Introduction
Esophagectomies is associated with significant
morbidity and mortality, although recent advances in surgical and
postoperative management techniques have improved the treatment
outcome (1). Respiratory
morbidity, in particular, remains the most common serious
complication following esophagectomy and a number of studies
demonstrated a respiratory complication rate of ∼20% (2–5).
Since the duration of one-lung ventilation is known to affect
postoperative immune reactions and cause respiratory complications,
it is crucial to reduce the intrathoracic operative time (6,7).
We first performed an esophagectomy preceded by the
laparoscopic transhiatal approach (LTHA) for patients with
esophageal cancer in 2009 (8–10).
With this method, carbon dioxide is introduced into the mediastinum
from the abdominal side of the diaphragm and middle and lower
mediastinal operations may be performed via a transhiatal approach.
The main advantages of this method are that the thoracic procedures
performed via right thoracotomy may be simplified and the duration
of one-lung ventilation may be shortened. In addition, a good
surgical view of the lower mediastinum is obtained and the quality
of the mediastinal surgery may be improved. By December, 2012, a
total of 121 patients with esophageal tumors had undergone LTHA
during a variety of esophageal surgical procedures, including
subtotal esophagectomy, middle and lower esophagectomy and tumor
resection (8–11).
In our previous study, we demonstrated the efficacy
of LTHA preceding subtotal esophagectomy with gastric tube
reconstruction via a retrosternal route, with regard to
peri-operative outcomes (8). In
this study, we aimed to analyze the perioperative treatment
outcomes of patients with distal esophageal cancer who underwent
middle and lower esophagectomy preceded by hand-assisted LTHA and
gastric tube reconstruction via the posterior mediastinal route
(anastomosis in the thoracic cavity). Our results revealed that our
method markedly shortened the duration of one-lung ventilation and
decreased intraoperative blood loss. Furthermore, this method
improved postoperative care by decreasing the extubation time
following surgery, the duration of thoracic drainage and the length
of the postoperative hospital stay.
Patients and methods
Surgical procedure
An abdominal operation was performed using
hand-assisted laparoscopic surgery (HALS). Subsequently, middle and
lower mediastinal operations were performed using LTHA. The
