Introduction
Mammography detection is a widely-used screening
technique for breast cancer (1).
Typical features characteristic of invasive malignant carcinoma
include evident mass, micro-calcification, architectural distortion
or asymmetric density. Tumors with various clinical and
pathological characteristics have different appearances on
mammography, leading to variable prognoses (2). It has been reported that HER2/neu is
a factor that influences specific mammographic appearances
(3). Regarding the image features
of certain special histology types, Yang et al (4) compared metaplastic breast cancer and
invasive ductal carcinomas (IDCs). Increasing attention has focused
on the clinical and pathological characteristics of breast
carcinomas, which may exhibit various types of biological behaviors
over the years of treatment and prognosis (5). However, few studies have been
conducted with regard to this aspect.
The aim of the present study was to investigate the
association between mammographic image features and
clinicopathological characteristics in IDC.
Materials and methods
Patient data and mammography studies
The clinical and pathological results and
mammography reports of 231 patients were retrospectively analyzed.
The mammographic appearances were assessed according to the
analytical criteria of the Breast Imaging Reporting and Data System
from the database of Tianjin Oncology Hospital Breast Cancer Center
(6). All the patients were female,
and underwent breast radical mastectomy between 2011 to 2013.
Mammography screening detection was obtained prior to surgery.
Study design and conduction
Pathological information was prospectively collected
according to patient age, estrogen2 level in circulation, tumor
size, the grade of IDC, the molecular type of carcinoma, estrogen
receptor (ER) and progesterone receptor (PR) status, HER2/neu
status, Ki-67 expression level, p53 status and lymph node
metastasis status. Mammograms were assessed by five radiologists
who specialize in breast radiology at the Department of Oncology
Center (Tianjin Medical University Cancer Institute and Hospital,
Tianjin, China), without any information of the pathological
results. The mammography studies were collected and divided into
five groups according to the traditional identification of
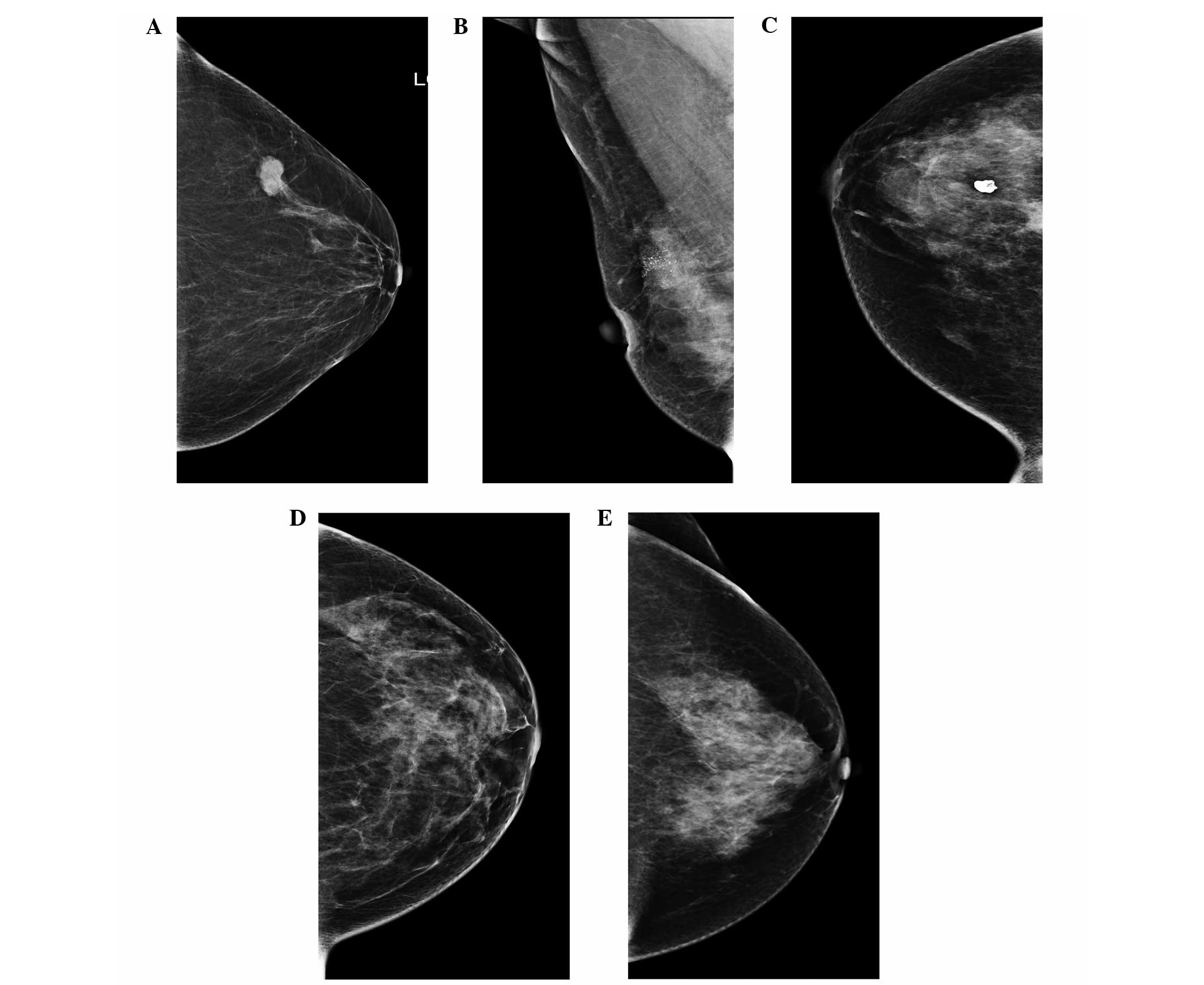
malignant breast cancer in general. The five groups were i) evident
mass without calcifications (Fig.
1A), ii) malignant calcifications without mass (Fig. 1B), iii) evident mass with
