Introduction
Colorectal cancer is one of the most common
malignancies in developed countries. Chemotherapy is the standard
treatment option for advanced colorectal cancer. Uracil plus
ftorafur (UFT) is an oral anticancer drug composed of
1-(tetrahydro-2-furanyl)-5-fluorouracil (ftorafur) and uracil, at a
molar ratio of 1:4 (1,2). Ftorafur, a prodrug of 5-fluorouracil
(5-FU), is converted to 5-FU by hepatic metabolism following
gastrointestinal absorption (3).
Uracil competitively inhibits the degradation of 5-FU by
dihydropyrimidine dehydrogenase. Thus, UFT increases 5-FU
concentration within the tumor site. Leucovorin
(5-formyltetrahydrofolate; LV) itself possesses no antitumor
activity; however, it enhances the anticancer activity of 5-FU by
providing a stable supply of 5,10-methylenetetrahydrofolate
(reduced folate; CH2THF) (4). UFT plus LV (UFT/LV) is widely
accepted as a chemotherapeutic regimen for advanced colorectal
cancer, due to its comparable efficacy to intravenous 5-FU plus LV
(5-FU/LV), its more favorable toxicity profile and its convenience
(by eliminating the need for repeated intravenous injections of
5-FU) (5–8). Although 5-FU/LV plus oxaliplatin
(FOLFOX) and 5-FU/LV plus irinotecan (FOLFIRI) have been widely
used, UFT/LV chemotherapy remains the backbone of colorectal cancer
treatment. Therefore, the identification of chemosensitivity
markers that may predict response to UFT/LV chemotherapy is a
useful approach to the individualization of colorectal cancer
treatment and it may help avoid the administration of inappropriate
chemotherapeutic regimens with unpleasant side effects.
The effectiveness of chemotherapy is dependent on
the intracellular accumulation of the anticancer drugs, which may
be altered by uptake and efflux transporters. Previously, the
majority of investigations on drug transporters has focused on the
drug efflux transporters and their ability to confer multidrug
resistance. However, mechanisms of uptake into the tumor cells may
prove even more important compared to efflux mechanisms in
predicting the efficacy of anticancer drugs, since they determine
intracellular drug concentrations (9,10).
The organic anion transporter (OAT) family of proteins are
essential for the uptake of endogenous compounds, a variety of
xenobiotics and clinically important drugs (11,12).
OAT2, also known as SLC22A7, mediates the sodium-independent uptake
of anticancer drugs, including 5-fluorouracil, methotrexate and
paclitaxel (13).
Reduced folate carrier 1 (RFC1), also referred to as
SLC19A1, is the major transporter of folates and methotrexate in
mammalian cells (14). LV is
preferred to folate as it is already reduced and may therefore
enter the cytoplasm via RFC1 (15). It is suggested that the expression
of OAT2 and RFC1 in tumor cells may be of predictive value for the
effectiveness of UFT/LV chemotherapy in colorectal cancer patients.
However, the role of the drug uptake transporters in UFT/LV
chemotherapy has not been elucidated. In the present study, we used
an immunohistochemical approach to investigate the correlation
between OAT2 and RFC1 expression and histological response to
preoperative UFT-based (UFT or UFT/LV) chemotherapy in colorectal
cancer, with the aim of identifying predictive biomarkers for the
efficacy of UFT/LV chemotherapy.
Materials and methods
Patients and specimens
The study population included 45 colorectal cancer
patients (28 male and 17 female), with a median age of 60 years
(range, 33–81 years). The patients had received preoperative
chemotherapy for 2 weeks, until 1 day prior to surgical resection
at the Fujita Health University Hospital, Aichi, Japan, between
2001 and 2009. The chemotherapeutic regimen was UFT (450–600
mg/body/day) for 24 patients and UFT (450–600 mg/body/day) plus LV
(75 mg/body/day) for the remaining 21 patients. No other treatment
was administered preoperatively. The clinicopathological
characteristics of the patients are provided in Table I. Informed consent for the
administration of preoperative chemotherapy as well as the use of
tumor tissue for analyzing protein expression was obtained from all
patients. This study was approved by the Ethics Committee of Kobe
University Graduate School of Health Sciences and Fujita Health
University School of Medicine.
 | Table ICorrelation of OAT2 and RFC1
expression with clinicopathological parameters. |
Table I
Correlation of OAT2 and RFC1
expression with clinicopathological parameters.
