Introduction
There is no curative therapy for persistent or
recurrent disease in ovarian carcinoma, despite the development of
novel chemotherapeutic drugs. Frequently, second- or third-line
therapies cannot be performed completely due to the accumulated
toxicities, emergence of drug resistance and the terminal declines
in performance status.
Radiation therapy has been previously challenged in
the treatment of epithelial ovarian tumors. Curative irradiation
strategies, including whole abdominal techniques for selected
patients with microscopic disease following debulking surgery, have
been reported (1–5). Effective palliative irradiation
following chemotherapy failure has also been reported, even in the
absence of a substantial survival advantage (6–8).
However, this treatment became almost obsolete due to the
remarkable progression of up-scaled platinum-containing systemic
chemotherapies and little or no guideline-based recommendations for
the use of irradiation for disseminated tumors. However, more
recently, revival of the irradiation has been argued and reported
in non-serous ovarian subtypes or locoregionally-recurrent ovarian
cancer (9–11).
As the chemotherapeutic drugs used in ovarian cancer
and radiation are the same DNA-damaging agent, it is natural that
the intrinsic drug-resistant cells could be resistant to radiation.
In vitro studies have also suggested the cross-resistance
between the platinum agents and irradiation through the
overexpression of the ras gene (12,13).
By contrast, the radiosensitization effect of the chemotherapeutic
drug could be expected when radiation is used concurrently or
sequentially similar to the case of cervical cancer (14,15).
The present study reviews the experience of
irradiation in patients with recurrent ovarian cancer, who have
been repeatedly treated with platinum-based chemotherapy in the
Jikei University School of Medicine (Tokyo, Japan). The
radiation-effect availability was questioned and the factors
impacting the efficacy were examined.
Patients and methods
Patients
The study is a retrospective analysis of 71 patients
evaluated for the relapse of epithelial ovarian cancer between 1997
and 2006. The patients were referred to the Department of
Obstetrics and Gynecology by one of the four affiliated hospitals
of the Jikei University School of Medicine when recurrent-focal
lesions developed following second- or third-line treatment. The
initial management included exploratory laparotomy, hysterectomy,
salpingo-oophorectomy and mostly platinum-containing chemotherapy
regimens. Nodal dissection was not routine.
Eligibility was identified by a stepwise process.
Metastatic tumors, other than ovarian cancer, and tumors of
non-epithelial histology or borderline tumors were excluded. The
patients were selected to receive irradiation on a case-by-case
basis following a discussion among gynecological and radiological
oncologists. The factors that were considered in deciding whether
to offer irradiation included the ability to encompass the
locoregional disease by the irradiation field, performance status,
no ascites and limitations of the other treatment options.
Irradiation was largely abandoned in the group of
hospitals after 2004, following the announcement of the consensus
statements on the management of ovarian cancer in the 3rd
International Gynecologic Cancer Intergroup Ovarian Cancer
Consensus Conference (16) and
when it was recognized that the combination of platinum and taxane
was a highly-active systemic agent. Due to its current infrequent
use, the clinical information was acquired from medical records
over a decade ago, and certain data were missing due to the paucity
of each record. In the study, the focus was on the association
between the irradiation efficacy and chemotherapy response, and the
treatment break following the completion of chemotherapy up to the
initiation of radiation therapy. Therefore, 10 out of 71 patients
with brain metastasis were excluded as the blood-brain barrier
disabled the evaluation of the chemotherapeutic effect. The study
was approved by the Institutional Review Board in Jikei University
School of Medicine and conformed to the policies and practices for
human subject research.
Chemotherapy
The clinical response to the initial chemotherapy
was assessed in 55 patients with a measurable disease based on the
classical WHO criteria (17). A
complete response (CR) was defined as the disappearance of all the
clinical evidence of the malignant disease, and a partial or minor
response (PR or MR) was a >50 or 25% decrease in the size of the
clinically measurable disease, respectively. In the study, a
positive response was designated as CR, PR or NC.