patients were placed in a supine position on the operating table.
An upper abdominal incision (70 mm) was performed and a Lap Disc
(regular) (Ethicon Endo-Surgery, Cincinnati, OH, USA) was placed
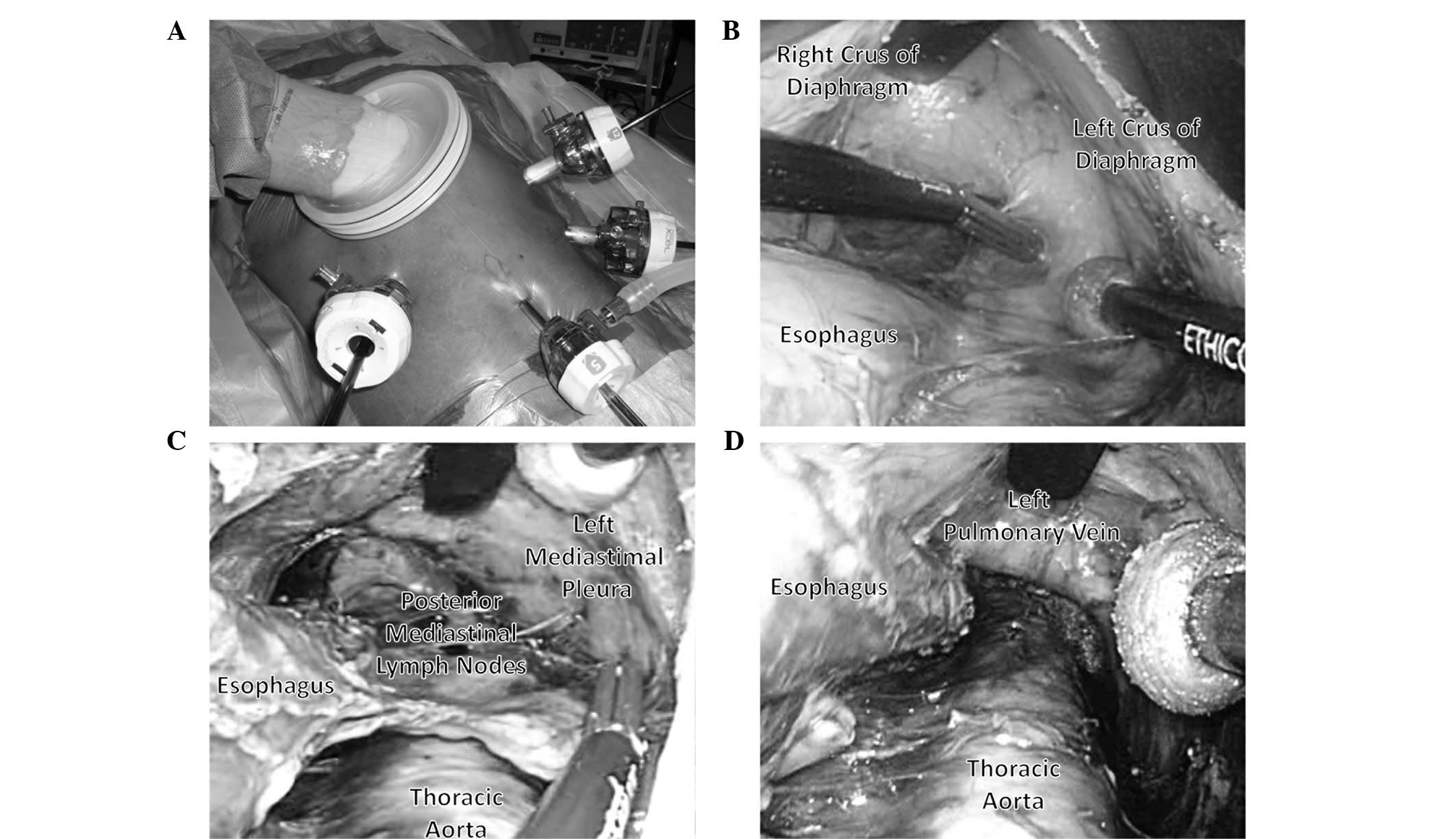
(Fig. 1A). Three 12-mm ports were
inserted, one in each flank and one in the left hypochondrium; one
5-mm port for the flexible laparoscope was inserted into the lower
abdomen (Fig. 1A). The operator
stood on the right side of the patient and inserted the Lap Disc
with their left hand. The 12-mm port in the right flank was mainly
used for the surgery. The assistant stood on the left side of the
patient and the ports in the left abdomen were used to provide
assistance. The scopist stood near the patient’s groin. Carbon
dioxide was introduced into the intra-abdominal space and the
pneumoperitoneum pressure was controlled at 10 mmHg (8–11).
The operator lifted up the stomach with their left
hand and the greater omentum, left gastroepiploic vessels and
gastrosplenic ligament were divided using the EnSeal device
(Ethicon Endo-Surgery). Subsequently, the esophageal hiatus was
opened and carbon dioxide was introduced into the mediastinum. The
assistant inserted an Endo Retract (Autosuture Norwalk, CT, USA)
and a blunt tip dissector through the ports on the left side and
the working space in the mediastinum was secured with these two
devices and 10 mmHg of pneumomediastinum pressure. Dissection of
the anterior and left side of the distal esophagus up to the level
of the tracheal bifurcation was performed with the EnSeal device
and the blunt tip dissector. Using this approach, dissection of the
anterior sides of the posterior mediastinal lymph nodes was easily
performed (Fig. 1B) (9).
Subsequently, the adventitia of the thoracic aorta
was exposed at the level of the crura of the diaphragm, followed by
dissection of the anterior side of the thoracic aorta to the
cranial side. The roots of the proper esophageal arteries were
confirmed and divided using the EnSeal device. Following these
procedures, the anterior and posterior sides of the posterior
mediastinal lymph nodes, including the thoracic paraaortic and left
pulmonary ligament lymph nodes, were dissected. While lifting these
lymph nodes like a membrane, they were cut along the borderline of
the left mediastinal pleura (Fig.
1C). In this manner, the posterior mediastinal lymph nodes were
dissected en bloc (Fig. 1D)
(8,9).