calcifications (Fig. 1C), iv)
architectural distortion or asymmetric density without mass or
calcifications (Fig. 1D) and v) no
visible changes (Fig. 1E),
respectively.
Statistical analysis
Pathological information was correlated with
mammographic appearances. The data were analyzed by the SPSS 17.0
statistical program (SPSS, Inc., Chicago, IL, USA). Statistical
analysis was performed to assess the association using Fisher’s
exact test, the χ2 test, Spearman’s correlation and
logistic regression, as appropriate.
Results
Several factors generally associated with
mammographic appearances
Table I
demonstrates that there were significant differences between
mammographic appearances in general and age, estrogen2 level in
circulation, tumor size, grade of IDC, HER2 expression status,
Ki-67 expression index and the molecular type of the tumor
(P=0.005, 0.044, 0.020, 0.037, 0.001, 0.001 and 0.013,
respectively).
 | Table IAssociation between mammographic
appearance and clinical characteristics. |
Table I
Association between mammographic
appearance and clinical characteristics.
| Mammographic
appearance, % | | |
|---|
|
| | |
|---|
| Characteristics | 1 | 2 | 3 | 4 | 5 | P-value | χ2 |
|---|
| Age, years | | | | | | 0.005 | 14.91 |
| >50 | 26.1 | 16.8 | 51.3 | 5.0 | 0.8 | | |
| ≤50 | 26.1 | 31.5 | 30.6 | 7.2 | 4.5 | | |
| Estrogen2 level in
circulation | | | | | | 0.044 | 9.794 |
| >40 | 26.2 | 31.0 | 32.1 | 6.0 | 4.8 | | |
| ≤40 | 25.9 | 19.6 | 47.6 | 6.3 | 0.7 | | |
| Tumor size, cm | | | | | | 0.020 | 11.668 |
| ≤2 | 29.9 | 23.9 | 32.5 | 9.4 | 4.3 | | |
| >2 | 21.9 | 23.7 | 50.0 | 2.6 | 1.8 | | |
| Lymph node
status | | | | | | 0.365 | 4.319 |
| Negative | 27.7 | 23.1 | 37.7 | 8.5 | 3.1 | | |
| Positive | 23.8 | 24.8 | 45.5 | 3.0 | 3.0 | | |
| Grade of IDC | | | | | | 0.037 | 16.393 |
| I | 37.5 | 16.7 | 29.2 | 16.7 | 0.0 | | |
| II | 24.8 | 28.0 | 37.9 | 5.6 | 3.7 | | |
| III | 23.9 | 13.0 | 58.7 | 2.2 | 2.2 | | |
| Estrogen
receptor | | | | | | 0.561 | 2.984 |
| Negative | 21.7 | 20.0 | 45.0 | 8.3 | 5.0 | | |
| Positive | 27.5 | 25.1 | 39.8 | 5.3 | 2.3 | | |
| Progesterone
receptor | | | | | | 0.726 | 2.054 |
| Negative | 23.7 | 19.7 | 46.1 | 6.6 | 3.9 | | |
| Positive | 27.1 | 25.8 | 38.7 | 5.8 | 2.6 | | |
| HER2 expression
status | | | | | | 0.001 | 19.993 |
| Negative | 36.3 | 27.5 | 22.5 | 10.0 | 3.8 | | |
| Positive | 20.5 | 21.9 | 51.0 | 4.0 | 2.6 | | |
| Ki-67 expression
index | | | | | | 0.001 | 17.650 |
