Introduction
Tumorigenesis is triggered by malignant cellular
development with the destruction of healthy gene regulation
systems. The cytogenetic change associated with unrecoverable
genomic aberrations induces the development of abnormal cell
phenotypes, such as the ability of cells to avoid undergoing
apoptosis, escalated proliferative signaling, insensitivity to
growth suppressors and irregularity of angiogenesis (1). Additionally, in the early phase of
tumorigenesis, stemness is considered to be an important property
of cancer cells. Cancer stem cells (CSCs) are, therefore, of key
importance in understanding the biological mechanisms of cancer
development, and are currently considered to be in the center of
malignant transformation, growth and metastasis (2,3).
CSCs are of increasing importance as a target for new anticancer
agents. The CSCs comprise a small subset of tumor cells with a
significant potential for tumorigenesis, and are identified by
their expression of specific cell surface markers, which are
different in the various cancer types (4–9).
CD133 is generally known to be a cooperatively
expressed marker of CD34+ hematopoietic stem cells
(10). The expression of CD133 is
observed in hematopoietic stem cells, as well as in stem cells
found in healthy tissues including the brain, kidneys, prostate and
pancreas (7). According to an
expression analysis of the cell surface antigens expressed in
various types of cancer cells, CD133 is considered to be a
promising CSCs marker for colon (2), pancreatic (5) and prostate cancers (11), as well as melanoma (4).
Bladder cancer is the second most common urological
malignancy. Globally, almost 40,000 new patients are diagnosed with
bladder cancer each year, with a mortality rate exceeding 150,000
per year (12). Numerous studies
have been conducted to identify promising diagnostic markers and
therapeutic targets for bladder cancer, in order to detect the
disease earlier and to develop better treatments.
Despite the clinical importance of urinary bladder
cancer, no experimental studies concerning the CD133 expression of
bladder cancer cells have been conducted. In this study, the
expression of CD133 and the phenotypic properties of a
CD133+ subpopulation present in the human urinary
bladder cancer cell line, J82, were examined.
Materials and methods
Cell culture
A human urinary bladder cancer cell line, J82, was
purchased from the American Type Culture Collection (Rockville, MD,
USA). The J82 cells were maintained in Dulbecco’s modified Eagle’s
medium (DMEM; Invitrogen, Carlsbad, CA, USA), supplemented with 10%
fetal bovine serum (FBS) at 37°C in a humidified 5% CO2
atmosphere, as previously described (13).
CD133 expression analysis in J82
cells
J82 cells were suspended at 5×105 and
incubated with an anti-CD133/APC antibody (Miltenyi Biotec,
Bergisch Gladbach, Germany) for 30 min at 4°C and analyzed by a
FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA)
and the CellQuest software program (Becton-Dickinson). Incubation
with the isotype control IgG (Miltenyi Biotec) was implemented to
provide a negative control.
Magnetic separation of J82 cells based on
their CD133 expression
J82 cells were suspended at 5×106 and
separated by immunomagnetic selection, on the basis of their CD133
expression. CD133+ cells were labeled by microbeads with
an anti-CD133 antibody (CD133 cell isolation kit; Miltenyi Biotec)
and separated by the magnetic cell separation system, according to
the manufacturer’s instructions. The separated CD133−
and CD133+ subpopulations of J82 cells were expanded and
stored in liquid nitrogen. The expression of CD133 in each subset
was also confirmed by flow cytometry, as described above.
Observation of the cell growth pattern
and cell proliferation analysis
The CD133− and CD133+
subpopulations were suspended in the complete medium and seeded in
96-well plates at 100 cells/well, in 100 μl of medium. Two
days after seeding, the cell growth pattern was observed in terms
of the cell capacity for colonization, and images of the cells in
the plates were captured by phase-contrast microscopy. For the cell
proliferation assay, each subset was seeded in 6-well plates at
1×104 cells/well, in 2 ml of complete medium. The total
cell number was counted at the indicated time points.
Western blot analysis
Western blot analysis was performed as previously
described (14). Briefly, the J82
cell subsets were lysed, and the extracted total proteins were
separated by gel electrophoresis and transferred onto
polyvinylidene difluoride (PVDF) membranes. After blocking in 10%
skimmed milk, the membranes were incubated overnight with the
following primary antibodies: anti-Oct-4 antibody (#2750; Cell
Signaling Technology, Inc., Danvers, MA), anti-Sox-2 antibody
(#3579; Cell Signaling Technology, Inc.), anti-β-actin antibody
(#4970, Cell Signaling Technology, Inc.). The membranes were washed
and incubated with secondary antibodies. The bound anti-bodies were
visualized using an enhanced chemiluminescence detection method
(ECL kit; Amersham Pharmacia Biotech, Chandler, AZ, USA).