Irradiation
For the prognostic-significance test of the
radiation dose, the dose fractionation schemes were converted to a
biologically-effective dose (BED) using the following formula: BED
= total dose [1 + fractional dose/(α/β)], α/β = 10 Gy.
The patients were followed up jointly by
gynecological and radiation oncologists. The response data were
extracted from all the records of these two oncological
disciplines. Due to the paucity of data, the exact response rate
was not calculated in the present retrospective study. The
responders were designated as the patients who showed ≥ no change
(NC) of documented recurrent tumor determined by computerized axial
tomography, magnetic resonance imaging or ultrasonography
approximately within 1 month after completion of irradiation. As
the radiation effect was often apparent 3–6 months after (18), the response criteria in the study
included the cases of NC. The toxicity by irradiation was not
assessed since the detailed clinical records were not
available.
Chemotherapy-irradiation break
Theoretically, chemotherapy and radiotherapy could
have a synergistic effect and the chemotherapy may function as a
radiosensitizer, similarly to the case of concurrent chemotherapy
in cervical cancer (14). By
contrast, the cancer cells surviving the previous
platinum-containing chemotherapy could acquire and maintain the
platinum resistance within 6 months after chemotherapy according to
the Gynecologic Oncology Group (GOG) criteria and the platinum
resistance may be cross-resistant to ionizing radiation as well. On
the bases of this cellular biology, the patients were categorized
into three groups by the treatment breaks prior to the initiation
of irradiation: Category I, ≤1 month; II, 1–6 months; and III,
>6 months.
Data were collected for age, histological type,
recurrent site, prior chemotherapeutic treatments, total dose, BED,
the treatment break between the last chemotherapy and irradiation,
response to irradiation as well as previous chemotherapy and
post-irradiation survival time.
Statistical analysis
The overall survival time was measured from the date
of the initiation of irradiation. The duration of the survival time
was measured up to the date of mortality or the date of the last
contact if the patient remained. A total of 46 eligible cases were
included in the survival-time analysis unless otherwise specified.
All the causes of mortality were used to calculate the survival
time, and the estimates of the cumulative proportion surviving were
based on Kaplan-Meier procedures (19). For the post-irradiation survival
time, the Cox proportional-hazards regression model was used to
estimate the treatment relative hazards (20). Pearson’s χ2 test was
used to test the independence of the response and treatment
(21). Data were analyzed using
Stata software (StataCorp, College Station, TX, USA). The primary
outcome was considered to indicate a statistically significant
difference when P<0.05.
Results
The demographics and disease characteristics of the
patients are summarized in Table
I. The total number of each factor was not exactly 61 due to
certain data being missing. Prior to irradiation, the patients
received a median of 12 chemotherapy courses (range, 5–14), and the
majority were composed of the platinum-taxane combination. The
median BED was 60.0 Gy (range, 15.6–72.0 Gy). The sites irradiated
included nodal recurrence (36 cases), abdominal (six cases), and
pelvic cavity (five cases). The majority of patients did not
complain of tumor-associated symptoms, however, the most common
symptoms of pain and vaginal bleeding were caused by disease in the
abdominal (six cases) or pelvic cavity (five cases).
Histologically, serous adenocarcinoma was the most common type of
the disease (23 cases, 38%) compared to mucinous (four cases, 7%),
endometrioid (three cases, 5%), and clear-cell types (six cases,
10%).
 | Table IPatient profiles. |
Table I
Patient profiles.
