Introduction
Unlike other odontogenic cystic lesions, the
odontogenic keratocyst (OKC) exhibits an intrinsic growth potential
and a marked ability to destroy bone (1,2).
Clinically, following conventional treatment such as enucleation,
OKC exhibits a high tendency for recurrence (1,3).
According to the 2005 World Health Organization classification, OKC
was reclassified as a benign neoplasm, referred to as keratocystic
odontogenic tumour (KCOT) (2,3).
In the field of molecular biology, several
immunohistochemical studies investigated the activity of the KCOT
epithelium by using various markers of proliferation and apoptosis
(4–7). The results of those studies
demonstrated that p53 (4), PCNA
(5) and Ki-67 (6) were more strongly expressed in the
KCOT epithelium compared to other types of odontogenic cysts. Thus,
it was concluded that the KCOT epithelium was highly proliferative,
which may explain the high recurrence rate of KCOT and suggests
that KCOTs have a different biological origin (7).
Recently, marsupialisation or decompression combined
with two-stage enucleation was proven to be an effective treatment
for large KCOTs (3). According to
data reported by Ninomiya et al (8), the size of the KCOT lesions was
significantly decreased following decompression. To improve the
outcome of decompression, investigation of the underlying molecular
mechanisms of this type of therapy is required.
Cyclooxygenase-2 (COX-2) levels were found to be
elevated in various types of tumours, such as esophageal and head
and neck tumours (9,10). Accumulating evidence suggests that
the overexpression of COX-2 is involved in tumour growth and spread
by interfering with different biological processes, such as cell
proliferation, adhesion, immune-surveillance, apoptosis and
angiogenesis (11). The regulation
of COX-2 expression is crucial for prostaglandin E2 (PGE2)
synthesis (11,12). The upregulation of COX-2 may
increase the synthesis of prostaglandins (PGs), thereby promoting
cell proliferation and angiogenesis and inhibiting
immune-surveillance (12,13). Recently, a study conducted by
Mendes et al (7)
demonstrated a mild to strong expression of COX-2 in the KCOT
epithelium and suggested that COX-2 is an important marker involved
in the biological behavior of KCOTs.
To the best of our knowledge, no studies are
currently available on the mechanism of change in COX-2 expression
following decompression. This is the first study to demonstrate the
significant downregulation of COX-2 expression in KCOT following
decompression, through the immunohistochemical investigation of 16
cases in the present series.
Materials and methods
Patients
This series comprised 16 patients with
histologically confirmed KCOT at Jiangsu Stomatological Hospital
and Shanghai Ninth People’s Hospital. Recurrent cases or those
associated with nevoid basal cell carcinoma syndrome were excluded
from this study. The patients underwent decompression surgery
followed by two-stage enucleation. The clinical information of the
patients is provided in Table I.
Postoperative follow-up comprised clinical and radiographic
examinations from January, 2004 to September, 2012. The average
duration of draining and irrigation prior to the two-stage surgery
was 19.5 months (range, 6.5–44 months). Paraffin specimens of the
tissue samples obtained at the time of decompression and
enucleation were collected from the Divisions of Oral Pathology of
Jiangsu Stomatological Hospital and Shanghai Ninth People’s
Hospital. All the patients provided signed informed consent prior
to enrollment and the study was approved by the Ethics Committees
of the Shanghai Ninth People’s Hospital and the Jiangsu
Stomatological Hospital.
 | Table I.Clinical information and intensity of
cyclooxygenase-2 (COX-2) expression. |
Table I.
Clinical information and intensity of
cyclooxygenase-2 (COX-2) expression.