| ≥45% | 22.4 | 13.4 | 59.7 | 1.5 | 3.0 | | |
| <45% | 27.8 | 28.4 | 32.7 | 8.0 | 3.1 | | |
| p53 status | | | | | | 0.066 | 8.794 |
| Negative | 29.3 | 25.6 | 35.4 | 7.3 | 2.4 | | |
| Positive | 18.5 | 20.0 | 53.8 | 3.1 | 4.6 | | |
| Molecular type | | | | | | 0.013 | 25.423 |
| Luminal A | 34.8 | 29.0 | 24.6 | 8.7 | 2.9 | | |
| Luminal B | 22.5 | 23.5 | 50.0 | 2.9 | 1.0 | | |
| HER2
overexpression | 18.4 | 18.4 | 53.1 | 6.1 | 4.1 | | |
| Triple
negative | 40.0 | 20.0 | 10.0 | 20.0 | 10.0 | | |
Comparison of mammographic features with
the immunohistochemistry labeling index of the tumor
In the HER2 group, significant differences were
identified between the presence and absence of malignant
calcifications and indistinct or amorphous calcifications on
mammography (P=0.001 and P=0.026). In the Ki-67 index group,
significant differences were identified between the presence and
absence of an evident mass (P=0.002). In the expression of the p53
group, significant differences were identified between the presence
and absence of pleomorphic calcifications on mammography (P=0.039).
These results are shown in Tables
II-V, respectively.
 | Table IIAssociation between malignant
calcification and clinical characteristics. |
Table II
Association between malignant
calcification and clinical characteristics.
| Malignant
calcification, % | | | | | | |
|---|
|
| | | | | | |
|---|
|
Characteristics | Positive | Negative | χ2 | P-value | r | Sig | HR (adjusted) | 95% CI |
|---|
| HER2 (n=231) | | | 11.989 | 0.001 | 0.228 | 0.000 | 3.205 | 1.617–6.354 |
| Negative | 50.0 | 50.0 | | | | | | |
| Positive | 72.8 | 27.2 | | | | | | |
| Molecular type
(n=230) | | | 13.496 | 0.004 | 0.080 | 0.227 | | |
| Luminal A | 53.6 | 46.4 | | | | | | |
| Luminal B | 73.5 | 26.5 | | | | | | |
| HER2
overexpression | 71.4 | 28.6 | | | | | | |
| Triple
negative | 30.0 | 70.0 | | | | | | |
| p53 (n=229) | | | 3.373 | 0.066 | 0.121 | 0.067 | | |
| Negative | 61.0 | 39.0 | | | | | | |
| Positive | 73.8 | 26.2 | | | | | | |
| Ki-67 (n=229) | | | 2.997 | 0.083 | 0.114 | 0.084 | | |
| ≥45% | 73.1 | 26.9 | | | | | | |
| <45% | 61.1 | 38.9 | | | | | | |
| Tumor size, cm
(n=231) | | | 7.567 | 0.006 | 0.181 | 0.006 | 1.913 | 1.075–3.405 |
| ≤2 | 56.4 | 43.6 | | | | | | |
| >2 | 73.7 | 26.3 | | | | | | |
 | Table VAssociation between indistinct and
amorphous calcifications and clinical characteristics. |
Table V
Association between indistinct and
amorphous calcifications and clinical characteristics.