| n (%) | Median (min-max) |
|---|
| Age, year | 58 | 54.0 (26.0–71.0) |
| Total dose, Gy | 58 | 47.7 (12.5–60.0) |
| BEDa, Gy | 55 | 60.0 (15.6–72.0) |
| Survivalb, Mo | 50 | 11.5 (0.5–82.4) |
| Intervalc, Mo | 53 | 3.2 (0.0–63.8) |
| Site | 61 | |
| Lymph node
(Virchow) | 19 (31.1) | |
| Lymph node | 17 (27.9) | |
| Abdomen | 6 (9.8) | |
| Pelvis | 5 (8.2) | |
| Mediastinum | 4 (6.6) | |
| Lung | 3 (4.9) | |
| Others | 4 (6.6) | |
| Unclear | 3 (4.9) | |
| Histology | 61 | |
| Serous | 23 (37.7) | |
| Mucinous | 4 (6.6) | |
| Endometrioid | 3 (4.9) | |
| Clear cell | 6 (9.8) | |
| Poorly
differentiated | 3 (4.9) | |
| Adenocarcinoma | 10 (16.4) | |
| Othersd | 4 (6.6) | |
| Unclear | 8 (13.1) | |
| Chemotherapy | 61 | |
| Platinum | 57 (93.4) | |
| Taxane | 42 (68.9) | |
| Irinotecan | 5 (8.2) | |
| Cytoxan | 5 (8.2) | |
| Doxorubicin | 18 (28.5) | |
Post-irradiation survival time
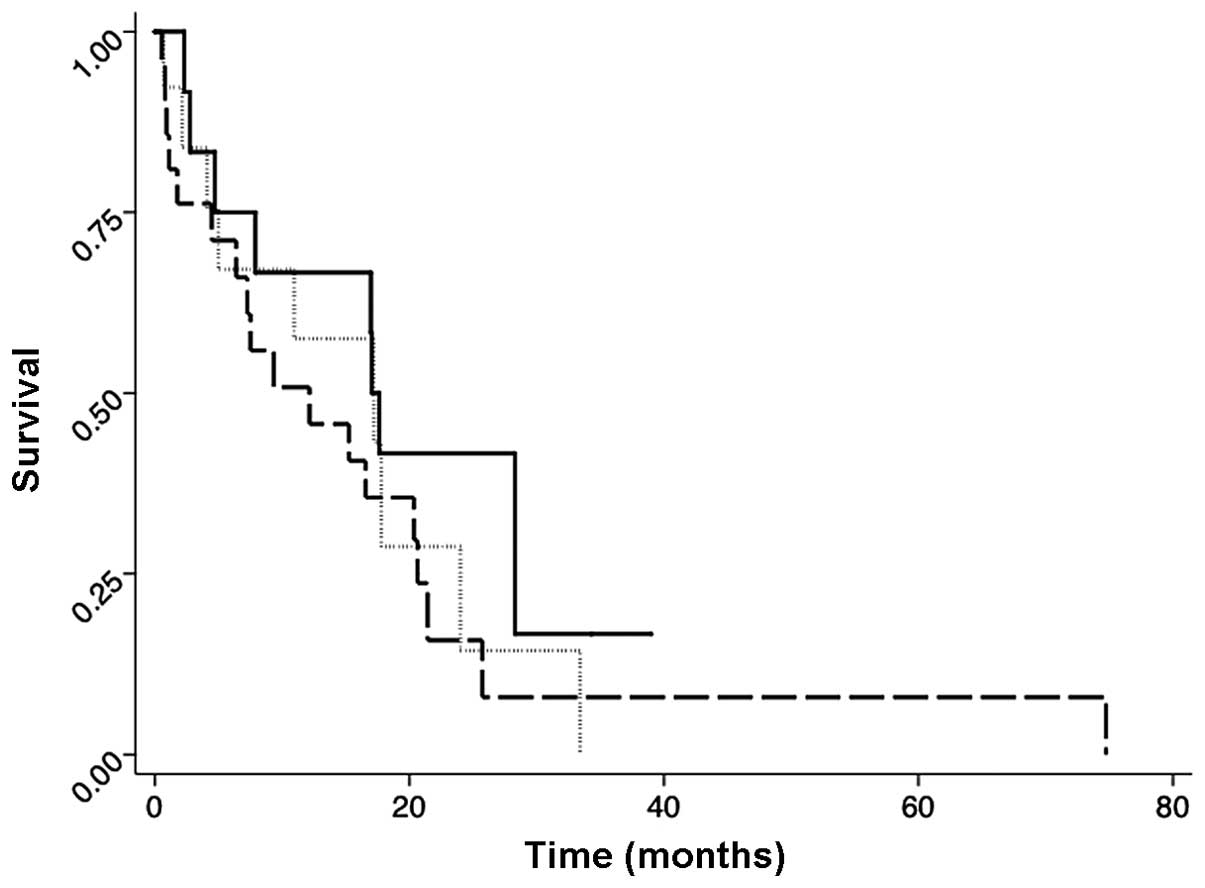
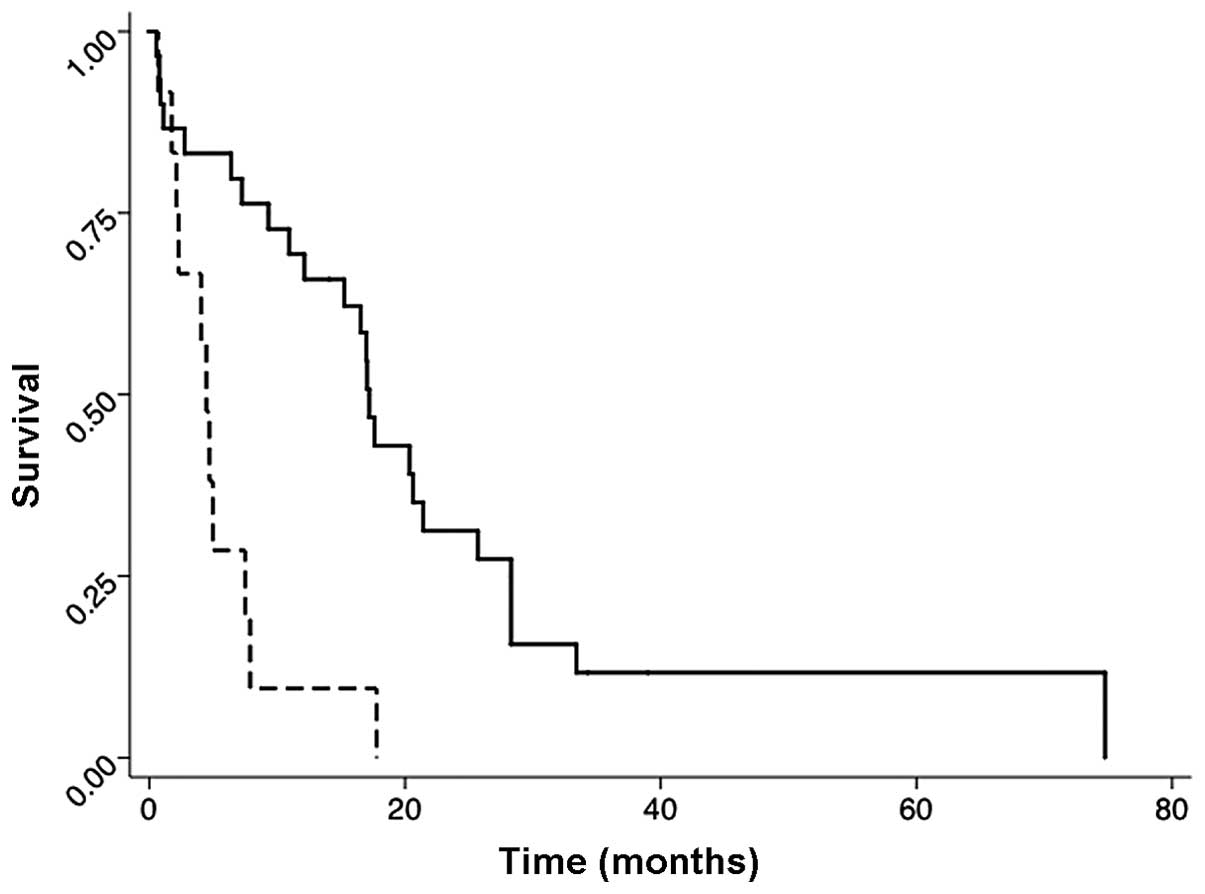
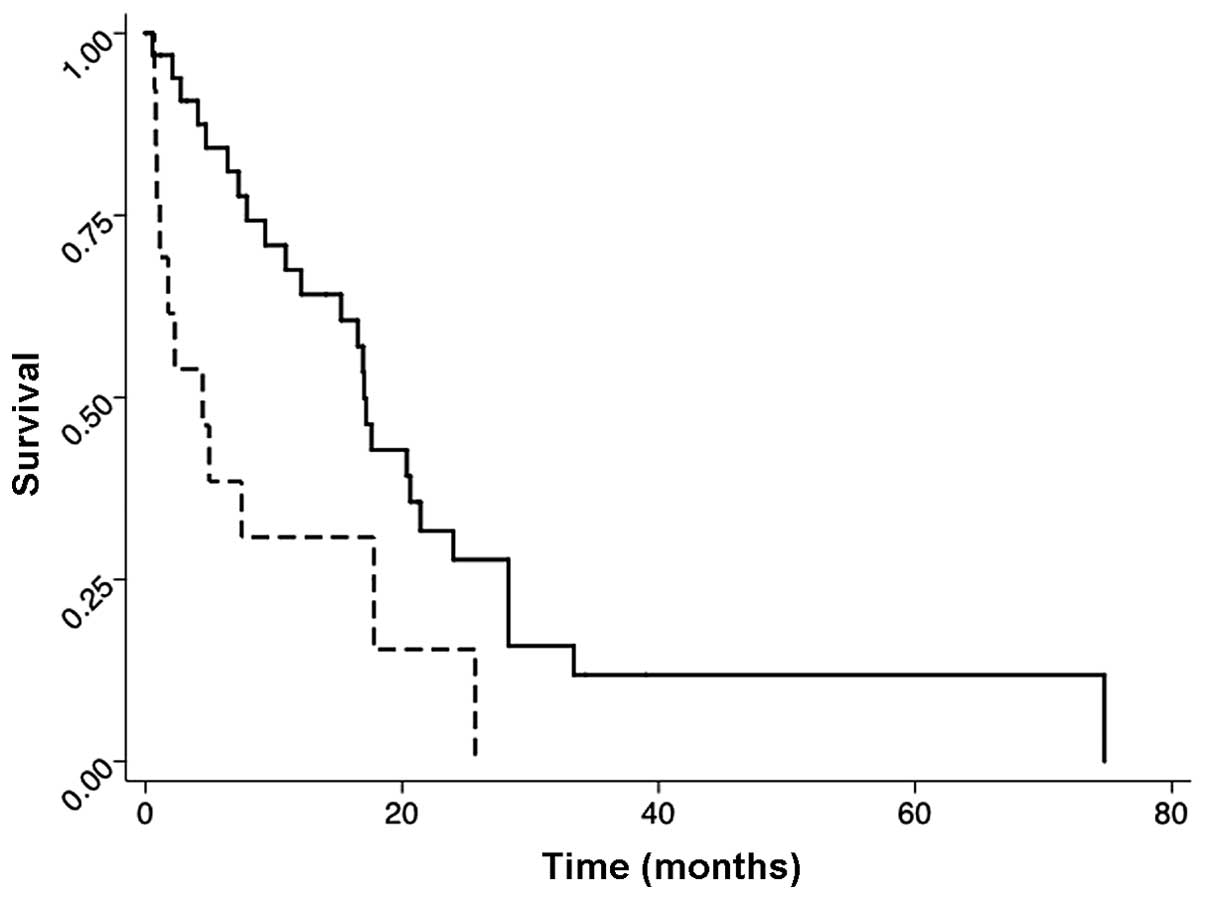
Figs. 1–3 show the post-irradiation survival
curves for all the eligible females by the category, radiation
response and drug sensitivity. The median duration of follow-up for
females who remained at the last contact point was 16 months
(range, 1–82 months). There was no statistically significant
difference among the treatment categories (Fig. 1). However, the patients whose
disease responded to the radiation therapy had a higher
post-irradiation survival rate than patients with
radiation-non-responded disease. The median survival time in the
responded group was 16 months, and in the non-responded group it
was 2 months [hazard ratio (HR), 0.39; P=0.013; 95% confidence
interval (CI), 0.19–0.82] (Fig.