| Case no. | Age (years) | Gender (M/F) | Duration of
decompression (months) | Location | Outcome (expression
degree of COX-2)
|
|---|
| Group I | Group II |
|---|
| 1 | 22 | M | 27 | Mandible;
Mol-Ram | 1 | 0 |
| 2 | 15 | M | 10 | Mandible;
Ang-Ram | 2 | 0 |
| 3 | 20 | M | 21 | Mandible;
Ang-Ram | 2 | 0 |
| 4 | 42 | M | 17 | Mandible;
Mol-Ram | 0 | 0 |
| 5 | 20 | F | 16 | Mandible;
Ang-Ram | 1 | 0 |
| 6 | 13 | F | 12 | Mandible;
Mol-Ram | 2 | 0 |
| 7 | 38 | F | 3 | Mandible;
Ang-Ram | 1 | 0 |
| 8 | 34 | F | 16 | Mandible;
Mol-Ram | 2 | 0 |
| 9 | 49 | F | 18 | Mandible;
Ang-Ram | 1 | 1 |
| 10 | 55 | M | 15 | Maxilla; Ant | 1 | 0 |
| 11 | 25 | F | 15 | Mandible;
Mol-Ram | 2 | 0 |
| 12 | 33 | F | 9 | Mandible;
Ang-Ram | 1 | 1 |
| 13 | 35 | M | 23 | Mandible;
Ang-Ram | 1 | 1 |
| 14 | 24 | F | 31 | Mandible;
Ang-Ram | 1 | 0 |
| 15 | 29 | M | 23 | Maxilla; Ant | 1 | 0 |
| 16 | 28 | F | 27 | Maxilla; Ant | 2 | 1 |
Immunohistochemical evaluation
Each of the 16 pairs of formalin-fixed,
paraffin-embedded samples was cut continuously into two 4-μm
sections and mounted on glass microscope slides. One section from
each pair was prepared for immunohistochemical COX-2 analysis and
the other section was used as a negative control by replacing the
anti-COX-2 primary antibody with phosphate-buffered saline.
Briefly, the deparaffinised sections were immersed in absolute
methanol containing 0.3% H2O2 for 15 min at
room temperature to block endogenous peroxidase activity. After
washing, the sections were immersed in 0.01 M citrate buffer (pH
6.0) and heated in a microwave oven at 95°C for 5 min. A properly
diluted (1:100) rabbit monoclonal anti-human COX-2 antibody
(product code, BS1076; Bioworld Technology, Inc., St. Louis Park,
MN, USA) was then applied to the sections at 4°C overnight,
followed by a prediluted anti-mouse IgG antibody conjugated with
peroxidase (Nichirei, Tokyo, Japan) for 1 h at room temperature.
The sections were immersed in 0.05% 3,3′-diaminobenzidine
tetrahydrochloride in 0.05 M Tris-HCl buffer (pH 8.5) containing
0.01% H2O2 for 8 min and then counterstained
with haematoxylin.
Immunohistochemical evaluation
Immunohistochemical reactivity for COX-2 was
evaluated using a semi-quantitative detection method as previously
described (7) and classified into
three groups according to the intensity score as follows: 0,
negative; 1, weakly to moderately positive; and 2, strongly
positive. The evaluation was performed by two experienced
pathologists who were blinded to the clinical data.
Statistical analysis
The Student’s paired t-test was used to evaluate the
differentiation of COX-2 immunoreactivity in KCOTs at the time of