Analysis of resistance to anticancer
agents
The resistance of the CD133− and
CD133+ subsets to anticancer agents was evaluated in
vitro. The subsets were seeded at 1×105 cells/well
on 6-well plates, in 2 ml of complete medium, and were cultured for
24 h. Either the chemotherapeutic agent, cisplatin (Sigma, St.
Louis, MO, USA) (final concentration, 5 μg/ml), or the
intravesical instillation agent, Bacillus Calmette-Guérin (BCG,
Connaught substrain; Nihon Kayaku, Co., Ltd., Tokyo, Japan) (final
concentration, 100 μg/ml), was added to the
CD133− and CD133+ cell cultures. The
CD133− and CD133+ cells were also cultured
without any anticancer drug, while the cell number was used as a
control. The relative cell number (%) was calculated for each
CD133− and CD133+ subset for 4 days,
subsequent to the treatment with the therapeutic agents.
Analysis of resistance to radiation
X-ray irradiation of the CD133− and
CD133+ subsets was performed in order to evaluate the
tolerance of the cells to radiation treatment. The cells were
seeded at 1×105 cells/well in 6-well plates, in 2
μl of complete medium, and incubated for 24 h. The subsets
were then subjected to a 6-Gy radiation dose. The CD133−
and CD133+ cells were also cultured without irradiation,
while the cell number was used as a control. The relative cell
number (%) was calculated for the treated CD133− and
CD133+ subpopulations 2 days subsequent to the
treatment.
Analysis of in vivo tumorigenicity
Male nude mice (BALB/c nu/nu, 6- to 8-weeks-old)
were purchased from the Charles River Laboratories (Tokyo, Japan).
The CD133− and CD133+ cells were suspended in
a 1:1 volume mixture of the medium and Matrigel (Becton-Dickinson),
as described previously (15).
Then, 1×106 cells of the CD133− and
CD133+ subsets were subcutaneously injected into the
left and right thighs of the nude mice, respectively. The tumor
sizes were measured with Vernier calipers, while the volume was
calculated using the following formula approximating the volume of
a sphere: [1/2 x (the shortest diameter) 2 x (the longest
diameter)].
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). An unpaired Student’s t-test was performed for the
statistical analysis of the differences in the two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of the CD133+
subset in J82 human bladder cancer cells
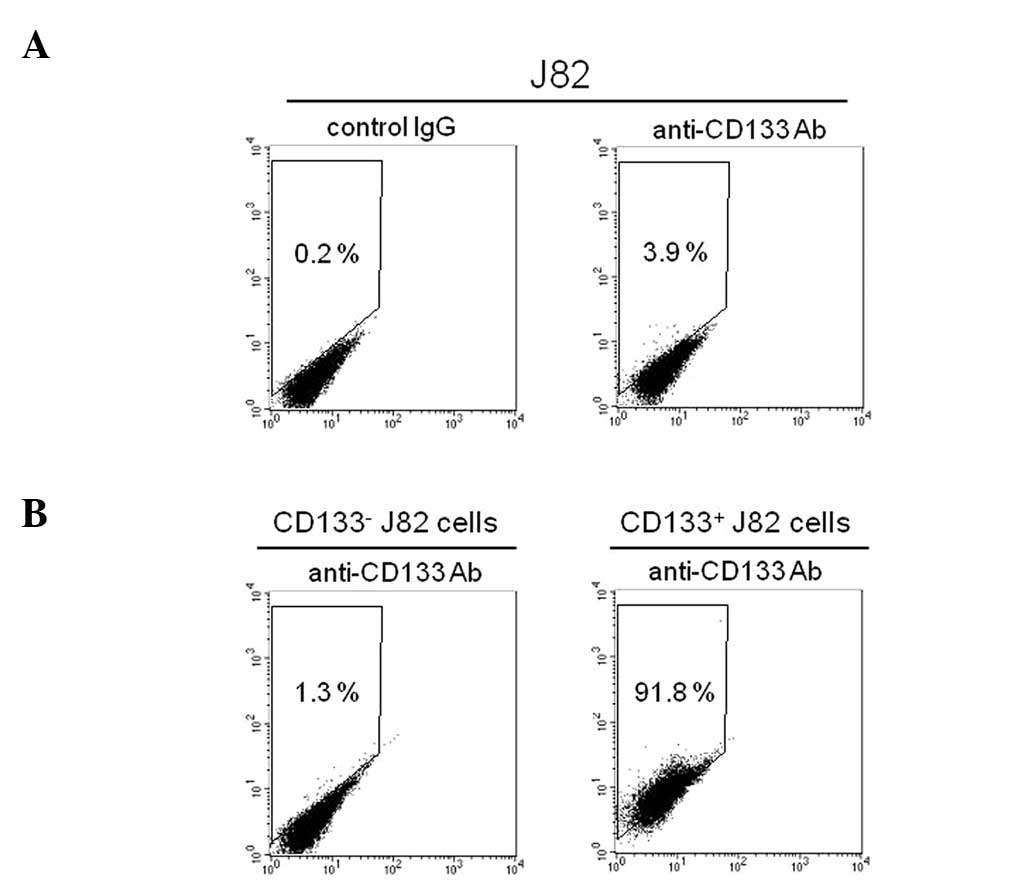
Flow cytometry was used to confirm the existence of
a CD133+ subpopulation in J82 cells. The