2). Similarly, patients whose disease responded to the initial
chemotherapy had a higher survival rate than patients without a
response. The median survival time in the chemo-responded group was
17 months, and in the non-responded group it was 4 months (HR,
0.23; P=0.001; 95% CI, 0.10–0.54) (Fig. 3).
Clinicopathological variables
Table II shows the
variables that were associated with the radiation effect. The
histotype of serous adenocarcinoma was not associated with the
radiation responsibility compared to the cluster of other
histotypes. Re-clustering clear cell, mucinous and endometrioid
into one group compared to serous or other types did not alter the
result. However, when the radiation-sensitivity of serous type (23
cases) was compared to the cluster of clear cell, endometrioid and
mucinous types (13 cases), serous type was more sensitive (P=0.033,
data not shown).
 | Table IIAssociation of the clinicopathological
variables to the radiation responsibility. |
Table II
Association of the clinicopathological
variables to the radiation responsibility.
| | Response | |
|---|
| |
| |
|---|
| Variable (n) | Category | −, n | +, n | % | Pearson’s
χ2 |
|---|
| Histology (61) | Serous | 3 | 19 | 86.4 | |
| Non-serous | 12 | 27 | 69.2 | 0.136 |
| Treatment break
(53) |
| Categorya |
| I vs. II | I | 1 | 14 | 93.3 | 0.026 |
| II vs. III | II | 9 | 14 | 60.9 | 0.357 |
| I vs. III | III | 4 | 11 | 75.0 | 0.165 |
| Site (61) | Lymph node | 6 | 30 | 83.3 | |
| Other | 9 | 16 | 64.0 | 0.085 |
| BEDb, Gy (58) | ≤60 | 8 | 20 | 71.4 | |
| >60 | 7 | 23 | 76.7 | 0.649 |
| Chemo response
(54) | − | 7 | 6 | 46.2 | |
| + | 6 | 35 | 85.4 | 0.007 |
Treatment break
A treatment break of ≤1 month (category I) is an
important factor associated with the positive-radiation response
compared to category II (93 vs. 61%; P=0.026). The radiation
response in category III (>6 months) appeared to be improved
compared to category II (1–6 months) (75 vs. 61%; P=0.357), however
this is not significant.
The comparison of the reported site of relapse for
irradiation and the amount of BED did not show any associations in
the rate of the radiation response. The patients whose disease
responded to the initial chemotherapy had a higher rate of
radiation responsibility compared to patients with chemotherapy
non-responded disease (85 vs. 46%; P=0.007).
Discussion
The results of the present study provide strong
evidence that irradiation can play an important role in curative
management of locoregionally-confined recurrent or persistent
ovarian cancer. The foundation of the data is the improved
post-irradiation survival rate in the patients with
radiation-responsive recurrent disease compared to those with
non-responsive disease, and the clear association between the
radiation effect with the treatment break or
chemo-responsibility.
Several points should be discussed with regards to
the study. First, the radiation-response criteria designated in the
study was not so strict that numerous cases were categorized as the
radiation-responsive group. This is due to the physicians being
unfamiliar with the response criteria in the solid tumor at the
time and due to various missing imaging data that did not allow for
the second chance of re-evaluation. Similarly, the
positive-chemotherapy response criteria in the study covered a
broad range of response, including NC, resulting in a large number
of responsive cases with the same reason as the
radiation-responsibility criteria. The present study demonstrated
that the chemo-responsibility associated well with the outcome of
the chemotherapy-responded patients having a longer
post-irradiation survival time than non-responded patients
(Fig. 3), and it was also well
associated with the radiation-responsibility (Table II). Similar findings were also
reported in two other studies (11,22),
showing that the platinum-sensitivity associated with the
prognostic benefit in locoregionally-recurrent ovarian cancer. In
the present study, 93% of the patients received platinum-containing
chemotherapy, indicating that chemo-sensitivity in the study could
be translated into platinum-sensitivity.