| Parameters | OAT2 expression
| RFC1 expression
|
|---|
| High | Low | P-value | High | Low | P-value |
|---|
| Age (years) | | | | | | |
| <60 | 6 | 16 | 0.13 | 6 | 16 | 1.00 |
| ≥60 | 12 | 11 | | 7 | 16 | |
| Gender | | | | | | |
| Male | 8 | 20 | 0.06 | 9 | 19 | 0.74 |
| Female | 10 | 7 | | 4 | 13 | |
| Tumor location | | | | | | |
| Colon | 10 | 5 | 0.02a | 5 | 10 | 0.73 |
| Rectum | 8 | 22 | | 8 | 22 | |
| Histological
grade | | | | | | |
| Low
(well-differentiated) | 6 | 12 | 0.75 | 5 | 13 | 1.00 |
| Intermediate
(moderately differentiated) | 11 | 15 | | 8 | 18 | |
| Depth of
invasion | | | | | | |
| Confined to the
muscularis propria | 6 | 6 | 0.50 | 3 | 9 | 1.00 |
| Invading or
exceeding the subserosa | 12 | 21 | | 10 | 23 | |
| Lymph node
metastasis | | | | | | |
| Negative | 8 | 12 | 1.00 | 9 | 11 | 0.05 |
| Positive | 10 | 15 | | 4 | 21 | |
| Distant
metastasis | | | | | | |
| Negative | 18 | 25 | 0.51 | 13 | 30 | 1.00 |
| Positive | 0 | 2 | | 0 | 2 | |
| Chemotherapeutic
regimen | | | | | | |
| UFT | 9 | 15 | 0.77 | 4 | 20 | 0.10 |
| UFT/LV | 9 | 12 | | 9 | 12 | |
Adequate biopsy material from ≥2 cancerous sections
was obtained from all patients prior to administration of
preoperative chemotherapy. The pre-treatment biopsies and
post-treatment resection specimens were routinely fixed in 10%
formalin and embedded in paraffin wax. Sections (3 μm) were
cut and mounted on aminopropyltriethoxysilane slides, then stained
with hematoxylin and eosin (H&E) to assess histopathological
features and chemotherapeutic effects.
Histological evaluation of
chemotherapeutic effects
Histological response was evaluated by grading the
post-treatment resection specimens according to the Japanese
Classification of Colorectal Carcinoma (16). Major grading (grades 0–3) and
additional minor grading for grade 1 (grades 1a and 1b) were
classified as follows: grade 0, no change; grade 1a, necrosis or
disappearance of the tumor in <1/3 of the whole lesion; grade
1b, necrosis or disappearance of the tumor in >1/3 but in
<2/3 of the whole lesion; grade 2, necrosis or disappearance of
the tumor in >2/3 of the whole lesion, with viable tumor cells;
and grade 3, necrosis of the whole lesion and/or replacement by
fibrotic tissue, with no viable tumor cells. The response of tumors
with grades 1b and 2 was classified as ‘good histological
response,’ and that of tumors with grades 0 and 1a as ‘poor
histological response’, according to a previous study (17). No tumors with grade 3 response were
identified in our study.
Immunohistochemistry
Immunohistochemical staining of formalin-fixed,
paraffin-embedded tumor sections was performed using a rabbit
polyclonal anti-OAT2 antibody (dilution 1:100, TransGenic,
Kumamoto, Japan) and a rabbit polyclonal anti-RFC antibody
(dilution 1:400, Atlas Antibodies, Stockholm, Sweden). Sections
were deparaffinized in xylene and rehydrated in graded alcohols.
Endogenous peroxidase activity was blocked by treatment with 0.3%
hydrogen peroxide in methanol for 30 min. For antigen retrieval,
pressure cooking was performed for 10 min at 120°C in optimal
soaking solutions: 0.001 mol/l ethylenediaminetetraacetic acid
(EDTA) (pH 8.0) for OAT2 and 0.01 mol/l Tris base containing 0.001
mol/l EDTA (pH 9.0) for RFC1. After pressure cooking, the sections
were cooled in the soaking solution at room temperature (RT) for 30
min. The sections were washed under running tap water, followed by
0.01 M phosphate-buffered saline (PBS, pH 7.2). After washing, the
sections were incubated with the primary antibodies overnight at
RT. The sections were then washed in PBS and incubated with
Histofine Simple Stain MAX PO (Nichirei, Tokyo, Japan) for 1 h at
RT. The reaction products were detected using a diaminobenzidine
solution (Dako, Glostrup, Denmark). Subsequently, the sections were
washed, counterstained with Mayer’s hematoxylin, dehydrated through
graded alcohols and xylene, and coverslipped. Negative controls
were set up by the omission of the primary antibodies. Positive
controls were formalin-fixed, paraffin-embedded tissue sections of
normal kidney and normal placenta for OAT2 and RFC1,
respectively.
