Introduction
Although eosinophils constitute only 1–4% of the
peripheral blood leukocytes, they may be important in various
inflammatory infectious and allergic diseases (1). Chronic graft-versus-host disease
(cGVHD) is a serious complication of allogeneic hematopoietic stem
cell transplantation (allo-HSCT). cGVHD can occur in almost every
organ and clinical diagnosis and staging are challenging (reviewed
in NIH cGVHD consensus conference guidelines) (2,3).
Although the pathophysiology of cGVHD remains ambiguous, previous
data have shown the involvement of a T helper (Th) 2 cell-mediated
process with overexpression of Th2 cytokines, such as IL-4 and IL-5
(4–6), and the correlation of eosinophilia
with the development of acute and cGVHD (7–17).
Th2-mediated immune responses linked to eosinophilia are regularly
identified in allograft rejection following solid organ
transplantation (18,19). Investigations of the course of
eosinophilia suggest a role of eosinophil counts as a predictive
marker for graft rejection in these patients (19).
The majority of studies investigating the role of
eosinophils in allo-HSCT have focused on the incidence of
eosinophilia in relation to overall survival (OS) and
relapse-related mortality in patients developing acute GVHD
(8,10,11,14,15,17).
A limited number of studies are available regarding the development
of chronic GHVD, and their focus is on eosinophilia as a risk
factor rather than on the course of peripheral blood eosinophils in
the context of time of diagnosis and initial treatment of cGVHD
(9,12–14,16).
The aim of this study was to investigate the role of
eosinophil counts in cGVHD. Our findings suggest that eosinophil
count changes constitute a possible indicator for overall cGVHD
activity, severity and treatment response, aiding clinicians in the
diagnosis and management of cGVHD patients.
Patients and methods
Patients
The clinical records of 64 patients developing cGVHD
after allo-HSCT at the University of Regensburg Medical Center
(Regensburg, Germany) between June, 1998 and December, 2003 were
included in this study and retrospectively analyzed. The
retrospective analysis for this study was approved by the ethics
committee of the University of Regensburg (Germany). Regular
follow-up was performed at least two years after allo-HSCT (median,
66.7 months).
The patients received standard infectious
prophylaxis with aciclovir, fluconazole, co-trimoxazole and
ciprofloxacin alone or in combination with metronidazole.
Intravenous immunoglobulins were administered in case of severe
hypogammaglobulinemia (<4 g/l). Patient characteristics are
provided in Table I.
 | Table I.Characteristics of the three cGVHD
patient groups (n=64) based on the course of eosinophil counts
seven days after cGVHD diagnosis. |
Table I.
Characteristics of the three cGVHD
patient groups (n=64) based on the course of eosinophil counts
seven days after cGVHD diagnosis.
| Patient
characteristics | Course of eosinophil
counts, n (%)
|
|---|
| Decreasing (n=26,
41%) | Intermediate (n=29,
45%) | Increasing (n=9,
14%) |
|---|
| Age (years) | 48.6±11.4 | 42.2±11.8 | 38.6±11.4 |
| Gender
(female/male) | 8 (30)/18 (70) | 14 (48)/15 (52) | 3 (33)/6 (66) |
| Disease | | | |
| AML | 9 (35) | 6 (21) | 1 (11) |
| ALL | 0 (0) | 3 (10) | 3 (33) |
| CML | 3 (12) | 5 (17) | 0 (0) |
| NHL | 6 (23) | 10 (35) | 5 (56) |
| CLL | 1 (4) | 3 (10) | 0 (0) |
| MM | 2 (8) | 1 (3) | 0 (0) |
| MF | 4 (15) | 0 (0) | 0 (0) |
| AA/PNH | 1 (4) | 0 (0) | 0 (0) |
| Other | 0 (0) | 1 (3) | 0 (0) |
| Sibling/unrelated
donor | 15 (58)/11 (42) | 13 (45)/16 (55) | 6 (66)/3 (33) |
| HLA identical/MM | 22 (85)/4 (15) | 27 (93)/2 (7) | 8 (89)/1 (11) |
| Stem cell source | | | |