CD133+ subset comprised ∼4% of the J82 bladder cancer
cells (Fig. 1A). By using
immunomagnetic selection on the basis of their differential
expression of CD133, the J82 cells were successfully separated into
CD133− and CD133+ subpopulations. The
expanded culture of the CD133− subpopulation showed that
∼99% of the cells were negative for CD133 expression (Fig. 1B). By contrast, the cytometric
analysis on the culture of CD133+ cells showed that
>90% of the subpopulation expressed CD133 (Fig. 1B). These results demonstrated that
the J82 human bladder cancer cells consist of CD133− and
CD133+ subpopulations.
Phenotypic analysis of the CD133-based
subsets of J82 cells
The phenotypes of the CD133− and
CD133+ subsets of J82 cells were investigated for the
clonogenic capacity, the expression of pluripotent stem cell
factors and their proliferation capacity. Fig. 2A shows images of the expanded
cultures of the CD133− and CD133+
subpopulations. The CD133+ cells had grown, indicating
their tendency to colonize, which depends on the clonogenic
capacity of the cells. By contrast, CD133− cells had a
diffuse growth pattern, which was identical to that of the parental
J82 cells. Fig. 2B shows the
results of the western blot analysis of the expression of the
pluripotent stem cell markers, Oct-4 and Sox-2. The analysis
demonstrated the upregulation of the pluripotent stem cell factors
in the CD133+ subset in comparison with the
CD133− cells. Since the transcription factor expression
might alter the cell growth potential, the in vitro
proliferation profiles of the subpopulations were monitored
(Fig. 2C). The proliferation
potential was different in the two subsets, and the
CD133+ subset was significantly increased compared to
that of the CD133− cells after 3 days of
cultivation.
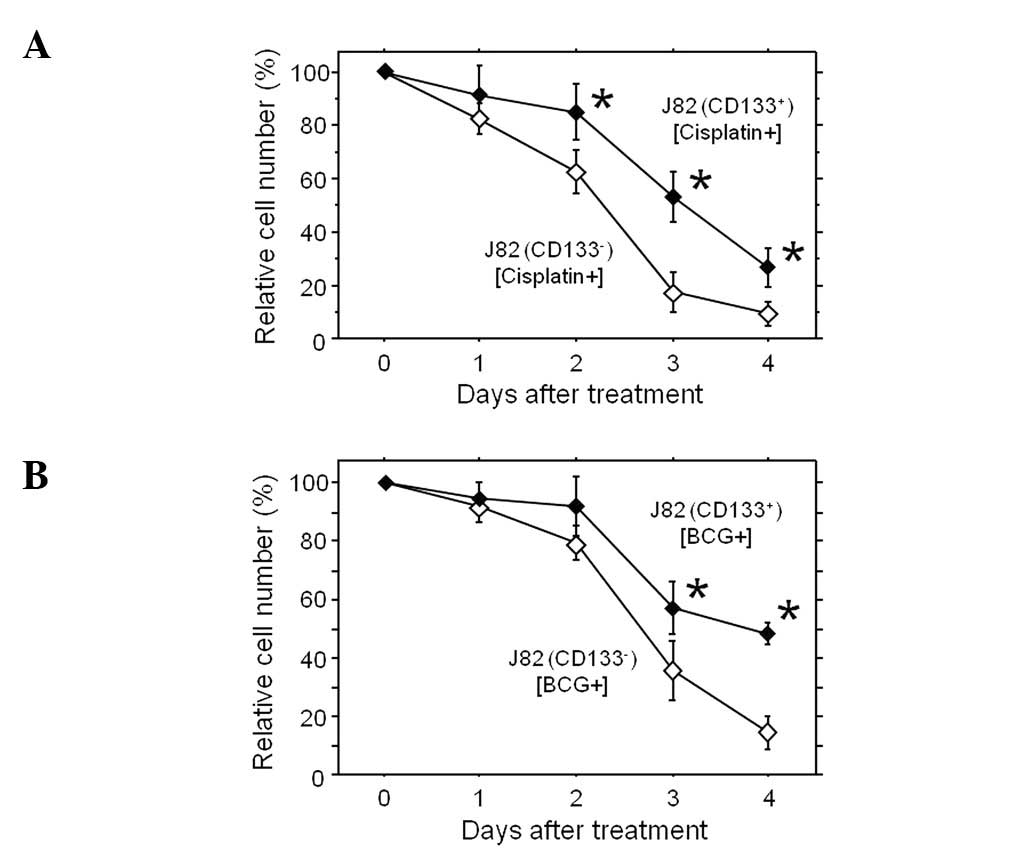
Resistance of the CD133+ cell
subset to anticancer agents and irradiation
To estimate the size of the CD133− and
CD133+ subsets when exposed to cisplatin and BCG,
commonly used as anti-bladder cancer drugs, these agents were added
to the culture medium of the subsets, and the cell growth was
analyzed (Fig. 3). The tolerance
to these agents was clearly different in the CD133− and
CD133+ subsets. In the treatment groups, the calculated
relative cell number (%) in the CD133+ subset was
significantly higher compared to that of the CD133−
cells 3–4 days subsequent to treatment. Thus, the CD133+
subpopulation of the J82 bladder cancer cells was demonstrated to
