Introduction
Noni (Morinda citrifolia) is a small
evergreen tree native to South Asia, with elongated leaves, white
tubular flower clusters and greenish fruits that ripen to
whitish-yellow. Its products have been used for centuries to treat
an array of maladies. A variety of major natural products have been
identified in the noni plant thus far (1,2). Two
novel glycosides and a new unusual iridoid, known as
citrifolinoside, isolated from noni were shown to have an
inhibiting effect on activator protein-1 (AP-1) transactivation and
cell transformation in the mouse epidermal JB6 cell line (3–7).
Although its traditional uses primarily involved topical
application of leaves and roots, the noni fruit has been more
popular for modern usage (8). The
documented effects of noni juice include anti-inflammatory,
antiangiogenic, anticancer, antibacterial and antioxidant
activities. Noni has been reported to modulate immune cells and has
been found to enhance the adaptive immune response by activating T
and B cells (9).
Noni has received much attention for its
antiangiogenic and anticancer properties in animal models and
clinical settings. The first attempt to identify the component of
noni responsible for its antitumor activities described a
water-soluble, ethanol-precipitating, polysaccharide-rich substance
from noni fruit, as an immunomodulator (10). Shortly thereafter, Wang and Su
demonstrated that noni inhibits 7,12-dimethylbenz[a]anthracene
(DMBA)-DNA adduct formation (11),
suggesting a protective role in chemically induced cancers. In
their study, Furusawa et al expanded their investigation to
demonstrate that the antitumor properties of noni precipitate (ppt)
were dependent on macrophages, NK and T cells and extended its
synergistic effects with even more chemotherapies (12). Additionally, noni was found to
inhibit new vessel sprouts at concentrations of 5% and induce
vessel degeneration at a concentration of 10% (13). In 2004, Wong developed a novel
technique of making fermented noni exudates (fNE) and reported that
two cancer patients had benefited by drinking fNE (14).
The intraperitoneal injection (i.p.) of C57BL/6J
mice with fNE has recently been reported (15) to significantly increase the
percentages of granulocytes and NK cells in the peripheral blood,
peritoneum and spleen. The fNE injection induced complete tumor
rejection in normal C57BL/6J mice in both preventive and
therapeutic treatment settings. Furthermore, that study also showed
that fNE injection induced partial tumor rejection in C57 nude mice
lacking functional lymphocytes, although no tumor rejection was
detected in NK cell-deficient beige mice. Over 85% of the C57BL/6J
mice that received fNE survived the first tumor injection and
rejected up to 5×106 tumor cells when re-challenged.
These data demonstrate that fNE is likely to stimulate the innate
and adaptive immune systems to reject tumor cells. NK cells exhibit
a rapid response and are fundamentally involved in the innate
immune system, while a response in the adaptive immune system
occurs later with a retained memory (15). This conclusion has been supported
in a recent study by Nayak and Mengi (16).
Therefore, to identify the active antitumor
components from fNE, the juice was partitioned into three fractions
and their antitumor activities were examined in a S180 sarcoma
tumor model using either i.p. injection or as a water supplement.
The in vivo animal study results showed that n-butanol
fraction of fNE (BuOH) is the most effective component in the two
experimental settings.
Materials and methods
Cells and mice
C57BL/6J mice (female) at 6–8 weeks of age were
purchased from Jackson Laboratories (Bar Harbor, ME, USA) and
housed in our pathogen-free animal facilities. The animal
experiments were carried out in accordance with the Guidelines for
the Care and Use of Laboratory Animals (NIH Publication No. 85-23)
and the institutional guidelines. Mouse sarcoma S180 tumor cells
were purchased from ATCC (Manassas, VA, USA) (TIB-66) and cultured
in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine
serum (FBS) and gentamicin (50 μg/ml).
Preparation of fNE and its fractions
Fermented noni exudates (fNE) were prepared as
described previously (15).
Briefly, physio logically mature, firm-yellow (ripe) noni fruits
were harvested from a commercial orchard on the island of Hawaii
and transported to the USDA-ARS Laboratory (Hilo, HI, USA) in a
cooler. Fruit were of excellent quality (disease-and injury-free).