| Bone marrow | 6 (23) | 5 (17) | 1 (11) |
| Selected PBSC | 8 (31) | 4 (14) | 0 (0) |
| Unselected
PBSC | 12 (46) | 20 (69) | 8 (89) |
| Conditioning
regimens | | | |
| TBI-based protocols
(>8 Gy) | 10 (39) | 16 (55) | 5 (56) |
| FBM protocol | 9 (35) | 9 (31) | 4 (44) |
| Other
protocols | 7 (27) | 4 (14) | 0 (0) |
| GVHD prophylaxis | | | |
| ATG 10 mg/20
mg | 4 (15)/5 (19) | 9 (31)/6 (21) | 2 (22)/2 (22) |
| CSA alone | 5 (19) | 3 (10) | 2 (22) |
| CSA/MTX | 9 (35) | 16 (55) | 5 (56) |
| CSA/MMF | 9 (35) | 8 (28) | 1 (11) |
| Other
immunosuppressive drugs | 3 (12) | 2 (7) | 1 (11) |
| Acute GVHD grade
II–IV | 15 (58) | 28 (97) | 9 (100) |
| Overlap acute to
cGVHD | 5 (19) | 8 (28) | 0 (0) |
| Relapse | 6/25 (24) | 10 (35) | 3/8 (38) |
| Survival, (months)
median | 75.9±40.0 | 62.7±42.0 | 48.6±47.2 |
| Start cGVHD
(day) | 215±89 | 184±56 | 188±67 |
| Eosinophilia
(>4) at diagnosis | 21 (81) | 2 (7) | 6 (66) |
| cGVHD severity
(limited disease) | 5 (19) | 9 (31) | 2 (22) |
| CSA/TAC Tx at cGVHD
diagnosis | 22 (85) | 24 (83) | 5 (55) |
| Mean prednisolone
dosage on days −14/−7 (mg) | 3.8/3.4 | 18.2/14.7 | 3.1/3.3 |
| Mean prednisolone
dosage on days 0/+7 (mg) | 50.6/44.5 | 28.7/26.3 | 28.9/30.1 |
| Prednisolone
escalation (1–2 mg/kg) | 16 (62) | 6 (21) | 4 (44) |
| cGHVD
aggravation | 3 (12) | 11 (38) | 6 (66) |
| Initiation of cGVHD
aggravation (days) | 463±358 | 360±138 | 373±241 |
| Organ involvement
in cGVHD | | | |
| Mucosa | 17 (66) | 21 (72) | 7 (78) |
| Skin | 9 (35) | 17 (59) | 4 (44) |
| Eyes | 8 (31) | 12 (41) | 2 (22) |
| Joints | 2 (8) | 5 (17) | 0 (0) |
|
Gastrointestinal | 5 (19) | 12 (41) | 3 (33) |
| Liver | 2 (8) | 2 (7) | 3 (33) |
| Lung | 9 (35) | 7 (24) | 2 (22) |
Acute GVHD was treated with prednisolone and other
immunosuppressive agents as needed. Cyclosporin A (CSA) was usually
discontinued 6 months after HSCT if there was no evidence of GVHD.
Acute and cGVHD were initially diagnosed and retrospectively
reassessed according to the respective official diagnosis criteria
(3,16). Among the 64 patients developing
cGVHD, localized mucosal/skin involvement or hepatic dysfunction
due to cGVHD was classified as limited cGVHD in 16 patients (25%)
and as extensive cGVHD in 48 patients (75%). Initial first-line
therapy for cGVHD was prednisolone (1–2 mg/kg body weight) or topic
steroids. Fifty patients (78%) additionally received systemic/topic
CSA or CSA dose escalation at diagnosis of cGVHD. Twenty-one
patients (33%) exhibited later clinical cGVHD aggravation during
follow-up defined by further intensification of steroid
treatment.
Methods
Eosinophil counts were retrospectively evaluated on
days −14, −7, 0 and +7 in relation to the first day of cGVHD
diagnosis with an allowed deviation of 2 days to the date of the
blood samples on days −14 and −7. Eosinophilia was defined as a
peripheral relative eosinophil count (REC) of >4% according to
the value used in recent publications and the internal reference
standards of our Institution (2,8,19).
In addition, clinically relevant changes in eosinophil counts were
assumed in an increase/decrease of at least 1%. For further
analysis, patients were stratified into three groups based on the
course of eosinophil counts 7 days after cGVHD diagnosis compared
to eosinophil counts at cGVHD diagnosis. The first group
(decreasing eosinophils, n=25) showed decreasing eosinophil counts
after cGVHD diagnosis of ≥1%. The second group (intermediate
eosinophils, n=30) comprised patients with intermediate eosinophil
counts, which were not increasing and not decreasing >1% at day
+7. Compared to group 1, the patients of the third group
(increasing eosinophils, n=9) exhibited increasing RECs of ≥1% 7
days after cGVHD diagnosis.
