Introduction
Nasopharyngeal carcinoma (NPC) occurs commonly in
the Asian population, particularly in Southern and Southeast China
(1). Due to the peculiar
characteristics in its epidemiology, pathology, clinical behavior
and response to treatment, NPC is different from other head and
neck squamous cell cancer and has a relatively high overall
survival rate with the integration of chemotherapy into
radiotherapy (2,3). However, the majority of NPC patients
present with a late stage disease accompanying neck nodal
metastases when diagnosed, and the cure rate for those advanced
NPCs remains unsatisfactory (4).
In addition, numerous NPC survivors are often affected by moderate
to severe late complications, resulting from the impact of
radiation on the organs that are adjacent to the nasopharynx and
neck nodes, and chemotherapy in advanced cases further exacerbates
these side-effects (5). Therefore,
exploring novel therapeutic regimens and improvements in disease
monitoring is required. If the therapeutic effect can be predicted
prior to or at the early course of the treatment, it is possible to
modify the therapeutic strategies for the remaining treatment.
Novel therapeutic alternatives, such as anti-epidermal growth
factor receptor monoclonal antibodies, including Cetuximab and
Nimotuzumab, could be advised as a combination for patients likely
to be resistant to conventional treatment.
Functional images, mainly including dynamic
contrast-enhanced-computed tomography (DCE-CT), positron emission
tomography (PET-CT), magnetic resonance spectrometry,
diffusion-weighted images (DWI) and dynamic
contrast-enhanced-magnetic resonance imaging (DCE-MRI), play an
important role in assessing the treatment effect on the solid
tumor. Considering the apparent diffusion coefficient value of DWI
and the perfusion parameter, Ktrans, of DCE-MRI as
examples, the aforementioned parameters may allow the possibility
to predict an early treatment response and prognosis for
chemotherapy and radiotherapy (6,7). The
aforementioned image modalities are mainly dependent on contrast
agents that may cause an allergic reaction. DCE-CT and PET use
ionizing radiation, which has known risks. Contrast-enhanced
ultrasound (CEUS) is another type of functional image. The medium
of CEUS is SonoVue, which contains micrometer-sized (1–10 μm in
diameter) bubbles of sulfur hexafluoride with a stabilizing shell.
The introduction of exogenous microbubbles into the vasculature
causes enhancement of the backscattered intensity of the blood and
can be used to assess tissue blood flow. Relevant analysis software
has been developed to analyze ultrasound signal intensity (SI)
patterns obtained by imaging continuously prior or subsequent to
treatment, and information regarding tissue blood flow and vascular
integrity, branching patterns and density will be assessed
(8). As reported previously, the
information extracted from the CEUS data was similar to that
obtained from DCE-MRI (9), and
CEUS has numerous benefits, including a sufficient high safety
profile that is acceptable for patients with renal failure or an
iodine allergy, absence of radiation, easy reproducibility and high
temporal resolution (10–13).
Thus, the present study was performed to evaluate
the potential utility of CEUS-derived parameters from the
metastatic cervical lymph nodes, prior or subsequent to the early
course of the treatment, in predicting the nodal treatment response
to radiation-based therapy in patients with NPC.
Patients and methods
Patient selection
Sixty-seven NPC with cervical lymph node metastases
patients who were treated at The Second Affiliated Hospital
(Zhejiang University School of Medicine, Hangzhou, China) between
December 2011 and February 2013, were enrolled in the study. The
study was approved by the Institutional review board, and written
informed consent was obtained from each participant prior to the
CEUS examination. All the subjects qualified for the following
criteria: i) NPC with metastatic lymph nodes proven by pathology;
ii) Eastern Cooperative Oncology Group performance status score,
≤2; iii) adequate organ function; and iv) no concomitant
malignancy. The stage of disease was classified according to the
7th edition of the Union for International Cancer Control (UICC)
and the American Joint Committee on Cancer (AJCC) staging system
(14). All the clinical
characteristics of the patient are listed in Table I.
 | Table IClinical data according to the
therapeutic response for nasopharyngeal carcinoma patients with
lymph node metastases, n=67. |
Table I
Clinical data according to the
therapeutic response for nasopharyngeal carcinoma patients with
lymph node metastases, n=67.