Scoring of immunostained tissue
The stained sections were independently reviewed by
two investigators (S.N. and S.K.), who were blinded to the
clinicopathological characteristics of the patients. Staining was
regarded as positive when the tumor cells exhibited cytoplasmic
and/or membrane staining. Semi-quantitative assessment of OAT2 and
RFC1 expression levels was scored according to the staining
intensity and percentage of positive tumor cells. Briefly, the
staining intensity was scored as follows: 0, no staining; 1, weakly
positive; 2, moderately positive; and 3, strongly positive. The
percentage of positive tumor cells was scored as follows: 0, no
positive tumor cells; 1, <40% positive cells; 2, 40–70% positive
cells; and 3, ≥71% positive cells. A composite score was obtained
by calculating the sum of the two scores. Any differences in the
scores were discussed between the two investigators and
consolidated into a final score.
Statistical analysis
Receiver operating characteristic (ROC) curve
analysis was used for selecting the optimal cut-off score to
determine the threshold for a high expression level. According to
the cut-off score determined by the optimal sensitivity and
specificity (maximum sum of sensitivity and specificity), scores of
0–5 represented a ‘low expression level’ and a score of 6
represented a ‘high expression level’.
The Fisher’s exact test was used to evaluate the
correlation between OAT2 and RFC1 expression levels with patient
age and gender, tumor location, histological grade, depth of
invasion, lymph node metastasis and distant metastasis. The
Fisher’s exact test was also used to determine the association of
chemotherapeutic response with patient age and gender, tumor
location, histological grade, lymph node metastasis, distant
metastasis, chemotherapeutic regimen and OAT2 and RFC1 expression.
The correlation of expression levels between OAT2 and RFC1 was
analyzed using Pearson’s test.
Variables with a P-value <0.35 in the Fisher’s
exact test were included in a logistic regression model for
univariate and multivariate analyses to assess the predictive
factors that may affect the efficacy of preoperative chemotherapy.
Statistical analyses were performed using a free statistical
software EZR (Easy R) on R commander version 2.13.0 (Saitama
Medical Center, Jichi Medical University, Saitama, Japan).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Immunohistochemical findings