Fruit were destemmed, sorted, washed, sanitized and kept at 22°C
until translucent. The jars, sieves and utensils used for juice
preparation were autoclaved for sterility. Fruit (1.5–1.7 kg/jar)
were placed into sterilized glass jars (3.8 liters) with sealed
lids to provide low oxygen conditions and allowed to ferment for 14
days at 22°C. For each batch, 20–25 jars of noni were prepared. Gas
concentrations (CO2 and O2) inside jars were
measured using a headspace analyzer (model 6600, Illinois
Instruments, Johnsburg, IL, USA). Jars were fitted with septa for
gas sampling. By day 14, the CO2 levels increased to
25%, whereas O2 levels decreased to 10%. The noni
exudates were decanted through a sterile mesh screen, collected in
sterile flasks, pasteurized at 85°C in a water bath for 3 min,
cooled and frozen at −20°C. The noni juice had a pH of 3.5 and a
total soluble solids content of 5.7%.
Fresh or thawed fNE were partitioned with ethyl
acetate to obtain the ethyl acetate-soluble residue. Then, the
remaining aqueous layer was partitioned three times with n-butanol.
Thus, two organic fractions designated as EtAc and BuOH and an
aqueous fraction (H2OL) were obtained. The three
fractions were freeze-dried in a lyophilizer and sent via overnight
air shipment to the Clemson University for evaluation in their
antitumor assays. Unfractionated fNE was also shipped. Prior to
injection, the noni fractions were thawed and dissolved with
phosphate-buffered saline (PBS) to one fourth of the original fNE
volume and adjusted to pH 7.0–7.4. The H2O fraction was
later found to be toxic. Therefore, fNE, BuOH and EtAc fractions
were used in the animal study.
Animal studies
Three animal studies were conducted with the noni
materials. In a tumor prevention study, four cages of female
C57BL/6J mice (n=8/cage; age, 10 weeks) were i.p. injected with 0.2
ml/mouse of fNE, BuOH and EtAc fractions or PBS on days 14 and 7.
On day 0, 5×105 S180 tumor cells were injected i.p. into
each mouse and their survival was monitored. In a tumor treatment
study, C57BL/6J mice were injected i.p. with live S180 tumor cells
(5×105 cells/mouse) on day 0. On days 3, 4 and 5, the
mice were i.p. injected with 0.2 ml of fNE, BuOH and EtAc fractions
or PBS (n=8 mice/group). The survival of the mice was monitored. In
a tumor prevention study with noni materials as water supplements,
female C57BL/6J mice (age, 8 weeks) were fed with water containing
fNE, BuOH or EtAc fractions at a concentration of 5% for 4 weeks
(n=4 mice/group). The mice were then injected i.p. with S180 tumor
cells (5×105 cells/mouse) and monitored for
survival.
Results
fNE fractions
Thawed fNE (400 ml) were partitioned with EtAc three
times (3×200 ml) to obtain the EtAc-soluble residue. Solvent was
thoroughly evaporated under reduced pressure using a rotary
evaporator. One gram (dry weight) of residue was obtained from this
process and labeled as EtAc fraction. The remaining aqueous layer
was partitioned with n-butanol three times (3×200 ml). Nineteen
grams (dry weight) of residue were obtained and labeled as BuOH
fraction. Twenty grams (dry weight) of residue were obtained from
the aqueous layer and labeled as H2OHL. The dry noni
materials were dissolved in 100 ml PBS (¼ of the original fNE
volume) and adjusted to pH 7.2–7.4. A pilot study showed that
H2OHL was toxic (data not shown), therefore, fNE, BuOh
and EtAc were used in the subsequent in vivo animal
studies.