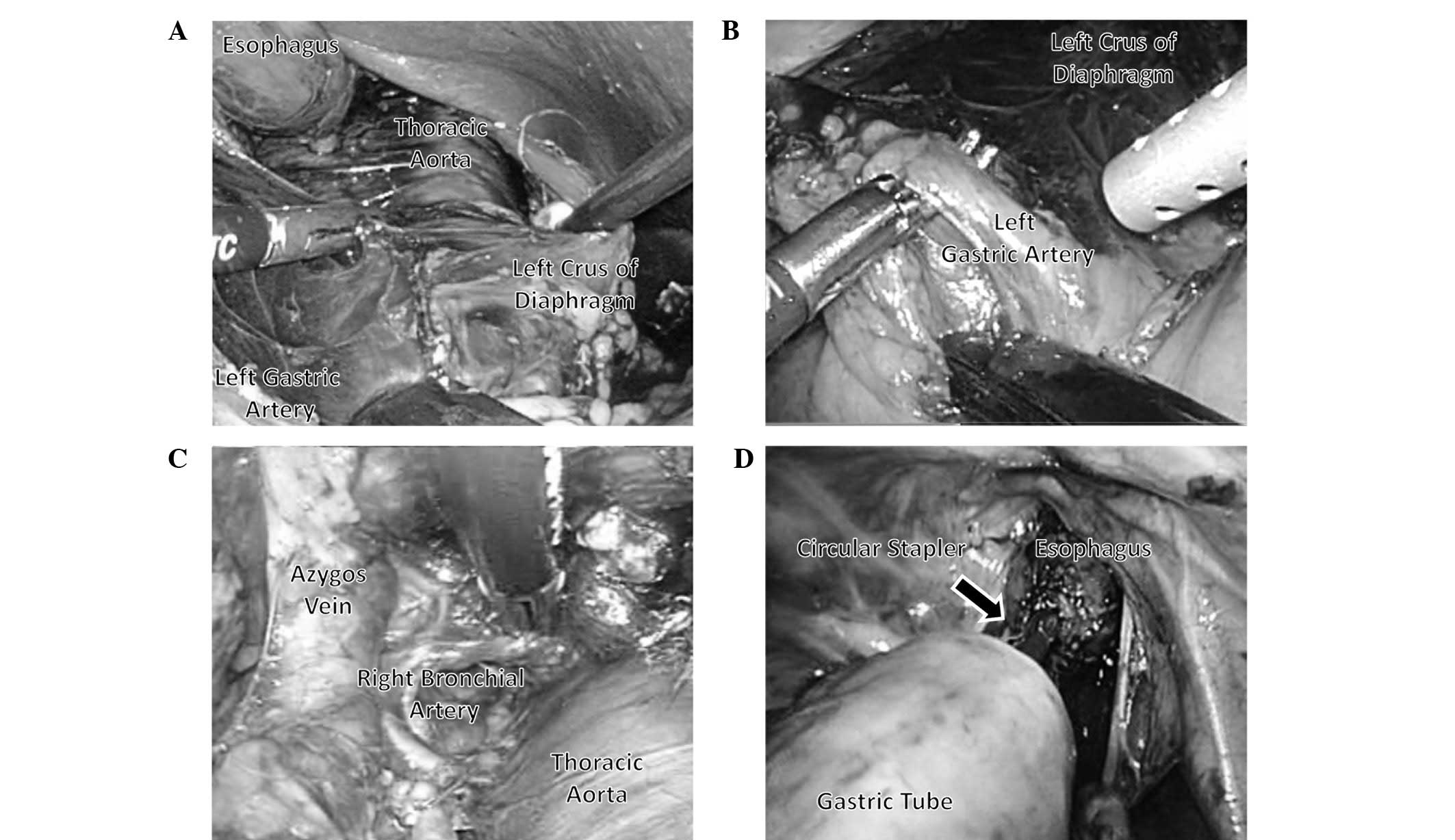
Following the dissection of posterior mediastinal
lymph nodes, the adventitia of the thoracic aorta and the crura of
the diaphragm were exposed. Therefore, the appropriate layer for
the dissection of the celiac lymph nodes was clearly identified and
the dissection of the posterior mediastinal lymph nodes was
extended towards the caudal side from the crura of the diaphragm to
the celiac artery (Fig. 2A). The
lymph nodes in the esophageal hiatus of the diaphragm, the
infradiaphragmatic lymph nodes and the lymph nodes along the celiac
artery were dissected en bloc from the left side approach.