| Therapeutic
response | |
|---|
|
| |
|---|
| Characteristics | CR | PR | P-value |
|---|
| Patients, n | 48 | 19 | |
| Age, years | 55.83±10.38 | 56.08±10.94 | 0.912 |
| Gender, n | | | 0.782 |
| Male | 26 | 11 | |
| Female | 22 | 8 | |
| PS, n | | | 0.492 |
| 0 | 39 | 14 | |
| 1 | 9 | 5 | |
| Treatment, n | | | 0.023 |
| RT | 6 | 7 | |
| RT+CT/T | 42 | 12 | |
| Differentiation,
n | | | 0.310 |
| Well/moderate | 4 | 2 | |
| Poor | 44 | 17 | |
| T stage, n | | | 0.686 |
| T1 | 5 | 3 | |
| T2 | 28 | 9 | |
| T3 | 9 | 6 | |
| T4 | 6 | 1 | |
| N stage, n | | | 0.021 |
| N1 | 27 | 4 | |
| N2 | 19 | 12 | |
| N3 | 2 | 3 | |
| Lymph node | 3.24±0.92 | 3.83±0.93 | 0.005 |
| Max. D, cm | | | |
Treatment protocol
The first CEUS examinations were performed prior to
any treatment, and the second CEUS examinations were arranged at
the 5th fraction of radiotherapy (using the CEUS methodology). All
the 67 patients underwent intensity-modulated radiotherapy (IMRT)
and among them, 52 patients received platinum-based concomitant
chemotherapy and 10 received weekly Nimotuzumab-targeted therapy at
a dose of 200 mg 1 week before and during the course of
radiotherapy and the other 5 cases received only IMRT. Nimotuzumab,
a monoclonal antibody against epidermal growth factor receptor, has
been officially approved by the State Food and Drug Administration
of China to treat advanced NPC (15). The target volume contouring was
made following the guideline of the Radiation Therapy Oncology
Group Contouring Atlas (http://www.rtog.org/CoreLab/ContouringAtlases/HNAtlases.aspx).
The planning target volume of the metastatic lymph nodes was
defined as PCTVnd. IMRT was performed using 6-MV photon
beams and the IMRT plan was normalized such that 95% of the
PCTVnd was covered with the prescription dose [60–70
Gy/30-32 fractions], and all the patients underwent IMRT once daily
and 5 fractions a week.
CEUS methodology
The first section focused exclusively on the
pre-treatment CEUS examination. All the ultrasound investigations
were performed using the Sequoia 512 Acuson sonographic system
(Siemens Healthcare, Erlangen, German) equipped with
CadenceTM contrast pulse-sequencing visualization
technology and a high-resolution broadband ultrasound transducer
(8L5; 5–8 MHz). Each dose of the intravenous contrast medium of
microbubbles (SonoVue; Bracco, Milan, Italy) was dissolved in 5 ml
of saline and a 2.4 ml bolus was injected into the superficial
elbow vein of the patient at the rate of 1 ml/sec, followed by a
5.0 ml saline flush (16). The
process of CEUS was performed by the same ultrasound investigator
with >5 years of experience in CEUS. The process of CEUS should
include the following: i) Scan parameters (depth, focus, pulse
repletion frequency, mechanical index and depth-gain compensation)
were optimized for a clear, artifact-free depiction; ii) the probe
was manually stabilized at the largest diameter of the target lymph
node; and iii) the duration of the video was ~90 sec for analysis
(17). The video was stored as a
digital archive (Audio Video Interleave) in the hard disc and was
transferred to a personal computer for off-line parametric
analysis.
The second section was in-treatment CEUS
examination, and each of the 67 patients underwent the
aforementioned CEUS examination at the 5th fraction radiotherapy
once again. To assure agreement of the lymph nodes examination with
the first examination, the ultrasound investigator reviewed the
previous CEUS video and subsequently performed the second
examination. All the patients underwent the CEUS examinations
twice; the former digital archive was the baseline as a control,
whereas the later digital archive was under the treatment as a
comparison.
Off-line parametric CEUS analysis
The aforementioned digital archives were processed
with the use of contrast-enhanced computer-assisted perfusion
analysis of the metastatic nodes using the QontrastTM
analysis software (Qontrast 4.0; Bracco SpA, Milan, Italy), which
is a post-processing computational tool and can be used to obtain
objective and quantitative parameters of the microvessels in
various organs, including the lymph node (6). A region of interest (ROI)
encompassing the whole area of the lymph node was manually drawn,
and subsequently the software automatically processed and a
