Introduction
Lung cancer is one of the most commonly encountered
human malignancies. Due to the increase in life expectancy and the
incidence of lung cancer, the incidence of senile lung cancer has
increased significantly and reaches a peak between the ages of 70
and 74 years. Adenocarcinoma accounts for ∼50% of lung cancer cases
in senile patients. Therefore, the combined modality treatment of
lung adenocarcinoma is important for senile patients. Radiotherapy
and biological targeted therapy have become important therapeutic
methods for senile patients with adenocarcinoma of the lung, due to
organ miopragia and decreased cell damage repair ability exhibited
by senile patients. Gefitinib (ZD1839, Iressa) is a selective
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor,
exhibiting satisfactory effects and low adverse reaction rates in
Asian non-small cell lung cancer patients (NSCLC) (1). Gefitinib has also been used in the
treatment of elderly or poor performance status patients with
advanced NSCLC (2). γ-ray
stereotactic radiotherapy (SBRT) exerts a satisfactory short-term
therapeutic effect in senile NSCLC patients. Furthermore, EGFR
inhibitors have been proven to be efficient radiosensitizers
(3,4). In order to evaluate the efficacy and
safety of gefitinib combined with γ-ray SBRT as the first-line
treatment for senile patients with adenocarcinoma of the lung, we
enrolled 122 senile lung adenocarcinoma patients between July, 2005
and June, 2007 and demonstrated that gefitinib combined with γ-ray
SBRT is feasible and exhibits long-term and short-term
efficacy.
Patients and methods
General
This study did not use randomization; instead, the
opinions of the patients and their family members were solicited
and the patients were subsequently assigned to the corresponding
therapeutic group.
Patient selection criteria
Criteria for enrollment in the study were as
follows: adenocarcinoma of the lung confirmed by histology or
cytology; operation and chemotherapy were rejected by the patients
and/or their family members; the age of patients was ≥70 years and
their performance status according to Zubrod-ECOG-WHO (ZPS) was
0–3; presence of at least one measurable lesion and a total number
of lesions <3; normal function of the heart, liver and kidneys;
no moderate or severe malignant hydrothorax; no typical
interstitial pneumonia or pulmonary fibrosis. This study was
approved by the ethics committee of Jiangsu University. Patients
and/or family members signed the informed consent.
Patient charcteristics
A total of 122 patients (54 males and 68 females)
were included in the 3 groups (average age, 74.5 years; range,
70–83 years). All 122 patients had adenocarcinoma of the lung
confirmed by histology or cytology. According to the American Joint
Committee on Cancer TNM Staging System for lung cancer, 27 patients
had stage II and 95 had stage III lung adenocarcinoma. The average
tumor diameter was 5.6 cm (range, 2–10 cm). The ZPS performance
status was 0–3 and the average score was 2.2. There was no
statistical variance in age and clinical stage among the 3 groups
(Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Group A | Group B | Group C |
|---|
| Average age
(years) | 74.6 | 74.4 | 74.5 |
| Male | 16 | 20 | 18 |
| Female | 19 | 25 | 24 |
| Average performance
status (ZPS) | 1.9 | 1.9 | 1.9 |
| Average score | 2.3 | 2.2 | 2.2 |
| Average diameter of
the lesions (cm) | 5.2 | 5.7 | 5.7 |
Therapeutic method
Group A included 35 patients treated with gefitinib
combined with γ-ray SBRT, group B included 45 patients treated with
γ-ray SBRT alone and group C included 42 patients treated with
gefitinib alone. The patients received 250 mg of gefitinib per day,
from the first day of the treatment until disease progression or
discontinuation due to other causes. The patients were treated with
γ-ray SBRT, initiated on the second day. The dose curve for this
case group was 50–80%. The encircled dose was 4.0–6.5 Gy per
fraction and the range of the total radiation dose was 36–48 Gy.
The total number of treatments was 8–12, at a frequency of 5 times
per week. Antiasthmatic, expectorant, anti-inflammatory and other
symptomatic treatments were used as supportive therapy in order to
manage symptoms such as cough, expectoration and shortness of
breath.
