Introduction
Treatment with the epidermal growth factor receptor
(EGFR) tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib
has led to significant clinical improvement in certain patients
with advanced non-small-cell lung cancer (NSCLC), particularly
those of Asian descent, non-smokers and those with adenocarcinoma
(1–4).
Despite prolonged survival, it should be noted that
discontinuation of EGFR inhibition may cause more rapid progression
of symptoms and lesions in certain patients, which is referred to
as ‘disease flare’ (5). The most
likely explanation for this phenomenon is oncogene addiction, which
is recognized in several types of cancer. Gastrointestinal stromal
tumors have a unique biology and exhibit rapid disease progression
when the kinase inhibitor imatinib is removed after prolonged
benefit (6). This series describes
a similar flare phenomenon in the setting of acquired resistance in
EGFR-mutant lung cancer when gefitinib or erlotinib are
discontinued due to disease progression.
An effective treatment for patients with disease
flare has not yet been established. Preclinical studies indicated
that continuous TKI administration may be a valuable strategy.
However, available published data on the clinical activity of
gefitinib combination chemotherapy following failure of gefitinib
are limited. Therefore, the role of combination treatment after
gefitinib failure remains remains debatable. This study was
retrospectively performed to evaluate the role of combination
treatment following gefitinib failure in patients with advanced
NSCLC.
Patients and methods
Patients
This retrospective study was conducted through a
review of medical records of patients with advanced NSCLC who
received gefitinib combined with chemotherapy following disease
progression due to gefitinib failure, between July, 2010 and June,
2012. The study was approved by the Ethics Committee of the
Zhejiang Cancer Hospital. Eligibility criteria included: i)
histological or cytological diagnosis of stage IIIb or IV NSCLC;
ii) at least one measurable tumor lesion; iii) initial gefitinib
treatment for >6 months and acquired resistance to gefitinib
according to Jackman’s criteria (7); and iv) discontinuation time between
the prior treatment and re-administration of gefitinib of ≤1 week.
The characteristics of the study population are shown in Table I.
 | Table IBaseline characteristics of the study
population (n=26). |
Table I
Baseline characteristics of the study
population (n=26).
| Variables | No. | Percentage |
|---|
| Gender |
| Male | 14 | 53.8 |
| Female | 12 | 46.2 |
| PS |
| 0–1 | 13 | 50.0 |
| 2 | 13 | 50.0 |
| Age |
| Median | 56 | |
| Mean | 57 | |
| <65 | 18 | 69.2 |
| ≥65 | 8 | 30.8 |
| Smoking status |
| Yes | 9 | 34.6 |
| No | 17 | 65.4 |
| Chemotherapy |
| Pemetrexed | 14 | 53.8 |
| Docetaxel | 12 | 46.2 |
| Stage |
| IIIb | 0 | 0 |
| IV | 26 | 100 |
| Histology |
| Adenocarcinoma | 22 | 84.6 |
|
Non-adenocarcinoma | 4 | 15.4 |
| Median duration of
prior gefitinib treatment | 9.6 months |
Methods
Patients were administered gefitinib orally from day
1 of the first cycle and pemetrexed or docetaxel as an intravenous
(i.v.) infusion on day 1. Pemetrexed was administered as a 10-min
i.v. infusion and docetaxel 75 mg/m2 as a 1-h i.v.
infusion once every 3 weeks. Chemotherapy (pemetrexed or docetaxel)
was discontinued if no progression occurred at the end of 4 cycles
and gefitinib was continuously administered until disease
progression.
Evaluation of response and toxicity
The tumor response was classified in accordance with
the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. The
patients were evaluated to determine the stage of their disease
prior to treatment initiation and at the time of disease
progression or relapse, by computed tomography (CT) of the chest
and abdomen and other staging procedures. Adverse events were
evaluated according to the Common Terminology Criteria for Adverse
Events (CTCAE) 3.0.