Subsequently, the left gastric vessels were exposed from the left
side, clipped and divided and the lymph nodes along the left
gastric artery were dissected (Fig.
2B). Thus, the posterior mediastinal and celiac lymph nodes
that were located around the border between the thoracic and the
abdominal cavity were continuously dissected with the LTHA.
Dissection of the posterior and right sides of the
distal esophagus was subsequently performed and an incision was
made in the right mediastinal pleura to allow ablation. The right
mediastinal pleural incision was then extended to the level of the
arch of the azygos vein (Fig. 2C).
Thus, the middle and lower thoracic esophagus was completely
detached from the surrounding tissue.
Subsequently, in the left semilateral-decubitus
position, the operating table was rotated to the left, a small
right thoracotomy was performed (∼10 cm) and a rib retractor was
used to maintain the small surgical field. The middle mediastinal
lymph nodes, including subcarinal and bilateral main bronchial
lymph nodes, were resected via the thoracic approach. Following
division of the azygos vein, the upper thoracic esophagus was
divided.
The operating table was then rotated to the right
and the middle and lower thoracic esophagus was extracted via an
upper abdominal incision. Finally, reconstruction via the posterior
mediastinal route with a gastric tube and anastomosis in the
thoracic cavity were performed using a CDH25 circular stapler
(Ethicon Endo-Surgery) via the thoracic approach (Fig. 2D).
Patients
We started to routinely perform LTHA-preceded middle
and lower esophagectomies in patients with distal esophageal cancer
in December, 2009. Between January, 2005 and December, 2012, a
total of 21 patients with esophageal cancer underwent middle and
lower esophagectomy combined with lymph node dissection and gastric
tube reconstruction via the posterior mediastinal route
(anastomosis in the thoracic cavity) at the Division of Digestive
Surgery, Department of Surgery, Kyoto Prefectural University of
Medicine. The patients were retrospectively divided into two groups
according to the operative method, with and without LTHA (10
patients from December, 2009 to December, 2012; and 11 patients
from January, 2005 to November, 2009, respectively). The two groups
were compared with respect to the perioperative treatment outcome.
The operative indication was identical in the two groups, namely
clinical T1–3, N0–3, M0 esophageal cancer, staged according to the
International Union Against Cancer TNM classification of malignant
tumours, 7th edition (12). All
patients were treated by two highly skilled surgeons and informed
consent was obtained from each participant.
Surgical procedure without LTHA
For the 11 patients who underwent middle and lower
esophagectomy without LTHA the procedure was as follows: the
patient was placed in the left semilateral-decubitus position, the
operating table was rotated to the right and abdominal surgery was
performed first via laparotomy (∼20 cm). The operating table was
then rotated to the left and thoracic surgery (including middle and
lower mediastinal operation) was performed via thoracotomy (∼20
cm). Finally, reconstruction via the posterior mediastinal route
with a gastric tube and anastomosis in the thoracic cavity were
performed using a CDH25 circular stapler, via the thoracic
approach.
All the patients underwent two-field
lymphadenectomy. A total of 6 patients (3 patients with and 3
without LTHA) received preoperative chemotherapy, including 2
courses of cisplatin (80 mg/m2/day on day 1) plus
5-fluorouracil (800 mg/m2/day on days 1–5) (13).
Clinicopathological characteristics
To compare the backgrounds of the two groups, we
analyzed their clinicopathological characteristics, such as age,
gender, primary tumor location, histological type, TNM category and
pathological stage. Histopathological examinations were performed
on the primary lesions and all the dissected lymph nodes using
serial sections. The histopathological diagnoses were confirmed by
experienced pathologists. The TNM category and pathological stage
were classified according to the pTNM pathological classification
(12). The effects of preoperative
comorbidities were analyzed using the Charlson comorbidity index
(14).
