washington MK and Black CC: Epithelial-mesenchymal transition
markers in pancreatic ductal adenocarcinoma. Pancreas. 38:e1–e6.
2009.
|