time-intensity curve and parametric graphs were produced. The
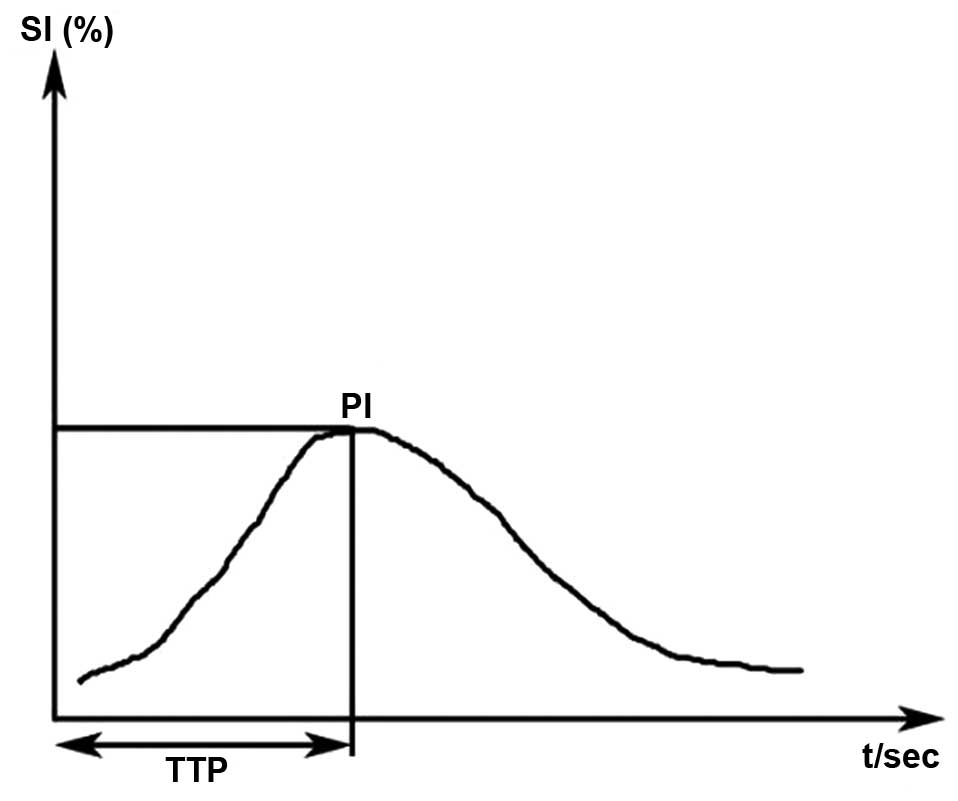
following parameters were automatically generated (Fig. 1): i) Peak intensity [PI; including
pre-treatment (PIpre) and in-treatment (PIin)
as percentages] defined as the increase in signal intensity (SI)
from baseline SI to the maximal SI measured in the selected ROI;
and ii) time to peak (TTP; including TTPpre and
TTPin, in sec) defined as the time period from the onset
of the lymph node enhancement to the moment the maximal SI is
reached. The aforementioned analyses were all performed by the same
investigator who was well experienced in the Qontrast software, and
was blinded to the clinical data. To maintain intra-investigator
agreement, >3 repeats of the aforementioned analysis were
carried out by the same investigator. Concordant measurements were
those that differed by no more than ±1 sec. Subsequently,
PIΔ and PIratio were calculated by the
algorithm that represented the change of the contrast agent
perfusion via treatment: PIΔ =
PIpre-PIin and PIratio =
PIin/PIpre.
Notably, the Qontrast software can compensate for
minor changes in the imaging plane (such as from extremely shallow
breathing). In the case of more pronounced changes in the imaging
plane, frame-by-frame editing can be performed, and the respective
frames can be manually selected and characterized as ‘wrong’
(18). By contrast,
Qontrast-assisted CEUS parameters exhibited high inter-investigator
reproducibility (11).
Evaluation of the therapeutic
response
CE-MRI was recommended as the tool for evaluating
the treatment effect of the metastatic cervical lymph nodes. Each
patient underwent the CE-MRI examination twice, the former arranged
prior to any treatment and the latter scheduled 1 month after the
completion of all the radiation fractions. MR images were
interpreted by the same experienced radiation oncologist with
>10 years of clinical experience in NPC, who was informed of the
target lymph nodes in advance. The Response Evaluation Criteria in
Solid Tumors (RECIST) 1.1 criteria was referred to for assessment
of the therapeutic response (19).
Statistical analysis
All the statistical analyses were carried out using
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). The descriptive
statistics were produced for the continuous variables and the
results are presented as mean ± standard deviation (SD). The
χ2 or Fisher’s exact tests were used to determine the
significance of the associations between the therapeutic effect and
categorical variables, whereas the correlation between the
continuous variables and the therapeutic effect was assessed by the
analysis of variance. The statistical significance of the changes
in PI and TTP was evaluated with the paired t-test. All the tests
were two-sided and P<0.05 was considered to indicate a
statistical significance difference. The Spearman’s correlation
coefficient between the situation of the lymph nodes and changes in
the perfusion parameters was calculated. The correlations were
interpreted according to Cohen’s standard, in which absolute
correlations of <0.3 were considered weak, 0.3–0.5 were moderate
and 0.5–1.0 were strong. A receiver-operating characteristic (ROC)
curve was constructed to assess the accuracy of the parameters for
the prediction of the therapeutic responses. The ROC was
constructed to illustrate the predicted probability of the
parameters.
Results
Clinical data and treatment response
Of the 67 patients, there were 37 males and 30
females, with a mean age of 55.93±10.55 years (range, 24–88 years).
The baseline patient and tumor characteristics are summarized in
Table I. All the patients
completed the entire radiation-therapy plan. The response
assessment at the lymph nodes revealed a complete response (CR) in
48 patients and partial response (PR) in 19 patients. No patients
showed stable or progressive disease at the lymph nodes or at the
primary site. Age, gender, performance status score, T stage or
pathological differentiation status were not associated with the
treatment response (P>0.05). However, N stage, the size of the
lymph node and the treatment modalities were significantly
different between the CR and PR groups, respectively (P<0.05).