be more resistant to the chemotherapeutic agent cisplatin and BCG
in comparison to the CD133− subset. The size of these
subsets was then examined against irradiation (Fig. 4). After a 2-day X-ray exposure at a
total dose of 6 Gy, the calculated relative cell number (%) in the
CD133+ subpopulation was significantly larger compared
to that of the CD133− cells, demonstrating that the
CD133+ subpopulation was markedly more resistant to
radiation. These findings indicated that CD133+ cells
were more resistant to anticancer treatment, while having more
malignant characteristics in comparison to the CD133−
subset.
In vivo tumorigenicity of the CD133-based
subsets of J82 cells
The in vivo tumorigenicity of
CD133− and CD133+ subsets was evaluated by
inoculating these subsets into nude mice (Fig. 5). The CD133− and
CD133+ cells were subcutaneously injected into the left
and right thighs of the mice, respectively. Seven days subsequent
to the inoculation, subcutaneous tumors were found only at the
injection site of the CD133+ cells, as opposed to the
injection site of the CD133− cells. Twenty-one days
after the inoculation, the tumor size was significantly larger in
the CD133+ subpopulation compared to that of the
CD133− cells. These results indicated that there was a
significant difference in the tumorigenic potential of the
CD133− and CD133+ subpopulations, and that
the CD133+ cells exhibited a more malignant
phenotype.
Discussion
Human CD133 was first cloned as a cell surface
protein expressed on CD34+ hematopoietic progenitor
cells. The open reading frame of CD133 encodes 865 amino acids (AA)
with a 19-AA signal peptide at its N-terminus. Its molecular weight
is almost 120 kDa due to the N-glycosylation of the protein after
post-translational modification (16). Although the biological functions,
structure and endogenous effectors of CD133 are still being
elucidated, it has been proven to be a useful marker of stemness.
In addition to using its expression to identify hematopoietic stem
cells, CD133 has been used to identify the tumorigenic cells in
various organs, such as the colon and prostate (2,11).
In the present study, the CD133+ subset of J82 human
bladder cancer cells has been demonstrated to upregulate the
pluripotent stem cell markers Oct-4 and Sox-2, while demonstrating
a more aggressive proliferation compared to the CD133−
subpopulation. The CD133+ subpopulation also had a
tendency to form colonies, indicating a strong clonogenic capacity.