Statistical methods
Kaplan-Meier survival curves were used to estimate
overall survival (OS) and progression-free survival (PFS). OS was
measured from the first day of combination treatment to the day the
patient succumbed or last follow-up. PFS was defined as the
interval from the initiation of combination treatment to treatment
failure or the date of the last follow-up. All the analyses were
performed with SPSS software version 16 (SPSS Inc., Chicago, IL,
USA).
Results
Patient characteristics
A total of 26 patients (14 males and 12 females)
were included in the present study. The median age of the patients
was 56 years (range, 42–71 years). The performance status (PS)
score was 0–1 in 13 patients (50%) and 2 in the remaining 50%. The
majority of tumors (84.6%) were adenocarcinomas with advanced stage
at presentation and 34.6% (9/26) of the patients had a history of
smoking. The median duration of the initial gefitinib treatment was
9.6 months [95% confidence interval (CI): 7.5–12.0]. Docetaxel was
administered to 12 and pemetrexed to 14 patients, concurrently with
gefitinib treatment.
Response data and survival analysis
The median follow-up period for the 26 patients was
8.0 months (range, 1.0–15 months). Sixteen patients had exhibited a
PR and 10 had SD during the prior gefitinib treatment. The response
to combination treatment included 6 cases of PR, 13 of SD and 7 of
progressive disease (PD), which accounted for a disease control
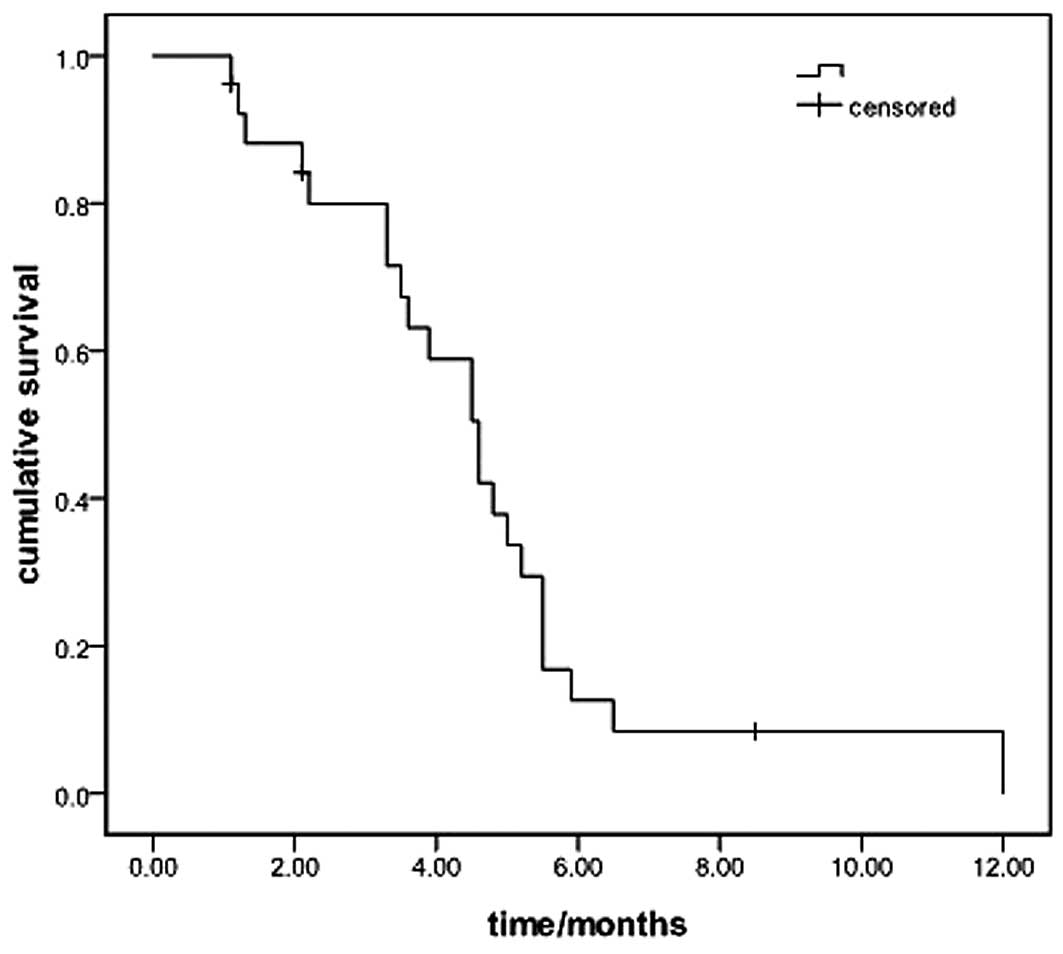
rate (DCR) of 73.1%. The median PFS was 4.6 months (95% CI:
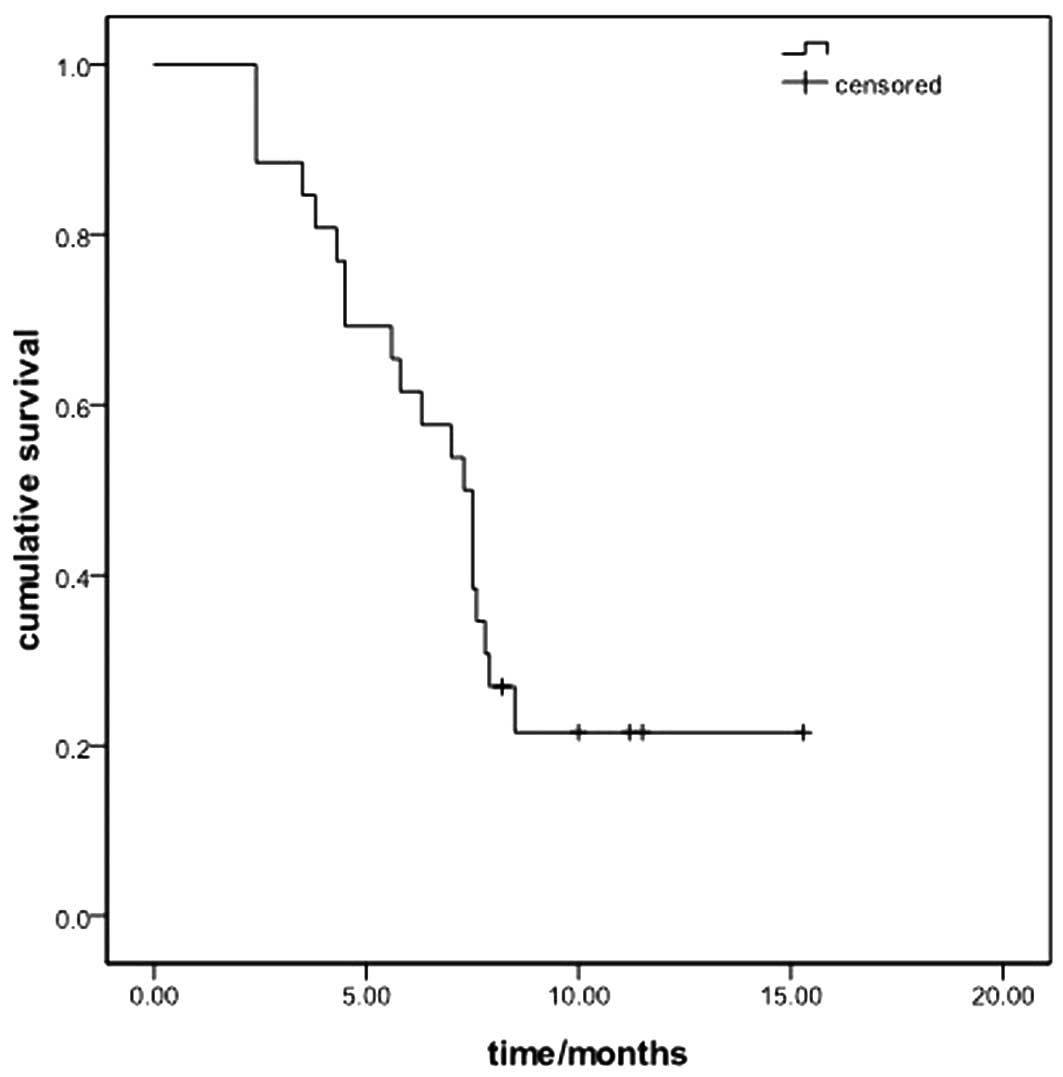
3.8–5.4; Fig. 1). The median OS of
the entire patient sample was 7.3 months (95% CI: 6.1–8.5 months)
(Fig. 2). Of the 26 patients, 13
underwent analysis of EGFR mutations and 10 were found to harbor
activating mutations, including 6 patients with exon 19 deletions
and 4 with exon 21 L858R mutations, whereas 3 had a negative
mutational status. The median PFS of the 10 patients harboring EGFR
mutation was 4.6 months, with 4.2 months for the EGFR wild-type
patients (P=0.86).