With respect to the chemo- or radiation-sensitivity,
the second important issue was the histological type-associated
radiation responsibility. The data from the present study and two
other studies (11,23) that contained a large number of
serous carcinoma, showed an apparent prognostic or symptomatic
benefit from irradiation. However, Swenerton et al (9) reported that the use of adjuvant
radiation following chemotherapy in microscopic disease showed an
incremental survival benefit for the non-serous carcinoma,
including clear cell, endometrioid and mucinous adenocarcinomas,
and not for the serous type (9,10).
In the present study, the irradiation was used for recurrent,
macroscopic and locoregional diseases, and radiation was used as
field-involved irradiation with a median dose of 48 Gy. However,
Swenerton et al (9) used
radiation for minimal, and usually disseminated, residual disease
as adjuvant whole abdominal irradiation, with 23–28 Gy. As the
present data contained a large number of unclassified or unclear
histotypes, the study is not adequate in showing the
histotype-specific radiation-sensitivity, however, the
aforementioned inequality of the targeting tumor, as well as the
total dose, makes it hard to conclude the histotype-specific
radiation sensitivity.
Finally, the treatment break associated with the
radiation-response should be noted. The present data showed that
irradiation after ≤1 month break (category I) was well associated
with the radiation-response compared to 1–6 months (category II),
despite the lack of a survival benefit. In category I, when the
tumor cells were not intrinsically resistant and therefore the
tumor responded to primary chemotherapy, the preceding chemotherapy
could have the potential to augment the effects of radiation,
similar to the case of concurrent chemoradiation with cisplatin in
cervical cancer (14). A similar
effect was also noted when the radiation was used sequentially
following paclitaxel plus carboplatin (15). In the study, sequential radiation
was initiated 2–3 weeks after the completion of the last
chemotherapy cycle, but the interval was allowed to be extended and
this is exactly the same as category I in the present study. The
present study demonstrated a poorer locoregional control when
irradiation was delayed to 1–6 months after the completion of
chemotherapy, but the response appeared to be recovered slightly
when the time break was extended to >6 months. This could be
attributable to the possible acquired cross-resistance to
chemotherapy and radiation therapy for the patients with a
treatment break of 1–6 months, which is consistent with the
treatment-free interval of absolute platinum resistant in the GOG
criteria.
The present study has several limitations, including
a comprising heterogeneous study population, lack of toxicity data
and tumor size. However, the study suggests that irradiation could
yield a survival benefit when the treatment is applied to
chemosensitive, locoregionally-recurrent ovarian cancer and when
the treatment is initiated ≤1 month after the preceding
chemotherapy.
Acknowledgements
The authors would like to thank Mr. G. Rupelle,
lecturer of Jikei Kashiwa Hospital (Tokyo, Japan), for the critical
review of the present study.
References
|
1
|
Dembo AJ: Radiotherapeutic management of
ovarian cancer. Semin Oncol. 11:238–250. 1984.PubMed/NCBI
|
|
2
|
Einhorn N, Tropé C, Ridderheim M, Boman K,
Sorbe B and Cavallin-Ståhl E: A systematic overview of radiation
therapy effects in ovarian cancer. Acta Oncol. 42:562–566. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomas GM and Dembo AJ: Integrating
radiation therapy into the management of ovarian cancer. Cancer.
71(Suppl 4): 1710–1718. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez A, Schray MF, Howes AE and
Bagshaw MA: Postoperative radiation therapy for epithelial ovarian
cancer: the curative role based on a 24-year experience. J Clin
Oncol. 3:901–911. 1985.PubMed/NCBI
|
|
5
|
Cardenes H and Randall ME: Integrating
radiation therapy in the curative management of ovarian cancer:
current issues and future directions. Semin Radiat Oncol. 10:61–70.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albuquerque KV, Singla R, Potkul RK, et
al: Impact of tumor volume-directed involved field radiation
therapy integrated in the management of recurrent ovarian cancer.