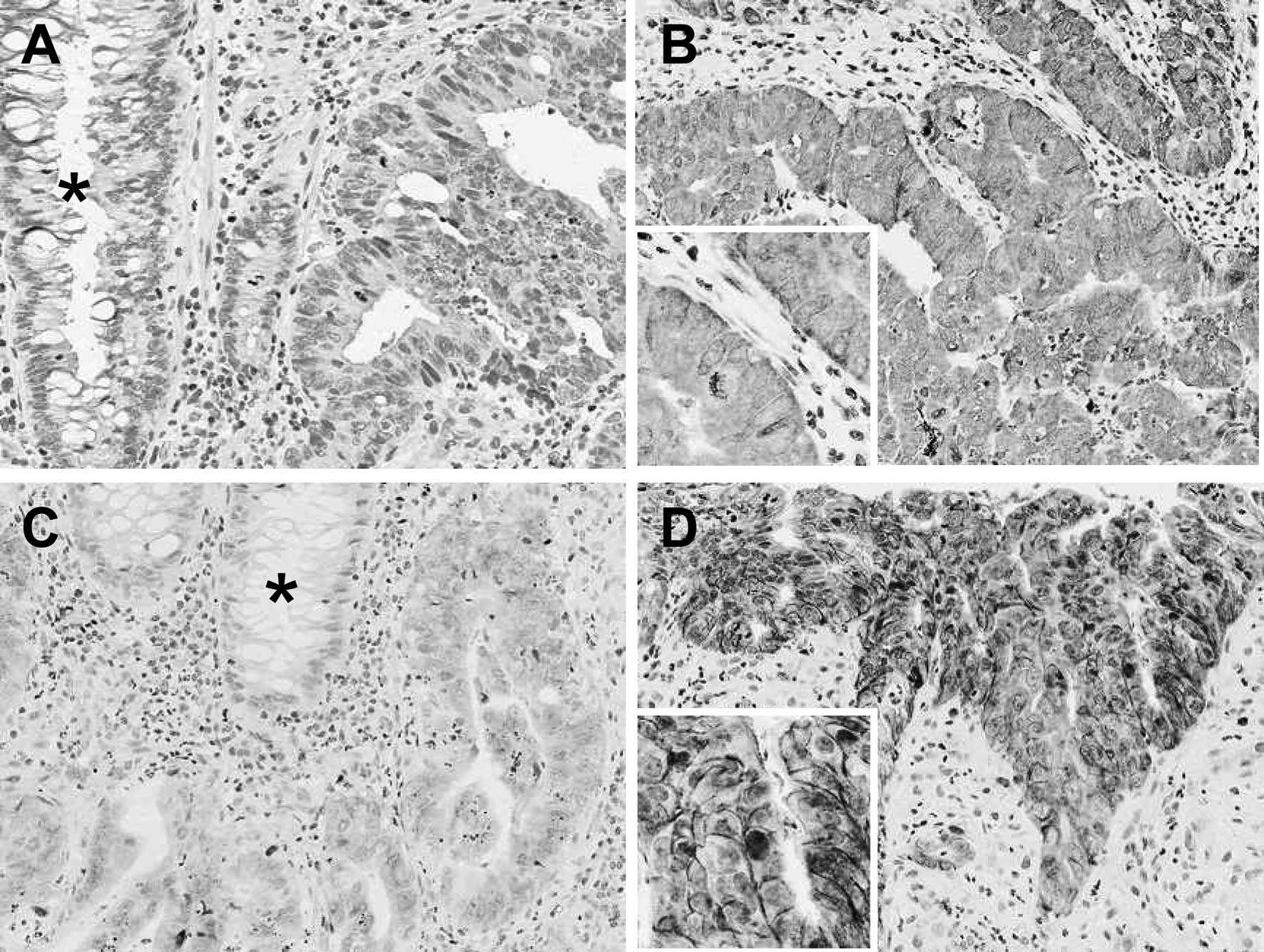
OAT2 was relatively homogeneously distributed
throughout the tumors; however, RFC1 exhibited heterogeneous
distribution. Of the 45 pre-treated biopsy specimens, 18 (40%)
exhibited high expression levels of OAT2 and 27 (60%) specimens
exhibited a low OAT2 expression. Thirteen (29%) of the biopsy
specimens exhibited high expression levels of RFC1 and 32 (71%)
specimens exhibited low RFC1 expression. No significant correlation
of expression levels was observed between OAT2 and RFC1 (r= 0.174;
P= 0.252). Normal colorectal epithelia exhibited negative or weak
staining for OAT2 and RFC1. The representative staining patterns of
OAT2 and RFC1 are shown in Fig.
1.
Correlation of expression levels of OAT2
and RFC1 with clinicopathological parameters
The correlation between expression levels of OAT2
and RFC1 in pre-treatment biopsy specimens and clinicopathological
parameters is shown in Table I.
High expression levels of OAT2 were observed in 10 (67%) of the 15
colon cancer patients and in 8 (27%) of the 30 rectal cancer
patients (P=0.02). No significant association of OAT2 expression
with the other clinicopathological parameters, including patient
age and gender, histological grade, depth of invasion, lymph node
metastasis, distant metastasis and chemotherapeutic regimen was
observed. No significant correlation was observed between
expression levels of RFC1 and any of the clinicopathological
parameters.
Correlation of clinicopathological
parameters and expression levels of OAT2 and RFC1 with histological
response to chemotherapy
Table II shows the
correlation of clinicopathological parameters and OAT2 and RFC1
expression levels with histological response to preoperative
chemotherapy. Good histological response was observed in 12 (27%)
of the 45 tumors [5 (21%) of the 24 UFT-treated tumors and in 7
(33%) of the 21 UFT/LV-treated tumors]. No significant association
was observed between histological response and clinicopathological
parameters, including patient age and gender, tumor location,
histological grade, depth of invasion, lymph node metastasis,
distant metastasis and chemotherapeutic regimen.
 | Table IICorrelation of clinicopathological
parameters and OAT2 and RFC1 expression with histological response
to UFT-based chemotherapy. |
Table II
Correlation of clinicopathological
parameters and OAT2 and RFC1 expression with histological response
to UFT-based chemotherapy.