| Indistinct and
amorphous calcifications, % | | | | | | |
|---|
|
| | | | | | |
|---|
|
Characteristics | Positive | Negative | χ2 | P-value | r | Sig | HR (adjusted) | 95% CI |
|---|
| Age, years
(n=230) | | | 3.319 | 0.071 | −0.120 | 0.068 | | |
| >50 | 20.2 | 79.8 | | | | | | |
| ≤50 | 30.6 | 69.4 | | | | | | |
| HER2 (n=231) | | | 5.085 | 0.026 | 0.149 | 0.024 | 2.155 | 1.077–4.315 |
| Negative | 16.2 | 83.8 | | | | | | |
| Positive | 29.8 | 70.2 | | | | | | |
| Tumor size, cm
(n=231) | | | 3.729 | 0.068 | 0.127 | 0.053 | | |
| ≤2 | 19.7 | 80.3 | | | | | | |
| >2 | 33.7 | 66.3 | | | | | | |
| p53 (n=229) | | | 3.657 | 0.066 | 0.127 | 0.056 | | |
| Negative | 6.1 | 93.9 | | | | | | |
| Positive | 13.8 | 86.2 | | | | | | |
| Ki-67 (n=229) | | | 3.497 | 0.061 | −0.124 | 0.061 | | |
| ≥45% | 3.0 | 97.0 | | | | | | |
| <45% | 10.5 | 89.5 | | | | | | |
Comparison of mammographic features with
the pathological index of the tumor
There were significant differences between the
presence and absence of malignant calcifications in the size of the
tumor group (P=0.006) (Table II).
Furthermore, in the group of an evident mass on mammogram, there
were significant differences between irregular and lobular or oval
mass shape in various grades of IDC (P=0.001) (Table VI).
 | Table VIAssociation between the shape of mass
and clinical characteristics. |
Table VI
Association between the shape of mass
and clinical characteristics.
| Shape of mass
(%) | | | | | | |
|---|
|
| | | | | | |
|---|
|
Characteristics | Regular | Irregular | χ2 | P-value | r | Sig | HR (adjusted) | 95% CI |
|---|
| Tumor size, cm
(n=155) | | | 4.841 | 0.036 | 0.177 | 0.027 | | |
| ≤2 | 61.6 | 38.4 | | | | | | |
| >2 | 43.9 | 56.1 | | | | | | |
| Grade of invasive
ductal carcinoma (n=155) | | | 10.604 | 0.001 | 0.268 | 0.001 | 2.365 | 1.263–4.430 |
| I | 62.5 | 37.5 | | | | | | |
| II | 60.4 | 39.6 | | | | | | |
| III | 26.3 | 73.7 | | | | | | |
Comparison of mammographic features with
the nodal involvement status of the tumor
In terms of the nodal involvement of the tumor, the
data in Table IV demonstrated
that there were significant differences between the presence and
absence of pleomorphic calcifications (P=0.048).
 | Table IVAssociation between pleomorphic
calcifications and clinical characteristics. |
Table IV
Association between pleomorphic
calcifications and clinical characteristics.
| Pleomorphic
calcifications, % | | | | | | |
|---|
|
| | | | | | |
|---|
|
Characteristics | Positive | Negative | χ2 | P-value | r | Sig | HR (adjusted) | 95% CI |
|---|
| HER2 (n=231) | | | 3.186 | 0.074 | 0.117 | 0.075 | | |
| Negative | 15.0 | 85.0 | | | | | | |
| Positive | 25.2 | 74.8 | | | | | | |
| p53 (n=229) | | | 4.246 | 0.039 | 0.136 | 0.040 | 2.049 | 1.051–3.997 |
| Negative | 18.3 | 81.7 | | | | | | |
| Positive | 30.8 | 69.2 | | | | | | |
| Ki-67 (n=229) | | | 3.556 | 0.059 | 0.125 | 0.059 | | |
| ≥45% | 29.9 | 70.1 | | | | | | |
| <45% | 18.5 | 81.5 | | | | | | |
| Tumor size, cm
(n=231) | | | 2.895 | 0.089 | 0.112 | 0.090 | | |
| ≤2 | 17.1 | 82.9 | | | | | | |
| >2 | 26.3 | 73.7 | | | | | | |
| Lymph node status
(n=231) | | | 3.909 | 0.048 | 0.130 | 0.048 | 1.993 | 1.049–3.785 |
| Negative | 16.9 | 83.1 | | | | | | |
| Positive | 27.7 | 72.3 | | | | | | |
Discussion
In previous years, the mammogram has become one of
the most significant diagnostic approaches of breast tumor.