Perioperative factors
To determine the efficacy of LTHA, the two groups
were compared with respect to several perioperative factors, such
as total operative time, duration of one-lung ventilation,
operative blood loss, number of resected lymph nodes, duration of
management by respirator, frequency of postoperative respiratory
complications, number of white blood cells, serum C-reactive
protein (CRP) level, duration of thoracic drainage, frequency of
anastomotic leakage and postoperative hospital stay. The number of
peripheral white blood cells and the serum CRP level were measured
on days 2 and 7 following surgery. According to our criteria, the
thoracic drainage tube was removed when the amount of thoracic
discharge was reduced to <150 m1/day. Postoperative respiratory
complications were defined as those involving major respiratory
insufficiency; such as, a need for reintubation or severe
pneumonia.
Statistical analysis
Statistical analysis was performed using the
Student’s t-test and Fisher’s exact test. P<0.05 was considered
to indicate a statistically significant difference. All analyses
were performed using JMP statistical software, version 5 (SAS
Institute Inc., Cary, NC, USA).
Results
Patients
A total of 21 patients with esophageal cancer who
underwent middle and lower esophagectomy combined with gastric tube
reconstruction in the thoracic cavity were divided into two groups
according to the operative procedure, such as, with and without
LTHA (10 and 11 patients, respectively). Although the percentage of
female patients was significantly higher in the LTHA group, there
were no significant differences in the other clinicopathological
parameters (age, primary tumor location, histological type, TNM
category, pathological stage or Charlson comorbidity index) between
the two groups (Table I). Two
patients (one with and one without LTHA) were pathologically
diagnosed as pT4 (invasion of the diaphragm).
 | Table I.Comparison of clinicopathological
parameters of the patients who underwent middle and lower
esophagectomy (anastomosis in the thoracic cavity) with and without
preceding laparoscopic transhiatal approach (LTHA). |
Table I.
Comparison of clinicopathological
parameters of the patients who underwent middle and lower
esophagectomy (anastomosis in the thoracic cavity) with and without
preceding laparoscopic transhiatal approach (LTHA).
| Variables | LTHA
| P-value |
|---|
| With | Without |
|---|
| Age, years (mean ±
SEM) | 66.1±3.2 | 67.4±3.2 | 0.781 |
| Gender | | | |
| Male | 6 | 11 | 0.035a |
| Female | 4 | 0 | |
| Location of primary
tumor | | | |
| Distal thoracic
esophagus | 5 | 8 | 0.387 |
| Abdominal
esophagus | 5 | 3 | |
| Histological
type | | | |
| SCC | 5 | 8 | 0.387 |
| Other | 5 | 3 | |
| pT category | | | |
| pT0–1 | 3 | 3 | 1.000 |
| pT2–4 | 7 | 8 | |
| pN category | | | |
| pN0 | 4 | 6 | 0.670 |
| pN1–3 | 6 | 5 | |
| Pathological
stage | | | |
| 0–II | 4 | 6 | 0.670 |
| III–IV | 6 | 5 | |
| Charlson comorbidity
index (mean ± SEM) | 2.0±0.1 | 2.2±0.1 | 0.172 |
Intraoperative factors
We performed a comparison of the intra-operative
factors between the two groups. The total operative time was
significantly shorter in the group treated with compared to that in
the group treated without LTHA (mean ± SEM: 304.3±23.3 vs.
485.0±22.3 min, respectively) (Table
II). The duration of one-lung ventilation was significantly
shorter in patients treated with compared to that in patients
treated without LTHA (mean ± SEM: 144.4±16.5 vs. 212.5±16.0 min,
respectively) (Table II). The
total operative blood loss was significantly reduced in patients
treated with compared to those treated without LTHA (mean ± SEM:
272.9±101.4 vs. 567.7±98.6 ml, respectively) (Table II). The total number of resected
lymph nodes did not differ significantly between the two groups
(24.3±3.6 and 27.4±3.3 with and without LTHA, respectively)
(Table II). The total number of
resected thoracic lymph nodes did not differ significantly between
the two groups (5.3±1.9 and 8.7±1.9 with and without LTHA,
respectively). These results suggest that LTHA-preceded
esophagectomy may be used to reduce the total operative time, the
duration of one-lung ventilation and total operative blood loss,
without compromising the quality of lymph node dissection.
 | Table II.Comparison of intraoperative factors
between patients who underwent middle and lower esophagectomy
(anastomosis in the thoracic cavity) with and without laparoscopic
transhiatal approach (LTHA). |
Table II.