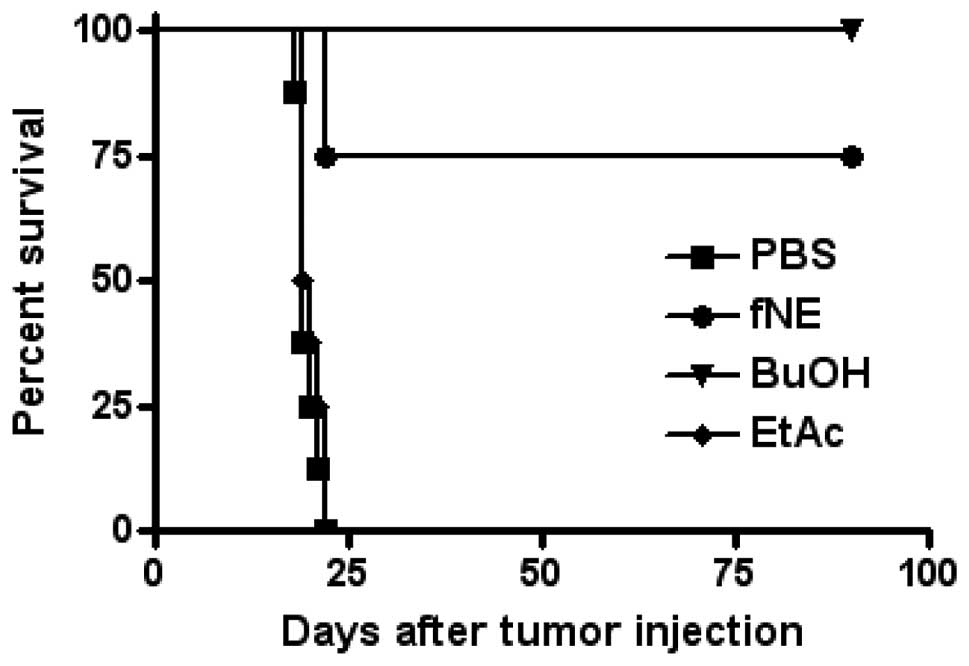
Intraperitoneal injection of noni
materials rejected S180 tumor challenge
Four cages of female C57BL/6J mice (n=8/cage; age,
10 weeks) were i.p. injected with 0.2 ml/mouse of fNE, BuOH and
EtAc fractions or PBS on days 14 and 7. On day 0, 5×105
S180 tumor cells were injected i.p. into each mouse and their
survival was monitored. While the injection of PBS or EtAc did not
provide any protection from S180 tumor challenge, the mice injected
with BuOH fraction completely rejected S180 tumor challenge. Six of
eight mice injected with fNE rejected S180 tumor cells (Fig. 1). These mice remained tumor-free
for their entire lifespan. Two months later, four tumor
cell-resistant mice from the fNE and BuOH groups were rechallenged
with 5×106 live S180 tumor cells/mouse i.p. The mice
completely rejected this second tumor cell challenge, indicating
that an effective immune response had been activated.
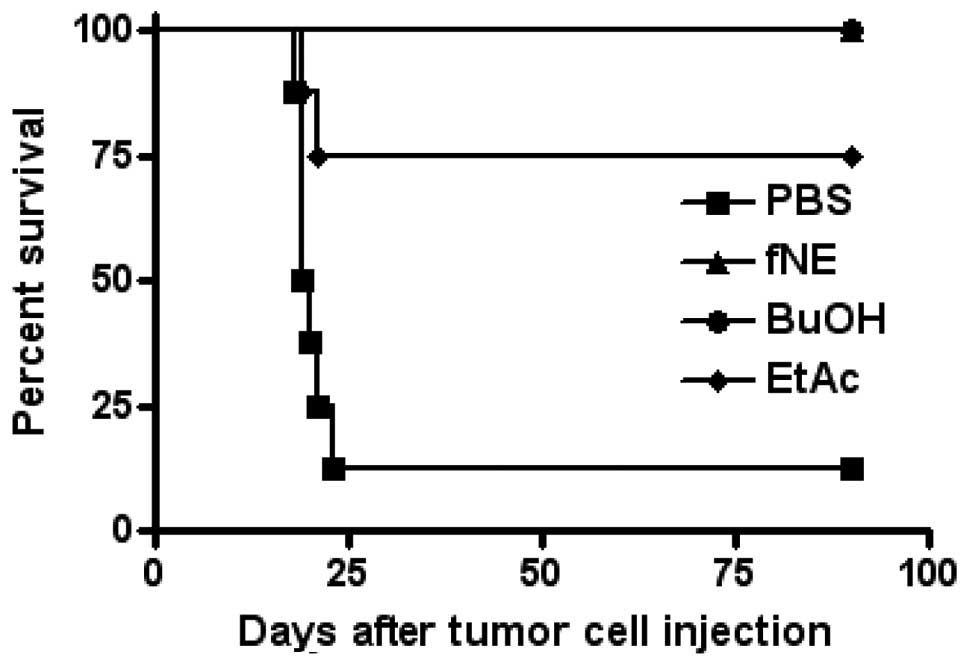
Intraperitoneal injection of noni
materials eradicated existing S180 tumor cells
In a therapeutic treatment experimental setting,
C57BL/6J mice were injected i.p. with live S180 tumor cells
(5×105 cells/mouse). On days 3, 4 and 5, the mice were
i.p. injected with 0.2 ml of fNE, BuOH fractions, EtAc fractions or
PBS (8 mice/group). The survival of the mice was monitored. The
i.p. injection of fNE and BuOH fraction completely eradicated the
existing S180 tumor cells, while six of the eight mice injected
with the EtAc fraction were tumor-free (Fig. 2).