enucleation compared to that at the time of decompression. SPSS
17.0 software (SPSS Inc., Chicago, IL, USA) was used for
statistical analysis. Specific data are provided in Table I. P<0.05 was considered to
indicate a statistically significant difference.
Results
In our study, the collection of samples obtained at
the time of decompression were referred to as group I and those
obtained at the time of two-stage enucleation as group II. The
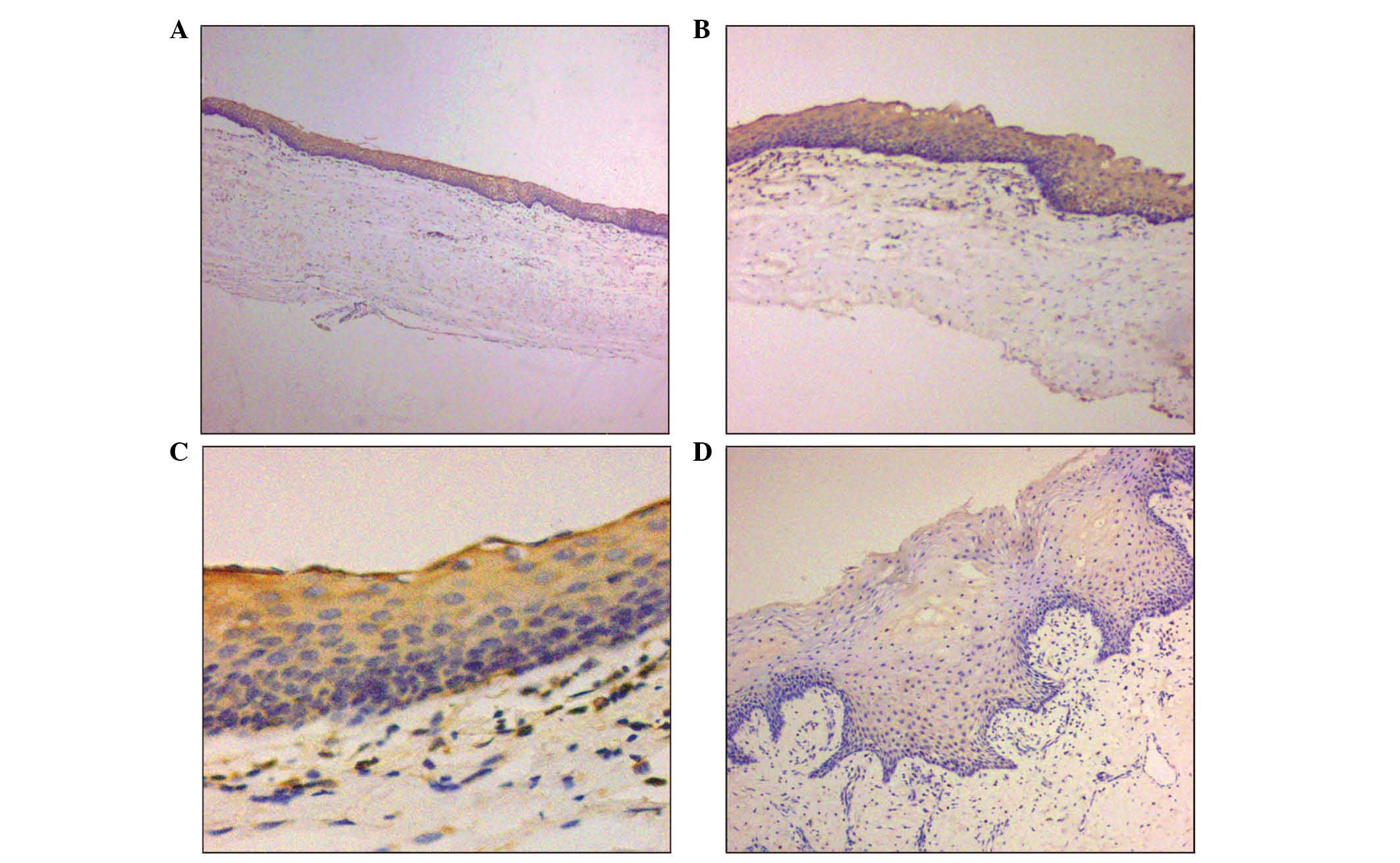
immunohistochemical staining pattern of COX-2 in KCOT presented as
membranous and cytoplasmic. In group I, positive COX-2
immunostaining was present in 15 of the 16 samples (93/8%) and
involved the full thickness of the epithelium, with 6 specimens
exhibiting strong expression and 9 mild positivity (Fig. 1A–C). In group II, 4 of the 16 cases
exhibited either mild (Fig. 1D) or
negative COX-2 expression, consistent with their corresponding
counterparts in group I. In the remaining 12 cases, COX-2
expression was significantly decreased (negative COX-2
expression).
The mean of intensity scores in group I was
1.31±0.60, whereas the mean of intensity scores in group II was
0.25±0.45. The P-value of group I vs. group II in COX-2 staining
intensity was 0.00004. The statistical analysis demonstrated a
significant decrease in COX-2 expression of group II compared to
that of group I (P<0.05).
Discussion
Marsupialisation or decompression is frequently
performed as a conservative therapy for large KCOTs, since this
therapy is considered beneficial in reducing the size and the
recurrence rate of the tumour (14). Nakamura et al (15) reported that 96% of the cases
included in their study exhibited a cystic reduction of ≥50%.
Histologically, the typical characteristics of KCOT were eliminated
following this type of therapy, with the lesion assuming the form
of hyperplastic epithelium, thickened fibrous lamina and increased
inflammatory infiltration (8,15).
Three factors are considered to lead to the
development of KCOT in the jaws: i) epithelial proliferation; ii)
increased intracystic fluid pressure; iii) molecules associated
with bone resorption, such as IL1, IL6 and PGE2 (16).
A series of biomarkers that are highly expressed in
KCOTs prior to decompression were previously reported to exhibit a
marked decrease following therapy, such as IL-1α-induced PGs and
collagenase (8), Ki-67 (8,18),
Bcl-2 (17) and KGF (19), indicating the attenuation of cell
proliferation, anti-apoptosis and local invasion of the KCOT
epithelial cells. These changes may be attributed to the release of
intracystic fluid pressure and the communication with the oral
environment. However, the precise mechanism has not been fully
elucidated.