| Variables | Good response | Poor response | P-value |
|---|
| Age (years) | | | |
| <60 | 5 | 17 | 0.74 |
| ≥60 | 7 | 16 | |
| Gender | | | |
| Male | 6 | 22 | 0.32 |
| Female | 6 | 11 | |
| Tumor location | | | |
| Colon | 4 | 11 | 1.00 |
| Rectum | 8 | 22 | |
| Histological
grade | | | |
| Low
(well-differentiated) | 3 | 15 | 0.30 |
| Intermediate
(moderately differentiated) | 9 | 17 | |
| Depth of
invasion | | | |
| Confined to the
muscularis propria | 5 | 7 | 0.25 |
| Invading or
exceeding the subserosa | 7 | 26 | |
| Lymph node
metastasis | | | |
| Negative | 7 | 13 | 0.32 |
| Positive | 5 | 20 | |
| Distant
metastasis | | | |
| Negative | 12 | 31 | 1.00 |
| Positive | 0 | 2 | |
| Chemotherapeutic
regimen | | | |
| UFT | 5 | 19 | 0.50 |
| UFT/LV | 7 | 14 | |
| OAT2
expression | | | |
| Low | 1 | 26 | <0.0001a |
| High | 11 | 7 | |
| RFC1
expression | | | |
| Low | 4 | 28 | 0.002a |
| High | 8 | 5 | |
A high OAT2 expression in the pre-treatment biopsies
was significantly correlated with good histological response to
UFT-based chemotherapy (P<0.0001): good histological response
was observed in 11 (61%) of the 18 tumors exhibiting a high OAT2
expression and in 1 (4%) of the 27 tumors exhibiting a low OAT2
expression. In addition, a high RFC1 expression was correlated with
good histological response (P= 0.002): good histological response
was observed in 8 (62%) of the 13 tumors exhibiting high RFC1
expression and in 4 (13%) out of the 32 tumors exhibiting a low
RFC1 expression.
Table III shows the
results of the logistic regression analysis of predictive factors
for histological response to UFT-based chemotherapy. OAT2
expression was identified as the most significant predictive factor
for good histological response in the univariate analysis [odds
ratio (OR): 0.03, 95% confidence interval (CI): 0.0006–0.24,
P<0.0001], as well as in the multivariate analysis (OR: 106, 95%
CI: 2.40–4650, P=0.02). RFC1 expression was identified as a
predictive factor for good histological response in the univariate
analysis (OR: 0.10, 95% CI: 0.02–0.51, P=0.002), but not in the
multivariate analysis (OR: 117, 95% CI: 0.85–16100, P= 0.06).
However, patient gender, histological grade, depth of invasion and
lymph node metastasis were not identified as predictive factors for
good histological response.
 | Table IIILogistic regression analysis of
predictive factors for histological response to UFT-based
chemotherapy. |
Table III
Logistic regression analysis of
predictive factors for histological response to UFT-based
chemotherapy.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Gender | 0.51
(0.11–2.40) | 0.32 | 7.70
(0.21–275) | 0.26 |
| Histological
grade | 0.39
(0.057–1.90) | 0.30 | 9.50
(0.47–190) | 0.14 |
| Depth of
invasion | 2.60 (0.49–13) | 0.25 | 0.03
(0.0005–2.3) | 0.12 |
| Lymph node
metastasis | 2.10 (0.46–10) | 0.32 | 0.19
(0.009–4.0) | 0.28 |
| OAT2
expression | 0.03
(0.0006–0.24) | <0.0001a | 106
(2.40–4650) | 0.02a |
| RFC1
expression | 0.10
(0.02–0.51) | 0.002a | 117
(0.85–16100) | 0.06 |
Correlation of OAT2 and RFC1 expression
with histological response according to chemotherapeutic
regimen
As shown in Table
IV, when separately analyzed according to the chemotherapeutic
regimen, OAT2 expression levels were significantly correlated with
good histological response in the UFT-treated and UFT/LV-treated
groups. In the UFT-treated group, 4 (44%) of the 9 patients with
high OAT2 expression levels and 1 (7%) of the 15 patients with low
OAT2 expression levels exhibited a good histological response
(P=0.04). In the UFT/LV-treated group, good histological response
was observed in 7 (78%) of the 9 patients with high OAT2 expression
levels and in none of the 12 patients with low OAT2 expression
levels (P<0.0005: higher level of statistical significance
compared to the UFT-treated group).
