Introduction
Melanocytes are derived from the neural crest and
are found scattered throughout the basal layer of the epidermis. In
response to hormones or ultraviolet (UV) light, the melanin
pigments contained in melanocytes are transferred to the
surrounding keratinocytes through the tips of the dendrites to
protect against UV light damage or carcinogenesis. This has been
considered a morphological indicator of melanocytes and melanoma
cells. During this process, dendrite extension is critical, since
melanocytes constitute a minor population in the epidermis
(1–3). Previous studies demonstrated that the
dendritic outgrowth of melanocytes and melanoma cells is promoted
by UV light irradiation, growth factors and cAMP-elevating agents
(4–6).
The rearrangement of the actin cytoskeleton
promoting dendritic outgrowth and the underlying signaling
mechanisms in melanocytes and melanoma cells were previously
investigated (3,4,7). The
identification of the small GTPases of the Rho family has
elucidated the molecular signaling underlying cell shape
alterations. The small GTPases of the Rho family regulate several
intracellular processes, including cytoskeletal reorganization,
cell motility, cell-cell adhesion and apoptosis (8–11),
as well as regulation of the cell cycle, oncogenesis and gene
transcription (12,13). Twenty genes encoding proteins
belonging to the Rho family of small GTPases have been described in
humans thus far. With regards to cell morphology, three of the
small GTPases appear to be more important: Rac1, RhoA and Cdc42
(14). The role of Rac1 and RhoA
in dendrite formation by human melanocytes and melanoma cells was
defined by several studies. Overexpression of the constitutive
active form of MKK6 resulted in significant elongation of the
dendrites via the upregulation of Cdc42 and Rac1 expression in the
SK-mel-24 human melanoma cell line (15). A study conducted by Ito et
al(16) demonstrated that
centaureidin may act via the Rho signaling pathway to inhibit
dendrite outgrowth in melanocytes. Furthermore, Scott and Leopardi
(17) reported that cAMP-mediated
dendrite formation in B16 melanoma cells occurs via the
upregulation of Rac and inhibition of Rho activity.
There is substantial evidence suggesting that UV
irradiation plays a pivotal role in regulating melanocyte
dendricity. In the development of melasma, the strongest predictors
are UV light exposure and genetic factors (18,19).
Furthermore, the histological analysis of melasma lesions revealed
that epidermal melanocytes exhibited a strong staining intensity
and more dendrites (20).
Reflectance confocal imaging of murine skin in vivo
following exposure to UVB light highlighted the dendricity of
melanocytes (3). Although it is
likely that UV light is a primary stimulus for melanocyte dendrite
formation, the possible molecular mechanisms have not been fully
elucidated. The effect of narrow-band UVB radiation on the
morphological changes and the actin cytoskeleton in B16 melanoma
cells was investigated, with the aim of elucidating the mechanism
underlying this process.
Materials and methods
Cell culture and narrow-band UVB
radiation treatment
B16 melanoma cells are transformed melanocytes and
suitable as a physiological model of normal melanocytes. In the
present study, B16 melanoma cells were investigated to elucidate
the mechanism of dendrite formation induced by narrow-band UVB
radiation. B16 melanoma cells (Institute of Biochemistry and Cell
Biology, Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences, Shanghai, China) were maintained in RPMI-1640
medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS) and antibiotics (100 IU/ml penicillin and 50
mg/ml streptomycin) (Invitrogen, Grand Island, NY, USA) in a
humidified atmosphere of 5% CO2 at 37ºC. The cells were
exposed to various doses of radiation by a specific UVB lamp
emitting a peak wavelength of 311 nm with an intensity of 1
mW/cm2 (Philips, Amsterdam, Netherlands). During
irradiation, the medium was replaced with phosphate-buffered saline
(PBS) to avoid the formation of medium-derived toxic photoproducts
induced by UVB exposure. Immediately after phototreatment, PBS was
removed and media were added to the cells. The subsequent
experiments were performed three times in triplicate.