Assessments of therapeutic effects
The patients were telephonically followed up monthly
and by a follow-up visit at the clinic once every 3 months. The
objective treatment effectiveness was assessed in the 3 groups by
evaluation of the double helical computed tomography (CT) performed
at 2 months. Efficacy was evaluated using the Response Evaluation
Criteria in Solid Tumors, version 1.1 (6). Patients were classified as exhibiting
complete response (CR), partial response (PR), stable disease (SD)
or progressive disease (PD). The response rate (RR) was calculated
using the formula RR = CR + PR. The disease control rate (DCR) was
calculated using the formula DCR = CR + PR + SD. Toxicity was
evaluated according to the standards for adverse reactions (grades
1–4), as issued by the National Cancer Institute of the USA
(7).
Statistical analysis
The therapeutic effect was evaluated as the time
period between treatment effectiveness and appearance of signs of
disease progression. The OS rate was estimated for the time period
between enrollment and death. Disease progression was evaluated
from enrollment to the appearance of signs of disease progression.
The objective therapeutic effect was calculated with the
χ2 test and the survival time was assessed using the
Kaplan-Meier method. All 122 patients enrolled in our study were
analyzed.
Results
Objective therapeutic effects
Table II shows the
effects exerted by different therapeutic methods, as assessed by
contrast-enhanced double helical CT at 2 months and expressed as
progression-free survival (PFS), median overall survival (OS) and
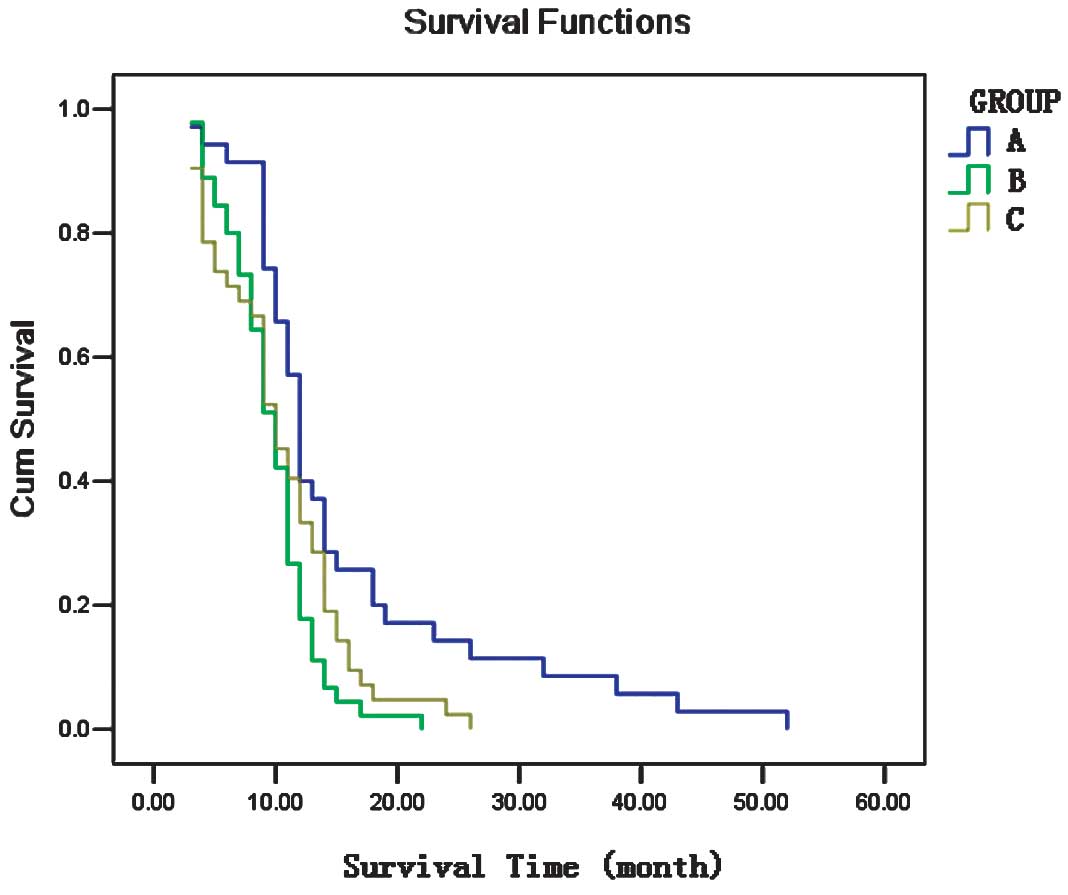
1-year OS rate. The survival analysis is shown in Fig. 1. Group A exhibited a better