Among the various biological markers involved in
KCOT, the number of available studies using an anti-COX-2 antibody
for the investigation of odontogenic tumours is limited. Mendes
et al (7) reported a
positive COX-2 expression in all the KCOT samples included in their
study, with mild to strong expression levels and a cytoplasmic
staining pattern. In the present study, a positive COX-2 expression
was observed in 15 of the 16 cases in group I and the
immunostaining involved the full thickness of the epithelial
lining. This result was consistent with that reported by Mendes
et al (7). As regards the
staining pattern, the present study demonstrated a cytoplasmic and
membranous staining pattern, whereas Mendes et al (7) reported only cytoplasmic staining. At
the time of enucleation, only 4 cases exhibited COX-2 expression
levels consistent with their corresponding counterparts in group I.
In the remaining 12 cases the staining was negative.
To the best of our knowledge, this is the first
study on the differentiation of COX-2 expression in KCOTs between
one-stage decompression and two-stage enucleation. However, the
exact mechanism of COX-2 involvement in the pathogenesis and
progression of KCOT has not been elucidated.
Cyclooxygenase is a key regulatory enzyme involved
in the conversion of arachidonic acid to PGs (10). As one of the isoforms, COX-2
expression is induced under physiopathological conditions, such as
inflammatory stimuli and oncogenes (10). By binding to a G protein-coupled
surface receptor and leading to changes of intracellular cyclic AMP
and calcium (20), PGs play a key
role in several important physiological processes such as
oncogenesis, inflammation, cell reproduction and apoptosis and bone
metabolism (9).
Mechanical stress is crucial in the regulation of
bone metabolism (21). According
to Kubota et al (22) the
intracystic fluid pressure of odontogenic cysts in the jaws is
>80 mmHg during the early growth stage. However, whether the
positive intracystic fluid pressure affects the growth of
odontogenic jaw cysts such as KCOT and the exact underlying
mechanism have not been fully elucidated. Interleukin-1α (IL-1α) is
a multifunctional proinflammatory cytokine (10,18).
A previous study by Oka et al (18) demonstrated that the positive
pressure upregulated the expression of IL-1α in KCOT epithelial
cells. Furthermore, in a co-culture of KCOT fibroblasts and
epithelial cells, the IL-1α secreted by the epithelial cells
further induced the secretion of MMP-1, MMP-2, MMP-3, PGE2 and
M-CSF by the fibroblasts, which ultimately stimulated
osteoclastogenesis and collagen degradation (18). Moreover, it was previously verified
that the transcription and expression levels of COX-2 were
significantly decreased in KCOT epithelial linings following
decompression (8). There appears
to be a strong correlation among intracystic pressure, expression
of IL-1α and bone resorption, with regulation of COX-2 and PGE2
being the key link in this process. Apart from the intracystic
fluid pressure, mechanical loading on bone generates a fluid flow
within the mineralized matrix, which may exert fluid shear stress
(FSS) on cell membranes (23). A
previous study by Wadhwa et al (23) reported that FSS may induce the
transcription of COX-2 in MC3T3-E1 osteoblasts through the PKA and
ERK signaling pathways.
The findings mentioned above may explain the
possible mechanism underlying the mechanical stress-dependent
growth of KCOT in the jaws. Following decompression, the release of
fluid pressure leads to the gradual reduction of bone resorption
(18) and shrinking of the tumour
size (8), which is at least
partially mediated by the COX-2/PGE2 regulation.
In conclusion, despite the small size of this
sample, our results combined with the current knowledge of the
effects of COX-2 on oncogenesis suggest that COX-2 may contribute
to the biological profile of KCOTs by affecting the biological
regulation of the tumoural epithelium. In the present study,
although a notable change was observed in COX-2 expression
following decompression, the potential association between this
change and the clinical characteristics of KCOT, such as the
duration of decompression, tumour location and recurrence rate,
could not be further assessed, due to the small sample size and
short follow-up period. These issues require further
investigation.
Acknowledgements
This study was funded by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD).
References
|
1.
|
Shear M: The aggressive nature of the
odontogenic keratocyst: is it a benign cystic neoplasm? Part 2.
Proliferation and genetic studies. Oral Oncol. 38:323–331. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Madras J and Lapointe H: Keratocystic
odontogenic tumour: reclassification of the odontogenic keratocyst
from cyst to tumour. J Can Dent Assoc. 74:165h. 2008.
|
|
3.