Cell viability assay
The cells were seeded in 96-well plates at a density
of 1×104 cells/well and incubated for 24 h.
Subsequently, the cells were exposed to various doses of
narrow-band UVB radiation (0, 25, 50, 100, 200 and 300
mJ/cm2) and were incubated for an additional 24 h. Cell
viability was assessed using a commercially available kit (Cell
Counting kit-8; Dojindo Co., Ltd., Kumamoto, Japan) according to
the manufacturer’s instructions to determine the appropriate dose
of narrow-band UVB radiation for the subsequent experiments. The
colorimetric absorbance was recorded at 490 nm using the ELx800
microplate reader (BioTek, Winooski, VT, USA).
Dendrite formation assay
B16 melanoma cells (1×105 cells/well)
growing in 6-well microplates were cultured for 24 h and were
subsequently irradiated with narrow-band UVB light (100
mJ/cm2). Following incubation for a further 24 h in an
FBS-free medium (in order to avoid the effect of growth factors in
FBS on dendrite formation), the morphological changes were observed
under an ELWD 0.3 phase contrast microscope (Nikon, Tokyo, Japan).
The number of dendrites per cell was determined for ~100 cells from
each experiment.
Immunofluorescence microscopy
B16 melanoma cells growing on coverslips were placed
within culture plates (1×105 cells), cultured for 24 h
and then irradiated with narrow-band UVB light (100
mJ/cm2). Following incubation for an additional 24 h in
FBS-free medium for immunofluorescence studies, the B16 melanoma
cells growing on glass coverslips were briefly rinsed with PBS and
fixed in 4% formaldehyde, then permeabilized with 0.5% Triton X-100
(Sigma-Aldrich, St. Louis, MO, USA) for 2 min for detection of
cytoskeletal F-actin. The cells were washed for 10 min in PBS and
exposed to rhodamine phalloidin staining (Cytoskeleton Inc.,
Denver, CO, USA) diluted in PBS containing 1% of BSA, applied for
30 min at 37ºC. The coverslips were rinsed with PBS for 10 min and
observed and photographed with a Leica TCS SP2 laser scanning
confocal microscope (LSCM) (Leica, Solms, Germany).
Pull-down assay
An equal number of B16 melanoma cells
(~1×106 cells) was cultured, followed by lysis in
ice-cold cell lysis buffer supplemented with 1X protease inhibitor
cocktail after narrow-band UVB irradiation for the indicated time.
The pull-down assay was implemented for the detection of GTP-Rac1
and -RhoA using a commercially available kit (Cytoskeleton Inc.)
according to the manufacturer’s protocol. In brief, a
pre-determined amount of PAK-PBD beads (20 μg) or GST-Rhotekin-RBD
beads (10 μg) was added to the cleared lysates and incubated at 4ºC
on a rotator for 1 h. GTP-bound proteins were captured onto the
beads and pelleted. The beads were rinsed with a wash buffer and
GTP-bound protein was eluted with 2X Laemmli sample buffer. Samples
were immunoblotted after 12.5% SDS-PAGE using standard procedures.
To assess loading equality among different lysates, a portion of
each lysate was removed prior to the addition of PAK-PBD beads or
GST-Rhotekin-RBD beads and blotted with anti-RhoA and anti-Rac1
antibodies. The expression of GTP-Rac1 and GTP-RhoA was detected
using the Plus-ECL method. Densitrometric analysis was performed
using LAS-3000 ECL image analysis system (Fujifilm Medical Systems,
Tokyo, Japan).
Results
Effects of narrow-band UVB radiation on
B16 melanoma cell viability
B16 melanoma cells were irradiated with different
doses of narrow-band UVB light. The results demonstrated that
irradiation with narrow-band UVB light at 25, 50 and 100
mJ/cm2 exerted no stimulatory effect on cell
proliferation compared to control groups. However, 200 and 300
mJ/cm2 of irradiation significantly reduced the cell
survival rate. According to these results, narrow-band UVB
irradiation at 100 mJ/cm2 was selected as the
appropriate dose for the subsequent experiments.