Statistical analysis
Statistical analysis was performed using SPSS
version 17. Univariate analysis was performed using the
χ2 test, Fisher’s exact test and paired Student’s t-test
as indicated. Multivariate analysis was performed by Cox’s
proportional hazard analysis. For OS, actuarial curves were
obtained using the Kaplan-Meier method and were statistically
compared using the log-rank test. In all analyses, a two-sided
significance level of P=0.05 was considered to indicate a
statistically significant difference.
Results
OS, RR and cGVHD aggravation
Previous studies suggested eosinophilia as an
indicative parameter for clinical outcome (8,11,14).
Therefore, we analyzed the OS, relapse rate (RR) and cGVHD
aggravation of the patients included in this study. A trend towards
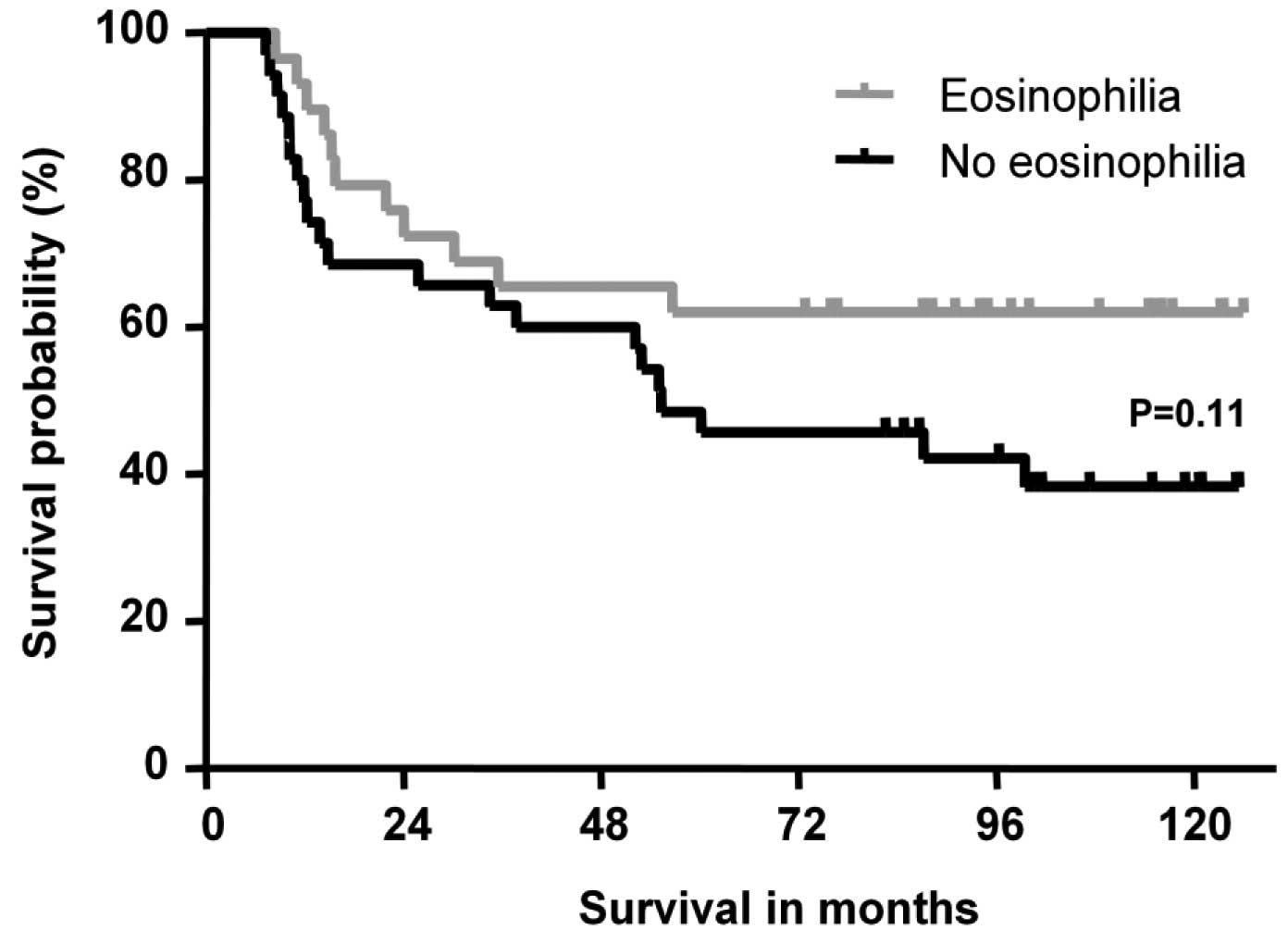
improved OS was observed in patients with eosinophilia compared to
those without eosinophilia (P=0.11, Fig. 1). No difference was observed
between the two patient groups with regard to relapse rate
(P=0.29), limited cGVHD (P=0.10) and later cGVHD aggravation
(P=0.60).