Notably, radiation therapy in combination with
chemotherapy/targeted therapy was superior to radiotherapy alone,
in regards to therapeutic response (Table I).
Data of CEUS parameters and therapeutic
response
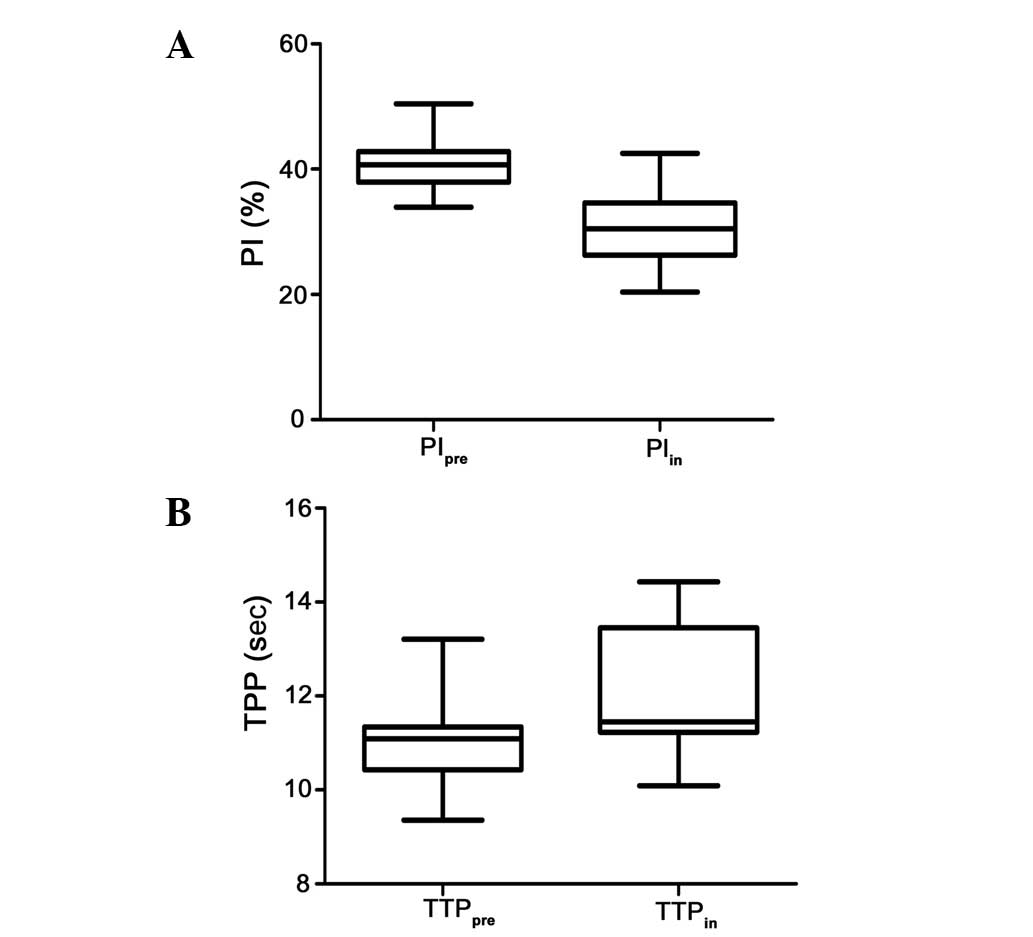
For all the 67 cases investigated, PIpre
ranged from 33.9–50.4% (40.9±3.4%), and the PI following 5
fractions of radiation (PIin) ranged from 20.4–42.5%
(30.7±5.3%). There was a significant difference in PIpre
between the patients who showed a CR or PR of the metastatic lymph
nodes, and the mean values of PIpre were higher in
patients with CR nodes compared to the patients with PR nodes
(41.90±3.62 vs. 39.39±2.48%; P=0.002). Following 5 fractions of
radiation, a decrement in PI was observed in all the patients
without any exception (Fig. 2A).
The mean PIin value of the lymph nodes that achieved CR
was 34.24±3.78%, which was significantly higher compared to the
PIin value for the PR, 25.62±2.30% (P<0.001). To
further standardize the data, a PI-quotient known as
PIratio, was calculated by dividing the PIin
by the corresponding PIpre for the same lymph node. A
higher PIratio was also observed in the CR lymph nodes
(0.81±0.01 vs. 0.66±0.01; P=0.001). As shown in Table II, the mean change in PI
(PIΔ; PIΔ = PIpre-PIin)
was smaller in the patients with CR nodes compared to the patients
with PR nodes (7.79±3.28 vs. 13.77±1.90%; P<0.001).
 | Table IICEUS parameters with different
therapeutic responses for lymph node metastases of nasopharyngeal
carcinoma patients, n=67. |
Table II
CEUS parameters with different
therapeutic responses for lymph node metastases of nasopharyngeal
carcinoma patients, n=67.