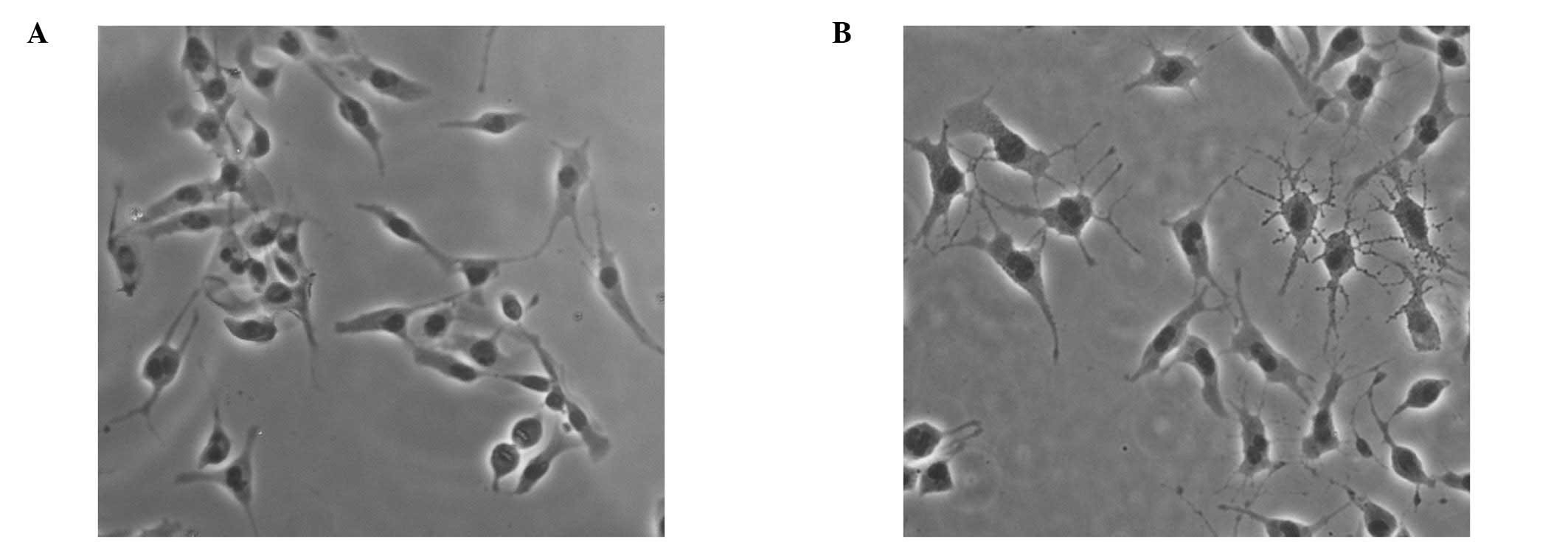
Morphological changes in B16 melanoma
cells following narrow-band UVB irradiation
To investigate the effects of narrow-band UVB
radiation on dendrite formation, B16 melanoma cells were irradiated
with 100 mJ/cm2 narrow-band UVB light for 24 h. The
dendrite number in 100 B16 melanoma cells from each experiment was
manually counted. The morphological changes in B16 melanoma cells
induced by narrow-band UVB radiation were evident, with markedly
globular cell bodies and significantly increased numbers of tree
branch-like dendrites (5.52±1.35/per cell) compared to untreated
cells, which exhibited 2–3 dendrites (2.39±0.36/per cell) (Fig. 1).