Course of peripheral blood eosinophils in
cGVHD patients
Since RECs potentially reflect the activity of
cGVHD, we investigated patients based on the development of the
eosinophil course one week after cGVHD diagnosis. The analysis of
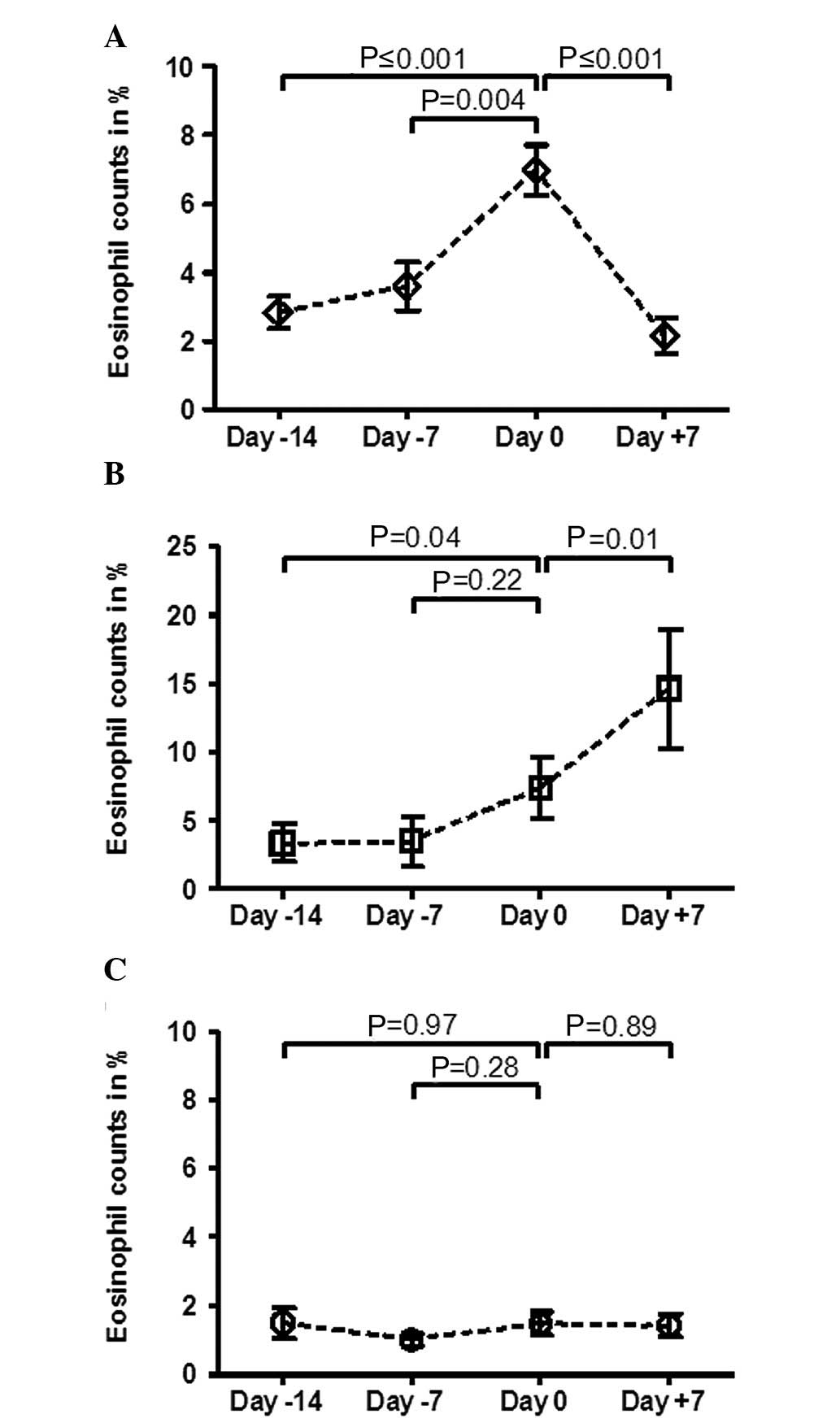
the course of RECs demonstrated that patients with a decrease of
>1% the week after diagnosis, exhibited a significant increase
of RECs the week prior to cGVHD diagnosis (Fig. 2A). In patients with an additional
increase of eosinophil counts the week following cGVHD diagnosis, a
significant increase of eosinophils to the same levels as in the
decreasing course of RECs group was observed (Fig. 2B). Notably, the analysis of
patients without a significant change of RECs after diagnosis did
not demonstrate any significant changes before and after cGVHD
diagnosis (Fig. 2C). In agreement
with these findings, significantly more patients with eosinophilia
were observed in the decreasing (88%, P≤0.001) and increasing (66%,
P≤0.001) groups compared to the intermediate course of RECs group
(7%).
Changes of eosinophil counts after cGVHD
diagnosis in the three groups
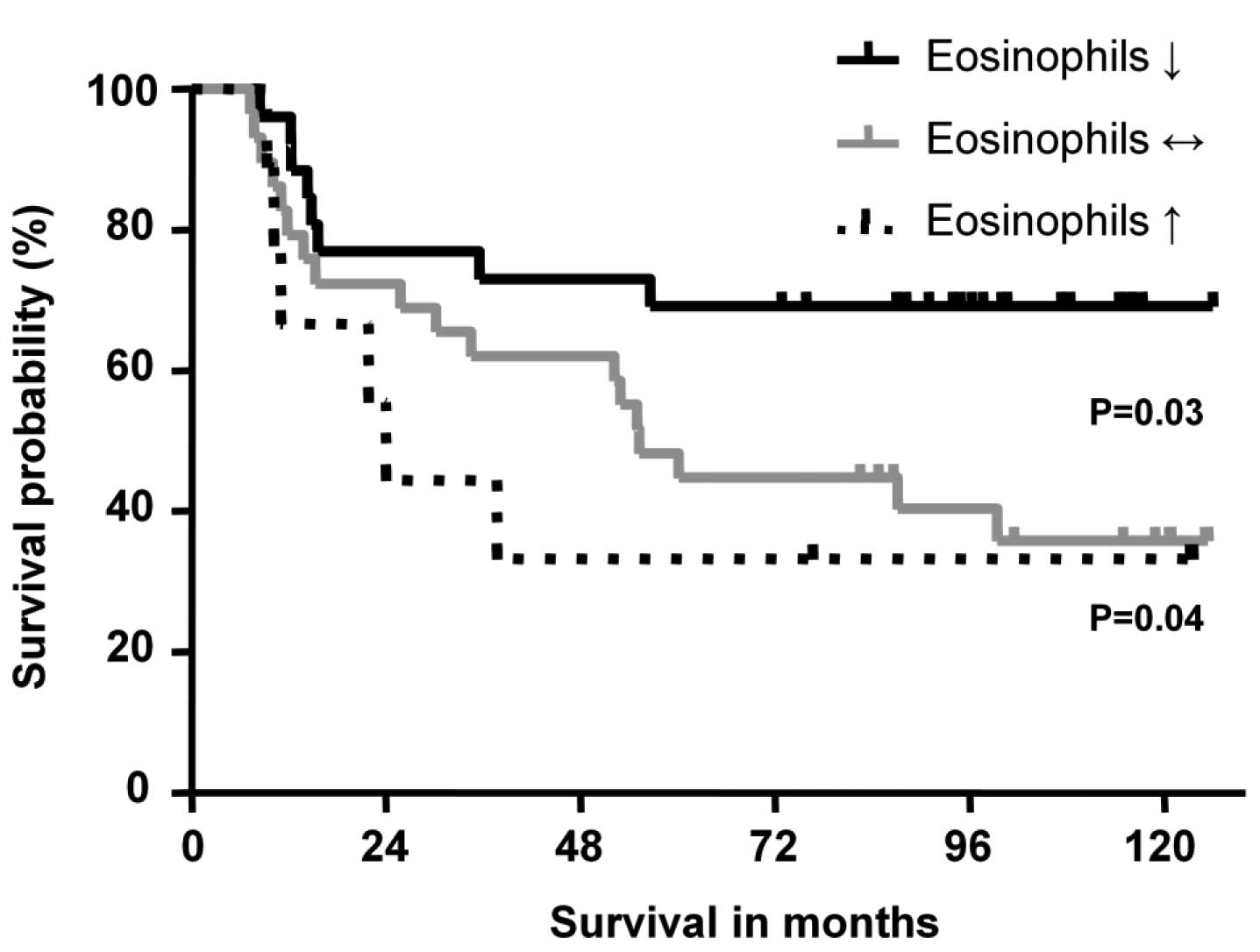
OS was then analyzed in relation to the eosinophil
course after diagnosis. The analysis of the Kaplan-Meier survival
curve demonstrated a significantly improved OS (P=0.04) in the
decreasing compared to the increasing course of RECs group
(Fig. 3). The survival curve of
patients without significant changes of RECs also showed a
significantly impaired OS compared to patients with increasing
(P=0.52) and decreasing eosinophil groups (P=0.03). Focusing on
potential factors in explaining the different OS rates, no