| Parameters | CR (n=48) | PR (n=19) | P-value |
|---|
| PIpre,
% | 41.90±3.62 | 39.39±2.48 | 0.002 |
| PIin,
% | 34.24±3.78 | 25.62±2.30 | 0.001 |
| TTPpre,
sec | 10.92±0.26 | 11.07±0.61 | 0.334 |
| TTPin,
sec | 12.42±1.49 | 12.13±1.40 | 0.356 |
| PIΔ,
% | 7.79±3.28 | 13.77±1.90 | 0.001 |
|
PIratio | 0.81±0.01 | 0.66±0.01 | 0.001 |
In the present study, TTPpre ranged from
9.36 to 13.21 sec (10.98±0.69 sec), and TTPin ranged
from 10.09 to 14.43 sec (12.30±1.46 sec), showing that the value of
TTP had a tendency to increase following treatment and the change
in TTP was statistically significant (P<0.05) (Fig. 2B). However, the mean
TTPpre was 10.92±0.26 sec in the CR nodes and 11.07±0.61
sec in the PR nodes. No significant difference was observed between
the two groups (P=0.334). Similarly, there was no significant
difference in TTPin between patients who showed a CR or
PR of the metastatic lymph nodes (12.42±1.49 vs. 12.13±1.40;
P=0.356), as shown in Table
II.
Correlation between the CEUS parameters
and therapeutic response
The Spearman’s correlation coefficient between the
lymph nodes therapeutic response and changes in CEUS perfusion
parameters was calculated. There was a strong-positive correlation
between the PIin, PIratio and therapeutic
response (ρ=0.81, ρ=0.734), a moderate-positive correlation between
the PIpre and therapeutic response (ρ=0.368) and a
strong-negative correlation between the therapeutic response and
PIΔ (ρ=−0.777) (Table
III). Logistic regression analysis of the CEUS parameters
indicated that PIin and PIratio were the
significant predictors of the therapeutic response (P<0.01).
 | Table IIISpearman’s rank correlation
coefficients for the CEUS parameters and therapeutic response. |
Table III
Spearman’s rank correlation
coefficients for the CEUS parameters and therapeutic response.
| Therapeutic
response |
PIpre |
PIin |
TTPpre |
TTPin | PIΔ |
PIratio |
|---|
| Therapeutic
response | 1 | | | | | | |
|
PIpre | 0.368a | 1 | | | | | |
|
PIin | 0.810a | 0.681a | 1 | | | | |
|
TTPpre | −0.141 | −0.159 | −0.156 | 1 | | | |
|
TTPin | 0.121 | 0.009 | 0.129 | 0.164 | 1 | | |
| PI | −0.777a | 0.120 | −0.785a | 0.066 | −0.188 | 1 | |
|
PIratio | 0.734b | −0.263a | 0.868b | 0.070 | −0.204 | −0.956b | 1 |
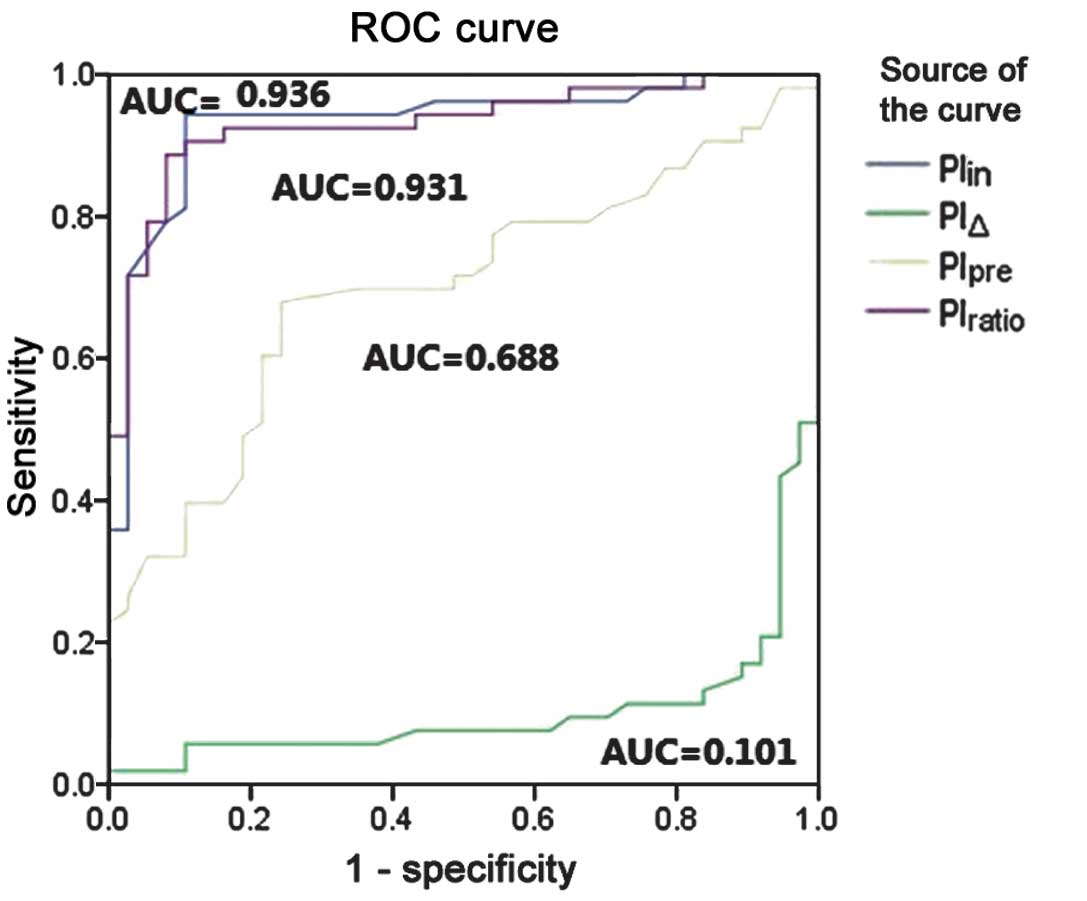
An ROC curve was constructed to assess the accuracy
of the parameters for the prediction of the therapeutic responses.