Comparison of intraoperative factors
between patients who underwent middle and lower esophagectomy
(anastomosis in the thoracic cavity) with and without laparoscopic
transhiatal approach (LTHA).
| Variables | LTHA
| P-value |
|---|
| With | Without |
|---|
| Total operative time
(min) | 304.3±23.3 | 485.0±22.3 | <0.001a |
| Duration of one-lung
ventilation (min) | 144.4±16.5 | 212.5±16.0 | 0.008a |
| Total operative blood
loss (ml) | 272.9±101.4 | 567.7±98.6 | 0.049a |
| Total number of
resected lymph nodes | 24.3±3.6 | 27.4±3.3 | 0.503 |
| Thoracic lymph
nodes | 5.3±1.9 | 8.7±1.9 | 0.206 |
| Abdominal lymph
nodes | 16.9±2.5 | 18.6±2.4 | 0.622 |
Postoperative factors
We then performed a comparison of the postoperative
factors between the two groups (Table
III). The extubation time after surgery was significantly
shorter in the patients treated with compared to that in patients
treated without LTHA (mean ± SEM: 0.9±1.1 vs. 4.1±1.0 days,
respectively). Postoperative respiratory complications occurred in
10.0% of the patients treated with the LTHA (pneumonia in 1
patient), whereas they occurred in 36.3% of the patients treated
without LTHA (pneumonia in 4 patients), without a statistically
significant difference. We analyzed the number of peripheral white
blood cells and the serum CRP level at 2 and 7 days after the
operation. Although the elevation in the number of peripheral white
blood cells at 2 days after the operation tended to be more limited
in patients treated with compared to that in patients treated
without LTHA (mean ± SEM: 9,820±1,749 vs. 12,718±1,710/μl,
respectively), the differences were not considered to be
significant. The duration of thoracic drainage was significantly
shortened in patients treated with compared to those treated
without LTHA (mean ± SEM: 6.9±2.9 vs. 19.2±2.7 days, respectively),
suggesting that this method decreased the amount of postoperative
thoracic discharge by preventing damage to the thoracic and
mediastinal tissues. The frequency of anastomotic leakage did not
differ significantly between the two groups. The postoperative
hospital stay was significantly shortened in patients treated with
compared to those treated without LTHA (mean ± SEM: 25.7±5.3 vs.
40.7±5.2 days, respectively). These results suggest that
LTHA-preceded esophagectomy may be used to improve postoperative
care without increasing the risk of major complications.
 | Table III.Comparison of postoperative factors
between the patients performed middle and lower esophagectomy
(anastomosis in thoracic cavity) with and without laparoscopic
transhiatal approach (LTHA). |
Table III.
Comparison of postoperative factors
between the patients performed middle and lower esophagectomy
(anastomosis in thoracic cavity) with and without laparoscopic
transhiatal approach (LTHA).
| Variables | LTHA
| P-value |
|---|
| With | Without |
|---|
| Extubation time after
surgery (days) | 0.9±1.1 | 4.1±1.0 | 0.044a |
| Postoperative
respiratory complications [n/total (%)] | 1/10 (10.0) | 4/11 (36.3) | 0.311 |
| White blood cells
(n/μl) | | | |
| 2 days after
surgery | 9,820±1,749 | 12,718±1,710 | 0.245 |
| 7 days after
surgery | 7,422±1,204 | 8,782±1,106 | 0.413 |
| Serum CRP
(mg/dl) | | | |
| 2 days after
surgery | 19.1±2.2 | 21.7±2.1 | 0.397 |
| 7 days after
surgery | 7.2±2.3 | 6.6±2.1 | 0.843 |
| Duration of
thoracic drainage (days) | 6.9±2.9 | 19.2±2.7 | 0.006a |
| Anastomotic leakage
(cases/total) | 1/10 (10.0%) | 1/11 (9.1%) | 1.000 |
| Postoperative
hospital stay (days) | 25.7±5.3 | 40.7±5.2 | 0.025a |
Discussion
The surgical trauma caused by esophagectomy is
greater compared to that caused by any other general surgical
operations. Therefore, pulmonary complications remain the most
common and serious complications following esophagectomy (2–4). It
was recently demonstrated that thoracoscopic (15–19)
or mediastinoscopic esophagectomy (20,21)
minimizes injury to the chest wall and reduces surgical
invasiveness. Furthermore, the duration of one-lung ventilation is
known to affect postoperative immune reactions and cause
respiratory complications (6,7). We
were able to perform middle and lower thoracic mediastinal surgery
from the abdominal side of the diaphragm via LTHA and the
subsequent thoracic operations, which were performed via a right
thoracotomy, were markedly simplified. Therefore, the thoracic
trauma was minimized and the duration of one-lung ventilation was
reduced. Furthermore, our results demonstrated that the total
intraoperative blood loss was significantly decreased and the
duration of thoracic drainage was significantly shortened using
LTHA. We consider that one of the reasons is that this method
enables dissection of the appropriate layer under a magnified
videoscopic view and, therefore, damage to the microvessels and
lymphatic ducts is avoided (8,9). In
our experience, there were no problems associated with applying a
10-mmHg pneumomediastinum pressure for intraoperative respiratory
care. All these factors may contribute to improved postoperative
outcome, regarding the extubation time following surgery and the
postoperative hospital stay.