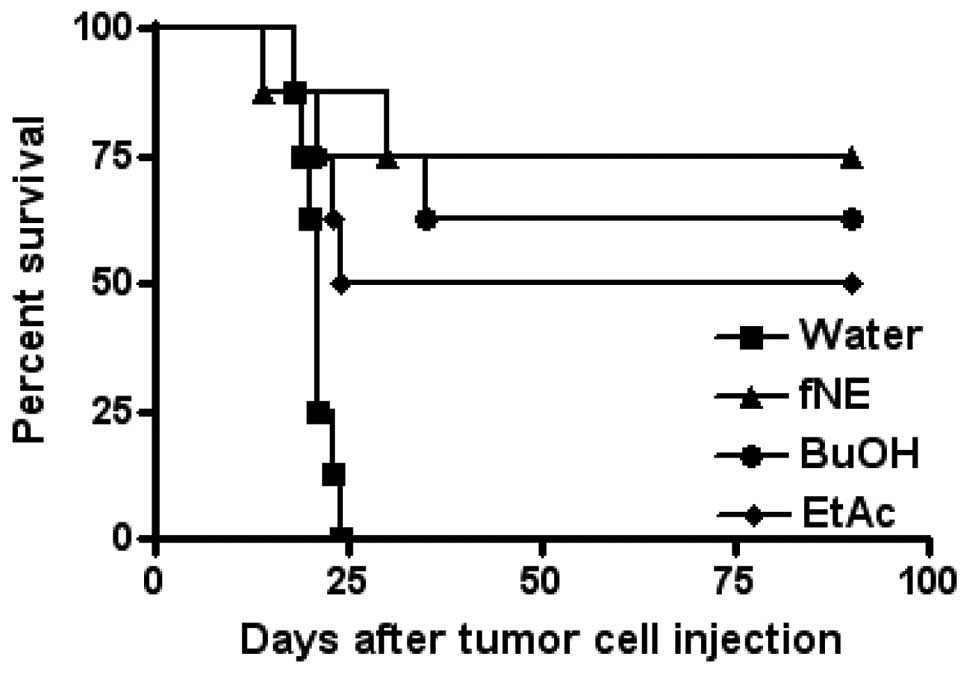
Mice fed with noni materials as a water
supplement rejected S180 tumor challenge
Female C57BL/6J mice (age, 8 weeks) were fed with
water containing fNE, BuOH or EtAc fractions at a concentration of
5% for 4 weeks (n=8 mice/group). The mice were then injected i.p.
with S180 tumor cells (5×105 cells/mouse) and monitored
for survival. Six of eight mice fed with fNE rejected S180 tumor
challenge, five of eight mice fed with BuOH or four of eight mice
fed with EtAc rejected S180 tumor challenge. The mice that survived
the first tumor challenge were tumor-free for their entire lifespan
(Fig. 3).
Discussion
Although noni products have been used as folk
medicines for thousands of years, it is only recently that the
mechanisms and the active components of noni have been understood,
with attention being focused on noni juice (10,12).
The method to produce noni juice was modified by the addition of
fermentation and the product was termed fermented noni exudates
(fNE) (14,15). Novel species were identified from
fNE (17). fNE was demonstrated to
be used to prevent tumor development as well as treat existing
tumors in a mouse sarcoma tumor model. This antitumor activity is
mediated by the activation of the immune system, especially by NK
cells (15). The fNE was also
shown to have the potential to activate dendritic cells that are
important in the initiation of the adaptive immunity (18).