The ROC curve showed that the therapeutic response could be well
predicted by the parameters of PIin and
PIratio compared to PIpre and PIΔ.
The PIin area under the ROC curve (AUC) was 0.936 (95%
confidence interval, 0.877–0.988), and the AUC of the
PIratio was 0.931 (95% confidence interval, 0.877–0.985)
(Fig. 3). When the cut-off value
of PIin was set at 29.4%, sensitivity and specificity in
predicting the lymph node therapeutic response was 94.3 and 88.2%.
The best cut-off value of the PIratio was ≥0.69 by
coordinating the points of the ROC curve, and the predicted
sensitivity and specificity of the PIratio was 92.5 and
83.8%, respectively (Table
IV).
 | Table IVSensitivity and specificity for the
CEUS parameters, PIpre, PIin and
PIratio. |
Table IV
Sensitivity and specificity for the
CEUS parameters, PIpre, PIin and
PIratio.
| Parameters | Cut-off value | Sensitivity, % | Specificity, % |
|---|
|
PIpre | ≥39.65% | 72.0 | 52.0 |
|
PIin | ≥29.40% | 94.3 | 88.2 |
|
PIratio | ≥0.69 | 92.5 | 83.8 |
Discussion
To the best of our knowledge, the present study is
the first to assess the early predictive value of parametric CEUS
for the therapeutic response in the metastatic cervical lymph nodes
of NPC patients treated with radiation-based therapy. The aim was
to evaluate whether any of these CEUS parameters are suitable as a
predictor for the nodal treatment response to radiation-based
therapy in NPC patients with cervical lymph nodes metastases.
In the present study, a higher mean value of
PIpre was observed in the lymph nodes that achieved CR
compared to PR, and the difference was statistically significant.
Functional images, including DCE-MRI, have been employed for the
prediction of the early treatment response and prognosis for head
and neck cancers (18,20,21).
DCE-MRI provides a perfusion parameter, Ktrans, which
reflects a combination of the tumor blood flow and microvascular
permeability. In the study reported by Chawla et al
(18), patients with head and neck
squamous cell carcinoma that was responsive to chemoradiation
therapy had significantly higher pre-treatment Ktrans
values from nodal masses than patients with a PR. In another cohort
of 33 patients with head and neck squamous cell carcinoma who were
treated with chemoradiotherapy, the average pre-treatment
Ktrans value of the CR group was found to be
significantly higher (P=0.001) than that of the PR group (21). The result of the present study is
partially consistent with the aforementioned DCE-MRI studies.
However, the majority of the PIpre values fell in a wide
region and there was a large range of PIpre values from
the CR and PR groups that overlapped with each other. The predicted
sensitivity and specificity of PIpre was relatively low,
and therefore the PIpre value alone is of less interest
for predicting the therapeutic response of the lymph node
metastases from NPC.
We hypothesized that alternations in the metastatic
nodal perfusion parameters during the early course of treatment for
NPC may improve the predictive value for the therapeutic response
than pre-treatment measurements alone. Thus, the second CEUS
examinations were arranged at the 5th fraction of radiotherapy for
all the 67 patients. A decrement in PI was observed in all the
investigated nodes regarding the PIpre. There was a
significant difference in the PIin between the patients
who showed CR or PR, and the values of PIin were much
higher in the patients with CR nodes (34.24±3.78%) compared to
those with PR nodes (25.62±2.30%). For each individual lymph node,
the PIratio was calculated by dividing the
PIin by the PIpre, and a higher
PIratio was also observed in the lymph nodes that
achieved a CR (0.81±0.01 vs. 0.66±0.01; P=0.001). The
PIΔ was found to be smaller in the CR lymph node. These
findings suggest that an improved blood supply and potentially
improved oxygenation during the early course of treatment may be a
positive indicator for the therapeutic response at the metastatic
lymph node of NPC. Based on the ROC curve, a cut-off for the
PIin and PIratio were established, which
predicted the response with a specificity of 88.2 and 83.8%, and a
sensitivity of 94.3 and 92.5%, respectively. To the best of our
knowledge, the present study describes for the first time the CEUS
parameters during the early course of chemo-radiotherapy that
reliably predict the metastatic lymph node response. These
parameters may help to optimize the patient selection, thereby
individualizing treatment and preventing non-responders from
undesirable side-effects.