Gynecol Oncol. 96:701–704. 2005.
|
|
7
|
May LF, Belinson JL and Roland TA:
Palliative benefit of radiation therapy in advanced ovarian cancer.
Gynecol Oncol. 37:408–411. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujiwara K, Suzuki S, Yoden E, Ishikawa H,
Imajo Y and Kohno I: Local radiation therapy for localized relapsed
or refractory ovarian cancer patients with or without symptoms
after chemotherapy. Int J Gynecol Cancer. 12:250–256. 2002.
View Article : Google Scholar
|
|
9
|
Swenerton KD, Santos JL, Gilks CB, et al:
Histotype predicts the curative potential of radiotherapy: the
example of ovarian cancers. Ann Oncol. 22:341–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas G: Revisiting the role of radiation
treatment for non-serous subtypes of epithelial ovarian cancer. In:
American Society of Clinical Oncology Educational Book/ASCO.
American Society of Clinical Oncology Meeting; pp. e205Toronto.
2013, PubMed/NCBI
|
|
11
|
Brown AP, Jhingran J, Klopp AH, Schmeler
KM, Ramirez PT and Eifel PJ: Involved-field radiation therapy for
locoregionally recurrent ovarian cancer. Gynecol Oncol.
130:300–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sklar MD: Increased resistance to
cis-diamminedichloroplatinum(II) in NIH 3T3 cells transformed by
ras oncogenes. Cancer Res. 48:793–797. 1988.PubMed/NCBI
|
|
13
|
Sklar MD: The ras oncogenes increase the
intrinsic resistance of NIH 3T3 cells to ionizing radiation.
Science. 239:645–647. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rose PG, Bundy BN, Watkins EB, et al:
Concurrent cisplatin-based radiotherapy and chemotherapy for
locally advanced cervical cancer. N Engl J Med. 340:1144–1153.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sehouli J, Runnebaum IB, Fotopoulou C, et
al: A randomized phase III adjuvant study in high-risk cervical
cancer: simultaneous radiochemotherapy with cisplatin (S-RC) versus
systemic paclitaxel and carboplatin followed by percutaneous
radiation (PC-R): a NOGGO-AGO Intergroup Study. Ann Oncol.
23:2259–2264. 2012. View Article : Google Scholar
|
|
16
|
Quinn M, Avall-Lundqvist E, du Bois A, et
al: History, scope and methodology of the 3rd international
consensus workshop on ovarian cancer 2004. Ann Oncol. 16(Suppl 8):
viii5–viii6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
WHO Handbook for Reporting Results of
Cancer Treatment. WHO Offset Publication no. 48. World Health
Organization; Geneva: 1979
|
|
18
|
Mayr NA, Taoka T, Yuh WT, Denning LM, Zhen
WK, Paulino AC, et al: Method and timing of tumor volume
measurement for outcome prediction in cervical cancer using
magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 52:14–22.
2002.PubMed/NCBI
|
|
19
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
20
|
Cox DR: Regression models and life-tables
(with discussion). J Royal Stat Soc, Series B. 34:187–220.
1972.
|
|
21
|
Agresti A: Categorical Data Analysis. John
Wiley & Sons; New York, NY: 1990
|
|
22
|
Lee SW, Park SM, Kim YM, Kim YS, Choi EK,
Kim DY, et al: Radiation therapy is a treatment to be considered
for recurrent epithelial ovarian cancer after chemotherapy. Tumori.
97:590–595. 2011.PubMed/NCBI
|
|
23
|
Choan E, Quon M, Gallant V and Samant R:
Effective palliative radiotherapy for symptomatic recurrent or
residual ovarian cancer. Gynecol Oncol. 102:204–209. 2006.
View Article : Google Scholar : PubMed/NCBI
|