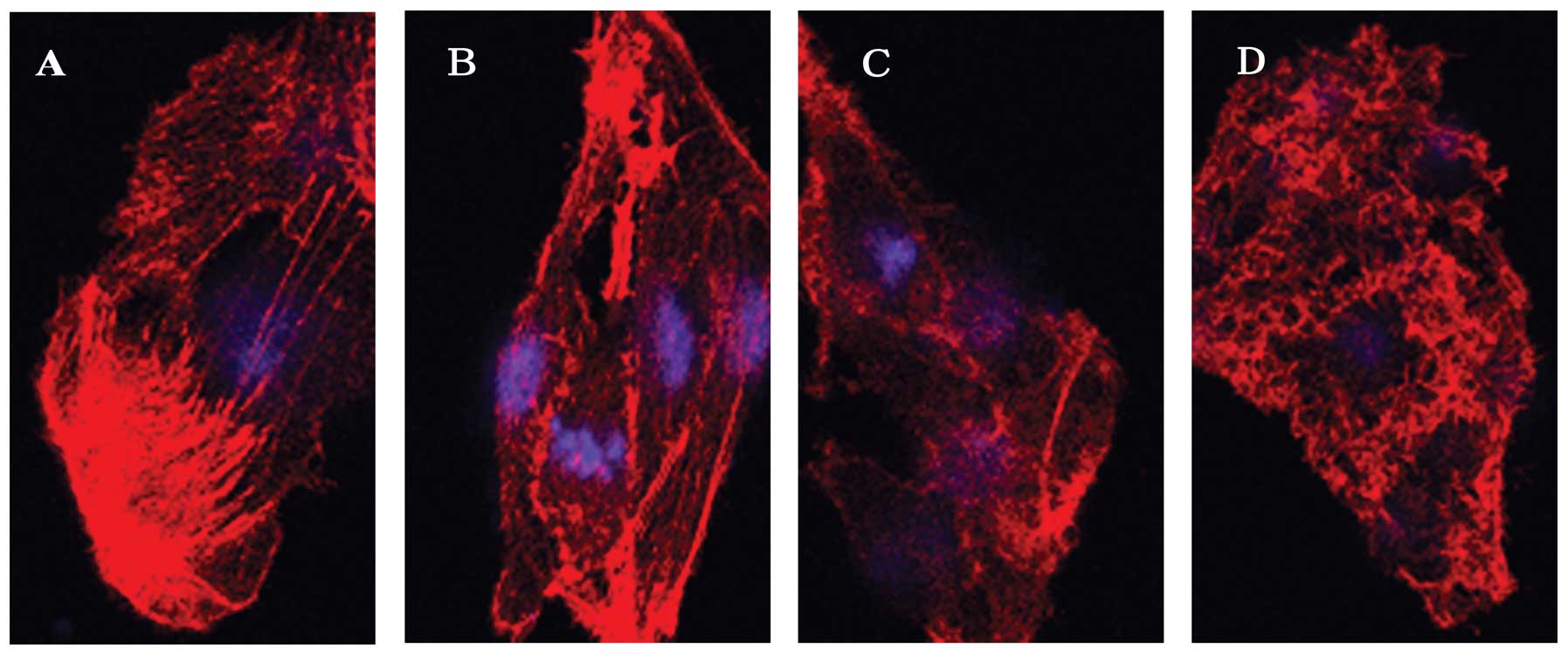
Effects of narrow-band UVB radiation on
cytoskeletal F-actin in B16 melanoma cells
The changes in the cytoskeletal F-actin were
observed using an LSCM. F-actin appeared to be organized in
numerous clear stress fibers crossing the cytoplasm in
non-irradiated cells (Fig. 2A).
However, these stress fibers became obscure as actin was
disassembled following narrow-band UVB irradiation (100
mJ/cm2) in a time-dependent manner. This event was
observed as early as 30 min (Fig.
2B) after irradiation and became more evident with the
appearance of punctate spots at 6 h (Fig. 2C and D).