As the first step in identifying the active
antitumor components or compounds from fNE in this study, the noni
juice was partitioned into three fractions: water
(H2OL), alcohol (BuOH) and acid (fractions EtAc). The
antitumor activities of these fractions were then examined in an
in vivo S180 sarcoma tumor model using either i.p. injection
or as a water supplement. H2OL was found to be extremely
toxic due to the contamination of lipopolysaccharide (LPS) (data
not shown) and not included in the in vivo assay.
In a preventive setting, fNE, BuOH or EtAc were i.p.
injected into experimental mice in order to determine whether or
not the noni fractions stimulated the immune system to reject tumor
cells. As shown in Fig. 1, BuOH
stimulated a stronger immune response compared to fNE, since the
eight mice that received BuOH injection rejected S180 tumor cells,
while six of the mice that received fNE rejected the tumor cells.
Of note, no immune response was stimulated subsequent to EtAc
injection. Consequently, the active antitumor compounds are very
likely present in the alcohol fraction of fNE. More importantly,
the mice that received fNE or BuOH and rejected the first tumor
challenge rejected a second and higher dose of tumor challenge,
indicating that long-term immunomemory was established (data not
shown).
To determine whether or not the antitumor immune
response activated by fNE and its fractions has the potential to
eradicate existing tumor cells, mice were injected with tumor cells
and then treated with fNE, BuOH or EtAc. As shown in Fig. 2, the mice treated with fNE or BuOH
eradicated tumor cells and remained tumor-free for their entire
lifespan. Notably, six of the eight mice treated with EtAc also
exhibited eradicated tumor cells. At this point of the experiment,
two phenomena were of interest. First, the therapeutic treatment
showed a better antitumor activity compared to the preventive
treatment. Secondly, EtAc treatment did not reject tumor challenge,
but was able to eradicate existing tumors. A possible explanation
for these noteworthy observations is the activation of the two arms
of the immune system: innate and adaptive immunity. Although the
immunities might be activated in the preventive setting, only
adaptive immunity has the potential to generate immunomemory, which
rejects a future tumor challenge. In the therapeutic treatment
setting, the immunities are activated and mount a rapid and
effective immune response against the tumor cells. In addition,
EtAc may not be effective in stimulating the adaptive immune
responses, but is able to activate the innate immune response,
especially the NK cells. These interesting findings require further
investigation.
Since the i.p. injection of fNE and its fractions
stimulate the adaptive immunity and reject a later tumor challenge,
it was then hypothesized that if the noni materials can be
delivered in a natural manner, such as oral delivery, an
immunomemory may be established. The fNE, BuOH or EtAc were added
into the feeding water for the mice. After a four-week feeding with
the noni material-containing water, the mice were i.p. injected
with live S180 tumor cells. Fig. 3
shows that the addition of fNE, BuOH or EtAc into the feeding water
effectively activated the immune system and rejected tumor
challenge, although not as effectively as the delivery by direct
i.p. injections. Notably, 50% of the mice fed with EtAc-containing
water rejected tumor cells. To determine the reason for which the
i.p. injection of the EtAc fraction did not reject tumor challenge
as opposed to its oral delivery, additional studies are
required.
In conclusion, this preliminary study confirms that
fNE is able to activate immune responses to reject tumor challenge
as well as eradicate existing tumors. The alcohol fraction (BuOH)
of fNE contains the active components that can activate both innate
and adaptive immune responses. More importantly, the noni materials
may be delivered orally and activate the immune system,
representing a novel approach for cancer prevention.
Acknowledgements
The authors would like to thank Eric
Holle and his staff for the professional care of mice used in this
study. This study was supported in part by the Patient-First Cancer
Foundation (2007639).
References
|
1.
|
Balakrishna S, Seshadri TR and
Venkataramani B: Special chemical component of commercial woods and
related plant materials: Part X-Heartwood of Morinda
citrifolia Linn. J Sci Industrial Res. 20B:331–333. 1961.
|
|
2.
|
Potterat O and Hamburger M: Morinda
citrifolia (noni) fruit -phytochemistry, pharmacology, safety.