 | Table IVCorrelation of OAT2 and RFC1
expression with histological response according to chemotherapeutic
regimens. |
Table IV
Correlation of OAT2 and RFC1
expression with histological response according to chemotherapeutic
regimens.
| Transporter
expression | Good response | Poor response | P-value |
|---|
| UFT-treated group
(n=25) | | | |
| OAT2-high | 4 | 5 | 0.04a |
| OAT2-low | 1 | 14 | |
| RFC1-high | 2 | 2 | 0.18 |
| RFC1-low | 3 | 17 | |
| UFT/LV-treated
group (n=20) | | | |
| OAT2-high | 7 | 2 | <0.0005a |
| OAT2-low | 0 | 12 | |
| RFC1-high | 6 | 3 | 0.02a |
| RFC1-low | 1 | 11 | |
No association between RFC1 expression levels and
histological response was observed in the UFT-treated group (P=
0.18). However, RFC1 expression levels correlated with good
histological response in the UFT/LV-treated group. Good
histological response was observed in 6 (67%) of the 9 patients
with high RFC1 expression and in 1 (8%) of the 12 patients with low
RFC1 expression (P=0.02). Six of the 7 patients in the
UFT/LV-treated group who responded well, demonstrated high
expression levels of both OAT2 and RFC1.
Discussion
Oral UFT/LV chemotherapy remains the backbone of
treatment for colorectal cancer; thus, the identification of
chemosensitivity markers for predicting the response to UFT/LV
chemotherapy may facilitate more effective and individualized
treatment of this disease. Previously identified predictive
chemosensitivity markers for UFT/LV chemotherapy in colorectal
cancer include high mRNA expression levels of 5-FU
metabolism-related enzymes, orotate phosphoribosyltransferase
(18) and thymidine phosphorylase
(19). However, although the
expression of drug uptake transporters has been considered to be
mechanistically and biologically associated with tumor
chemosensitivity (9,10), the role of drug uptake transporters
in colorectal cancer chemotherapy has not been elucidated. Since
OAT2 and RFC1 are the major transporters of 5-FU and LV (13,15),
respectively, the expression levels of OAT2 and RFC1 may be
candidate biomarkers for the prediction of tumor response to UFT/LV
chemotherapy. In the present study, we immunohistochemically
investigated the correlation between expression levels of OAT2 and
RFC1 and histological response to UFT-based chemotherapy.
High expression levels of OAT2 and RFC1 were
observed in 40 and 29% of pre-treated colorectal cancer specimens,
respectively, whereas the corresponding normal colorectal epithelia
were negative or weakly positive for the two transporters. In
accordance with our results, Seithel et al reported that
OAT2 mRNA is absent in the normal human colon (20). In addition, Odin et al
reported that mean expression levels of the RFC-1 gene were
significantly higher in colorectal cancer tissues compared to the
adjacent normal mucosa (21). The
higher frequency of OAT2 and RFC1 expression in colorectal cancer
specimens, compared to the corresponding normal tissues, may
indicate that an increased expression of OAT2 and RFC1 is
associated with the development of colorectal cancer.
We also demonstrated that high expression levels of
OAT2 occurred significantly more frequently in colon cancer
compared to rectal cancer (P= 0.02). Recently, Hlavata et al
(22) reported that certain
ATP-binding cassette (ABC) transporters exhibited significantly
differential mRNA expression between colon and rectal cancer
tissues. The mRNA levels of ABCA12, ABCC7 and
ABCC8 increased in direction from the colon to the rectum,
whereas ABCB9, ABCB11, ABCG5 and ABCG8
exhibited a significant reverse trend, i.e., a decrease in the
levels in direction from the colon to the rectum. However, although
we identified OAT2 to be differentially distributed between colon
and rectal cancer tissues, this distribution pattern has not yet
been investigated. Further investigations are required to elucidate
the underlying mechanisms and biological significance of the
differential transporter distribution.
In the univariate logistic regression analysis
following adjustment for several clinical factors, high expression
levels of OAT2 and RFC1 were identified as significant predictive
factors of good histological response to UFT-based chemotherapy
(P<0.0001 and P= 0.002, respectively). In the multivariate
logistic regression analysis, the association between high
expression levels of OAT2 and good response to UFT-based
chemotherapy was also found to be significant, independent of the
other clinicopathological factors (P=0.02). However, RFC1
expression was not confirmed as an independent predictive factor
for tumor response to treatment.