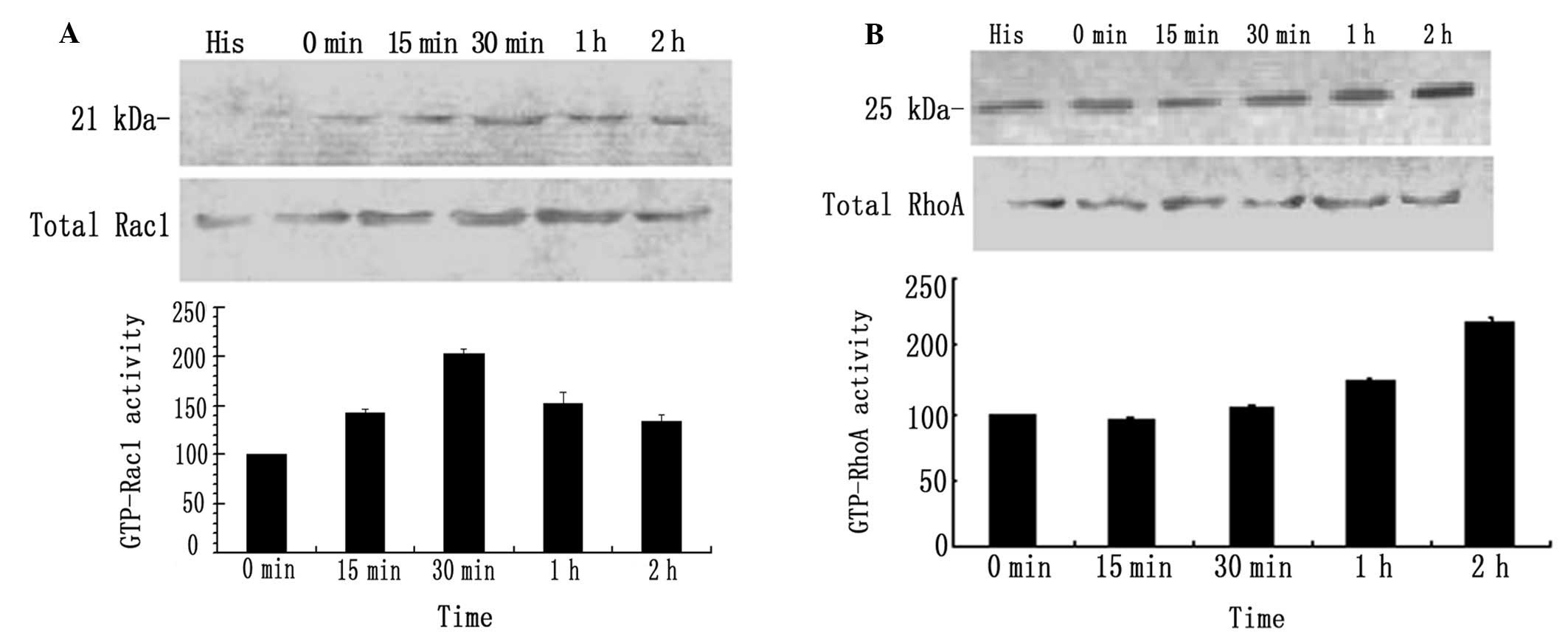
Effects of narrow-band UVB radiation on
the activity of GTP-Rac1 and -RhoA in B16 melanoma cells
The pull-down analysis (Fig. 3A) revealed that GTP-Rac1 protein
expression was significantly increased at 15 min and reached twice
the baseline levels at 30 min after narrow-band UVB irradiation
(100 mJ/cm2), followed by a minor decrease, although the
protein levels remained elevated at 60 and 120 min compared to the
control cells. The GTP-RhoA levels were marginally altered within
the first 30 min after narrow-band UVB irradiation (100
mJ/cm2), were elevated at 60 min and by 1.6-fold at 120
min compared to the control cells (Fig. 3B).
Discussion
Over the last few years, the molecular mechanisms
responsible for melanocyte dendricity have attracted increasing
attention in the field of melanocyte research. By examining the
cytoskeletal components during cAMP-induced dentricity in B16F10
cells, it was observed that the cAMP-dependent dendricity was
accompanied by a significant reorganization of the actin
cytoskeleton (21). It has been
verified previously that UV light irradiation is involved in the
regulation of dendricity in human or mice melanocytes, as described
above (3,18–20).