TTP represents the arrival time of the contrast
agent to reach its maximum. In the present study, TTP had a
tendency to increase 1 week after the initiation of the
radiation-based treatment. However, TTPpre and
TTPin were found to have no significant difference
regarding the CR or PR lymph node response status. In a study by
Knieling et al (22),
hepatocellular carcinoma patients were treated with sorafenib and
the TTP increased as early as 1 month after the initiation of the
treatment in the responder group compared to the non-responder
group. In the study by Schirin-Sokhan et al (23), non-primary resectable liver
metastases from colorectal cancer were treated with
bevacizumab-based chemotherapy and it was demonstrated that the
baseline TTP was significantly lower in the responder group
compared to the non-responders, suggesting that low baseline TTP
significantly correlates with tumor response according to RECIST.
Furthermore, correlating to the antiangiogenic effect of
bevacizumab, a strong increase in TTP was observed during
chemotherapy, which was restricted to the responder group. The data
concerning TTP and radiation-therapeutic response are sparse and
should be investigated further in a larger patient population with
more CEUS examinations during the course of the radiation-based
therapy.
The patients treated with radiotherapy combined with
chemotherapy and/or targeted therapy had a higher CR rate of lymph
node metastases compared to those who received radiotherapy alone
in the present study. A conventional radiotherapy course is usually
6–7 weeks, and may be followed by adjuvant chemotherapy and
targeted therapy. If the early changes in nodal perfusion could
help to predict the therapeutic response, it will be possible to
alter the intensity of the treatment regimen, thus individualizing
the remaining treatment.
The present study is, to the best of our knowledge,
the first to investigate CEUS as a predictor for the therapeutic
response in NPC cervical lymph nodes metastases. The data suggests
that the CEUS parameters during the early course of
chemo-radiotherapy, PIin and PIratio, are
associated with the therapeutic response of the lymph node
metastases from NPC, thus yielding conceivable predictors with the
potential to modify and individualize treatment.
Acknowledgements
The authors would like to thank all the colleagues
in the Department of Radiation Oncology and Ultrasound for their
good cooperation. The present study was supported by the National
Natural Science Foundation of China (grant no. 81071823 and
81201811) and the Zhejiang University Research Foundation.
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Razak AR, Siu LL, Liu FF, Ito E,
O‘Sullivan B and Chan K: Nasopharyngeal carcinoma: the next
challenges. Eur J Cancer. 46:1967–1978. 2010. View Article : Google Scholar
|
|
3
|
Xiao WW, Huang SM, Han F, Wu SX, Lu LX,
Lin CG, Deng XW, Lu TX, Cui NJ and Zhao C: Local control, survival,
and late toxicities of locally advanced nasopharyngeal carcinoma
treated by simultaneous modulated accelerated radiotherapy combined
with cisplatin concurrent chemotherapy: long-term results of a
phase 2 study. Cancer. 117:1874–1883. 2011. View Article : Google Scholar
|
|
4
|
Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW,
Millward MJ, Hong RL, Whang-Peng J, Ma BB, To KF, Mueser M, Amellal
N, Lin X and Chang AY: Multicenter, phase II study of cetuximab in
combination with carboplatin in patients with recurrent or
metastatic nasopharyngeal carcinoma. J Clin Oncol. 23:3568–3576.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee AW, Tung SY, Chan AT, Chappell R, Fu
YT, Lu TX, Tan T, Chua DT, O‘sullivan B, Xu SL, Pang ES, Sze WM,
Leung TW, Kwan WH, Chan PT, Liu XF, Tan EH, Sham JS, Siu L and Lau
WH: Preliminary results of a randomized study (NPC-9902 Trial) on
therapeutic gain by concurrent chemotherapy and/or accelerated
fractionation for locally advanced nasopharyngeal carcinoma. Int J
Radiat Oncol Biol Phys. 66:142–151. 2006. View Article : Google Scholar
|
|
6
|
Cao Y, Popovtzer A, Li D, Chepeha DB,
Moyer JS, Prince ME, Worden F, Teknos T, Bradford C, Mukherji SK
and Eisbruch A: Early prediction of outcome in advanced
head-and-neck cancer based on tumor blood volume alterations during
therapy: a prospective study. Int J Radiat Oncol Biol Phys.
72:1287–1290. 2008.PubMed/NCBI
|
|
7
|
Korpanty G, Carbon JG, Grayburn PA,
Fleming JB and Brekken RA: Monitoring response to anticancer
therapy by targeting microbubbles to tumor vasculature. Clin Cancer
Res. 13:323–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen JJ, Fu SY, Chiang CS, Hong JH and Yeh
CK: A preclinical study to explore vasculature differences between
primary and recurrent tumors using ultrasound Doppler imaging.