Planta Med. 73:191–199. 2007.
|
|
3.
|
Wang M, Kikuzaki H, Jin Y, Nakatani N, Zhu
N, Csiszar K, et al: Novel glycosides from noni (Morinda
citrifolia). J Nat Prod. 63:1182–1183. 2000.
|
|
4.
|
Sang S, He K, Liu G, Zhu N, Cheng X, Wang
M, et al: A new unusual iridoid with inhibition of activator
protein-1 (AP-1) from the leaves of Morinda citrifolia L.
Org Lett. 3:1307–1309. 2001.
|
|
5.
|
Sang S, Cheng X, Zhu N, Wang M, Jhoo JW,
Stark RE, et al: Iridoid glycosides from the leaves of Morinda
citrifolia. J Nat Prod. 64:799–800. 2001.
|
|
6.
|
Sang S, Cheng X, Zhu N, Stark RE, Badmaev
V, Ghai G, et al: Flavonolglycosides and novel iridoid glycoside
from the leaves of Morinda citrifolia. J Agric Food Chem.
49:4478–4481. 2001.
|
|
7.
|
Liu G, Bode A, Ma WY, Sang S, Ho CT and
Dong Z: Two novel glycosides from the fruits of Morinda
citrifolia (noni) inhibit AP-1 transactivation and cell
transformation in the mouse epidermal JB6 cell line. Cancer Res.
61:5749–5756. 2001.
|
|
8.
|
Pawlus AD and Kinghorn AD: Review of the
ethnobotany, chemistry, biological activity and safety of the
botanical dietary supplement Morinda citrifolia (noni). J
Pharm Pharmacol. 59:1587–1609. 2007.
|
|
9.
|
Schafer M, Sharp P, Brooks VJ, Xu J, Cai
J, Keuler NS, Peek SF, Godbee RG, Schultz RD and Darien BJ:
Enhanced bactericidal activity against Escherichia coli in
calves fed Morinda citrifolia (noni) puree. J Vet Int Med.
22:499–502. 2008.
|
|
10.
|
Hirazumi A and Furusawa E: An
immunomodulatory polysaccharide-rich substance from the fruit juice
of Morinda citrifolia (noni) with antitumour activity.
Phytother Res. 13:380–387. 1999.
|
|
11.
|
Wang MY and Su C: Cancer preventive effect
of Morinda citrifolia (noni). Ann N Y Acad Sci. 952:161–168.
2001.
|
|
12.
|
Furusawa E, Hirazumi A, Story S and Jensen
J: Antitumour potential of a polysaccharide-rich substance from the
fruit juice of Morinda citrifolia (noni) on sarcoma 180
ascites tumour in mice. Phytother Res. 17:1158–1164. 2003.
|
|
13.
|
Hornick CA, Myers A, Sadowska-Krowicka H,
Anthony CT and Woltering EA: Inhibition of angiogenic initiation
and disruption of newly established human vascular networks by
juice from Morinda citrifolia (noni). Angiogenesis.
6:143–149. 2003.
|
|
14.
|
Wong DK: Are immune responses pivotal to
cancer patient’s long term survival? Two clinical case-study
reports on the effects of Morinda citrifolia (noni). Hawaii
Med J. 63:182–184. 2004.
|
|
15.
|
Li J, Stickel S, Bouton-Verville H, Burgin
KE, Yu X, Wong DK, Wagner TE and Wei Y: Fermented noni exudate
(fNE): A mediator between immune system and anti-tumor activity.
Oncol Rep. 20:1505–1510. 2008.
|
|
16.
|
Nayak S and Mengi S: Immunostimulant
activity of noni (Morinda citrifolia) on T and B
lymphocytes. Pharmal Biol. 48:724–731. 2010.
|
|
17.
|
Nishijima KA, Wall MM, Chang LC, Wei Y and
Wong DKW: First report of association of Mucor
circinelloides on noni (Morinda citrifolia) in Hawaii.
Plant Dis. 95:3602011.
|
|
18.
|
Zhang X, Li J, Wong DK, Wagner TE and Wei
Y: Fermented noni exudate-treated dendritic cells directly
stimulate B lymphocyte proliferation and differentiation. Oncol
Rep. 21:1147–1152. 2009.
|