significant correlation was observed between organ manifestation of
cGVHD and eosinophil counts in the three groups (P>0.05).
Regarding the rates of limited cGVHD disease and the overlap of
acute and cGVHD, no significant differences were observed among the
three groups (P>0.05, Table I).
Proceeding acute GVHD was found to occur more frequently in the
increasing (100%, P=0.04) and intermediate groups (93%, P=0.007)
compared to the decreasing course of RECs group (58%). Notably, the
analysis of relapse rate did not differ among the three groups
(P>0.05), while cGVHD aggravation after diagnosis occurred
significantly less frequently in patients with decreasing (12%)
compared to patients with an increasing course of RECs (66%,
P=0.004). A strong tendency towards a higher incidence of later
cGVHD aggravation was observed in patients with decreasing compared
to patients with no significant changes in the course of RECs (37%,
P=0.06).
Steroid treatment
The role of steroid treatment in the three groups
was also analyzed, possibly affecting the eosinophil course after
cGVHD diagnosis. The percentage of patients administered steroid
dose escalation (1–2 mg/kg body weight) did not differ between
patients with decreasing (60%) and increasing RECs (44%, P=0.46).
Patients without significant changes in the course of RECs
exhibited a significantly lower frequency of steroid escalation
(23%) compared to the decreasing (P=0.01) group, while this was not
the case compared to the increasing course of RECs group
(P=0.24).
Results of multivariate analysis
A multivariate analysis was performed with
parameters including age, relapse, previous history of acute GVHD,