An additional advantage of this surgical procedure
is that it enables easy access to the posterior mediastinal lymph
nodes, including the paraaortic and left pulmonary ligament lymph
nodes (9). The left side of the
mediastinum is a difficult space to approach via a right
thoracotomy. In particular, complete resection of the left
pulmonary ligament lymph nodes via a right thoracic approach
carries an increased risk of serious complications. Furthermore,
when performing thoracoscopic esophagectomy in the left lateral
decubitus position, it is difficult to maintain the surgical field
in the lower mediastinum (15–17).
With this method, the gas supply during dissection of the
mediastinum makes the layers between the esophagus and the
surrounding organs easy to identify and the separation of these
layers helps minimize bleeding. Accordingly, the separation of the
anterior and posterior sides of the posterior mediastinal lymph
nodes is easy to perform and, by lifting up these lymph nodes like
a membrane, the border of the left mediastinal pleura may be
clearly identified, enabling en-bloc dissection (9). Furthermore, by exposing the
adventitia of the thoracic aorta and the crura of the diaphragm,
the appropriate layer for the dissection of the celiac lymph nodes
may be clearly identified and the lymph nodes along the celiac
artery may be dissected continuously.
We previously demonstrated the efficacy of
LTHA-preceded subtotal esophagectomy with gastric tube
reconstruction via the retrosternal route with regard to the
perioperative outcomes of patients with esophageal cancer (8). Namely, LTHA-preceded esophagectomy
shortened the intrathoracic operative time, decreased
intraoperative bleeding and reduced the amount of postoperative
thoracic discharge, without increasing the risk of major
postoperative complications (8).
In the present study, similar tendencies were observed in patients
treated with LTHA-preceded middle and lower esophagectomy. In both
studies, the elevation in the number of peripheral white blood
cells 2 days after the operation tended to be more limited in
patients treated with LTHA, suggesting that this method
downregulated excessive immune responses (8). As previously demonstrated, we
performed operations using HALS to avoid injury of the
reconstructed organ (8–10). The abdominal and the mediastinal
operation may be easily performed and, thus far, there has been no
report of reconstructed organs suffering intraoperative injuries.
Furthermore, we have mainly been using the EnSeal device to avoid
damaging the surrounding organs (8–10).
The tip of the EnSeal blade is not too sharp and is therefore
suitable for safe dissection. In the present study, the same
procedure was used for distal esophageal cancer and the extubation
time following surgery and postoperative hospital stay were
significantly shortened, suggesting that the advantages of the
LTHA-preceded method are more distinct in middle and lower
esophagectomy.
In conclusion, LTHA-preceded middle and lower
esophagectomy, significantly shortened the total operative time and
the duration of one-lung ventilation and decreased intraoperative
blood loss. Furthermore, the extubation time after surgery, the
duration of thoracic drainage and postoperative hospital stay were
significantly shortened. Additionally, this procedure ensured a
good surgical view of the posterior and left mediastinum, enabling
safe en bloc lymph node dissection around these areas, without
increasing the risk of major perioperative complications.