Prognostic factors
In the univariate analysis, PS had a statistically
significant effect on PFS (Table
II). No significant differences in PFS were observed with
respect to other factors. The Cox regression model was constructed
with the incorporation of age, gender, histological grade, smoking
history, chemotherapeutic regimen and PS. PS was identified as the
only independent prognostic factor (P=0.043).
 | Table IIUnivariate analysis of PFS in the 26
patients. |
Table II
Univariate analysis of PFS in the 26
patients.
| Variables | PFS | 95% CI | P-value |
|---|
| Gender | | | 0.013 |
| Male | 4.5 | 2.9–6.3 | |
| Female | 4.6 | 4.2–6.8 | |
| Age (years) | | | 0.57 |
| ≥65 | 4.5 | 3.2–6.4 | |
| <65 | 4.8 | 1.3–7.7 | |
| PS | | | 0.043 |
| 0–1 | 5.5 | 5.2–5.8 | |
| 2 | 3.3 | 1.9–4.7 | |
| Chemotherapy | | | 0.86 |
| Pemetrexed | 3.9 | 1.5–6.3 | |
| Docetaxel | 4.6 | 3.9–5.3 | |
| Smoking history | | | 0.40 |
| Yes | 4.6 | 3.4–5.8 | |
| No | 5.2 | 4.0–6.4 | |
| Histology | | | 0.86 |
|
Non-adenocarcinoma | 3.3 | 1.1–6.4 | |
| Adenocarcinoma | 4.6 | 4.2–5.0 | |
| Treatment line | | | 0.91 |
| Third-line | 4.6 | 1.1–8.1 | |
| Further-line | 4.5 | 3.3–5.7 | |
Treatment toxicities
Grade 1/2 skin and hematological toxicities were
observed in 13 and 18 patients, respectively. Grade 2 diarrhea
developed in 6 patients. Toxicities were considered acceptable,
with grade 3/4 skin toxicity in 5 and neutropenia in 9 patients.
Two patients had a dosage reduction due to grade 4 neutropenia and
2 patients developed hepatic function abnormalities following
gefitinib treatment.
Discussion
In the present study, the response rate and DCR with
gefitinib and chemotherapy following failure of gefitinib treatment
were 23.1 and 73.1%, respectively. The median PFS was 4.6 months,
which was considered to be favorable compared to previous third- or
further-line treatments. The outcome indicated that this treatment
was an optimal choice for the patients after failure of gefitinib
therapy.
According to the guidelines of the National
Comprehensive Cancer Network (8),
EGFR-TKIs are recommended as a second- or third-line treatment
regimen for patients with NSCLC. However, there were no established
treatment protocols for patients following failure of previous
gefitinib or erlotinib treatment and discontinuation of EGFR
inhibition may cause more rapid progression of symptoms and lesions
in certain patients. Chaft et al(5) observed a 23% flare rate during the
EGFR TKI washout period following disease progression under TKI
treatment. Therefore, discontinuation of TKI treatment may not be
suitable for patients who benefited from gefitinib or
erlotinib.