short-term therapeutic effect (RR) compared to that of group C
(P=0.014); however, there was no significant difference compared to
group B (P=0.116). The PFS of group A was higher compared to that
of groups B and C (7.8 vs. 5.9, P=0.018 and 7.8 vs. 5.1, P=0.013,
respectively). The OS of group A was higher compared to that of
groups B and C (15.5 vs. 9.6, P=0.002 and 15.5 vs. 10.3, P=0.017,
respectively). The short-term therapeutic effect in group B was
better compared to that in group C, although this finding was of no
statistical significance (P=0.320). There were no significant
differences in PFS and OS between groups B and C (5.9 vs. 5.1,
P=0.329 and 9.6 vs. 10.3, P=0.633, respectively).
 | Table II.Therapeutic effects in patients
treated by different therapeutic methods. |
Table II.
Therapeutic effects in patients
treated by different therapeutic methods.
| Therapeutic
method | CR n (%) | PR n (%) | SD n (%) | PD n (%) | RR n (%) | DCR n (%) | PFS (months) | OS (months) | 1-year OS rate
(%) |
|---|
| Group A | 3 (8.6) | 21 (60.0) | 7 (20.0) | 4 (11.4) | 24 (68.6) | 31 (88.6) | 7.8 | 15.5 | 40.0 |
| Group B | 1 (2.2) | 22 (48.9) | 9 (20.0) | 13 (28.9) | 23 (51.1) | 32 (71.1) | 5.9 | 9.6 | 15.6 |
| Group C | 3 (7.1) | 14 (33.3) | 9 (21.4) | 16 (38.1) | 17 (40.5) | 26 (61.9) | 5.1 | 10.3 | 35.7 |
Adverse effects
All 122 patients were included in the toxicity
evaluation (Table III). Adverse
effects experienced by patients were considered acceptable and they
included skin rash and diarrhea (grades 1–3) that were manageable
by symptomatic treatment.
 | Table III.Adverse effects experienced by
patients treated by different therapeutic methods. |
Table III.
Adverse effects experienced by
patients treated by different therapeutic methods.