Findings of previous studies (7–10)
have indicated that various types of breast tumor present different
appearances on mammogram. Several typical mammographic appearances
can reflect the tumor attributes and its biological behaviors,
which may provide valuable information to the clinicians.
Mammographic features can be used as predictors of prognosis and
pathological characteristics, which influence the subsequent
treatment. Therefore, the mammographic pattern is considered a risk
factor for subsequent development of breast cancer (11).
It is known that specific types of breast tumor,
including colloid or tubular, manifest particular appearances on
mammogram (12,13). However, the IDC is the most
frequent histological type among breast tumors. In general, breast
tumors exhibit up to five different radiological patterns
corresponding to the biological heterogeneity of these tumors.
The data of the present study have demonstrated that
several typical mammographic features are correlated with certain
indices of immunohistochemistry and pathology, and lymph node
metastasis status. The aforementioned evidence may reflect tumor
attributes to a certain extent.
In terms of the HER2 expression level, the data
indicated that the ratio of malignant calcifications on mammogram
was significantly high in HER2-positive cases. In particular,
differences existed between the HER2-positive or -negative group on
mammogram. A study by Gajdos et al (14) suggested that calcifications were
associated with HER2 overexpression. The presence of calcifications
in a mass or segmental calcifications on mammography were
significantly associated with a positive HER2 status. Studies have
been conducted on ER-negative breast cancer patients, which
demonstrated that in the ER-negative group, HER2-positive breast
cancers are more likely to be irregular masses, with spiculated
margins associated with pleomorphic calcifications, whereas the
HER2-negative breast cancers have been more frequently identified
as round/variform-shaped masses with indistinct margins and have
shown a great diversity of morphological types of calcifications
comparatively (15). Thus far, a
few studies have reported the association between HER2
overexpression of various types of tumor and malignant-appearing
calcifications with regard to ductal carcinoma in situ
(DCIS), non-palpable breast carcinomas and invasive breast
carcinomas (3,13,16).
These studies demonstrated that, regardless of the type, the
malignant calcifications on mammography were correlated with HER2
status, tending to exist in the HER2 overexpression cases. As for
the IDCs, the results were concordant with previous studies
conducted concerning this aspect (3). Based on the aforementioned evidence,
it may be inferred that patients who exhibit the malignant
calcifications on mammography tend to be HER2 overexpressed when
they are newly diagnosed. As is widely known, the HER2 status is an
important prognostic factor for overall survival and disease-free
survival of patients with breast cancer (17), which is closely associated with the
HER2 receptor-targeted trastuzumab therapy.
Previous studies have demonstrated the association
between p53 and Ki-67 expression with mammography. Gilliland et
al (18) concluded that
rapidly growing and aggressive tumors are responsible for a
considerable amount of breast cancer detection failure by
mammography. The identification of cancer within 12 months
following a negative mammogram is defined as ‘interval breast
cancer.’ The dysregulation of the cell cycle and potential genetic
instability were measured by p53 expression, whereas the
proliferation rate of the tumor was measured by the Ki-67 index.
The results of the study indicated that the proportion of
pleomorphic calcifications on mammography in p53-positive
expression cases was significantly higher compared with the
negative cases. The rates of evident breast mass on mammography in
the high Ki-67-expression level group were significantly higher
than those with low expression levels. The study by Porter et
al (19) also indicated that
screening mammography may miss certain rapidly proliferating,
high-grade tumors. Thus, the studies highlight that more concerns
should be taken for patients with pleomorphic calcifications or
evident breast mass on mammogram. It is likely that these
mammographic appearances are correlated with p53 or Ki-67
expression status, which are prognostic factors of great importance
(20,21).
In addition, the present study examined the
correlation between the mammographic feature of nipple retraction
and PR expression status. No specific mammographic findings were
significantly associated with ER or PR status in the study by
Gajdos et al (14). Another
study found that non-spiculated margins or hyperdense masses were
associated with a negative ER status (22). The results of the data in the
present study showed that the presentation ratio of the nipple
retraction symptom was significantly higher in PR-positive
expression patients compared with negative expression of PR.