Since CSCs were reported to have the potential for colonization in
conditioned medium (2,17), while expressing the pluripotent
transcription factors (18–20)
in the other types of cancer, the CD133+ subset of the
human bladder cancer cell line, J82 exhibited typical phenotypic
features associated with CSCs.
The CD133+ subpopulation of the bladder
cancer cells was also demonstrated to be more tolerant to the
chemotherapeutic agent cisplatin compared to that of the
CD133− subpopulation. Cisplatin exerted a genotoxic
effect by means of crosslinking the cell genomic DNA and triggering
cell death. The excretion of chemical compounds by ABC (ATP-binding
cassette) transporters is a mechanism whereby the cells become
resistant to chemotherapeutic agents (21). A previous study has found that the
Oct-4 transcription factor directly binds to the promoter regions
of the ABC-B1, ABC-G2 and ABC-C1 transporters, while being involved
in regulating their expression (22). Since our studies demonstrated that
the Oct-4 expression level was significantly upregulated in the
CD133+ subset of cells, and these cells were more drug
resistant than their CD133− counterparts, the resistance
of the CD133+ subpopulation is likely to be linked to
the upregulation of Oct-4. In this study, the CD133+
subpopulation was also demonstrated to be more tolerant to the
intravesical instillation agent, BCG. Although the precise
mechanism of the resistance against this anticancer agent has yet
to be elucidated, the differential analysis of the molecular
signaling between the CD133− and CD133+
subsets is likely to be a promising approach to identify this
mechanism.
Notably, the significant radiation-resistant
properties of the CD133+ subpopulation of J82 human
bladder cancer cells were also demonstrated. Radiation tolerance
has also been observed in the CD133+ cells derived from
clinical specimens of teratoid/rhabdoid tumor (23) and medulloblastoma (24). Since findings of another study
suggested that the Oct-4 and Sox-2 transcription factors provide a
molecular scaffold for the activation of the DNA repair complex on
the genomic DNA in stem cells (25), it is possible that these factors,
which are upregulated in the CD133+ subset of J82 cells,
are directly related to radioresistance as a result of an increased
DNA repair after irradiation.
The in vivo tumorigenesis of the cells was
also analyzed by subcutaneously transplanting CD133− and
CD133+ subsets of J82 cancer cells into nude mice. The
tumor growth was more aggressive in the CD133+
subpopulation compared to that of the CD133− cells,
showing a significant difference in the tumorigenic potential in
these subsets. The stronger in vivo tumorigenic potential of
the CD133+ cells is consistent with the more aggressive
in vitro proliferation of this subpopulation. Since in
vivo environmental factors may affect the cell growth of the
CD133− and CD133+ subsets, it would be of
note to determine the ratio of these subpopulations in the tumors
derived from the CD133 subsets of J82 cancer cells in a future
experiment.
In conclusion, this is the first study to
demonstrate the cancer stem cell-like characteristics of the
CD133+ subpopulation in the human bladder cancer cell
line, J82. The human bladder cancer cells were demonstrated to
comprise CD133− and CD133+ subsets, while the
CD133+ cells demonstrated a tendency for colonization,
with an upregulated expression of the pluripotent stem cell factors
Oct-4 and Sox-2, and an increased proliferation potential. The
CD133+ subset was more resistant to anticancer drugs and
radiation therapy in vitro, while exhibiting a more
aggressive tumorigenicity in vivo compared to the
CD133− subset. These results suggest that the CD133
molecule is, not only a potential marker of the malignancy of
bladder cancer, but also a promising therapeutic target potentially
used to develop novel anticancer drugs against refractory bladder
cancer.
Acknowledgements
This study was financed by a
scientific research grant (KAKENHI 23390382) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan. The
authors would like to thank Dr Shun-Ai Li (Okayama University,
Okayama, Japan) for her valuable assistance.
References
|
1.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011.
|
|
2.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007.
|
|
3.
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA,
Parmiani G, Castelli C and Clarke MF: Phenotypic characterization
of human colorectal cancer stem cells. Proc Natl Acad Sci USA.