| Adverse effect | Group A, n (%)
| Group B, n (%)
| Group C, n (%)
|
|---|
| 0 | 1 | 2 | 3a | 0 | 1 | 2 | 3a | 0 | 1 | 2 | 3a |
|---|
| Leukopenia | 25 (71) | 7 (20) | 3 (9) | 0 (0) | 36 (80) | 5 (11) | 4 (9) | 0 (0) | 40 (95) | 2 (5) | 0 (0) | 0 (0) |
| Thrombocytopenia | 32 (91) | 3 (9) | 0 (0) | 0 (0) | 43 (96) | 2 (4) | 0 (0) | 0 (0) | 41 (98) | 1 (2) | 0 (0) | 0 (0) |
| Anemia | 29 (83) | 5 (14) | 1 (3) | 0 (0) | 40 (89) | 3 (7) | 2 (4) | 0 (0) | 40 (95) | 2 (5) | 0 (0) | 0 (0) |
| Rash | 10 (29) | 14 (40) | 9 (26) | 2 (6) | 43 (96) | 2 (4) | 0 (0) | 0 (0) | 15 (36) | 14 (33) | 12 (29) | 1 (2) |
| Diarrhea | 19 (54) | 7 (20) | 6 (17) | 3 (9) | 43 (96) | 2 (4) | 0 (0) | 0 (0) | 24 (57) | 9 (21) | 8 (19) | 1 (2) |
| Nausea/vomiting | 26 (74) | 5 (14) | 3 (9) | 1 (3) | 41 (91) | 4 (9) | 0 (0) | 0 (0) | 37 (88) | 3 (7) | 2 (5) | 0 (0) |
| Dyspnea | 24 (69) | 7 (20) | 3 (9) | 1 (3) | 39 (87) | 5 (11) | 1 (2) | 0 (0) | 36 (86) | 4 (10) | 2 (5) | 0 (0) |
Discussion
Gefitinib is an aniline quinazoline compound that
may be used to promote apoptosis, limit angiogenesis and restrict
differentiated proliferation or migration of cancer cells via the
potent inhibition of EGFR tyrosine kinase. It is an oral
pharmaceutic and may be administered at home. Therefore, it is
widely used as second- or third-line treatment in patients with
advanced NSCLC, following failure of platinum-based therapy
(7). Although a previous ISEL
study (8) did not demonstrate a
distinct effect on the prolongation of the patient life span, the
subordinate analysis demonstrated a significant life span
prolongation in patients of Asiatic origin. The median survival
time of the gefitinib and placebo groups was 9.5 and 5.5 months,
respectively. Wu et al (9)
reported that the parameters resulting from gefitinib
administration to Chinese patients with advanced NSCLC were as
follows: CR, 4.3% (5/115); PR, 39.1% (45/115); NC, 27.0% (31/115);
PD, 29.6% (34/115) and RR, 43.5% (50/115); the median time of
symptomatic response was 8 days. These results demonstrated that
gefitinib exerted satisfactory therapeutic and few adverse effects
in Chinese patients with advanced NSCLC. Recently, Gao et al
(10) reported their clinical
observations from a study investigating gefitinib as a first-line
treatment in 68 patients with advanced NSCLC and reached the
conclusion that first-line gefitinib treatment is an effective and
well-tolerated treatment regimen for advanced NSCLC. The
complications of conventional radiotherapy are limiting to its
application in senile lung cancer patients, due to their poor
overall health status. With the development of stereotactic
radiological technology, we may focus the delivery of the
appropriate radiation dose to the diseased region, avoiding
irradiation of surrounding normal tissues and organs. Thus, we may
increase irradiation dose of the tumor while protecting the normal
tissues. Therefore, the complications of radiotherapy are reduced,
total treatment time is shortened and SBRT may be successfully used
in senile lung cancer patients. To the best of our knowledge, SBRT
is effective regarding locoregional tumor control; however, its
therapeutic effect regarding tumor recurrence or metastasis is
limited. Therefore, we attempted to combine gefitinib and SBRT in a
treatment regimen that is locally and systemically effective for
the senile NSCLC patients. Four previously conducted large
(>4,000 patients) randomized phase III trials on chemotherapy
with or without concomitant administration of an EGFR tyrosine
kinase inhibitor in unselected patients with advanced-stage NSCLC,
did not demonstrate any correlation between combination treatment
and improvement in clinical outcome (11). However, a preclinical study
conducted by Tanaka et al (12) demonstrated that gefitinib enhances
the radioresponse of NSCLC cells by suppressing cell DNA repair
capacity, thereby prolonging the presence of radiation-induced DNA
double-strand breaks (DSBs). Their findings suggested that the
combination of gefitinib and radio-therapy may be of value in
clinical practice. A previous study by Zhuang et al
(13) demonstrated that optimal
radiosensitization was achieved when gefitinib was administered
prior to irradiation. In our study, gefitinib treatment was
initiated on the first day and radiation treatment was initiated on
the second day, allowing for the position fixing of γ-ray SBRT on
the first day. Therefore, gefitinib may have significantly enhanced
the efficiency of radiotherapy in our study. Stinchcombe et
al (14) reported that
induction chemotherapy with carboplatin, irinotecan and paclitaxel,
followed by high-dose three-dimensional conformal thoracic
radiotherapy (74 Gy) with concurrent administration of carboplatin,
paclitaxel and gefitinib, elicited a good response in unresectable
stage IIIA and IIB NSCLC. However, the combination of gefitinib,
radiotherapy and chemotherapy was associated with severe adverse
effects, such as radiation esophagitis and cardiac toxicity.