Ultrasound Med Biol. 39:860–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yankeelov TE, Niermann KJ, Huamani J, Kim
DW, Quarles CC, Fleischer AC, Hallahan DE, Price RR and Gore JC:
Correlation between estimates of tumor perfusion from microbubble
contrast-enhanced sonography and dynamic contrast-enhanced magnetic
resonance imaging. J Ultrasound Med. 25:487–497. 2006.
|
|
10
|
Beaton C, Cochlin D and Kumar N: Contrast
enhanced ultrasound should be the initial radiological
investigation to characterise focal liver lesions. Eur J Surg
Oncol. 36:43–46. 2010. View Article : Google Scholar
|
|
11
|
Ridolfi F, Abbattista T, Busilacchi P and
Brunelli E: Contrast-enhanced ultrasound evaluation of hepatic
microvascular changes in liver diseases. World J Gastroenterol.
18:5225–5230. 2012.PubMed/NCBI
|
|
12
|
Rissanen TT, Korpisalo P, Karvinen H,
Liimatainen T, Laidinen S, Gröhn OH and Ylä-Herttuala S:
High-resolution ultrasound perfusion imaging of therapeutic
angiogenesis. JACC Cardiovasc Imaging. 1:83–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Badea AF, Tamas-Szora A, Clichici S,
Socaciu M, Tăbăran AF, Băciut G, Cătoi C, Mureşan A, Buruian M and
Badea R: Contrast enhanced ultrasonography (CEUS) in the
characterization of tumor microcirculation. Validation of the
procedure in the animal experimental model. Med Ultrason. 15:85–94.
2013. View Article : Google Scholar
|
|
14
|
Shah JP, Ang K, Baatenburg De Jong RJ, et
al: Head and neck. AJCC Cancer Staging Manual. Edge SB, Byrd DR,
Compton CC, Fritz AG, Greene FL and Trotti A: 7th edition.
Springer; New York: pp. 21–100. 2010
|
|
15
|
Ramakrishnan MS, Eswaraiah A, Crombet T,
Piedra P, Saurez G, Iyer H and Arvind AS: Nimotuzumab, a promising
therapeutic monoclonal for treatment of tumors of epithelial
origin. MAbs. 1:41–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ang J, Hu L, Huang PT, Wu JX, Huang LN,
Cao CH, Zheng YX and Chen L: Contrast-enhanced ultrasonography
assessment of gastric cancer response to neoadjuvant chemotherapy.
World J Gastroenterol. 18:7026–7032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moschouris H, Malagari K, Marinis A,
Kornezos I, Stamatiou K, Nikas G, Papadaki MG and Gkoutzios P:
Hepatocellular carcinoma treated with transarterial
chemoembolization: Evaluation with parametric contrast-enhanced
ultrasonography. World J Radiol. 4:379–386. 2012. View Article : Google Scholar
|
|
18
|
Chawla S, Kim S, Dougherty L, Wang S,
Loevner LA, Quon H and Poptani H: Pretreatment diffusion-weighted
and dynamic contrast-enhanced MRI for prediction of local treatment
response in squamous cell carcinomas of the head and neck. AJR Am J
Roentgenol. 200:35–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and
Verweij J: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
20
|
Shukla-Dave A, Lee NY, Jansen JF, Thaler
HT, Stambuk HE, Fury MG, Patel SG, Moreira AL, Sherman E, Karimi S,
Wang Y, Kraus D, Shah JP, Pfister DG and Koutcher JA: Dynamic
contrast-enhanced magnetic resonance imaging as a predictor of
outcome in head-and-neck squamous cell carcinoma patients with
nodal metastases. Int J Radiat Oncol Biol Phys. 82:1837–1844. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim S, Loevner LA, Quon H, Kilger A,
Sherman E, Weinstein G, Chalian A and Poptani H: Prediction of
response to chemoradiation therapy in squamous cell carcinomas of
the head and neck using dynamic contrast-enhanced MR imaging. AJNR
Am J Neuroradiol. 31:262–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knieling F, Waldner MJ, Goertz RS, Zopf S,
Wildner D, Neurath MF, Bernatik T and Strobel D: Early response to
anti-tumoral treatment in hepatocellular carcinoma - can
quantitative contrast-enhanced ultrasound predict outcome?
Ultraschall Med. 34:38–46. 2013.PubMed/NCBI
|
|
23
|
Schirin-Sokhan R, Winograd R, Roderburg C,
Bubenzer J, do Ó NC, Guggenberger D, Hecker H, Trautwein C and
Tischendorf JJ: Response evaluation of chemotherapy in metastatic
colorectal cancer by contrast enhanced ultrasound. World J
Gastroenterol. 18:541–545. 2012. View Article : Google Scholar : PubMed/NCBI
|