104:10158–10163. 2007.
|
|
4.
|
Monzani E, Facchetti F, Galmozzi E,
Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D,
Santinami M, Invernici G, Parati E, Alessandri G and La Porta CA:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007.
|
|
5.
|
Immervoll H, Hoem D, Sakariassen PØ,
Steffensen OJ and Molven A: Expression of the ‘stem cell marker’
CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC
Cancer. 8:482008.
|
|
6.
|
Marhaba R, Klingbeil P, Nuebel T,
Nazarenko I, Buechler MW and Zoeller M: CD44 and EpCAM:
cancer-initiating cell markers. Curr Mol Med. 8:784–804. 2008.
|
|
7.
|
Wu Y and Wu PY: CD133 as a marker for
cancer stem cells: progresses and concerns. Stem Cells Dev.
18:1127–1134. 2009.
|
|
8.
|
An Y and Ongkeko WM: ABCG2: the key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009.
|
|
9.
|
Lathia JD, Gallagher J, Heddleston JM,
Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE,
Hjelmeland AB and Rich JN: Integrin alpha 6 regulates glioblastoma
stem cells. Cell Stem Cell. 6:421–432. 2010.
|
|
10.
|
Yin AH, Miraglia S, Zanjani ED,
Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J and Buck
DW: AC133, a novel marker for human hematopoietic stem and
progenitor cells. Blood. 90:5002–5012. 1997.
|
|
11.
|
Guzmán-Ramírez N, Völler M, Wetterwald A,
Germann M, Cross NA, Rentsch CA, Schalken J, Thalmann GN and
Cecchini MG: In vitro propagation and characterization of
neoplastic stem/progenitor-like cells from human prostate cancer
tissue. Prostate. 69:1683–1693. 2009.
|
|
12.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
|
|
13.
|
Ueki H, Watanabe M, Kaku H, Huang P, Li
SA, Ochiai K, Hirata T, Noguchi H, Yamada H, Takei K, Nasu Y,
Kashiwakura Y and Kumon H: A novel gene expression system for
detecting viable bladder cancer cells. Int J Oncol. 41:135–140.
2012.
|
|
14.
|
Hirata T, Watanabe M, Kaku H, Kobayashi Y,
Yamada H, Sakaguchi M, Takei K, Huh NH, Nasu Y and Kumon H: REIC/
Dkk-3 encoding adenoviral vector as a potentially effective
therapeutic agent for bladder cancer. Int J Oncol. 41:559–564.
2012.
|
|
15.
|
Watanabe M, Ueki H, Ochiai K, Huang P,
Kobayashi Y, Nasu Y, Sasaki K, Kaku H, Kashiwakura Y and Kumon H:
Advanced two-step transcriptional amplification as a novel method
for cancer-specific gene expression and imaging. Oncol Rep.
26:769–775. 2011.
|
|
16.
|
Bidlingmaier S, Zhu X and Liu B: The
utility and limitations of glycosylated human CD133 epitopes in
defining cancer stem cells. J Mol Med (Berl). 86:1025–1032.
2008.
|
|
17.
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005.
|
|
18.
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008.
|
|
19.
|
Chen YC, Hsu HS, Chen YW, Tsai TH, How CK,
Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH and Chiou SH:
Oct-4 expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PloS One. 3:e26372008.
|
|
20.
|
Walter D, Satheesha S, Albrecht P,
Bornhauser BC, D’Alessandro V, Oesch SM, Rehrauer H, Leuschner I,
Koscielniak E, Gengler C, Moch H, Bernasconi M, Niggli FK and
Schäfer BW; CWS Study Group: CD133 positive embryonal
rhabdomyosarcoma stem-like cell population is enriched in
rhabdospheres. PloS One. 6:e195062011.
|
|
21.
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
|
|
22.
|
Marques DS, Sandrini JZ, Boyle RT, Marins
LF and Trindade GS: Relationships between multidrug resistance
(MDR) and stem cell markers in human chronic myeloid leukemia cell
lines. Leuk Res. 34:757–762. 2010.
|
|
23.
|
Chiou SH, Kao CL, Chen YW, Chien CS, Hung
SC, Lo JF, Chen YJ, Ku HH, Hsu MT and Wong TT: Identification of
CD133-positive radioresistant cells in atypical teratoid/rhabdoid
tumor. PloS One. 3:e20902008.
|
|
24.
|
Blazek ER, Foutch JL and Maki G: Daoy
medulloblastoma cells that express CD133 are radioresistant
relative to CD133− cells, and the CD133+
sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys.
67:1–5. 2007.
|
|
25.
|
Fong YW, Inouye C, Yamaguchi T, Cattoglio
C, Grubisic I and Tjian R: A DNA repair complex functions as an
Oct4/Sox2 coactivator in embryonic stem cells. Cell. 147:120–131.
2011.
|