limited or extended cGVHD and eosinophil behaviour. Age (P=0.01)
and relapse (P=0.001) were identified as independent factors for
OS, in contrast to eosinophil counts (P=0.45), previous history of
acute GVHD (P=0.89) and limited or extended cGVHD (P=0.49).
Discussion
cGVHD significantly contributes to morbidity and
mortality after allo-HSCT. Therefore, the identification of risk
factors and clinical markers predicting cGVHD and its clinical
course are important to enable possible pre-emptive therapy and
treatment changes (16).
The role of eosinophilia in cGVHD patients remains
ambiguous. Two previous studies have shown a correlation of
eosinophilia with improved OS and reduced non-relapse mortality,
and have demonstrated no difference in the rate of relapse
(8,14). In agreement with findings of those
studies, the present study has shown that cGVHD patients with
eosinophilia demonstrated a tendency towards an improved outcome in
OS, while no difference was identified for the relapse rate or
limited cGVHD as reported elsewhere (8,14).
By contrast, a previous study by Ahmad et al (7) did not show improved OS in patients
with cGVHD and eosinophilia in patients administered only
reduced-intensity conditioning (RIC) with peripheral blood stem
cell transplant as stem cell source for allogeneic recipients, of
which the vast majority had myeloma or non-Hodgkin lymphoma (NHL)
as the underlying disease. Similarly, a direct comparison between
the results of Ahmad et al (7), the results of the present study and
other studies (8,14) is difficult due to the heterogeneity
of the patients and transplant characteristics, such as
conditioning intensity, stem cell source and underlying
disease.