When separately analyzed according to the
chemotherapeutic regimen, high OAT2 expression levels were
significantly correlated with good response in the UFT-treated
(P=0.04) as well as in the UFT/LV-treated groups (P<0.0005:
higher level of statistical significance compared to the
UFT-treated group). However, high RFC1 expression was associated
with good response in the UFT/LV-treated group (P=0.02) but not in
the UFT-treated group. Furthermore, the majority of responders in
the UFT/LV-treated group exhibited high expression levels of both
OAT2 and RFC1. These results suggest that the predictive power of
OAT2 and RFC1 expression was stronger for the UFT/LV combination
therapy compared to the UFT monotherapy.
Based on the data of the present study, we
hypothesized on the mechanism by which OAT2 and RFC1 expression
levels are predictive of the response to UFT/LV chemotherapy. 5-FU
is imported into the tumor cells via OAT2 and then metabolized to
its active metabolite, 5-fluorodeoxyuridine monophosphate (FdUMP)
(4). FdUMP binds to
CH2THF and thymidylate synthase (TS) to form a ternary
complex, resulting in the suppression of DNA synthesis through the
inhibition of TS activity (4).
High intracellular levels of CH2THF are required for
optimal formation of the ternary complex. LV enters the cell via
RFC1, increases the intracellular concentration of
CH2THF and thus potentiates ternary complex formation
(4). In patients with high OAT2
expression levels, large amounts of 5-FU are imported following UFT
administration. In the UFT/LV-treated group, in patients with high
expression levels of RFC1 as well as OAT2, increased LV uptake may
lead to sufficient levels of CH2THF. Consequently,
ternary complex formation and subsequent TS inhibition may be more
efficient. However, in patients with a low RFC1 expression,
CH2THF does not reach sufficient levels and the ternary
complex may not be able to form as efficiently, resulting in poor
TS inhibition, despite the administration of LV.
However, the contribution of OAT2 expression to the
antitumor effect of UFT monotherapy was less significant compared
to that of the UFT/LV combination therapy. Even if a sufficient
amount of 5-FU enters the tumor cells via OAT2, the ternary complex
may not be able to form sufficiently if the tumor cells are in a
folate-deficient condition, resulting in poor TS inhibition.
Furthermore, RFC1 expression levels may be irrelevant to the effect
of UFT alone.
In conclusion, high expression levels of OAT2 and
RFC1 in pre-treatment biopsy specimens were significantly
correlated with the antitumor effect of UFT-based chemotherapy,
particularly of UFT/LV regimens. Immunohistochemical analysis of
OAT2 and RFC1 expression may be a useful tool to identify
colorectal cancer patients who may benefit from treatment with an
UFT/LV regimen. However, our findings were based on a retrospective
analysis of a limited patient sample; thus, a large-scale
prospective trial is required to confirm OAT2 and RFC1 expression
as prognostic indicators in colorectal cancer patients receiving
oral UFT/LV chemotherapy in the adjuvant setting.
References
|
1.
|
Fujii S, Ikenaka K, Fukushima M and
Shirasaka T: Effect of uracil and its derivatives on antitumor
activity of 5-fluorouracil and
1-(2-tetrahydrofuryl)-5-fluorouracil. Gann. 69:763–772.
1978.PubMed/NCBI
|
|
2.
|
Milano G, Ferrero JM and François E:
Comparative pharmacology of oral fluoropyrimidines: a focus on
pharmacokinetics, pharmacodynamics and pharmacomodulation. Br J
Cancer. 91:613–617. 2004.PubMed/NCBI
|
|
3.
|
Anttila MI, Sotaniemi EA, Kairaluoma MI,
Mokka RE and Sundquist HT: Pharmacokinetics of ftorafur after
intravenous and oral administration. Cancer Chemother Pharmacol.
10:150–153. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Borner MM, Schöffski P, de Wit R, et al:
Patient preference and pharmacokinetics of oral modulated UFT
versus intravenous fluorouracil and leucovorin: a randomised
crossover trial in advanced colorectal cancer. Eur J Cancer.