|
Bhargava D, Deshpande A and Pogrel MA:
Keratocystic odontogenic tumour (KCOT) - a cyst to a tumour. Oral
Maxillofac Surg. 16:163–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Li TJ, Browne RM, Prime SS, Paterson IC
and Matthews JB: p53 expression in odontogenic keratocyst
epithelium. J Oral Pathol Med. 25:249–255. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Piattelli A, Fioroni M, Santinelli A and
Rubini C: Expression of proliferating cell nuclear antigen in
ameloblastomas and odontogenic cysts. Oral Oncol. 34:408–412. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kim DK, Ahn SG, Kim J and Yoon JH:
Comparative Ki-67 expression and apoptosis in the odontogenic
keratocyst associated with or without an impacted tooth in addition
to unilocular and multilocular varieties. Yonsei Med J. 44:841–846.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mendes RA, Carvalho JF and van der Waal I:
A comparative immunohistochemical analysis of COX-2, p53, and Ki-67
expression in keratocystic odontogenic tumours. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 111:333–339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ninomiya T, Kubota Y, Koji T and Shirasuna
K: Marsupialization inhibits interleukin-1α expression and
epithelial cell proliferation in odontogenic keratocysts. J Oral
Pathol Med. 31:526–533. 2002.PubMed/NCBI
|
|
9.
|
Gallo O, Masini E, Bianchi B, Bruschini L,
Paglierani M and Franchi A: Prognostic significance of
cyclooxygenase-2 pathway and angiogenesis in head and neck squamous
cell carcinoma. Hum Pathol. 33:708–714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mendes RA, Carvalho JF and Waal Iv: An
overview on the expression of cyclooxygenase-2 in tumours of the
head and neck. Oral Oncol. 45:e124–e128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Suyama Y, Kubota Y, Ninomiya T and
Shirasuna K: Immunohistochemical analysis of interleukin-1α, its
type I receptor and antagonist in keratocystic odontogenic tumours.
J Oral Pathol Med. 37:560–564. 2008.
|
|
12.
|
Lin DT, Subbaramaiah K, Shah JP,
Dannenberg AJ and Boyle JO: Cyclooxygenase-2: a novel molecular
target for the prevention and treatment of head and neck cancer.
Head Neck. 24:792–799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Williams CS, Tsujii M, Reese J, Dey SK and
DuBois RN: Host cyclooxygenase-2 modulates carcinoma growth. J Clin
Invest. 105:1589–1594. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shudou H, Sasaki M, Yamashiro T,
Tsunomachi S, Takenoshita Y, Kubota Y, Ninomiya T, Kawazu T and
Mori Y: Marsupialisation for keratocystic odontogenic tumours in
the mandible: longitudinal image analysis of tumour size using 3D
visualised CT scans. Int J Oral Maxillofac Surg. 41:290–296. 2012.
View Article : Google Scholar
|
|
15.
|
Nakamura N, Mitsuyasu T, Mitsuyasu Y,
Taketomi T, Higuchi Y and Ohishi M: Marsupialization for
odontogenic keratocysts: long-term follow-up analysis of the
effects and changes in growth characteristics. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 94:543–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
August M, Faquin WC, Troulis MJ and Kaban
LB: Differentiation of odontogenic keratocyst epithelium after cyst
decompression. J Oral Maxillofac Surg. 61:678–683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Pogrel MA and Jordan RC: Marsupialization
as a definitive treatment for the odontogenic keratocyst. J Oral
Maxillofac Surg. 62:651–655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Oka S, Kubota Y, Yamashiro T, Ogata S,
Ninomiya T, Ito S and Shirasuna K: Effects of positive pressure in
odontogenic keratocysts. J Dent Res. 84:913–918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Suyama Y, Kubota Y, Yamashiro T, Ninomiya
T, Koji T and Shirasuna K: Expression of keratinocyte growth factor
and its receptor in odontogenic keratocysts. J Oral Pathol Med.
38:476–480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mohan S and Epstein JB: Carcinogenesis and
cyclooxygenase: the potential role of COX-2 inhibition in upper
aerodigestive tract cancer. Oral Oncol. 39:537–546. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kanzaki H, Chiba M, Shimizu Y and Mitani
H: Periodontal ligament cells under mechanical stress induce
osteoclastogenesis by receptor activator of nuclear factor κB
ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner
Res. 17:210–220. 2002.
|
|
22.
|
Kubota Y, Ninomiya T and Oka S:
Interleukin-1α-dependent regulation of matrix
metalloproteinase-9(MMP-9) secretion and activation in the
epithelial cells of odontogenic jaw cysts. J Dent Res.
79:1423–1430. 2000.
|
|
23.
|
Wadhwa S, Choudhary S, Voznesensky M,
Epstein M, Raisz L and Pilbeam C: Fluid flow induces COX-2
expression in MC3T3-E1 osteoblasts via a PKA signaling pathway.
Biochem Biophys Res Commun. 297:46–51. 2002. View Article : Google Scholar : PubMed/NCBI
|