Conflicting results have not only been reported for
eosinophilia and the overall outcome after allo-HSCT, but also for
the correlation of eosinophilia with the development of cGVHD. A
number of studies have shown no correlation of eosinophilia to
cGVHD, whereas according to other studies eosinophilia precedes or
parallels the development of cGVHD (9,12–14,16,17).
Kim et al (14) reported a
biphasic pattern in the course of eosinophilia following allo-HSCT
with two peaks, one prior to day +100 and one ∼200 days after HSCT,
possibly explaining the conflicting results concerning eosinophilia
and GVHD in previous studies.
As a result, the exact time course of eosinophil
counts in cGVHD patients before and after diagnosis, taking into
consideration steroid treatment, was investigated in this study.
Two different patterns of the relative eosinophil course were
identified. Patients exhibiting later increasing or decreasing RECs
following diagnosis showed relatively stable counts from day −14 to
day −7, but showed a similar increase at the day of diagnosis. The
other pattern was observed in patients without significant RECs
changes. Eosinophil counts remained stable over time and did not
show any increase at the day of the diagnosis most likely due to
the higher administered steroid dose observed in this group.
Previous studies in solid organ transplantation have demonstrated
that eosinophilia frequently occurs 2–4 days prior to the onset of
graft rejection (19,20). In agreement with the patient groups
in the present study, Ahmad et al (7) reported an increase of the
eosinophilia counts 4.5 days prior to diagnosis and a prompt
decrease of the eosinophil counts after the initiation of cGVHD
treatment.
However, not all patients with cGVHD developed
eosinophilia with RECs >4% or relevant changes of the eosinophil
counts, explaining the potentially insufficient value of
eosinophilia as a sensitive predictive marker for cGVHD diagnosis
due to low sensitivity. However, a high specificity of eosinophilia
for acute rejection in solid organ transplantation has been
reported (19,20).
Focusing on eosinophil counts as a marker for cGVHD
activity including changes of subclinical RECs not being classified
as eosinophilia, we investigated patients depending on the course
of the eosinophil counts the week after diagnosis. A significant
survival advantage of patients with decreasing RECs compared to
patients with additional increasing RECs the week after diagnosis
was identified. The two groups did not differ in terms of relapse
rate and limited cGVHD, while patients with further increasing RECs
exhibited a significantly increased rate of later cGVHD
aggravation, possibly contributing to the impaired OS. In agreement
with this observation, patients without significant RECs changes
also exhibited a strong tendency to later cGVHD aggravation.
Concerning additional factors affecting OS (16), the role of significant higher
percentage of previous history of acute GVHD in the group with
increasing RECs compared to other groups remains ambiguous since
none of the patients exhibited cGVHD overlapping from acute GVHD.
In addition, the multivariate analysis suggested only age and
relapse as independent factors for survival.
Notably, during the analysis of steroid treatment as
a possible bias on eosinophil counts, the percentage of patients
administered steroid dosage escalation as part of the cGVHD therapy
did not differ in the decreasing and increasing course of RECs
groups. However, higher steroid doses already applied prior to
cGVHD diagnosis could cover changes of eosinophil counts as
observed in patients without significant RECs changes. In addition,
the possibility that other factors apart from the supposed Th2
cytokine pattern inducing eosinophilia may contribute to cGVHD
pathophysiology could not be excluded (4,5).
In conclusion, the results of this study support the
role of eosinophil counts changes as a marker for cGVHD activity
and future severity. A further increase of RECs the week after
cGVHD diagnosis indicates uncontrolled disease activity as observed
in a higher percentage of later cGVHD aggravation and this may
contribute to impaired OS. By contrast, spontaneous or possibly
steroid-induced decreasing eosinophil counts account for better
control of cGVHD disease activity, indicated by a reduced frequency
of later cGVHD aggravation and, consequently, an improved OS. Due
to the need for further improvement of therapy and treatment
decision making in cGVHD patients, eosinophil decrease the week
following diagnosis may help identify those patients that may
benefit from graft versus leukemia effects in the presence of
controllable cGVHD. For more detailed investigation, prospective
clinical trials on larger patient cohorts with well-defined testing
interventions directed to the pathophysiology of the disease, may
help to identify additional groups of patients that could benefit
from cGVHD and gain a better understanding of the correlation
between eosinophils, Th2 cytokines, cGVHD and its treatment.