Therefore, we did not include chemotherapy in our combination
treatment, since our clinical trial involved senile patients.
There was no reported patient mortality associated
with treatment-related adverse effects. Furthermore, the adverse
effects experienced by the patients were tolerable and treatable.
The RR of group A (gefitinib combined with γ-ray SBRT), group B
(γ-ray SBRT alone) and group C (gefitinib alone) was 68.6, 51.1 and
40.5%, respectively, when we assessed tumors with contrast-enhanced
double helical CT at 2 months. The RR demonstrated by our study is
higher compared to that observed in non-Asiatic patients, a finding
that may be attributed to the fact that RR is positively correlated
with the mutation rate of EGFR, which, to the best of our
knowledge, is higher in Chinese compared to non-Asiatic patients
(15,16). Group A exhibited better short-term
therapeutic effects compared to group C (P=0.014) and had higher
PFS and OS rates compared to groups B and C. It suggested that
gefitinib combined with γ-ray SBRT exhibited better short- as well
as long-term therapeutic effects when used as a first-line regimen
for the treatment of senile patients with adenocarcinoma of the
lung. Therefore, this trial demonstrated that the combination of
micromolecular-targeted drug therapy and SBRT may be beneficial.
Furthermore, group A (gefitinib combined with γ-ray SBRT) exhibited
better short-term therapeutic effects compared to group B (γ-ray
SBRT alone), although the difference was of no statistical
significance (P=0.116). This finding may be attributed to our
relatively small patient sample and further studies, including
larger patient samples, are required. Previous studies demonstrated
that the EGFR gene mutation status was associated with the efficacy
of gefitinib in patients with advanced NSCLC (17,18).
A recent NEJ 003 study suggested that first-line gefitinib
treatment may be preferable to standard chemotherapy for advanced
NSCLC patients aged ≥75 years harboring EGFR mutations (19). However, we did not screen all
patients for EGFR and KRAS mutations at the initial enrollment in
2005. Therefore, we were not able to elucidate the relationship
between gene mutation status and treatment efficacy for different
treatment protocols.
We are currently observing the therapeutic effects
of SBRT in patients not previously treated with gefitinib, using
gefitinib only in the case of tumor recurrence following treatment
with SBRT. At present, the therapeutic effects of a treatment
protocol combining erlotinib and SBRT are under evaluation. Whether
the combination of molecular-targeted therapy and radiotherapy is
preferable to the current standard combination of chemo- and
radiotherapy in non-senile patients with advanced NSCLC, requires
further investigation.
In conclusion, gefitinib combined with γ-ray SBRT
appears to be feasible and effective as the first-line treatment
regimen for senile patients with adenocarcinoma of the lung,
although further investigations are required.
Acknowledgements
This study was supported by a grant
from the Clinical Medicine Sciences Foundation of Jiangsu
University (no. JLY20080085).
References
|
1.
|
Cappuzzo F, Ligorio C, Jänne PA, et al:
Prospective study of gefitinib in epidermal growth factor receptor
fluorescence in situ hybridization-positive/phospho-Akt-positive or
never smoker patients with advanced non-small-cell lung cancer: the
ONCOBELL trial. J Clin Oncol. 25:2248–2255. 2007. View Article : Google Scholar
|
|
2.
|
Lin CC and Yang CH: Epidermal growth
factor receptor tyrosine kinase inhibitors in elderly or poor
performance status patients with advanced non-small cell lung
cancer. Target Oncol. 4:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chang BW and Saif MW: Combining epidermal
growth factor receptor inhibitors and radiation therapy in
pancreatic cancer: small step or giant leap? JOP. 10:231–236.