38:349–358. 2002. View Article : Google Scholar
|
|
6.
|
Carmichael J, Popiela T, Radstone D, et
al: Randomized comparative study of tegafur/uracil and oral
leucovorin versus parenteral fluorouracil and leucovorin in
patients with previously untreated metastatic colorectal cancer. J
Clin Oncol. 20:3617–3627. 2002. View Article : Google Scholar
|
|
7.
|
Douillard JY, Hoff PM, Skillings JR, et
al: Multicenter phase III study of uracil/tegafur and oral
leucovorin versus fluorouracil and leucovorin in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
20:3605–3616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lembersky BC, Wieand HS, Petrelli NJ, et
al: Oral uracil and tegafur plus leucovorin compared with
intravenous fluorouracil and leucovorin in stage II and III
carcinoma of the colon: results from National Surgical Adjuvant
Breast and Bowel Project Protocol C-06. J Clin Oncol. 24:2059–2064.
2006. View Article : Google Scholar
|
|
9.
|
Dobson PD and Kell DB: Carrier-mediated
cellular uptake of pharmaceutical drugs: an exception or the rule?
Nat Rev Drug Discov. 7:205–220. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sissung TM, Baum CE, Kirkland CT, Gao R,
Gardner ER and Figg WD: Pharmacogenetics of membrane transporters:
an update on current approaches. Mol Biotechnol. 44:152–167. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Marzolini C, Tirona RG and Kim RB:
Pharmacogenomics of the OATP and OAT families. Pharmacogenomics.
5:273–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
You G: The role of organic ion
transporters in drug disposition: an update. Curr Drug Metab.
5:55–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kobayashi Y, Ohshiro N, Sakai R, Ohbatashi
M, Kohyama N and Yamamoto T: Transport mechanism and substrate
specificity of human organic anion transporter 2 (hOat2 [SLC22A7]).
J Pharm Pharmacol. 57:573–578. 2005.
|
|
14.
|
Sirotnak FM and Tolner B: Carrier-mediated
membrane transport of folates in mammalian cells. Annu Rev Nutr.
19:91–122. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Blehaut H, Mircher C, Ravel A, et al:
Effect of leucovorin (folinic acid) on the developmental quotient
of children with Down’s syndrome (trisomy 21) and influence of
thyroid status. PLoS One. 5:e83942010.PubMed/NCBI
|
|
16.
|
Japanese Society for Cancer of the Colon
and Rectum: Japanese Classification of Colorectal Carcinoma. 2nd
English edition. Kanehara Shuppan Co. Ltd.; Tokyo: 2009
|
|
17.
|
Kamoshida S, Matsuoka H, Shiogama K, et
al: Immunohistochemical analysis of thymidylate synthase,
p16INK4a, cyclin-dependent kinase 4 and cyclin D1 in
colorectal cancers receiving preoperative chemotherapy:
significance of p16INK4a-mediated cellular arrest as an
indicator of chemosensitivity to 5-fluorouracil. Pathol Int.
54:564–575. 2004.PubMed/NCBI
|
|
18.
|
Ichikawa W, Uetake H, Shirota Y, et al:
Both gene expression for orotate phosphoribosyltransferase and its
ratio to dihydropyrimidine dehydrogenase influence outcome
following fluoropyrimidine-based chemotherapy for metastatic
colorectal cancer. Br J Cancer. 89:1486–1492. 2003. View Article : Google Scholar
|
|
19.
|
Sadahiro S, Suzuki T, Tanaka A, Okada K,
Nagase H and Uchida J: Association of right-sided tumors with high
thymidine phosphorylase gene expression levels and the response to
oral uracil and tegafur/leucovorin chemotherapy among patients with
colorectal cancer. Cancer Chemother Pharmacol. 70:285–291. 2012.
View Article : Google Scholar
|
|
20.
|
Seithel A, Karlsson J, Hilgendorf C,
Björquist A and Ungell AL: Variability in mRNA expression of ABC-
and SLC-transporters in human intestinal cells: comparison between
human segments and Caco-2 cells. Eur J Pharm Sci. 28:291–299. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Odin E, Wettergren Y, Nilsson S, et al:
Altered gene expression of folate enzymes in adjacent mucosa is
associated with outcome of colorectal cancer patients. Clin Cancer
Res. 9:6012–6019. 2003.PubMed/NCBI
|
|
22.
|
Hlavata I, Mohelnikova-Duchonova B,
Vaclavikova R, et al: The role of ABC transporters in progression
and clinical outcome of colorectal cancer. Mutagenesis. 27:187–196.
2012. View Article : Google Scholar : PubMed/NCBI
|