In the present study, the effect of narrow-band UVB
radiation on morphological changes in B16 melanoma cells in
vitro was observed. Marked morphological changes were evident
in B16 melanoma cells treated with 100 mJ/cm2
narrow-band UVB radiation, with globular cell bodies and increased
numbers of tree branch-like dendrites as compared to untreated
cells, which merely exhibited 2–3 dendrites. Furthermore, the
changes in the cytoskeletal F-actin microfilament in response to
narrow-band UVB radiation were investigated using LSCM. LSCM
revealed that F-actin appeared organized in numerous clear stress
fibers crossing the cytoplasm in non-irradiated cells. However,
these stress fibers became obscure as actin was disassembled
following narrow-band UVB irradiation in a time-dependent manner.
This event was observed as early as 30 min following irradiation
and became more evident with the appearance of punctate spots at 6
h. These results suggest that narrow-band UVB radiation may promote
the disorganization of cytoskeletal F-actin, leading to
morphological changes such as globular cell bodies and increased
numbers of dendrites in B16 melanoma cells.
The small GTPases, including RhoA, Rac1 and Cdc42,
have been shown to regulate the assembly and disassembly of the
actin cytoskeleton in neural crest-derived cells (3,21).
Another possible explanation for the morphological changes
exhibited by B16 melanoma cells exposed to narrow-band UVB
radiation is the up- or downregulation of these small GTPases,
which is based on the findings of previous studies, according to
which Rac1 activation and RhoA inhibition induce elongation of the
dendrites and the activated RhoA stimulates dendrite retraction
(5,22–24).
In this study, we investigated the effect of
narrow-band UVB radiation on Rac1 and RhoA activity using the
pull-down assay. The analysis revealed that GTP-Rac1 protein
expression was significantly increased at 15 min and had doubled at
30 min following narrow-band UVB irradiation, which was followed by
a minor decrease, although the protein levels remained elevated at
1 and 2 h compared to non-irradiated cells. GTP-RhoA expression
exhibited minor alterations in the first 30 min following
narrow-band UVB irradiation, was elevated at 1 h and by 1.6-fold at
2 h compared to control cells. Unlike the overexpression of Rac1,
RhoA activity in B16 melanoma cells exhibited no significant change
in the short period after narrow-band UVB irradiation, although a
delayed elevation was observed. The reason for this finding has not
been elucidated. Ridley et al(25) concluded that a linear hierarchy
exists, with Rac activating Rho, which appears to be consistent
with the results of our study. It was hypothesized that
Rac-dependent Rho activation in response to narrow-band UVB
irradiation may serve as a negative feedback to avoid exorbitant
dendrite extension promoted by the activated Rac1, according to the
role of RhoA activation in dendrite retraction in B16 melanoma
cells (23). In summary, our
results suggest that narrow-band UVB radiation specifically
activates the Rac1 signaling pathway, leading to F-actin
rearrangement and resulting in increased dendrite formation in B16
melanoma cells.
Acknowledgements
The authors would like to thank Wang Li and Wang Lei
(Department of Neurobiology, Fudan University Medical School) for
performing the laser scanning confocal microscope test and Zhang
Guo-Ping (Institute of Biomedical Research, Fudan University) for
providing assistance in the laboratory.
References
|
1
|
Chakraborty AK, Funasaka Y, Araki K,
Horikawa T and Ichihashi M: Evidence that the small GTPase Rab8 is
involved in melanosome traffic and dendrite extension in B16
melanoma cells. Cell Tissue Res. 314:381–388. 2003. View Article : Google Scholar
|
|
2
|
Fitzpatrick B, Miyamoto M and Ishikawa K:
The evolution of concepts of melanin biology. Arch Dermatol.