2009.PubMed/NCBI
|
|
4.
|
Magné N, Chargari C, Castadot P, et al:
The efficacy and toxicity of EGFR in the settings of radiotherapy:
focus on published clinical trials. Eur J Cancer. 44:2133–2143.
2008.PubMed/NCBI
|
|
5.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
6.
|
Wiffen P, Mitchell M, Snelling M and
Stoner N: Oxford Handbook of Clinical Pharmacy. 2nd edition. Oxford
University Press; New York: 2007
|
|
7.
|
Yoneda S: Outpatient chemotherapy for lung
cancer. Gan To Kagaku Ryoho. 34:533–537. 2007.(In Japanese).
|
|
8.
|
Chang A, Parikh P, Thongprasert S, et al:
Gefitinib (IRESSA) in patients of Asian origin with refractory
advanced non-small cell lung cancer: subset analysis from the ISEL
study. J Thorac Oncol. 1:847–855. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wu YL, Yang JJ, Lin JY, et al: Gefitinib
target treatment in non-small cell lung cancer. Chin J Tuber Respir
Dis. 30:98–102. 2007.(In Chinese).
|
|
10.
|
Gao Z, Han B, Wang H, et al: Clinical
observation of gefitinib as a first-line therapy in sixty-eight
patients with advanced NSCLC. Oncol Lett. 3:1064–1068.
2012.PubMed/NCBI
|
|
11.
|
Gandara DR, Davies AM, Gautschi O, et al:
Epidermal growth factor receptor inhibitors plus chemotherapy in
non-small-cell lung cancer: biologic rationale for combination
strategies. Clin Lung Cancer. 2:S61–S67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tanaka T, Munshi A, Brooks C, et al:
Gefitinib radiosensitizes non-small cell lung cancer cells by
suppressing cellular DNA repair capacity. Clin Cancer Res.
14:1266–1273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhuang HQ, Sun J, Yuan ZY, et al:
Radiosensitizing effects of gefitinib at different administration
times in vitro. Cancer Sci. 100:1520–1525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Stinchcombe TE, Morris DE, Lee CB, et al:
Induction chemo-therapy with carboplatin, irinotecan, and
paclitaxel followed by high dose three-dimension conformal thoracic
radiotherapy (74 Gy) with concurrent carboplatin, paclitaxel, and
gefitinib in unresectable stage IIIA and stage IIIB non-small cell
lung cancer. J Thorac Oncol. 3:250–257. 2008.
|
|
15.
|
Fischer JR, Geiger D, Haffner UJ and Lahm
H: Successful individualized and targeted therapy of an NSCLC
patient with gefitinib based on a predictive assessment of the
EGF-receptor mutation status. Pneumologie. 61:264–269. 2007.(In
German).
|
|
16.
|
Yoshida K, Yatabe Y, Park JY, et al:
Prospective validation for prediction of gefitinib sensitivity by
epidermal growth factor receptor gene mutation in patients with
non-small cell lung cancer. J Thorac Oncol. 2:22–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fukuoka M, Wu Y, Thongprasert S, et al:
Biomarker analyses from a phase III, randomized, open-label,
first-line study of gefitinib versus carboplatin/paclitaxel in
clinically selected patients with advanced non-small cell lung
cancer in Asia (IPASS). J Clin Oncol. 29:2866–2874. 2011.
View Article : Google Scholar
|
|
18.
|
Zhong W, Wang M, Li L, et al: EGFR gene
mutation statuses in advanced non-small cell lung cancer patients
and their influence on effect of gefitinib. Chin J Lung Cancer.
15:513–520. 2012.(In Chinese).
|
|
19.
|
Maemondo M, Minegishi Y, Inoue A, et al:
First-line gefitinib in patients aged 75 or older with advanced
non-small cell lung cancer harboring epidermal growth factor
receptor mutations: NEJ 003 study. J Thorac Oncol. 7:1417–1422.
2012. View Article : Google Scholar : PubMed/NCBI
|