References
|
1.
|
Tachibana M, Kinugasa S, Yoshimura H, et
al: Clinical outcomes of extended esophagectomy with three-field
lymph node dissection for esophageal squamous cell carcinoma. Am J
Surg. 189:98–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Whooley BP, Law S, Murthy SC, Alexandrou A
and Wong J: Analysis of reduced death and complication rates after
esophageal resection. Ann Surg. 233:338–344. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Law S, Wong KH, Kwok KF, Chu KM and Wong
J: Predictive factors for postoperative pulmonary complications and
mortality after esophagectomy for cancer. Ann Surg. 240:791–800.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ferguson MK and Durkin AE: Preoperative
prediction of the risk of pulmonary complications after
esophagectomy for cancer. J Thorac Cardiovasc Surg. 123:661–669.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bailey SH, Bull DA, Harpole DH, et al:
Outcomes after esophagectomy: a ten-year prospective cohort. Ann
Thorac Surg. 75:217–222. 2003.PubMed/NCBI
|
|
6.
|
Zingg U, Forberger J, Frey DM, et al:
Inflammatory response in ventilated left and collapsed right lungs,
serum and pleural fluid, in transthoracic esophagectomy for cancer.
Eur Cytokine Netw. 21:50–57. 2010.PubMed/NCBI
|
|
7.
|
De Conno E, Steurer MP, Wittlinger M, et
al: Anesthetic-induced improvement of the inflammatory response to
one-lung ventilation. Anesthesiology. 110:1316–1326.
2009.PubMed/NCBI
|
|
8.
|
Shiozaki A, Fujiwara H, Murayama Y, et al:
Perioperative outcomes of esophagectomy preceded by the
laparoscopic transhiatal approach for esophageal cancer. Dis
Esophagus. Oct 22–2012.(Epub ahead of print).
|
|
9.
|
Shiozaki A, Fujiwara H, Murayama Y, et al:
Posterior mediastinal lymph node dissection using the
pneumomediastinum method for esophageal cancer. Esophagus. 9:58–64.
2012. View Article : Google Scholar
|
|
10.
|
Shiozaki A, Fujiwara H, Daisuke I, Okamoto
K, Komatsu S and Otsuji E: Pneumomediastinum method for esophageal
cancer. Operation. 65:1277–1280. 2011.
|
|
11.
|
Shiozaki A, Fujiwara H, Murayama Y, et al:
Hand-assisted laparoscopic transhiatal approach for mediastinal
esophageal duplication cyst resection. Esophagus. 9:247–251. 2012.
View Article : Google Scholar
|
|
12.
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of malignant tumors. 7th edition. John Wiley
& Sons, Inc; Hoboken, NJ: 2009
|
|
13.
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar
|
|
14.
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chron Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Osugi H, Takemura M, Lee S, et al:
Thoracoscopic esophagectomy for intrathoracic esophageal cancer.
Ann Thorac Cardiovasc Surg. 11:221–227. 2005.PubMed/NCBI
|
|
16.
|
Osugi H, Takemura M, Higashino M, Takada
N, Lee S and Kinoshita H: A comparison of video-assisted
thoracoscopic oesophagectomy and radical lymph node dissection for
squamous cell cancer of the oesophagus with open operation. Br J
Surg. 90:108–113. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Thomson IG, Smithers BM, Gotley DC, et al:
Thoracoscopic-assisted esophagectomy for esophageal cancer:
analysis of patterns and prognostic factors for recurrence. Ann
Surg. 252:281–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Noshiro H, Iwasaki H, Kobayashi K, et al:
Lymphadenectomy along the left recurrent laryngeal nerve by a
minimally invasive esophagectomy in the prone position for thoracic
esophageal cancer. Surg Endosc. 24:2965–2973. 2010. View Article : Google Scholar
|
|
19.
|
Cadière GB, Dapri G, Himpens J and Rajan
A: Thoracoscopic esophagectomy in prone position. Ann Surg Oncol.
18:8382011.
|
|
20.
|
Tangoku A, Yoshino S, Abe T, et al:
Mediastinoscope-assisted transhiatal esophagectomy for esophageal
cancer. Surg Endosc. 18:383–389. 2004. View Article : Google Scholar
|
|
21.
|
Mimatsu K, Oida T, Kawasaki A, et al:
Mediastinoscopy-assisted esophagectomy is useful technique for poor
surgical-risk patients with thoracic esophageal cancer. Surg
Laparosc Endosc Percutan Tech. 19:e17–e20. 2009. View Article : Google Scholar : PubMed/NCBI
|