96:305–323. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scott G: Rac and Rho: the story behind
melanocyte dendrite formation. Pigment Cell Res. 15:322–330. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hara M, Yaar M and Gilchrest BA:
Endothelin-1 of keratinocyte origin is a mediator of melanocyte
dendricity. J Invest Dermatol. 105:744–748. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Busca R, Bertolotto C, Abbe P, Engalo W,
Ishizaki T, Narumiya S, Boquet P, Ortonne JP and Ballotti R:
Inhibition of Rho is required for cAMP-induced melanoma cell
differentiation. Mol Biol Cell. 9:1367–1378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshida M, Takahashi Y and Inoue S:
Histamine induces melanogenesis and morphologic changes by protein
kinase A activation via H2 receptors in human normal melanocytes. J
Invest Dermatol. 114:334–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hata K, Hori K, Murata J and Takahashi S:
Remodeling of actin cytoskeleton in lupeol-induced B16 2F2 cell
differentiation. J Biochem. 138:467–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nowak JM, Grzanka A, Zuryń A and Stepień
A: The Rho protein family and its role in the cellular
cytoskeleton. Postepy Hig Med Dosw (Online). 62:110–117. 2008.(In
Polish).
|
|
9
|
Parri M and Chiarugi P: Rac and Rho
GTPases in cancer cell motility control. Cell Commun Signal.
8:232010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haass NK, Smalley KS, Li L and Herlyn M:
Adhesion, migration and communication in melanocytes and melanoma.
Pigment Cell Res. 18:150–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ongusaha PP, Kim HG, Boswell SA, Ridley
AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA and Lee SW: RhoE is a
pro-survival p53 target gene that inhibits ROCK I-mediated
apoptosis in response to genotoxic stress. Curr Biol. 16:2466–2472.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benitah SA, Valerón PF and Lacal JC: ROCK
and nuclear factor-κB-dependent activation of cyclooxygenase-2 by
Rho GTPases: effects on tumor growth and therapeutic consequences.
Mol Biol Cell. 14:3041–3054. 2003.
|
|
13
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
14
|
Burridge K and Wennerberg K: Rho and Rac
take center stage. Cell. 116:167–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim MY, Choi TY, Kim JH, Lee JH, Kim JG,
Sohn KC, Yoon KS, Kim CD, Lee JH and Yoon TJ: MKK6 increases the
melanocyte dendricity through the regulation of Rho family GTPases.
J Dermatol Sci. 60:114–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito Y, Kanamaru A and Tada A: Centaureidin
promotes dendrite retraction of melanocytes by activating Rho.
Biochim Biophys Acta. 1760:487–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scott G and Leopardi S: The cAMP signaling
pathway has opposing effects on Rac and Rho in B16F10 cells:
implications for dendrite formation in melanocytic cells. Pigment
Cell Res. 16:139–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jimbow M and Jimbow K: Pigmentary
disorders in oriental skin. Clin Dermatol. 7:11–27. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pichardo R, Vallejos Q, Feldman SR, Schulz
MR, Verma A, Quandt SA and Arcury TA: The prevalence of melasma and
its association with quality of life in adult male Latino migrant
workers. Int J Dermatol. 48:22–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang WH, Yoon KH, Lee ES, Kim J, Lee KB,
Yim H, Sohn S and Im S: Melasma: histopathological characteristics
in 56 Korean patients. Br J Dermatol. 146:228–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hall A: Rho GTPases and the control of
cell behaviour. Biochem Soc Trans. 33(Pt 5): 891–895. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scott GA and Cassidy L: Rac1 mediates
dendrite formation in response to melanocyte stimulating hormone
and ultraviolet light in a murine melanoma model. J Invest
Dermatol. 111:243–250. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katoh H, Aoki J, Ichikawa A and Negishi M:
p160 RhoA-binding kinase induces neurite retraction. J Biol Chem.
273:2489–2492. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sander EE, ten Klooster JP, van Delft S,
van der Kammen RA and Collard JG: Rac downregulates Rho activity:
reciprocal balance between both GTPases determines cellular
morphology and migratory behavior. J Cell Biol. 147:1009–1022.
1999. View Article : Google Scholar
|
|
25
|
Ridley AJ, Paterson HF, Johnston CL,
Diekmann D and Hall A: The small GTP-binding protein Rac regulates
growth factor-induced membrane ruffling. Cell. 70:401–410. 1992.
View Article : Google Scholar : PubMed/NCBI
|