Acknowledgements
The authors would like to thank
Christoph P. Beier for reviewing the manuscript.
References
|
1.
|
Rothenberg ME: Eosinophilia. N Engl J Med.
338:1592–1600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Akpek G, Zahurak ML, Piantadosi S, et al:
Development of a prognostic model for grading chronic
graft-versus-host disease. Blood. 97:1219–1226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Filipovich AH, Weisdorf D, Pavletic S, et
al: National Institutes of Health consensus development project on
criteria for clinical trials in chronic graft-versus-host disease:
I. Diagnosis and staging working group report. Biol Blood Marrow
Transplant. 11:945–956. 2005. View Article : Google Scholar
|
|
4.
|
Baird K and Pavletic SZ: Chronic graft
versus host disease. Curr Opin Hematol. 13:426–435. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ferrara JL, Levine JE, Reddy P and Holler
E: Graft-versus-host disease. Lancet. 373:1550–1561. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Landfried K, Wolff D and Holler E:
Pathophysiology and management of graft-versus-host disease in the
era of reduced-intensity conditioning. Curr Opin Oncol. 21(Suppl
1): S39–S41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ahmad I, Labbe AC, Chagnon M, et al:
Incidence and prognostic value of eosinophilia in chronic
graft-versus-host disease after nonmyeloablative hematopoietic cell
transplantation. Biol Blood Marrow Transplant. 17:1673–1678. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Aisa Y, Mori T, Nakazato T, et al: Blood
eosinophilia as a marker of favorable outcome after allogeneic stem
cell transplantation. Transpl Int. 20:761–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Arslan O, Akan H, Koc H, et al:
Eosinophilia after allogeneic bone marrow transplantation using
busulfan and cyclophosphamide conditioning regimen. Bone Marrow
Transplant. 18:2611996.PubMed/NCBI
|
|
10.
|
Basara N, Kiehl MG and Fauser AA:
Eosinophilia indicates the evolution to acute graft-versus-host
disease. Blood. 100:30552002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Imahashi N, Miyamura K, Seto A, et al:
Eosinophilia predicts better overall survival after acute
graft-versus-host-disease. Bone Marrow Transplant. 45:371–377.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jacobsohn DA, Schechter T, Seshadri R,
Thormann K, Duerst R and Kletzel M: Eosinophilia correlates with
the presence or development of chronic graft-versus-host disease in
children. Transplantation. 77:1096–1100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kalaycioglu ME and Bolwell BJ:
Eosinophilia after allogeneic bone marrow transplantation using the
busulfan and cyclophosphamide preparative regimen. Bone Marrow
Transplant. 14:113–115. 1994.PubMed/NCBI
|
|
14.
|
Kim DH, Popradi G, Xu W, et al: Peripheral
blood eosinophilia has a favorable prognostic impact on transplant
outcomes after allogeneic peripheral blood stem cell
transplantation. Biol Blood Marrow Transplant. 15:471–482. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
McNeel D, Rubio MT, Damaj G, et al:
Hypereosinophilia as a presenting sign of acute graft-versus-host
disease after allogeneic bone marrow transplantation.
Transplantation. 74:1797–1800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Przepiorka D, Anderlini P, Saliba R, et
al: Chronic graft-versus-host disease after allogeneic blood stem
cell transplantation. Blood. 98:1695–1700. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sato T, Kobayashi R, Nakajima M, Iguchi A
and Ariga T: Significance of eosinophilia after stem cell
transplantation as a possible prognostic marker for favorable
outcome. Bone Marrow Transplant. 36:985–991. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Alegre ML, Florquin S and Goldman M:
Cellular mechanisms underlying acute graft rejection: time for
reassessment. Curr Opin Immunol. 19:563–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nagral A, Ben-Ari Z, Dhillon AP and
Burroughs AK: Eosinophils in acute cellular rejection in liver
allografts. Liver Transpl Surg. 4:355–362. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Foster PF, Sankary HN, Hart M, Ashmann M
and Williams JW: Blood and graft eosinophilia as predictors of
rejection in human liver transplantation. Transplantation.
47:72–74. 1989. View Article : Google Scholar : PubMed/NCBI
|