According to an ASCO 2012 retrospective study,
continuation of erlotinib and chemotherapy following failure of
erlotinib treatment enhanced the overall response rate (ORR) and
achieved a PFS of 4.4 months [Goldberg et al(9)]. In the present study the ORR was
23.1%, which was similar to that reported by Goldberg et
al(9). The outcome indicated
that patients may also benefit from the gefitinib combination
treatment.
Treatment with gefitinib-pemetrexed or docetaxel was
generally well-tolerated and the adverse events (AEs) were similar
to those observed in previous studies of each agent alone (10–13).
The most common AEs were grade 1/2 skin rash and hematological
toxicity.
The present study indicated the efficacy and safety
of combined pemetrexed/docetaxel therapy as subsequent treatment in
patients with gefitinib-resistant tumors that had exhibited an
initial response to gefitinib monotherapy. However, the small
sample size of this study may not be sufficient to accurately
interpret the study results. Further assessment in a large-scale
prospective study is required to obtain definitive evidence. A
phase II trial (NCT01707329) has been initiated in our hospital to
investigate the efficacy of combination treatment following failure
of icotinib, another EGFR-TKI, the efficacy of which was shown to
be similar to that of gefitinib in a phase III trial (14).
In conclusion, gefitinib combined with chemotherapy
for Chinese patients with advanced NSCLC achieved promising ORR,
DCR and PFS, with an acceptable toxicity profile.
References
|
1
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shepherd FA, Pereira JR, Ciuleanu T, et
al: Erlotinib in previously treated non-small-cell lung cancer. N
Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaft JE, Oxnard GR, Sima CS, Kris MG,
Miller VA and Riely GJ: Disease flare after tyrosine kinase
inhibitor discontinuation in patients with EGFR-mutant lung cancer
and acquired resistance to erlotinib or gefitinib: implications for
clinical trial design. Clin Cancer Res. 17:6298–6303. 2011.
View Article : Google Scholar
|
|
6
|
Van den Abbeele AD, Badawi RD, Manola J,
et al: Effects of cessation of imatinib mesylate (IM) therapy in
patients (pts) with IM-refractory gastrointestinal stromal tumors
(GIST) as visualized by FDG-PET scanning. J Clin Oncol. 22:abs.
30122004.
|
|
7
|
Jackman D, Pao W, Riely GJ, et al:
Clinical definition of acquired resistance to epidermal growth
factor receptor tyrosine kinase inhibitors in non-small-cell lung
cancer. J Clin Oncol. 28:357–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Comprehensive Cancer Network
(NCCN). NCCN Clinical Practice Guidelines in Oncology. Available
at: http://www.nccn.org/index.asp.
Accessed January 4, 2013
|
|
9
|
Goldberg SB, Oxnard OR, Digumarthy S,
Muzikansky A, Jackman DM, Lennes IT and Sequist LV: Chemotherapy
with erlotinib or chemotherapy alone in advanced NSCLC with
acquired resistance to EGFR tyrosine kinase inhibitors (TKI). J
Clin Oncol. 30(Suppl): abs. 75242012.PubMed/NCBI
|
|
10
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.
|
|
11
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim ES, Hirsh V, Mok T, et al: Gefitinib
versus docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III trial. Lancet. 22:1809–1818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maruyama R, Nishiwaki Y, Tamura T,
Yamamoto N, et al: Phase III study, V-15-32, of gefitinib versus
docetaxel in previously treated Japanese patients with
non-small-cell lung cancer. J Clin Oncol. 26:4244–4252. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Shi Y, Zhang L, et al: A
randomized, double-blind phase III study of icotinib versus
gefitinib in patients with advanced non-small cell lung cancer
(NSCLC) previously treated with chemotherapy (ICOGEN). J Clin
Oncol. 29(Suppl 15): abs. 75222011.PubMed/NCBI
|