Therefore, this finding may indicate that patients who showed
symptoms of nipple retraction on mammogram prior to surgery were
likely to exhibit PR-positive expression, which may provide
particular guidance for further endocrine treatment (23).
As for the pathological characteristics of tumors,
the results of the present study demonstrated significant
differences between the tumor size, various histological types and
grades of IDC with mammographic features, malignant or pleomorphic
calcifications, and the shape of the breast mass on the mammogram.
Among the type of evident mass on mammogram, irregular shapes of
mass were more frequently present in tumors with grade 3 IDC. By
contrast, the studies by Rotstein and Neerhut (24) and Lamb et al (25) indicated that high-grade IDCs may
paradoxically exhibit features similar to those of benign breast
masses, including a well-defined margin. Masses with non-spiculated
margins on mammography were associated with a higher histological
grade (22).
To a certain extent, it can account for the
phenomenon that tumor attributes contribute to the variety of
mammographic appearance types. However, due to the fact that the
majority of patients in the present study were IDC type, the number
of IDC accompanied by DCIS or other histological types was
relatively small. Therefore, further studies are required to
clarify this aspect. The aforementioned information on mammography
can offer accurate pre-operative evaluation for breast-conserving
surgery.
Regarding the nodal involvement, the data have
demonstrated that pleomorphic calcifications, overlying skin
thickening or dimpling on mammogram were more frequently present in
the positive lymph node status group. Awareness of this information
prior to surgery would aid clinicians in formulating the most
suitable choices of surgical treatment modality for patients,
including mastectomy or breast-conserving surgery (26). Another study revealed that the
presence of calcifications alone or masses associated with
calcifications on mammography was significantly associated with
positive extensive intraductal component, and this contraindicates
breast-conserving surgery and other mammographic features,
including irregular shape, indistinct margin, calcifications within
a mass and segmental calcifications (23).
In conclusion, it is of note that there are
significant differences between the mammographic appearances with
breast carcinoma attributes. The correlation of mammography image
features and clinical and pathological characteristics exist in
IDCs. Based on these findings, we believe that the mammography
image appearances may reflect certain biological behaviors of
tumors prior to surgery, which are useful for future evaluation and
treatment of patients.
Acknowledgements
The present study was supported by the Program for
the Applied Basic Research and Cutting-edge Technology Project of
Tianjin Science and Technology Commission to Professor Cao (grant
no. 11JCZDJC28000).
References
|
1
|
Tabar L, Yen MF, Vitak B, Chen HH, Smith
RA and Duffy SW: Mammography service screening and mortality in
breast cancer patients: 20-year follow-up before and after
introduction of screening. Lancet. 361:1405–1410. 2003.PubMed/NCBI
|
|
2
|
Tabar L, Tony Chen HH, Amy Yen MF, Tot T,
Tung TH, Chen LS, Chiu YH, Duffy SW and Smith RA: Mammographic
tumor features can predict long-term outcomes reliably in women
with 1–14-mm invasive breast carcinoma. Cancer. 101:1745–1759.
2004.PubMed/NCBI
|
|
3
|
Seo BK, Pisano ED, Kuzimak CM, Koomen M,
Pavic D, Lee Y, Cole EB and Lee J: Correlation of HER-2/neu
overexpression with mammography and age distribution in primary
breast carcinomas. Acad Radiol. 13:1211–1218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang WT, Hennessy B, Broglio K, Mills C,
Sneige N, Davis WG, Valero V, Hunt KK and Gilcrease MZ: Imaging
differences in metaplastic and invasive ductal carcinomas of the
breast. AJR Am J Roentgenol. 189:1288–1293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao J, Liu H, Wang M, Gu L, et al:
Characteristics and prognosis for molecular breast cancer subtypes
in Chinese women. J Surg Oncol. 100:89–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Breast Imaging Research Centre, American
College of Radiology and American College of Breast Imaging
Reporting and Data System. American College of Breast Imaging
Reporting and Data System. Am Coll Radiol. 1998.
|
|
7
|
Ildefonso C, Vazquez J, Guinea O, et al:
The mammographic appearance of breast carcinomas of invasive ductal
type: relationship with clinicopathological parameters, biological
features and prognosis. Eur J Obstet Gynecol Repord Biol.
136:224–231. 2008. View Article : Google Scholar
|
|
8
|
Broberg A, Glas U, Gustafsson SA,
Hellström L and Somell A: Relationship between mammographic pattern
and estrogen receptor content in breast cancer. Breast Cancer Res
Treat. 3:201–207. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paradiso A, Ventrella V, Farchi G, et al:
Mammographic aspect, cell kinetics and hormone receptor status of
operable breast cancer. Oncology. 50:104–109. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson TE, Helvie MA, Oberman HA and Joynt
LK: Pure and mixed mucinous carcinoma of the breast: pathologic
basis for differences in mammographic appearance. AJR Am J
Roentgenol. 165:285–289. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ciatto S and Zappa M: A prospective study
of the value of mammographic patterns as indicators of breast
cancer risk in a screening experience. Eur J Radiol. 17:122–125.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuda M, Yoshimoto M, Iwase T, et al:
Mammographic and clinicopathological features of mucinous carcinoma
of the breast. Breast Cancer. 7:65–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Günhan-Bilgen I and Oktay A: Tubular
carcinoma of the breast: mammographic, sonographic, clinical and
pathologic findings. Eur J Radiol. 61:158–162. 2007.PubMed/NCBI
|
|
14
|
Gajdos C, Tartter PI, Bleiweiss IJ, et al:
Mammographic appearance of nonpalpable breast cancer reflects
pathologic characteristics. Ann Surg. 235:246–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enache DE, Georgescu CV and Pătrană N:
Negative estrogen-receptor invasive breast carcinoma: mammographic
aspects, correlations with HER2/neu oncoprotein status. Rom J
Morphol Embryol. 53(3 Suppl): 755–762. 2012.PubMed/NCBI
|
|
16
|
Evans AJ, Pinder SE, Ellis IO, et al:
Correlations between the mammographic features of ductal carcinoma
in situ (DCIS) and C-erbB-2 oncogene expression. Nottingham Breast
Team. Clin Radiol. 49:559–562. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilliland FD, Joste N, Stauber PM, Hunt
WC, Rosenberg R, Redlich G and Key CR: Biologic characteristics of
interval and screen-detected breast cancers. J Natl Cancer Inst.
92:743–749. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Porter PL, El-Bastawissi AY, Mandelson MT,
Lin MG, Khalid N, Watney EA, Cousens L, White D, Taplin S and White
E: Breast tumor characteristics as predictors of mammographic
detection: comparison of interval- and screen-detected cancers. J
Natl Cancer Inst. 91:2020–2028. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sirvent JJ, Fortuño-Mar A, Olona M and
Orti A: Prognostic value of p53 protein expression and
clinicopathological factors in infiltrating ductal carcinoma of the
breast. A study of 192 patients. Histol Histopathol. 16:99–106.
2001.PubMed/NCBI
|
|
21
|
Jalava P, Kuopio T, Juntti-Patinen L,
Kotkansalo T, Kronqvist P and Collan Y: Ki67 immunohistochemistry:
a valuable marker in prognostication but with a risk of
misclassification: proliferation subgroups formed based on Ki67
immunoreactivity and standardized mitotic index. Histopathology.
48:674–682. 2006. View Article : Google Scholar
|
|
22
|
Shin HJ, Kim HH, Huh MO, Kim MJ, Yi A, Kim
H, Son BH and Ahn SH: Correlation between mammographic and
sonographic findings and prognostic factors in patients with
node-negative invasive breast cancer. Br J Radiol. 84:19–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Chia SK, Mehl E, Leung S, Rajput A,
Cheang MC and Nielsen TO: Progesterone receptor is a significant
factor associated with clinical outcomes and effect of adjuvant
tamoxifen therapy in breast cancer patients. Breast Cancer Res
Treat. 119:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rotstein AH and Neerhut PK: Ultrasound
characteristics of histologically proven grade 3 invasive ductal
breast carcinoma. Australas Radiol. 49:476–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lamb PM, Perry NM, Vinnicombe SJ and Wells
CA: Correlation between ultrasound characteristics, mammographic
findings and histological grade in patients with invasive ductal
carcinoma of the breast. Clin Radiol. 55:40–44. 2000. View Article : Google Scholar
|
|
26
|
Kollias J, Gill PG, Beamond B, et al:
Clinical and radiological predictors of complete excision in
breast-conserving surgery for primary breast cancer. Aust N Z J
Surg. 68:702–706. 1998. View Article : Google Scholar
|